Abstract
Hypophosphatemia can lead to muscle weakness and respiratory and heart failure, but the mechanism is unknown. To address this question, we noninvasively assessed rates of muscle ATP synthesis in hypophosphatemic mice by using in vivo saturation transfer [31P]-magnetic resonance spectroscopy. By using this approach, we found that basal and insulin-stimulated rates of muscle ATP synthetic flux (VATP) and plasma inorganic phosphate (Pi) were reduced by 50% in mice with diet-induced hypophosphatemia as well as in sodium-dependent Pi transporter solute carrier family 34, member 1 (NaPi2a)-knockout (NaPi2a−/−) mice compared with their wild-type littermate controls. Rates of VATP normalized in both hypophosphatemic groups after restoring plasma Pi concentrations. Furthermore, VATP was directly related to cellular and mitochondrial Pi uptake in L6 and RC13 rodent myocytes and isolated muscle mitochondria. Similar findings were observed in a patient with chronic hypophosphatemia as a result of a mutation in SLC34A3 who had a 50% reduction in both serum Pi content and muscle VATP. After oral Pi repletion and normalization of serum Pi levels, muscle VATP completely normalized in the patient. Taken together, these data support the hypothesis that decreased muscle ATP synthesis, in part, may be caused by low blood Pi concentrations, which may explain some aspects of muscle weakness observed in patients with hypophosphatemia.—Pesta, D. H., Tsirigotis, D. N., Befroy, D. E., Caballero, D., Jurczak, M. J., Rahimi, Y., Cline, G. W., Dufour, S., Birkenfeld, A. L., Rothman, D. L., Carpenter, T. O., Insogna, K., Petersen, K. F., Bergwitz, C., Shulman, G. I. Hypophosphatemia promotes lower rates of muscle ATP synthesis.
Keywords: [31P]MRS, saturation transfer, inorganic phosphate
Inorganic phosphate (Pi) is essential for membrane structure, energy storage, and signal transduction in all cells (1), and homeostatic mechanisms exist to maintain extracellular Pi concentration in the physiologic range, which varies with age and from species to species (2). Acute hypophosphatemia can cause rhabdomyolysis and respiratory and heart failure, which often complicates care of patients in the intensive care setting (3, 4). Chronic hypophosphatemia leads to rickets or osteomalacia, which is often complicated by impaired muscle function and early fatigue, a feature that significantly affects quality of life (5). A possible connection between blood Pi, muscle energy metabolism, and vitamin D status has been suggested by studies that have demonstrated a delayed phosphocreatine recovery time in patients who are hypophosphatemic secondary to vitamin D deficiency, although it is unclear from these studies whether these changes could also be attributed to effects of vitamin D deficiency (6).
The contribution of Pi to regulation of respiratory control and mitochondrial substrate oxidation was recognized some decades ago (7–9), but ADP has been considered the most important regulator of mitochondrial respiration, with Pi acting only as a coregulator (10), which binds to specific sites on ATP synthase (11). Tanaka et al (12) showed that Pi is rate limiting for mitochondrial function in the perfused liver and Beard et al (13) suggested that Pi controls mitochondrial respiration, in particular, during low-to-moderate oxygen consumption.
Type III transporters SLC20A1/Pit1 and SLC20A2/Pit2 serve as ubiquitous Pi transporters in all tissues (14). Genetic ablation of Pit1 in all tissues is lethal at embryonic d 12.5 and causes abnormal liver development and defects in erythroid and B-lymphocyte differentiation (15); however, Pit1-knockout mice have normal development of skeletal muscle, likely as a result of compensation by Pit2, as shown by [32P] uptake experiments using mutant Pit1-null mouse embryonic fibroblasts (15). Mice and humans who lack Pit2 are viable and their skeletal muscle seems to be normal, but they develop brain calcifications later in life (16, 17). As there is no obvious muscle phenotype in the transporter knockouts, it remains unclear whether extracellular Pi modifies muscle function via homeostatic mechanisms, that is, synthesis of 1,25(OH)2-D (18), or via changes in intracellular Pi.
Muscular energy requirements are met through hydrolysis of ATP, which is mainly synthesized by mitochondrial oxidative phosphorylation. Rates of muscle ATP synthetic flux (VATP) can be noninvasively assessed by using saturation transfer [31P]-magnetic resonance spectroscopy (ST-[31P]MRS) (19–23). By using this method, we have previously demonstrated that muscle VATP is reduced in healthy elderly subjects and in insulin-resistant offspring of parents with type 2 diabetes (20, 21). In addition, it has been shown that muscle VATP doubles during insulin stimulation in insulin-sensitive individuals and that this response is blunted in the insulin-resistant offspring (22). However, how alterations in Pi affect muscle function and muscle ATP synthetic flux is poorly understood. To further explore the association between Pi, muscle function, and ATP synthetic flux, we studied the relation between plasma Pi and muscle VATP measured by ST-[31P]MRS in diet- and genetically induced hypophosphatemic mice and in a patient with chronic hypophosphatemia as a result of hereditary hypophosphatemic rickets who had a 50% reduction in serum Pi content.
MATERIALS AND METHODS
Reagents
Chemicals were obtained from commercial sources and used as received, unless noted otherwise.
Animals
All rodent protocols were approved by the Yale University School of Medicine Animal Care and Use Committee. Mice were housed under controlled temperature (22 ± 2°C) and lighting (12 h of light, 0700–1900 h; 12 h of dark, 1900–0700 h), with free access to water and food (0.7% Pi, 1.0% Ca, TD 2018S; Harlan Teklad, Madison, WI, USA).
NaPi2a−/− mouse model
Mice homozygous for a mutation in the gene of sodium-dependent Pi transporter solute carrier family 34, member 1 (NaPi2a) were obtained from The Jackson Laboratory (Bar Harbor, ME, USA) and bred with C57BL/6J mice. Resulting heterozygotes (HETs) were bred HET by HET, and NaPi2a−/− and wild-type (WT) littermate controls were used for experiments.
For experiments shown in Fig. 1 and Table 1, NaPi2a−/− mice were evaluated at age 2–3 mo, placed on a low-Pi diet (LPD; 0.02% Pi, 0.6% Ca, egg white–based, TD 140659; Harlan Teklad) or a high-phosphate diet (HPD; 1.2% Pi, 0.6% Ca, egg white–based, TD 85349; Harlan Teklad), and evaluation was repeated at 2 and 10 wk. Plasma Pi and lactate were measured at baseline, 2 wk, and 10 wk on LPD and HPD. Comprehensive animal metabolic monitoring system (CLAMS; see below) was used to determine activity and respiratory exchange ratio over a period of 72 h of mice that were fed ad libitum with LPD and HPD.
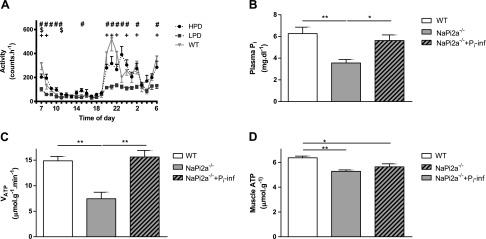
Figure 1.
NaPi2a−/− mice were studied at age 5–6 mo after receiving LPD (0.02% Pi, 0.6% Ca) or HPD (1.2% Pi, 0.6% Ca) for 10 wk. A) CLAMS was used to determine activity over a period of 72 h for LPD and HPD, which was compared with WT littermate controls on regular chow (RC). Activity was reduced in mice that received LPD and was mostly normalized after administering HPD. B, C) Mice with genetically induced hypophosphatemia (NaPi2a−/−) were hypophosphatemic compared with WT littermate controls (B) and showed reduced VATP determined by ST-[31P]MRS (C). D) Actual muscle ATP concentration measured by LC-MS was decreased in NaPi2a−/− mice compared with WT littermate controls. Plasma Pi levels and VATP normalized to euphosphatemic levels after infusion of a bolus of 25 μmol Pi (B, C), whereas muscle ATP concentration was not changed after infusion (D). All data are means ± sem; n = 5–8 in each group. +P < 0.05, NaPi2a−/− on LPD vs. WT on RC; $P < 0.05, NaPi2a−/− on HPD vs. WT on RC; #P < 0.01, NaPi-2a−/− on LPD vs. HPD; *P < 0.05; **P < 0.01 by double-sided Student’s t test.
TABLE 1.
Biometric and metabolic data assessed by CLAMS of NaPi2a−/− mice on LPD and HPD for 10 wk and of WT mice on RC and LPD for 2 wk
| Parameter |
NaPi2a−/− diet for 10 wk |
WT diet for 2 wk |
|||||
|---|---|---|---|---|---|---|---|
| WT on RC | LPD | HPD | P (LPD vs. HPD) | RC | LPD | P (RC vs. LPD) | |
| Body weight (g) | 27.4 ± 0.5 | 25.7 ± 0.9 | 28.8 ± 1.3 | 28.1 ± 0.9 | 26.1 ± 0.3 | ||
| Muscle mass (g) | 20.5 ± 0.5 | 17.4 ± 0.5 | 19.1 ± 0.6 | 0.05 | 21.4 ± 0.3 | 20.3 ± 0.2 | 0.03 |
| Fat mass (g) | 1.5 ± 0.2 | 2.9 ± 0.2 | 4.3 ± 0.7 | 3.0 ± 0.3 | 2.1 ± 0.3 | ||
| Muscle mass (%) | 74.9 ± 0.7 | 67.8 ± 0.5 | 67.0 ± 1.2 | 76.4 ± 2.0 | 77.6 ± 0.9 | ||
| Fat mass (%) | 5.5 ± 0.8 | 11.0 ± 0.7 | 14.0 ± 1.7 | 10.6 ± 0.8 | 8.1 ± 1.0 | ||
| VO2 (ml/kg/h) | 2972.4 ± 38.0 | 3439.4 ± 93.6 | 3350.0 ± 136.8 | 3300.9 ± 27.7 | 3422.2 ± 25.0 | <0.01 | |
| VCO2 (ml/kg/h) | 2719.7 ± 38.0 | 3145.5 ± 79.3 | 3112.7 ± 149.0 | 3166.9 ± 24.8 | 3408.5 ± 27.7 | <0.01 | |
| RER | 0.91 ± 0.01 | 0.91 ± 0.01 | 0.92 ± 0.01 | 0.96 ± 0.01 | 0.99 ± 0.01 | <0.01 | |
| Energy expenditure (Kcal/kg/h) | 14.6 ± 0.2 | 17.0 ± 0.4 | 16.2 ± 0.5 | 16.5 ± 0.1 | 17.2 ± 0.1 | <0.01 | |
| Energy intake (Kcal/kg/h) | 17.5 ± 0.5 | 23.2 ± 2.4 | 23.2 ± 1.2 | 26.7 ± 1.5 | 36.6 ± 2.5 | <0.01 | |
| Activity index (counts/min) | 158.5 ± 19.4 | 78.1 ± 8.9 | 109.7 ± 7.8 | 0.01 | 52.5 ± 2.4 | 44.1 ± 1.5 | <0.05 |
All data are means ± sem; n = 8–10 in each group. P values are indicated when significant by double-sided Student’s t test. RC, regular chow (0.7% Pi, 1.0% Ca). See Fig. 1 for composition of LPD and HPD.
LPD-induced hypophosphatemic mouse model
Male C57BL/6J mice (The Jackson Laboratory) between 2 and 3 mo of age were individually housed and maintained on either standard mouse chow diet with 0.6% Pi (RC; TD2018; Harlan Teklad) or LPD with 0.02% Pi and 0.6% calcium (TD96817; Harlan Teklad). Mice were matched for age, weight, and food withdrawal time within each experiment, and, if possible, between experiments (Fig. 2 and Table 1).
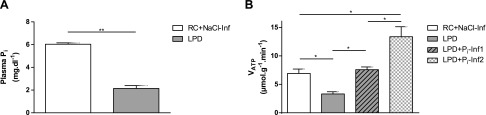
Figure 2.
A) WT mice 12–18 wk old maintained on LPD for 2 wk become hypophosphatemic. Mice received a bolus of 30 μmol Pi (Pi-Inf1), 60 μmol (Pi-Inf2), and saline for control group (NaCl-Inf) while in the magnet. B) VATP determined by ST-[31P]MRS was reduced in LPD mice, was increased in these animals after Pi-Inf1, and further increased after Pi-Inf2 compared with NaCl-infused mice. See Fig. 1 and Table 1 for composition of LPD and regular chow (RC). All data are means ± sem; n = 5–8 in each diet group. *P < 0.05, **P < 0.01 by double-sided Student’s t test.
Mouse ST-[31P]MRS
Muscle mitochondrial ATP synthetic flux in mice was assessed by ST-[31P]MRS by using a 9.4 T superconducting magnet system (horizontal/10-cm diameter bore magnet, 400.55 MHz for proton; Magnex Scientific, Palo Alto, CA, USA) interfaced to a Varian console as described earlier (19, 24, 25). Proof-of-principle studies have been performed in rat hind limb (24, 25) and humans (26) to validate the method. In brief, the animal was anesthetized by using 1.5% isoflurane to prevent movement during the experiment, the left hind limb was shaved, and the mouse was positioned prone in a stereotactic frame to ensure consistent, reproducible positioning for each study using a homemade holder that exposed the muscle to concentric surface coils [an outer 1H coil (25 mm) and an inner [31P] coil (15 mm)]. This stable experimental setup eliminates movement of the region of interest and guarantees acquisition of high signal/noise [31P] spectra. Body temperature was maintained at 37°C by using a heated cradle incorporated into the MR probe, and optimal signals were acquired by using localizers to ensure accurate positioning of the animal in the magnet. To monitor cardiopulmonary status of the animal throughout the experiment and control depth of anesthesia to maintain stable physiologic function, a multiparameter pulse oximeter was used (MouseOx Plus; Starr Life Sciences, Holliston, MA, USA). For shimming, a FastMap sequence over a 2.8- × 2.8- × 2.8-mm volume of the leg was applied. Coil geometries were optimized to match the size of the mouse and to ensure close proximity of the muscle to the coil for a high signal-to-noise ratio while avoiding occlusion of blood flow to the mouse extremity. By using Nuts software (Acorn NMR, Livermore, CA, USA) for spectra processing, we are able to calculate VATP. With the help of fully relaxed [31P] spectra, we estimated muscle Pi content. Parameters were calibrated against ATP and Pi measurements that were obtained from muscle tissue after dissection of animals (Fig. 1D). ST-[31P]MRS measurements were started 1 h after Pi infusion. Each animal was measured before and after Pi infusion. See Supplemental Data for more information.
Pi infusion of NaPi2a−/− and LPD mice
NaPi2a−/− mice at age 2–3 mo were studied after 2 wk on LPD (0.02% Pi, 0.6% Ca, TD96817; Fig. 3). Before ST-[31P]MRS experiment, mice were infused with a bolus of 25 μmol NaH2PO4 (pH 7.4) on the basis of a 72 ml/kg blood volume for a mouse to restore normophosphatemia (∼5.6 mg/dl).
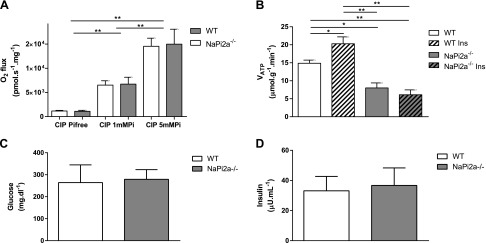
Figure 3.
A) In vitro measurement of oxygen flux in isolated skeletal muscle mitochondria of WT and NaPi2a−/− mice increased in a dose-dependent manner upon addition of 1 and 5 mM Pi to medium; however, mitochondrial function per se is not impaired in NaPi2a−/− mice compared with WT. B) Insulin increased VATP in WT but not in NaPi2a−/− mice when mice were infused with 5 mU/kg/min insulin, followed by 3 mU/kg/min of insulin and 12.5 mg/kg/min of 20% glucose at a rate of 2.1 μl/min on the basis of body weight of the animal to maintain euglycemia and hyperinsulinemia during ST-[31P]MRS (hyperinsulinemic-euglycemic clamp). C, D) Plasma glucose (C) and insulin (D) values measured after the experiment were not different between the 2 groups. CIP, oxidative phosphorylation capacity of complex I–related substrates. All data are means ± sem; n = 5–8 in each group. *P < 0.05; **P < 0.01 by double-sided Student’s t test.
WT mice were divided into 3 groups that each received a phosphate infusion on the basis of the protocol used for NaPi2a−/− mice while inside the MRS instrument. The LPD group received a bolus of 30 μmol NaH2PO4 and the HPD group received 60 μmol NaH2PO4 at pH 7.4. Control group received 60 μmol of iso-osmotic NaCl infusion. Blood samples were obtained at end of study by cardiac puncture for plasma [Pi] measurements.
Hyperinsulinemic-euglycemic clamp conditions
VATP was assessed during hyperinsulinemic-euglycemic conditions as shown in Fig. 3B. At the beginning of this study, we infused a bolus of 5 mU/kg/min of insulin, followed by 3 mU/kg/min of insulin (Novo Nordisk, Bagsværd, Denmark) at a rate of 2.1 μl/min and 12.5 mg/kg/min of 20% dextrose to maintain euglycemia during hyperinsulinemia. Blood samples were taken retro-orbitally after the clamp. At study completion, mice were anesthetized and tissues were harvested within 3 min with liquid N2–cooled aluminum tongs and stored at –80°C for subsequent analysis.
Case report of the patient with hypophosphatemic rickets with hypercalciuria
The study was approved by the Yale University Human Investigation Committee and written informed consent was obtained from the participant after explanation of the purpose, nature and potential complications of the study. We measured VATP and serum Pi before, after 5 mo, and again after a total of 8 mo oral Pi treatment to normalize plasma phosphorous concentrations in a 28-yr-old male individual with hereditary hypophosphatemic rickets with hypercalciuria (HHRH), a genetic form of hypophosphatemia as a result of loss-of-function of the proximal tubular sodium-Pi cotransporter NaPi2c, as previously reported by us (27). The patient initially presented at age 14 yr with gradual onset of bilateral knee and ankle pain. At age 13 yr, he had passed his first calcium oxalate kidney stone. There were bilateral genua valga on initial examination and he walked with a limp. He was found to have hypophosphatemia, elevated urinary calcium to creatinine ratio, and an increased 1,25(OH)2D level, whereas the concentration of 25-hydroxyvitamin D and midmolecule parathyroid hormone were within normal limits [see Bergwitz et al. (27) for more details]. Because he was poorly compliant with Pi supplements, his serum phosphorus level remained in the 1.5–2.0 mg/dl range, his adult height was 159 cm well below midparental height, and he has had multiple episodes of symptomatic nephrolithiasis. He had not been taking Pi supplements for at least 12 mo before initial evaluation. The patient presented before diagnosis and treatment with moderately severe muscle weakness. He tired rapidly after attempting to play sports with his peers and responded well with resolution of these complaints after 2–4 wk of supplemental phosphate therapy, after which time he could maintain normal activity. Spectroscopy studies were performed after being treated for several years, but in the midst of a limited period of nonadherence with phosphate medication, and, unfortunately, no formal muscle strength quantification was available at the time of study. Subjectively, he noticed improvement with therapy after periods of missing medication. Hypophosphatemia was restored by oral Pi supplementation with 500 mg KPhos MF 3 times/d, with a total daily dose of 1500 mg for a period of 8 mo. Serum Pi and VATP were assessed in 5 healthy age-matched males who served as control group.
CLAMS
CLAMS (Columbus Instruments, Columbus, OH, USA) was used to evaluate oxygen consumption (VO2), carbon dioxide production (VCO2), and respiratory exchange ratio (RER) as well as energy expenditure, food consumption, and activity for body weight and fat-matched animals as previously described (28). Fat and lean body masses were assessed by 1H-MRS (Bruker BioSpin, Billerica, MA, USA). Energy expenditure and RER were calculated from gas exchange data (energy expenditure = [3.815 + 1.232 × RER] × VO2). RER is the ratio of VCO2 to VO2, which changes depending on the substrate that the animal is using. When carbohydrates are the only substrate being oxidized, RER will approximate 1.0, and when only fatty acids are metabolized, 0.7.
Human ST-[31P]MRS
ST-[31P]MRS was performed as previously described (26) in a 4.0 Tesla whole-body MR scanner interfaced to a Bruker Avance console (Bruker Biospin). In brief, patients were positioned supine in the magnet with an MR probe that consisted of a 9-cm diameter [31P] surface coil and a concentric 13-cm diameter 1H surface coil placed over the right calf muscle. B0 field of the magnet was optimized by shimming using the FastMap procedure, and the voxel of interest was positioned inside the soleus muscle after imaging of the calf. Analysis of [31P] data was performed by using MatLab software (MathWorks, Natick, MA, USA); Free induction decays were zero-filled and apodized using a 5 Hz Lorentzian function before Fourier transformation. Spectra were manually phased and baseline corrected by fitting to a fifth-order polynomial. Peak areas of [31P] metabolites were determined by integration. Concentrations of [31P] metabolites were determined from fully relaxed spectra assuming a constant 5.5 mmol/kg muscle ATP concentration.
Calculation of muscle mitochondrial ATP synthetic flux
T1 of Pi under conditions of γ-ATP saturation (T1′ - spin lattice relaxation time) was measured by using an adiabatic 7-point inversion recovery pulse sequence in the presence of continuous γ-ATP saturation to calculate k′ (Eq. 1). Unidirectional ATP synthetic flux was calculated as k′ × [Pi] (Eq. 2). Pi concentration was assessed from a fully relaxed baseline MRS spectrum obtained at a TR of 35 s (comparing peak integrals from Pi and γ-ATP). Saturation-transfer parameters were: 2 ms adiabatic half passage excitation pulse (centered between Pi and γ-ATP), 15 s soft saturation pulse, sweep width = 10 kHz, 4096 points, effective repetition time = 15 s.
where M′ is Pi signal during γ-ATP saturation; and M0 is Pi signal without γ-ATP saturation.
In vitro ATP synthesis from isolated mitochondria
For isolation of mitochondria from the hind limb of NaPi2a−/− and WT mice (Fig. 3A), excised muscle was placed in 1 ml mitochondrial isolation buffer MiB06 (100 mM sucrose, 100 mM KCl, 50 mM Tris-HCl, 1 mM KH2PO4, 0.2 mM EFTA, fatty acid–free albumin from bovine serum) and cut into pieces. After washing 3–4 times with 1 ml MiB06, 1 ml of protease solution (2 mg/10 ml MiB06) was added and incubated for 2 min. Sample was then homogenized, transferred to a centrifuge tube, and centrifuged at 800 g for 10 min at 4°C. Supernatant was transferred to a new centrifuge tube after filtering through a cheesecloth and centrifuged at 10,000 g for 10 min at 4°C. Supernatant was discarded and mitochondrial pellet was resuspended in MiB06. After determining mitochondrial protein concentration by Bradford assay, intact mitochondria corresponding to 10 mg of mitochondrial protein were added to the chamber of an a oxygraph 2K (Oroboros Instruments, Innsbruck, Austria) that contained Pi-free mitochondrial respiration buffer MiR05 (0.5 mM EGTA, 3 mM MgCl20.6H2O, 60 mM potassium-lactobionate, 20 mM taurine, 20 mM HEPES, 110 mM sucrose, and 1 g/L bovine serum albumin at pH 7.1) and oxygen flux (pmol/s/ml) was measured at 37°C. Standardized instrumental calibrations were performed to correct for back-diffusion of oxygen into the chamber from various components, leak from exterior, oxygen consumption by chemical medium, and sensor oxygen consumption. Glutamate (10 mM), malate (2 mM) and ADP (1 mM) was added to the Pi-free medium to favor complex I respiration. Oxygen flux by complex I in dependence of different concentrations of Pi was determined by using DatLab (Oroboros).
Cell culture
L6 cells (passage number 5–20) were grown at 37°C under 5% CO2 in 150 cm2 flasks in 15-ml α-minimal essential medium with 1% penicillin/streptomycin and 2% fetal bovine serum (FBS) after growth to confluency (myocytes). RC13 cells (passage number 5–10) were grown at 37°C under 5% CO2 in 150 cm2 flasks in 15 ml DMEM that was supplemented with 10% FBS and 1% penicillin/streptomycin. For experiments, cells were grown to confluency in 24-well plates. Medium was purchased from Thermo Fisher Scientific Life Sciences (Waltham, MA, USA). Medium was changed every 3–4 d and cells were passaged (1:10) every 5–7 d after removal from the plate with 1 ml trypsin/EDTA. For experiments, cells were grown to confluency in 12- or 24-well plates in medium that contained 2% FBS and were differentiated into myotubes for 10–20 d.
Western blots
Cell lysates were analyzed by PAGE and immunoblotted for phosphorylated Akt (p-Akt) and total Akt as previously described (28) by using an antibody that detects p-Ser473 of Akt (Cell Signaling Technology, Danvers, MA, USA), which is the amino acid phosphorylated by PI3K and activates Akt.
Phosphate and glucose uptake assays
Cells were starved in serum-free α-minimal essential medium for 14–16 h before the experiment. Cells were then treated with indicated concentrations of inhibitors for 20 min: wortmannin (500 nM) and LY294002 (50 µM; Calbiochem, San Diego, CA, USA), KP372-1 (100 nM; Echelon Biosciences, Salt Lake City, UT, USA), and Akt-I-1/2 (100 nM; Sigma-Aldrich, St. Louis, MO, USA). ETOH and DMSO were used as vehicle controls. Inhibitors were delivered to each well in 1-µl volumes. After treatment with an inhibitor or vehicle control, cells were treated with 100 nM insulin (Novo Nordisk) or 5 µl H2O for 30 min. Wells were then rinsed with 500 µl buffer A 3 times. Each well received 500 µl of transport buffer B and was incubated for exactly 10 min. Reactions were quenched by washing each well with 500 µl of ice-cold buffer A for Pi transport and buffer A + 5 mM d-glucose for glucose transport. Cells were lysed by treatment with 400 µl of 1 M NaOH and neutralized with 400 µl of 1 M HCl. In both assays, radioactivity was quantified by liquid scintillation counting, normalized to protein content determined by BCA protein assay (Thermo Fisher Scientific Life Sciences), and reported as moles of Pi or glucose transported per minute per mg protein.
Data analysis
All results are presented as means ± sem. Multiple comparisons were analyzed by ANOVA—followed, when appropriate, by Turkey post hoc test—by using Prism 6 for Windows software (GraphPad Software, La Jolla, CA, USA). Single comparisons were performed by 2-tailed unpaired Student’s t test. For statistical analysis of human patient data, means of replicate measurements were compared with reference range. A value of P ≤ 0.05 was considered statistically significant.
RESULTS
Reduced spontaneous exercise capacity in NaPi2a−/− mice
Excessive renal loss of Pi in NaPi2a−/− mice was reflected by a 50% reduction in plasma Pi (3.6 ± 0.1 compared with 6.3 ± 0.2 mg/dl in WT littermates; P < 0.001) and decreased further to 2.1 ± 0.3 mg/dl (P < 0.001 vs. baseline) after 2–10 wk on LPD (see Materials and Methods). Plasma Pi levels normalized after 3–10 wk on HPD (see Materials and Methods; 5.8 ± 1.5 mg/dl; P < 0.001 vs. baseline). Spontaneous physical activity, as assessed over 72 h using CLAMS, was reduced by ∼30% in NaPi2a−/− after 10 wk on LPD compared with HPD (LPD: 78 ± 9 counts/h vs. HPD: 110 ± 8 counts/h; P = 0.014; Table 1 and Fig. 1A). Similarly, after 2 wk on LPD, WT mice had reduced spontaneous activity (LPD WT: 44.1 ± 1.5 counts/h vs. WT: 52.5 ± 2.4 counts/h; P = 0.006) and increased RER (WT LPD: 0.99 ± 0.01 vs. WT: 0.96 ± 0.01; P = 0.001; Table 1). On LPD, WT mice lost 5% muscle mass (P = 0.03 vs. WT) and NaPi2a−/− mice lost 15% (P = 0.05 vs. NaPi2a−/− on HPD; Table 1). Furthermore, both caloric intake and energy expenditure were increased in WT mice on LPD. Food intake and energy expenditure were similar between groups (Table 1). All mice appeared well as determined by normal grooming behavior and body weight.
NaPi2a−/− mice are hypophosphatemic and have reduced muscle VATP in vivo
On the basis of our prior ST-[31P]MRS studies in humans that showed that insulin stimulation of muscle mitochondrial VATP correlated with intracellular Pi concentrations (22), we next tested the hypothesis that hypophosphatemia decreases muscle Pi uptake and VATP in NaPi2a−/− mice. Higher magnetic field strengths now allow for in vivo ST-[31P]MRS in the small volumes of hind limb muscle of mice (∼0.8 cm3) (19, 29), and by further optimizing acquisition of [31P]MRS of the muscle, insulin-clamp studies could be performed over 4–6 h.
NaPi2a−/− mice were hypophosphatemic with a 43% reduction in plasma Pi (3.6 ± 0.1 vs. 6.3 ± 0.2 mg/dl in WT; P < 0.01; Fig. 1B). Muscle mitochondrial VATP was 50% lower in NaPi2a−/− mice than in WT (7.4 ± 1.3 vs. 14.9 ± 0.9 µmol/g/min; P = 0.001; Fig. 1C). Correspondingly, muscle ATP concentration measured by liquid chromatography–tandem mass spectrometry was reduced in NaPi2a−/− mice compared with their WT littermate controls (6.4 ± 0.1 vs. 5.3 ± 0.1 µmol/g; P = 0.007; Fig. 1D). There was a positive correlation between VATP and muscle Pi concentrations in WT mice (R2 = 0.72; P = 0.03) and a similar trend for NaPi2a−/− mice (R2 = 0.65; P = 0.10; Fig. 4A). On the basis of observed rate constant k′ reductions in NaPi2a−/− mice, the diminished VATP seemed to be a result of reduced ATP synthase activity rather than its level of expression in the mitochondrial membrane (Supplemental Fig. S3F).
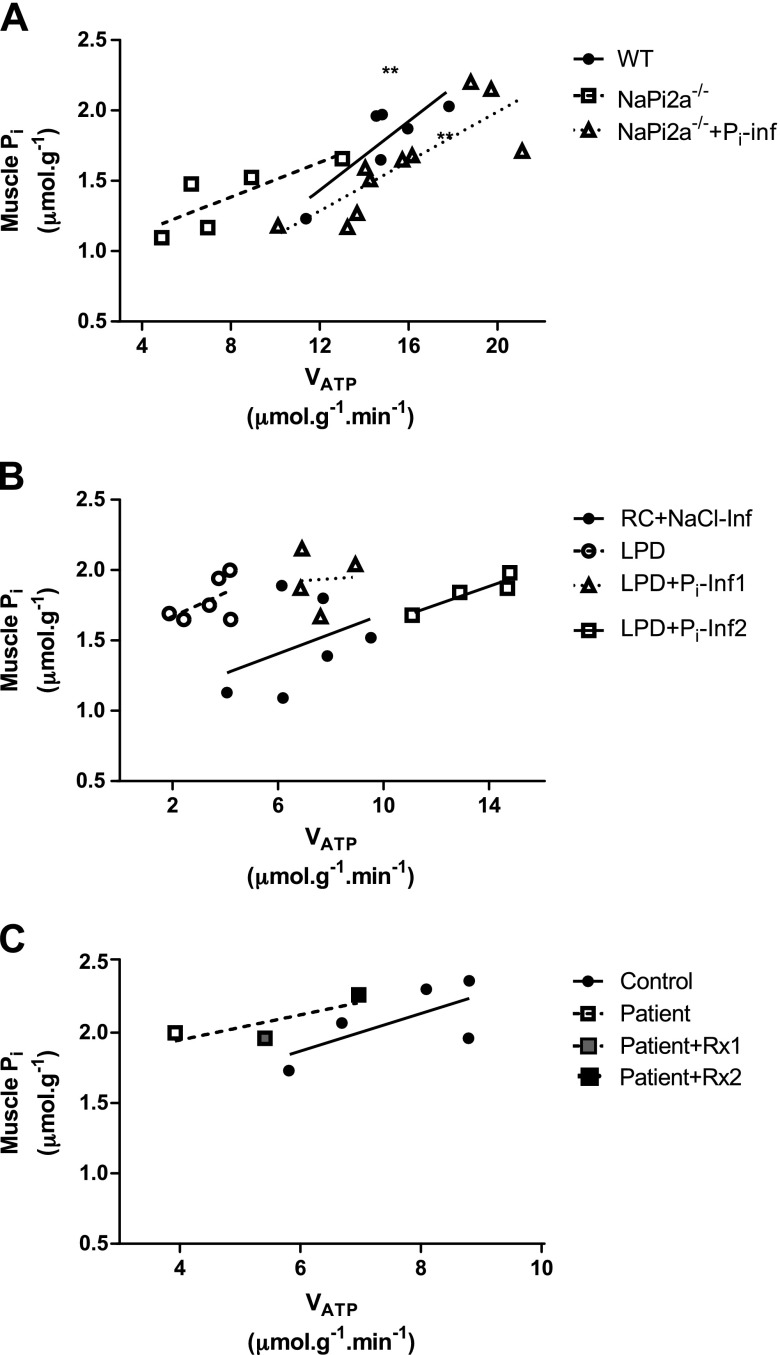
Figure 4.
Muscle Pi concentration and VATP are positively correlated. Shown is a regression analysis of muscle Pi and VATP obtained in WT mice, NaPi2a−/− mice, and NaPi2a−/− mice after continuous Pi infusion (A), in the LPD model before and after Pi infusion (B), and in the patient with hypophosphatemic rickets (C). Pi infusion (Pi-Inf) in panels A, B and oral phosphate supplementation after 5 (Rx1) and 8 (Rx2) mo improves VATP in all 3 models. Shown are individual animals with regression lines for each treatment group. Pooled regression analysis of all treatment groups was significant with **P < 0.01 in each panel.
Phosphate infusion restores plasma and muscle Pi and normalizes VATP in NaPi2a−/− mice
Bolus infusion of NaPi2a−/− mice with 25 µmol NaH2PO4 (pH 7.4) resulted in normalization of plasma Pi from 3.6 ± 0.1 to 5.6 ± 0.2 mg/dl (P < 0.001) and led to complete normalization of muscle mitochondrial VATP (from 7.4 ± 1.3 to 15.6 ± 1.3 µmol/g/min; P = 0.001; Fig. 1B, C). Muscle ATP concentrations were not changed after infusion (Fig. 1D). Muscle Pi concentrations correlated with VATP in NaPi2a−/− mice after NaH2Po4 infusion (R2 = 0.68; P = 0.004; Fig. 4A). Pooled analyses of all 3 groups (WT and NaPi2a−/− pre- and post-NaH2PO4) show that muscle VATP is directly correlated with muscle Pi concentrations (R2 = 0.55; P < 0.001; Fig. 4A).
Muscle mitochondrial VATP is low in mice on LPD
When WT C57Bl/6 mice were fed LPD for 2 wk to induce hypophosphatemia, similar results were observed, with a 65% reduction in plasma Pi concentrations from 6.0 ± 0.1 to 2.1 ± 0.3 mg/dl on LPD (P < 0.001). VATP in these mice was reduced by 52% compared with the regular chow-fed group (3.3 ± 0.4 vs. control: 6.9 ± 0.8 µmol/g/min; P = 0.002; Fig. 2).
Phosphate infusion restores plasma Pi and normalizes VATP in hypophosphatemic mice
To reverse hypophosphatemia and examine the effects on muscle mitochondrial VATP, mice on LPD were given bolus infusions of 30 and 60 µmol NaH2P04 (pH 7.4) at 2 µl/min. Basal VATP in hypophosphatemic mice on LPD was 6.9 ± 0.8 µmol/g/min and increased in a dose-dependent manner to 7.6 ± 0.5 µmol/g/min (P < 0.001 vs. preinfusion) after low-dose Pi infusion and 1.9-fold after high-dose Pi infusion to 13.4 ± 0.9 µmol/g/min (P < 0.01 vs. preinfusion; Fig. 2B). Linear regression analysis of pooled data for all groups again showed a positive correlation between muscle Pi and VATP (R2 = 0.55; P < 0.001; Fig. 4B). On the basis of the observed changes of the rate constant k′, this increase in VATP likely reflects increased physiologic ATP synthase activity rather than higher expression of ATP synthase in the inner mitochondrial membrane, which would be reflected by an increase in Vmax (Supplemental Fig. S3C).
Oxygen flux depends on external Pi concentration in isolated mouse mitochondria
To directly test the impact of Pi concentration on oxidative phosphorylation capacity in vitro, we measured the rate of oxygen flux in isolated quadriceps mitochondria from WT and NaPi2a−/− mice in a Pi-free medium in the presence of glutamate, malate, and ADP. Oxygen flux increased upon addition of 1 mM Pi and further increased upon addition of 5 mM Pi to medium (Fig. 3A). Increase in oxygen flux was similar between the 2 groups, which suggests that loss of NaPi2a and hypophosphatemia per se does not impair muscle mitochondrial function.
Insulin stimulation of VATP is impaired in NaPi2a−/− mice
To examine the potential role of plasma Pi levels on insulin stimulation of muscle mitochondrial VATP a hyperinsulinemic-euglycemic clamp was performed in WT and NaPi2a−/− mice during ST-[31P]MRS to directly measure basal and insulin-stimulated muscle VATP, which increased by 23% in the WT group (basal: 14.9 ± 0.9 vs. insulin-stimulated: 20.3 ± 1.9 µmol/g/min; P = 0.03). In contrast, insulin stimulation had no effect on VATP in NaPi2a−/− mice (Fig. 3B). Plasma glucose and insulin values were not different between the 2 groups (Fig. 3C, D). Saturation transfer effect (M′/M0), effective MR relaxation time of Pi (T1′) and the rate constant k′ were not changed in response to insulin stimulation but were different between WT and NaPi2a−/− mice (Supplemental Fig. S3G–I).
Insulin increases Pi uptake into L6 and RC13 myocytes that are dependent on PI3K-Akt signaling
We have previously observed a strong relationship between insulin stimulation of intramyocellular Pi concentrations and muscle VATP in healthy, lean, insulin-sensitive control subjects and a severe blunting of this effect in otherwise healthy, young, lean, insulin-resistant offspring of parents with type 2 diabetes (T2D) (22). To further examine this relationship between muscle Pi concentrations and VATP, [32P]i uptake was stimulated with 100 nM insulin in L6 human myocytes in the presence of sodium (0.012 ± 0.001 vs. 0.025 ± 0.001 pmol PO4/mg/min with insulin; P < 0.001; Supplemental Fig. S1A). Stimulation of Pi uptake was dose dependent and inhibited by wortmannin, a potent and specific PI3K inhibitor. Similar results were obtained by using the PI3K inhibitor LY294002 (50 μmol/min) and the PDK1 inhibitor KP372-1 (100 nM; Supplemental Fig. S1B, C), whereas Akt-I-1/2, an inhibitor of Akt, had no effect on blocking Pi uptake (Supplemental Fig. S1D) when used at a dose of 120 nM, which is sufficient to inhibit glucose uptake by GLUT4 in these cells (Supplemental Fig. S2D). These data suggest that insulin-stimulated glucose transport involves activation of PI3K, PDK1, and Akt, whereas insulin-stimulated Pi transport only involves PI3K and PDK1. Similar results were obtained in human RC13 myocyte cells (Supplemental Fig. S1E, F).
Muscle VATP in a patient with a mutation in the NAPI2C gene
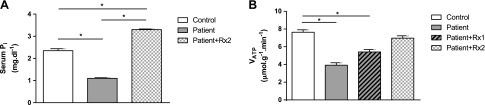
Hereditary HHRH is a genetic form of hypophosphatemia that is caused by loss of the proximal tubular sodium Pi cotransporter NaPi2c (30). We measured serum Pi concentrations and VATP by using ST-[31P]MRS after an overnight fast in 5 healthy volunteers and in 1 patient with HHRH before and after 8 mo of oral treatment with 500 mg KPhos MF administered 3 times/d. Baseline serum Pi concentrations in the patient with HHRH were 1.1 mg/dl (normal range, 2.5–4.5 mg/dl) and increased to 3.3 mg/dl after Pi supplementation. At baseline, muscle VATP was reduced by 50% in the patient with HHRH (3.9 vs. 7.6 ± 0.3 µmol/g/min for healthy controls) and normalized with serum Pi after treatment to 5.4 and, finally, 7.0 µmol/g/min (Fig. 5). Muscle Pi concentrations were 2.0 μmol/g before and increased to 2.3 μmol/g after chronic oral Pi supplementation.
Figure 5.
An individual with hypophosphatemic rickets has reduced VATP. A) Serum Pi was measured before and again after restoration of hypophosphatemia after a total of 8 mo (Rx2) oral phosphate supplementation with 500 mg KPhos MF 3 times/d, which comprised a total daily dose of 1500 mg. B) VATP was measured before, after 5 mo (Rx1), and again after a total of 8 mo (Rx2) treatment. The patient had reduced VATP measured by ST-[31P]MRS before treatment compared with 5 healthy controls, but VATP was restored after 8 mo treatment. All data are means ± sem; n = 5 in the control group. *P < 0.01 by double-sided Student’s t test.
DISCUSSION
By using ST-[31P]MRS technology, we found that mice with both genetically and diet-induced hypophosphatemia have reduced spontaneous activity and that this is accompanied by an ∼50% decrease in plasma Pi concentrations and muscle VATP. We also found that muscle VATP is directly related to cellular and mitochondrial Pi uptake in L6 and RC13 rodent myocytes and isolated muscle mitochondria, and demonstrate that both serum Pi concentrations and muscle VATP were reduced by 50% compared with healthy control individuals in a patient with hereditary HHRH and that muscle VATP completely normalized after oral Pi supplementation. Therefore, decreased muscle ATP synthesis, in part, may explain muscle weakness associated with hypophosphatemia and may serve as a noninvasive marker for hypophosphatemic myopathy.
Hypophosphatemia causes severe muscle weakness in many organisms, including humans (31, 32), dogs (33), and rodents (34). As muscle function in hypophosphatemic individuals rapidly returns to normal when physiologic Pi levels are restored (35), muscle weakness is likely directly related to blood Pi concentration, although it is possible that other changes in bone and mineral metabolism caused by hypophosphatemia are involved (36). Effects of Pi on muscle function as a substrate for biologic processes, such as energy metabolism and signal transduction, are not well understood. It is also not known which metabolic sensing pathways regulate uptake of Pi into cells and subcellular compartments, such as mitochondria.
We used murine knockout of NaPi2a, which is hypophosphatemic as a result of renal loss of Pi (37), as a model of hypophosphatemic myopathy. These mice have reduced spontaneous activity and impaired ATP synthesis, which leads to a metabolic shift toward glycolytic ATP generation (38).
To directly determine rates of muscle ATP synthetic rate in NaPi2a−/− mice, we adapted ST-[31P]MRS to mice (24, 25, 39). By using ST-[31P]MRS, we found that muscle VATP is markedly reduced in NaPi2a−/− mice. We reproduced these findings in a patient with hypophosphatemic myopathy and in WT mice in which hypophosphatemia was induced by LPD. Furthermore, VATP in all our models studied was rapidly restored after Pi supplementation consistent with the fact that VATP is measuring ATP synthetic flux but not steady state levels, and therefore a more sensitive to measure Pi effects on muscle metabolism. These findings suggest that VATP is likely directly related to blood Pi concentration.
Pi enters muscle cells via type III Na/Pi cotransporters (PiT1 and PiT2) (40). We therefore next evaluated whether blood Pi influences VATP. We observed that muscle Pi correlates with VATP (Fig. 4) before and after Pi repletion. This suggests that muscle Pi, which is the sum of interstitial and intracellular, but probably mostly intramyocellular, is an important determinant of mitochondrial phosphorylation activity.
To further test whether VATP is related to intramyocellular Pi in NaPi2a−/− mice, we examined isolated muscle mitochondria from these mice. Oxygen flux, a measure of VATP, is stimulated when Pi is added to the assay buffer; however, no difference was observed between mitochondria isolated from WT and NaPi2a−/− mice, which suggests that intramyocellular Pi regulates VATP and that intramyocellular Pi as a result of hypophosphatemia is likely the cause of reduced VATP in these mice.
In support of our earlier observations that insulin sensitivity correlates with muscle Pi and VATP in insulin-sensitive and insulin-resistant individuals (22), we demonstrated that insulin stimulates Pi uptake into cultured myocytes—evident in 2 cell lines—in a PI3K- and PDK1-dependent fashion. Furthermore, by using ST-[31P]MRS, we demonstrated that insulin stimulates muscle Pi and VATP in WT mice by 21%, whereas this stimulation was absent in hypophosphatemic NaPi2a−/− mice. This finding also supports the notion that muscle Pi measured by ST-[31P]MRS predominantly reflects intramyocellular Pi, extends our observation in isolated mitochondria, and further supports the hypothesis that muscle Pi uptake is important for VATP.
Finally, we showed in a patient with chronic hypophosphatemia as a result of a loss-of-function mutation in the NaPi2c renal phosphate transporter gene (SLC34A3) that the 50% reduction in plasma Pi was accompanied by a 50% reduction in muscle VATP and also that normalization of plasma Pi levels with oral phosphate repletion resulted in complete normalization of muscle VATP.
Taken together, our data strongly suggest that cellular Pi uptake is an important determinant of VATP and hypophosphatemic myopathy in mice and men. Future studies in mice in which Pi transporters are selectively deleted in muscle will further clarify the role of Pi uptake for mitochondrial and muscle function.
Our findings may have broader implications for 2 common clinical conditions: refeeding syndrome and myopathy commonly observed in T2D. Refeeding syndrome is a potentially fatal complication that occurs in patients with starvation, malnutrition or alcohol abuse (41). Although well recognized, this condition remains underdiagnosed and the exact mechanisms are not fully understood. Our data suggest that Pi transport into myocytes is stimulated by insulin, a hormone markedly induced during refeeding, which may provide a pathophysiologic explanation to the hypophosphatemia observed.
Muscle strength and function are also impaired in T2D (42, 43). Although peripheral diabetic neuropathy, in part, explains this weakness, exact reasons remain unclear. We recently showed that these individuals have decreased muscle Pi content and decreased insulin-stimulated rates of ATP synthesis (22), which suggests that insulin requires normophosphatemia to maintain normal VATP. Furthermore, in RC13 rhabdomyosarcoma cells and L6 myocytes, insulin regulates Pi uptake upstream of Akt, which involves activation of PI3K and PDK1. Similar observations were made by Wang et al (44), who reported increased rat vascular smooth muscle cell calcification after selective inhibition of the PI3K pathway while intact insulin signaling attenuated vascular smooth muscle cell calcification induced by high Pi conditions by the ability of insulin to reduce serum Pi concentrations and promote redistribution of Pi from the circulation into the cell. On the basis of these considerations, decreased muscle ATP synthetic flux may serve as a noninvasive marker to monitor therapy of patients with refeeding syndrome and T2D.
Our findings suggest that decreased muscle ATP synthetic flux, in part, may explain muscle weakness in hypophosphatemia, and VATP may serve as a noninvasive marker for hypophosphatemic myopathy. Our findings further establish insulin as a potential regulator of muscle Pi transport and of VATP. These findings might help to better understand the pathophysiology of refeeding hypophosphatemia and of the myopathy commonly observed in individuals with T2D.
ACKNOWLEDGMENTS
This work was supported by the Austrian Science Fund (FWF; J-3267); the German Science Association (DFG; BI1292/4-2); U.S. National Institutes of Health Grants DK-40936, DK-059635, and DK-45735 (National Institute of Diabetes and Digestive and Kidney Diseases), and AG-23686 (National Institute on Aging); and the Novo Nordisk Foundation Center for Basic Metabolic Research (University of Copenhagen). The authors thank Mario Kahn, Blas Guigni, Tomas Jelenik, John Stack, Gina Butrico, Ali Nasiri, Bryce Perler, and Maria Batsu for technical assistance and Oroboros Instruments for the loan of an Oxygraph-2k. The authors also thank the Hospital Research Unit (Yale–New Haven Hospital, New Haven, CT, USA) for help with clinical studies. The authors declare no conflicts of interest.
Glossary
- CLAMS
comprehensive animal metabolic monitoring system
- FBS
fetal bovine serum
- HET
heterozygote
- HHRH
hypophosphatemic rickets with hypercalciuria
- HPD
high-phosphate diet
- LPD
low-phosphate diet
- NaPi2a
sodium-dependent Pi transporter solute carrier family 34, member 1
- Pi
inorganic phosphate
- RER
respiratory exchange ratio
- ST-[31P]MRS
saturation transfer [31P]-magnetic resonance spectroscopy
- T2D
type 2 diabetes
- VATP
ATP synthetic flux rate
- WT
wild-type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
D. H. Pesta, D. N. Tsirigotis, D. E. Befroy, D. L. Rothman, T. O. Carpenter, K. Insogna, K. F. Petersen, C. Bergwitz, and G. I. Shulman designed the experimental protocols; D. H. Pesta, D. N. Tsirigotis, D. E. Befroy, D. Caballero, M. J. Jurczak, Y. Rahimi, G. W. Cline, S. Dufour, A. L. Birkenfeld, K. F. Petersen, C. Bergwitz, and G.W.C. performed the studies; D. H. Pesta, D. N. Tsirigotis, D. E. Befroy, M. J. Jurczak, Y. Rahimi, G. W. Cline, S. Dufour, A. L. Birkenfeld, K. F. Petersen, C. Bergwitz., and G. I. Shulman analyzed the data; and all authors contributed to the writing of the manuscript.
REFERENCES
- 1.Berndt T., Kumar R. (2007) Phosphatonins and the regulation of phosphate homeostasis. Annu. Rev. Physiol. 69, 341–359 [DOI] [PubMed] [Google Scholar]
- 2.Kuro-o M. (2010) A potential link between phosphate and aging--lessons from Klotho-deficient mice. Mech. Ageing Dev. 131, 270–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amanzadeh J., Reilly R. F. Jr (2006) Hypophosphatemia: an evidence-based approach to its clinical consequences and management. Nat. Clin. Pract. Nephrol. 2, 136–148 [DOI] [PubMed] [Google Scholar]
- 4.Camp M. A., Allon M. (1990) Severe hypophosphatemia in hospitalized patients. Miner. Electrolyte Metab. 16, 365–368 [PubMed] [Google Scholar]
- 5.Veilleux L. N., Cheung M., Ben Amor M., Rauch F. (2012) Abnormalities in muscle density and muscle function in hypophosphatemic rickets. J. Clin. Endocrinol. Metab. 97, E1492–E1498 [DOI] [PubMed] [Google Scholar]
- 6.Smith R., Newman R. J., Radda G. K., Stokes M., Young A. (1984) Hypophosphataemic osteomalacia and myopathy: studies with nuclear magnetic resonance spectroscopy. Clin. Sci. 67, 505–509 [DOI] [PubMed] [Google Scholar]
- 7.Lardy H. A., Wellman H. (1952) Oxidative phosphorylations; rôle of inorganic phosphate and acceptor systems in control of metabolic rates. J. Biol. Chem. 195, 215–224 [PubMed] [Google Scholar]
- 8.Lemasters J. J., Sowers A. E. (1979) Phosphate dependence and atractyloside inhibition of mitochondrial oxidative phosphorylation. The ADP-ATP carrier is rate-limiting. J. Biol. Chem. 254, 1248–1251 [PubMed] [Google Scholar]
- 9.Wilson D. F., Stubbs M., Veech R. L., Erecińska M., Krebs H. A. (1974) Equilibrium relations between the oxidation-reduction reactions and the adenosine triphosphate synthesis in suspensions of isolated liver cells. Biochem. J. 140, 57–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tager J. M., Wanders R. J., Groen A. K., Kunz W., Bohnensack R., Küster U., Letko G., Böhme G., Duszynski J., Wojtczak L. (1983) Control of mitochondrial respiration. FEBS Lett. 151, 1–9 [DOI] [PubMed] [Google Scholar]
- 11.Khananshvili D., Gromet-Elhanan Z. (1985) Characterization of an inorganic phosphate binding site on the isolated, reconstitutively active. beta. subunit of F0.cntdot.F1 ATP synthase. Biochemistry 24, 2482–2487 [Google Scholar]
- 12.Tanaka A., Chance B., Quistorff B. (1989) A possible role of inorganic phosphate as a regulator of oxidative phosphorylation in combined urea synthesis and gluconeogenesis in perfused rat liver. A phosphorus magnetic resonance spectroscopy study. J. Biol. Chem. 264, 10034–10040 [PubMed] [Google Scholar]
- 13.Beard D. A. (2006) Modeling of oxygen transport and cellular energetics explains observations on in vivo cardiac energy metabolism. PLOS Comput. Biol. 2, e107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Segawa H., Aranami F., Kaneko I., Tomoe Y., Miyamoto K. (2009) The roles of Na/Pi-II transporters in phosphate metabolism. Bone 45, S2–S7 [DOI] [PubMed] [Google Scholar]
- 15.Beck L., Leroy C., Beck-Cormier S., Forand A., Salaün C., Paris N., Bernier A., Ureña-Torres P., Prié D., Ollero M., Coulombel L., Friedlander G. (2010) The phosphate transporter PiT1 (Slc20a1) revealed as a new essential gene for mouse liver development. PLoS One 5, e9148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jensen N., Schrøder H. D., Hejbøl E. K., Füchtbauer E. M., de Oliveira J. R., Pedersen L. (2013) Loss of function of Slc20a2 associated with familial idiopathic basal ganglia calcification in humans causes brain calcifications in mice. J. Mol. Neurosci. 51, 994–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lemos R. R., Ramos E. M., Legati A., Nicolas G., Jenkinson E. M., Livingston J. H., Crow Y. J., Campion D., Coppola G., Oliveira J. R. (2015) Update and mutational analysis of SLC20A2: a major cause of primary familial brain calcification. Hum. Mutat. 36, 489–495 [DOI] [PubMed] [Google Scholar]
- 18.Sinha A., Hollingsworth K. G., Ball S., Cheetham T. (2013) Improving the vitamin D status of vitamin D deficient adults is associated with improved mitochondrial oxidative function in skeletal muscle. J. Clin. Endocrinol. Metab. 98, E509–E513 [DOI] [PubMed] [Google Scholar]
- 19.Choi C. S., Befroy D. E., Codella R., Kim S., Reznick R. M., Hwang Y. J., Liu Z. X., Lee H. Y., Distefano A., Samuel V. T., Zhang D., Cline G. W., Handschin C., Lin J., Petersen K. F., Spiegelman B. M., Shulman G. I. (2008) Paradoxical effects of increased expression of PGC-1alpha on muscle mitochondrial function and insulin-stimulated muscle glucose metabolism. Proc. Natl. Acad. Sci. USA 105, 19926–19931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petersen K. F., Befroy D., Dufour S., Dziura J., Ariyan C., Rothman D. L., DiPietro L., Cline G. W., Shulman G. I. (2003) Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science 300, 1140–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petersen K. F., Dufour S., Befroy D., Garcia R., Shulman G. I. (2004) Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N. Engl. J. Med. 350, 664–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petersen K. F., Dufour S., Shulman G. I. (2005) Decreased insulin-stimulated ATP synthesis and phosphate transport in muscle of insulin-resistant offspring of type 2 diabetic parents. PLoS Med. 2, e233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hettleman B. D., Sabina R. L., Drezner M. K., Holmes E. W., Swain J. L. (1983) Defective adenosine triphosphate synthesis. An explanation for skeletal muscle dysfunction in phosphate-deficient mice. J. Clin. Invest. 72, 582–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jucker B. M., Dufour S., Ren J., Cao X., Previs S. F., Underhill B., Cadman K. S., Shulman G. I. (2000) Assessment of mitochondrial energy coupling in vivo by 13C/31P NMR. Proc. Natl. Acad. Sci. USA 97, 6880–6884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jucker B. M., Ren J., Dufour S., Cao X., Previs S. F., Cadman K. S., Shulman G. I. (2000) 13C/31P NMR assessment of mitochondrial energy coupling in skeletal muscle of awake fed and fasted rats. Relationship with uncoupling protein 3 expression. J. Biol. Chem. 275, 39279–39286 [DOI] [PubMed] [Google Scholar]
- 26.Lebon V., Dufour S., Petersen K. F., Ren J., Jucker B. M., Slezak L. A., Cline G. W., Rothman D. L., Shulman G. I. (2001) Effect of triiodothyronine on mitochondrial energy coupling in human skeletal muscle. J. Clin. Invest. 108, 733–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bergwitz C., Roslin N. M., Tieder M., Loredo-Osti J. C., Bastepe M., Abu-Zahra H., Frappier D., Burkett K., Carpenter T. O., Anderson D., Garabedian M., Sermet I., Fujiwara T. M., Morgan K., Tenenhouse H. S., Juppner H. (2006) SLC34A3 mutations in patients with hereditary hypophosphatemic rickets with hypercalciuria predict a key role for the sodium-phosphate cotransporter NaPi-IIc in maintaining phosphate homeostasis. Am. J. Hum. Genet. 78, 179–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang D., Christianson J., Liu Z. X., Tian L., Choi C. S., Neschen S., Dong J., Wood P. A., Shulman G. I. (2010) Resistance to high-fat diet-induced obesity and insulin resistance in mice with very long-chain acyl-CoA dehydrogenase deficiency. Cell Metab. 11, 402–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brooks K. J., Hill M. D., Hockings P. D., Reid D. G. (2004) MRI detects early hindlimb muscle atrophy in Gly93Ala superoxide dismutase-1 (G93A SOD1) transgenic mice, an animal model of familial amyotrophic lateral sclerosis. NMR Biomed. 17, 28–32 [DOI] [PubMed] [Google Scholar]
- 30.Jones A., Tzenova J., Frappier D., Crumley M., Roslin N., Kos C., Tieder M., Langman C., Proesmans W., Carpenter T., Rice A., Anderson D., Morgan K., Fujiwara T., Tenenhouse H. (2001) Hereditary hypophosphatemic rickets with hypercalciuria is not caused by mutations in the Na/Pi cotransporter NPT2 gene. J. Am. Soc. Nephrol. 12, 507–514 [DOI] [PubMed] [Google Scholar]
- 31.Geerse D. A., Bindels A. J., Kuiper M. A., Roos A. N., Spronk P. E., Schultz M. J. (2010) Treatment of hypophosphatemia in the intensive care unit: a review. Crit. Care 14, R147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Soyoral Y., Aslan M., Ebinc S., Dirik Y., Demir C. (2014) Life-threatening hypophosphatemia and/or phosphate depletion in a patient with acute lymphoblastic leukemia: a rare case report. Am. J. Emerg. Med. 32, 1437.e3-e5. [DOI] [PubMed] [Google Scholar]
- 33.Fuller T. J., Carter N. W., Barcenas C., Knochel J. P. (1976) Reversible changes of the muscle cell in experimental phosphorus deficiency. J. Clin. Invest. 57, 1019–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schubert L., DeLuca H. F. (2010) Hypophosphatemia is responsible for skeletal muscle weakness of vitamin D deficiency. Arch. Biochem. Biophys. 500, 157–161 [DOI] [PubMed] [Google Scholar]
- 35.Gravelyn T. R., Brophy N., Siegert C., Peters-Golden M. (1988) Hypophosphatemia-associated respiratory muscle weakness in a general inpatient population. Am. J. Med. 84, 870–876 [DOI] [PubMed] [Google Scholar]
- 36.Imel E. A., Econs M. J. (2012) Approach to the hypophosphatemic patient. J. Clin. Endocrinol. Metab. 97, 696–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beck L., Karaplis A. C., Amizuka N., Hewson A. S., Ozawa H., Tenenhouse H. S. (1998) Targeted inactivation of Npt2 in mice leads to severe renal phosphate wasting, hypercalciuria, and skeletal abnormalities. Proc. Natl. Acad. Sci. USA 95, 5372–5377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haran M., Gross A. (2014) Balancing glycolysis and mitochondrial oxphos: lessons from the hematopoietic system and exercising muscles. Mitochondrion 19 Pt A, 3–7 [DOI] [PubMed] [Google Scholar]
- 39.Cline G. W., Vidal-Puig A. J., Dufour S., Cadman K. S., Lowell B. B., Shulman G. I. (2001) In vivo effects of uncoupling protein-3 gene disruption on mitochondrial energy metabolism. J. Biol. Chem. 276, 20240–20244 [DOI] [PubMed] [Google Scholar]
- 40.Khoshniat S., Bourgine A., Julien M., Weiss P., Guicheux J., Beck L. (2011) The emergence of phosphate as a specific signaling molecule in bone and other cell types in mammals. Cell. Mol. Life Sci. 68, 205–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gariballa S. (2008) Refeeding syndrome: a potentially fatal condition but remains underdiagnosed and undertreated. Nutrition 24, 604–606 [DOI] [PubMed] [Google Scholar]
- 42.Andersen H., Nielsen S., Mogensen C. E., Jakobsen J. (2004) Muscle strength in type 2 diabetes. Diabetes 53, 1543–1548 [DOI] [PubMed] [Google Scholar]
- 43.Andreassen C. S., Jakobsen J., Andersen H. (2006) Muscle weakness: a progressive late complication in diabetic distal symmetric polyneuropathy. Diabetes 55, 806–812 [DOI] [PubMed] [Google Scholar]
- 44.Wang C. C., Sorribas V., Sharma G., Levi M., Draznin B. (2007) Insulin attenuates vascular smooth muscle calcification but increases vascular smooth muscle cell phosphate transport. Atherosclerosis 195, e65–e75 [DOI] [PMC free article] [PubMed] [Google Scholar]