Abstract
Although single nucleotide polymorphisms (SNPs) in folate-mediated pathways predict susceptibility to choline deficiency during severe choline deprivation, it is unknown if effects persist at recommended intakes. Thus, we used stable isotope liquid chromatography-mass spectrometry (LC-MS) methodology to examine the impact of candidate SNPs on choline metabolism in a long-term, randomized, controlled feeding trial among pregnant, lactating, and nonpregnant (NP) women consuming 480 or 930 mg/d choline (22% as choline-d9, with d9 indicating a deuterated trimethyl amine group) and meeting folate-intake recommendations. Variants impairing folate metabolism, methylenetetrahydrofolate reductase (MTHFR) rs1801133, methionine synthase (MTR) rs1805087 [wild-type (WT)], MTR reductase (MTRR) rs1801394, and methylenetetrahydrofolate dehydrogenase-methenyltetrahydrofolate cyclohydrolase-formyltetrahydrofolate synthetase (MTHFD1) rs2236225, influenced choline dynamics, frequently through interactions with reproductive state and choline intake, with fewer genotypic alterations observed among pregnant women. Women with these variants partitioned more dietary choline toward phosphatidylcholine (PC) biosynthesis via the cytidine diphosphate (CDP)-choline pathway at the expense of betaine synthesis even when use of betaine as a methyl donor was increased. Choline intakes of 930 mg/d restored partitioning of dietary choline between betaine and CDP-PC among NP (MTHFR rs1801133 and MTR rs1805087 WT) and lactating (MTHFD1 rs2236225) women with risk genotypes. Overall, our findings indicate that loss-of-function variants in folate-metabolizing enzymes strain cellular PC production, possibly via impaired folate-dependent phosphatidylethanolamine-N-methyltransferase (PEMT)-PC synthesis, and suggest that women with these risk genotypes may benefit from choline intakes exceeding current recommendations.—Ganz, A. B., Shields, K., Fomin, V. G., Lopez, Y. S., Mohan, S., Lovesky, J., Chuang, J. C., Ganti, A., Carrier, B., Yan, J., Taeswuan, S., Cohen, V. V., Swersky, C. C., Stover, J. A., Vitiello, G. A., Malysheva, O. V., Mudrak, E., Caudill, M. A. Genetic impairments in folate enzymes increase dependence on dietary choline for phosphatidylcholine production at the expense of betaine synthesis.
Keywords: nutrigenetics, single nucleotide polymorphisms, 1-carbon metabolism, pregnancy, lactation
Single nucleotide polymorphisms (SNPs) can alter metabolism and use of the micronutrients folate and choline (1, 2). Inadequacy of these nutrients is a risk factor for cancer (3) and birth defects (4–6), which are pathologies that result from complex interactions between genetic and environmental risk factors. methylenetetrahydrofolate reductase (MTHFR) rs1801133, methionine synthase (MTR) rs1805087, MTR reductase (MTRR) rs1801394, and methylenetetrahydrofolate dehydrogenasemethenyltetrahydrofolate cyclohydrolase-formyltetrahydrofolate synthetase (MTHFD1) rs2236225 are common SNPs in enzymes involved in a folate-mediated, 1-carbon metabolism that predict increased risk of birth defects, as well as risk of choline deficiency and onset of fatty liver disease in humans deprived of choline (6, 7).
The relationship between folate enzyme SNPs and choline requirement may arise from the overlapping roles of folate and choline in methionine and phosphatidylcholine (PC) biosynthesis. In extrahepatic tissue, folate is responsible for the remethylation of homocysteine to methionine which generates the methyl donor S-adenosylmethionine (SAM). In the liver however, both folate and the choline oxidation product betaine contribute to methionine biosynthesis (8). An increased contribution by choline-derived betaine to methionine biosynthesis may spare folate cofactors for nucleotide biosynthesis. Furthermore, whereas choline is used for PC synthesis via the cytidine diphosphate (CDP)-choline pathway, PC produced by the phosphatidylethanolamine-N-methyltransferase (PEMT) uses SAM as a methyl donor, and therefore is dependent on both folate- and choline-derived methyl groups (9, 10). Genetic risk factors that increase the nutritional requirement for choline may contribute to the pathogenesis of diseases related to a 1-carbon metabolism (6, 11).
Physiologic factors, such as reproductive state, can similarly influence choline requirements. The demand for choline is uniquely high during pregnancy and lactation as a result of rapid membrane biogenesis, methylation of placental and fetal DNA, and brain development. Experimental data in humans have shown altered choline partitioning that indicates an increased requirement for choline during pregnancy and lactation (12–14). For example, compared with nonprenant (NP) women, pregnant women use more choline to synthesize PC through both the CDP-choline and PEMT pathways and use more of choline-derived betaine as a methyl donor (12). Furthermore, maternal choline intakes exceeding current dietary recommendations appear to have favorable effects on several physiologic outcomes during pregnancy, including placental functioning (15) and the fetal response to stress (16).
At present, it is unknown whether the increased need for choline during pregnancy and lactation can be compounded by genetic risk. Furthermore, although genetic variation modifies disease risk and choline requirements during severe choline deprivation, and the MTHFR rs1801133 variant is known to alter the metabolic flux of choline under conditions of folate inadequacy (17), it is unknown whether these variants are functionally relevant under conditions of nutrient sufficiency. Thus, we sought to evaluate the influence of risk genotypes in folate enzymes on choline metabolism among women of differing reproductive states and meeting dietary choline and folate intake recommendations (Fig. 1).
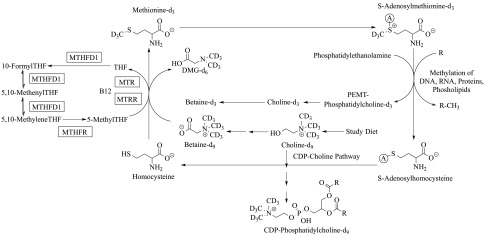
Figure 1.
Overview of the metabolic fate of isotopically labeled choline-d9 consumed by study participants. SNPs, in squared enzymes, were examined: MTHFD1 [dbSNP: rs2236225; c.1958 G > A; p.Arg653Gln; RefSeq NT_026437.13], MTHFR (dbSNP: rs1801133; c.677 C > T; p.Ala223Val; RefSeq NT_021937.17), MTR (dbSNP: rs1805087; c.2756 A > G; p.Asp919Gly; RefSeq NG_008959.1), MTRR (dbSNP: rs1801394; c.66 A > G; p.Ile22Met; RefSeq NG_008856.1). Note that a deuterated methyl group from DMG-d6 could be added to tetrahydrofolate (THF) by MTHFD1 to produce 5,10-methenyl-THF-d2 (loss of a deuterium occurs during addition), which might be converted to 5-methyl THF-d2 and used to methylate homocysteine to produce methionine-d2. Alternatively, the 5,10-methenyl-THF-d2 might be converted to 10-formyl-THF-d1 (loss of additional deuterium) and ultimately to methionine-d1. However, these scenarios result in methionine-d2 or methionine-d1 rather than methionine-d3 and therefore, do not confound our analysis of methionine-d3 and its downstream metabolites.
MATERIALS AND METHODS
Participants and study design
A long-term, randomized, controlled feeding study was carried out among women in different reproductive states, as described by Yan et al. (18). In brief, healthy NP, lactating, and third-trimester pregnant women were recruited from the Ithaca, New York, USA, area and assessed for eligibility using a blood chemistry profile, complete blood count, and health history questionnaire. Exclusion criteria included chronic disease; alcohol or tobacco use; and indication of liver, kidney, or pregnancy complications (18). Eligible participants (pregnant, n = 26; NP, n = 21; lactating, n = 28) consumed the study diet (18) for 10–12 wk, which provided 380 mg/d choline and 100 or 550 mg/d choline from supplemental choline chloride (Balchem, New Hampton, NY, USA). In addition, all participants consumed a daily prenatal multivitamin (PregnancyPlus; Fairhaven Health, Bellingham, WA, USA) containing 600 μg folic acid, a daily docosahexaenoic acid (DHA) supplement (200 mg; Neuromins; Nature’s Way, Lehi, UT, USA), and a thrice-weekly potassium and magnesium supplement (General Nutrition, Pittsburgh, PA, USA). During study wk 6–10 (lactating women) or 6–12 (pregnant and NP women), participants consumed 22% of their total choline in the form of choline chloride-(trimethyl-d9) (98%; Cambridge Isotope Laboratories, Tewksbury, MA, USA). The study was approved by the Institutional Review Boards at Cornell University (Ithaca, NY, USA) and Cayuga Medical Center (Ithaca, NY, USA) and was registered at clinicaltrials.gov as NCT01127022. All participants provided informed consent.
Sample collection and processing
Fasting blood (10 h) and breast-milk samples were collected and processed as described previously (13, 18). In brief, blood was collected into EDTA-coated tubes at baseline and study wk 3, 6, 9, 10, and 12 and placed on ice immediately. After centrifugation (2200 rpm, 15 min, 4°C), plasma and buffy coat were dispensed into 1.5 μl centrifuge tubes. Similar to blood, breast-milk samples were placed on ice, processed without delay, and stored at −80°C until analysis. One lactating participant provided wk 9 samples at wk 8. Another provided samples at wk 9.5 that were used for wk 9 measurements in plasma and wk 10 measurements in breast milk.
Genotyping
DNA for genotyping was extracted from blood sample buffy coats using the commercially available DNeasy Blood & Tissue kit (Qiagen, Valencia, CA, USA). The genotyping of MTR [U.S. National Center for Biotechnology Information (NCBI; Bethesda, MD, USA) Single Nucleotide Polymorphism Database (dbSNP; http://www.ncbi.nlm.nih.gov/snp): rs1805087; c.2756 A > G; p.Asp919Gly; NCBI Reference Sequence (RefSeq) database (http://www.ncbi.nlm.nih.gov/refseq) NG_008959.1; NCBI Online Mendelian Inheritance in Man (OMIM) database (http:www.ncbi.nlm.nih.gov/omim) 156570] and MTRR (dbSNP: rs1801394; c.66 A > G; p.Ile22Met; RefSeq NG_008856.1; OMIM 602568) was performed using Endpoint Genotyping on a LightCycler 480 (Roche, Indianapolis, IN, USA) in our facility. In brief, a commercially available mix (Applied Biosystems TaqMan Genotyping Master Mix and Assay Mix; Thermo Fisher Scientific, Waltham, MA, USA), using a dual hydrolysis probe with VIC and FAM dyes, was used to quantify the fluorescence and presence of the variant and familial alleles. High fluorescence of VIC or FAM was considered to be a homozygous variant or homozygous wild-type (WT), respectively, whereas samples with moderate fluorescence in VIC and FAM channels were considered heterozygous. Samples were run in duplicate with in-run standards and a negative water control. Cycling conditions were 95°C for 10 min, followed by 34 cycles of 92°C for 15 s and 62°C for 90 s.
The genotyping of MTHFD1 (dbSNP: rs2236225; c.1958 G > A; p.Arg653Gln; RefSeq NT_026437.13; OMIM 172460) and MTHFR (dbSNP: rs1801133; c.677 C > T; p.Ala223Val; RefSeq NT_021937.17; OMIM 607093) was performed by Sanger-DNA genotyping PCR, as described previously (19) in Cornell University’s Life Sciences Core Laboratories Center. The forward and reverse primers used for MTHFD1 rs2236225 were 5′-GCATCTTGAGAGCCCTGAC-3′ and 5′-CACTCCAGTGTTTGTCCATG-3′, respectively (Thermo Fisher Scientific) (20). Cycling conditions were 95°C for 5 min, 35 cycles (95°C for 30 s, 50°C for 30 s, 72°C for 30 s), 72°C for 10 min, and 4°C hold. The MTHFR forward and reverse primers were 5′-AGGACGGTGCGGTGAGAGTG-3′ and 5′-TGAAGGAGAAGGTGTCTGCGGGA-3′, respectively (Thermo Fisher Scientific) (21). Cycling conditions were 95°C for 5 min, 30 cycles (95°C for 30 s, 60°C for 30 s, 72°C for 30 s), 72°C for 10 min, 4°C hold. Double-stranded DNA obtained from PCR was purified using a commercially available QiaQuick PCR Purification kit (Qiagen) and sequenced using an automated 3730 DNA Analyzer (Thermo Fisher Scientific). Internal standards for each genotype were included, and all results were duplicated.
Enrichment of choline metabolites
Metabolite enrichments of choline-d9, choline-d3, betaine-d9, betaine-d3, and dimethylglycine (DMG)-d6, as well as PC-d3, PC-d6, and PC-d9, were measured at study wk 9 for plasma and study wk 10 for breast milk. Enrichments were measured on our TSQ Quantum Access Triple Quadrupole liquid chromatography-mass spectrometry (LC-MS) system (Thermo Fisher Scientific), operated in positive-ion mode using electrospray ionization, as described previously (12, 17). Internal standards were not added to samples designated for enrichment measurements to allow for the measurement of isotopic enrichments. Enrichments were calculated by dividing the amount of each isotopically labeled choline metabolite by the total amount of all isotopomers and multiplying by 100%.
Enrichment of methionine-d3
Acidified plasma (wk 9; 250 μl) was thawed, and 150 μl 25 mM ammonium acetate buffer was added to each sample. Proteins were precipitated with 125 μl 1 M perchloric acid, and samples were centrifuged at 14,000 rpm for 10 min at 4°C. Supernatant was transferred to a new 1.5 μl Eppendorf tube (Eppendorf, Hamburg, Germany) and neutralized with 90 μl 10 M ammonium hydroxide (final pH ∼4.5). Neutralized samples were applied to C18 solid-phase extraction columns (200 mg, 3 cc, Sep-Pak Vac; Waters, Milford, MA, USA) with a monofunctionally bonded silica phase (preconditioned with 1 ml methanol, 750 μl 0.1 N NaOH, and 1 ml H2O) on a vacuum manifold (Gast, Benton Harbor, MI, USA). Milli-Q water (1 ml; Millipore, Billerica, MA, USA) was used to wash the samples before elution with 1.5 ml 0.1% formic acid in 85:15 H2O:methanol. Samples were evaporated in a speed vacuum and subsequently resuspended in 50 μl water, diluted with 50 μl acetonitrile/methanol (1:1, v/v), and centrifuged (20,000 g, 4°C, 5 min) before injection into the LC-MS.
LC-MS for methionine-d3
Enrichment measurements of methionine-d3 were performed on a UltiMate 3000 ultra-high performance LC (UHPLC) (Thermo Fisher Scientific) and Q Exactive-MS (QE-MS; Thermo Fisher Scientific) in the Locasale Laboratory (Cornell University). Methods were adapted from Kim et al. (22). The purified extracts containing methionine-d3 were injected (5 μl) into an XBridge Amide Column (100 × 2.1 mm intradermally, 3.5 μm; Waters) and separated by elution (in acetonitrile and 10 mM ammonium acetate and 2.5 mM ammonium hydroxide in water with 3% acetonitrile, pH 8.6) at a rate of 150 µl/min from 0 to 7.9 min and 19 to 20 min and 180 µl/min from 8 to 10.6 min. The linear elution gradient was as follows: 0 min, 85% B; 1.5 min, 85% B; 10.6 min, 35% B; 12.5 min, 10% B; 14 min, 10% B; 15 min, 85% B; and 20 min, 85% B. All solvents were LC-MS grade and purchased from Thermo Fisher Scientific. The QE-MS is equipped with a heated electrospray ionization (HESI) probe, and the relevant parameters are as follows: heater temperature, 120°C; sheath gas, 30; auxiliary gas, 10; sweep gas, 3; spray voltage, 3.6 kV for positive mode and 2.5 kV for negative mode. The capillary temperature was set at 320°C, and the S-lens was 55. The maximum injection time was 200 ms. Automated gain control was targeted at 3 × 106 ions, and isolation width was 2.0. The QE-MS was operated in positive full-scan mode with a resolution of 70,000 and a scan range of 70–900 m/z, and ions were monitored at m/z 150.058 and 153.077 for identification of methionine and methionine-d3, respectively.
Quantification of choline metabolites
Total choline, betaine, DMG, and PC pool sizes were quantified at baseline and study wk 6, 9, and 10 for plasma and urine and at study wk 10 for breast milk on our TSQ Quantum Access Triple Quadrupole LC-MS system (Thermo Fisher Scientific), operated in positive-ion mode using electrospray ionization, as described previously (12, 17). Serum methionine pool sizes were quantified at baseline, study wk 6, and study end by gas chromatography-MS (18).
Metabolic flux calculations
When an isotopically labeled metabolic precursor is converted to an isotopically labeled metabolic product (precursor → product), the isotopic enrichment percentages of a metabolic precursor and a metabolic product can be calculated by Eqs. 1 and 2, respectively.
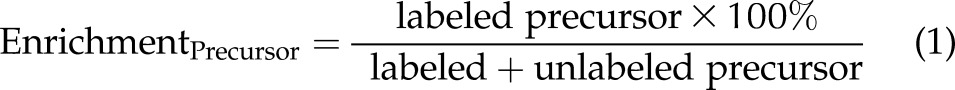 |
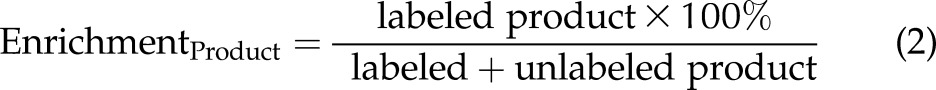 |
In the present study, plasma choline metabolite pools were at steady state by study wk 6 (18), when labeled choline was introduced into the study diet. Under conditions of steady state, labeled and unlabeled metabolites within a given pool are turned over at the same rate. Thus, EnrichmentProduct can be calculated from EnrichmentPrecursor, given Rate (the net conversion of precursor to product, in micromolars of metabolite product of plasma, over the study period) and Pool SizeProduct (the plasma pool size in micromolars of metabolite product of plasma; Eq. 3). We used Eq. 3 to derive the rate of conversion of metabolic precursors → products in micromolars per study period, over the 3-wk period of label exposure (Eq. 4).
 |
 |
Statistical analysis
We examined 6 metabolic outcomes in plasma as primary response variables. These outcomes were chosen to reflect partitioning between metabolic pathways (enrichment ratios of betaine-d9/PC-d9, PC-d3 + 6/PC-d9) or flux through metabolic pathways (metabolic flux calculations for turnover of betaine → methionine, betaine → DMG, and choline → betaine within the study period). A secondary analysis of individual metabolites in plasma, urine, and breast milk was used to support interpretation.
The effect of genetic variation on choline metabolites was assessed using linear models. Heterozygous and homozygous variant individuals were grouped together to examine the effect of variant allele presence. Outcomes were modeled as a function of SNP genotype. Reproductive status and choline intake group (480 or 930 mg/d choline) were included as covariates. A backward selection was used in which body mass index was retained at an α-cutoff of 0.05, and interactions were retained at an α-cutoff of 0.1. The 0.1 cutoff for interactions was selected to ensure that interactions were detected and prevent the interpretation of main effects in the presence of an interaction. SNPs with a low presence of the variant allele were evaluated for the effect of minor allele presence (a 2-level categorical variable for presence or absence of minor allele) on each outcome (Table 1). Standard diagnostic methods were used to assess model assumptions and the fit of the model to the data. Reported P values include Bonferroni corrections for multiple comparisons. When evaluating the 3-way interaction in MTHFD1 models, several groups had only one participant, which precluded the use of statistical tests for several comparisons. To avoid pair-wise comparisons with such groups, for this one genotype, we chose to compare representative individuals to 95% confidence intervals (CIs) constructed around groups with adequate sample size. This approach did not allow for the determination of statistical significance of these few comparisons but was a conservative and statistically valid alternative given the circumstances. In such cases, we report whether the representative individual had metabolite enrichment levels outside or inside the 95% constructed around groups with adequate n, to which we wished to draw comparisons. Two lactating participants had choline-d9 enrichment values >2 sd from the mean and were excluded from the entire analysis. All statistical analysis was performed using the lsmeans package (21) in The R Project for Statistical Computing environment, available from Comprehensive R Archive Network (CRAN) 2014 (23). Data are presented as predicted least-squares means, unless noted otherwise. Tables and figures show values for every genotype, reproductive state, and choline intake group, whereas data in the text report average over choline intake groups when there is not a choline intake by genotype interaction, and average over reproductive states when there is not a reproductive state by genotype interaction.
TABLE 1.
Genotype distribution (no. of participants) among reproductive states and choline intake groups
| Choline intake and variant alleles (n) | ||||||
|---|---|---|---|---|---|---|
| Genotype and reproductive state | 480 mg/d |
930 mg/d |
||||
| 0 | 1 | 2 | 0 | 1 | 2 | |
| MTHFR rs1801133 | ||||||
| Lactating | 4 | 7 | 1 | 5 | 6 | 1 |
| NP | 3 | 4 | 3 | 6 | 5 | 0 |
| Pregnant | 5 | 8 | 0 | 6 | 5 | 2 |
| MTR rs1805087 | ||||||
| Lactating | 8 | 4 | 0 | 8 | 4 | 0 |
| NP | 6 | 3 | 1 | 8 | 3 | 0 |
| Pregnant | 10 | 3 | 0 | 8 | 5 | 0 |
| MTHFD1 rs2236225 | ||||||
| Lactating | 4 | 7 | 1 | 1 | 8 | 3 |
| NP | 1 | 7 | 2 | 2 | 8 | 1 |
| Pregnant | 6 | 6 | 1 | 4 | 5 | 4 |
| MTRR rs1801394 | ||||||
| Lactating | 6 | 3 | 3 | 3 | 7 | 2 |
| NP | 2 | 6 | 2 | 5 | 3 | 3 |
| Pregnant | 2 | 9 | 2 | 2 | 7 | 4 |
Because of the relatively low prevalence of the variant allele, heterozygous and homozygous variant individuals were combined to examine the effect of variant allele presence on metabolic outcomes.
RESULTS
MTHFR rs1801133 (c.677 C > T; p.Ala223Val; RefSeq NT_021937.17)
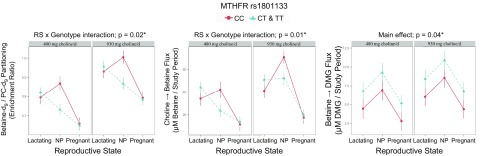
Genotype interacted with reproductive state to influence the partitioning of dietary choline between betaine synthesis and CDP-PC synthesis (P = 0.02), as well as the metabolic flux of choline → betaine (P = 0.01; Fig. 2, Table 2, and Supplemental Table 1). Among NP women, variant women exhibited a lower betaine-d9/PC-d9 enrichment ratio compared with nonvariant women (0.8 ± 0.03 vs. 0.9 ± 0.04; P = 0.01) and a lower turnover of choline → betaine (38 ± 5 vs. 56 ± 5 µM betaine/study period; P = 0.05; Table 2).
Figure 2.
Effect of MTHFR (dbSNP: rs1801133; c.677 C > T; p.Ala223Val; RefSeq NT_021937.17) genotype on the metabolic flux and partitioning of plasma choline metabolites. RS, reproductive state.
TABLE 2.
MTHFR rs1801133 genotype alters plasma choline metabolite partitioning and flux
| MTHFR rs1801133 group | 480 mg/d choline |
930 mg/d choline |
||||
|---|---|---|---|---|---|---|
| WT | Variant | P | WT | Variant | P | |
| Betaine-d9/PC-d9 | ||||||
| RS × gene interaction; P = 0.02* | ||||||
| Lactating | 0.79 ± 0.04 | 0.82 ± 0.03 | >0.99 | 0.93 ± 0.03 | 0.96 ± 0.03 | >0.99 |
| NP | 0.87 ± 0.04 | 0.73 ± 0.03 | 0.01** | 1.0 ± 0.03 | 0.87 ± 0.03 | 0.01** |
| Pregnant | 0.66 ± 0.03 | 0.65 ± 0.03 | >0.99 | 0.79 ± 0.03 | 0.79 ± 0.03 | >0.99 |
| Choline → betaine | ||||||
| RS × gene interaction; P = 0.01** | ||||||
| Lactating | 34 ± 6 | 44 ± 5 | 0.6 | 41 ± 6 | 51 ± 5 | 0.6 |
| NP | 42 ± 7 | 24 ± 5 | 0.05* | 71 ± 6 | 52 ± 6 | 0.05* |
| Pregnant | 11 ± 6 | 14 ± 5 | >0.99 | 18 ± 5 | 20 ± 5 | >0.99 |
| Betaine → DMG | ||||||
| Main effect; P = 0.04** | ||||||
| Lactating | 4.4 ± 1.4 | 6.8 ±1.2 | 0.04** | 6.0 ± 1.3 | 8.4 ± 1.2 | 0.04** |
| NP | 6.9 ± 1.4 | 9.3 ± 1.2 | 0.04** | 8.5 ± 1.3 | 10.9 ± 1.3 | 0.04** |
| Pregnant | 2.8 ± 1.3 | 5.1 ± 1.1 | 0.04** | 4.4 ± 1.2 | 6.8 ± 1.2 | 0.04** |
Values are least-squares means ± se. Betaine-d9/PC-d9 values are ratios, choline → betaine values are in millimolar betaine per study period, and betaine → DMG values are in micromolar DMG/study period. RS, reproductive state. P values represent the highest order interaction or main effect; symbols denote significance at the indicated α-cutoff. *P ≤ 0.05, **P ≤ 0.01.
Notably, NP variant women in the higher choline intake group (930 mg/d) exhibited metabolic profiles reminiscent of NP nonvariant women at the lower (recommended) choline intake (Table 3). Specifically, differences in betaine-d9/PC-d9 enrichment ratios and turnover of choline → betaine were not observed between variants in the higher intake group and nonvariants in the lower intake group (P > 0.99; Table 3).
TABLE 3.
Comparison of plasma betaine-d9/PC-d9 partitioning and choline → betaine turnover between NP MTHFR rs1801133 and MTR rs1805087 non-risk genotype women consuming 480 mg/d choline and risk genotype women consuming 930 mg/d choline
| NP group | 480 mg/d choline, risk genotype |
930 mg/d choline, non-risk genotype |
P |
|---|---|---|---|
| MTHFR rs1801133 | Nonvariant | Variant | |
| Betaine-d9/PC-d9 | 0.87 ± 0.04 | 0.87 ± 0.03 | >0.99 |
| Choline → betaine | 42 ± 7 | 52 ± 6 | >0.99 |
| MTR rs1805087 | Variant | Nonvariant | |
| Betaine-d9/PC-d9 | 0.86 ± 0.04 | 0.90 ± 0.03 | >0.99 |
Values are least-squares means ± se. Betaine-d9/PC-d9 values are ratios, and choline → betaine values are in micromolar betaine per study period.
Finally, across reproductive states, variant women exhibited a greater flux of betaine → DMG within the study period than nonvariant women (7.9 ± 0.7 vs. 5.5 ± 0.9 µM DMG/study period; P = 0.04; Fig. 2 and Table 2).
MTR rs1805087 (c.2756 A > G; p.Asp919Gly; RefSeq NG_008959.1)
MTR nonvariant women exhibited metabolic profiles similar to women with the MTHFR risk genotype.
First, genotype had a near-significant interaction with reproductive state to influence the partitioning of dietary choline between betaine synthesis and CDP-PC synthesis (P = 0.06; Fig. 3 and Table 4). Although multiple comparisons diminished significance, NP nonvariant women exhibited a lower betaine-d9/PC-d9 enrichment ratio compared with NP variant women (0.8 ± 0.03 vs. 0.9 ± 0.04; P = 0.07; Table 4).
Figure 3.
Effect of MTR (dbSNP: rs1805087; c.2756 A > G; p.Asp919Gly; RefSeq NG_008959.1) genotype on the metabolic flux and partitioning of plasma choline metabolites.
TABLE 4.
MTR rs1805087 genotype alters plasma choline metabolite partitioning and flux
| MTR rs1805087 group | 480 mg/d choline |
930 mg/d choline |
||||
|---|---|---|---|---|---|---|
| WT | Variant | P | WT | Variant | P | |
| Betaine-d9/PC-d9 | ||||||
| RS × gene interaction; P = 0.06 | ||||||
| Lactating | 0.81 ± 0.03 | 0.80 ± 0.04 | >0.99 | 0.96 ± 0.03 | 0.95 ± 0.04 | >0.99 |
| NP | 0.74 ± 0.03 | 0.86 ± 0.04 | 0.07 | 0.90 ± 0.03 | 1.01 ± 0.04 | 0.07 |
| Pregnant | 0.65 ± 0.03 | 0.63 ± 0.04 | >0.99 | 0.81 ± 0.03 | 0.78 ± 0.04 | >0.99 |
| Choline → betaine | ||||||
| 3-Way interaction; P = 0.008** | ||||||
| Lactating | 41 ± 5 | 40 ± 7 | >0.99 | 46 ± 5 | 48 ± 7 | >0.99 |
| NP | 33 ± 6 | 23 ± 7 | >0.99 | 50 ± 5 | 94 ± 9 | <0.001** |
| Pregnant | 12 ± 5 | 16 ± 9 | >0.99 | 19 ± 5 | 18 ± 7 | >0.99 |
| Betaine → methionine | ||||||
| Cho × gene interaction; P = 0.001*** | ||||||
| Lactating | 1.31 ± 0.09 | 1.54 ± 0.11 | 0.1 | 1.44 ± 0.09 | 1.18 ± 0.11 | 0.05* |
| NP | 1.42 ± 0.10 | 1.65 ± 0.11 | 0.1 | 1.76 ± 0.09 | 1.49 ± 0.11 | 0.05* |
| Pregnant | 1.79 ± 0.08 | 2.02 ± 0.11 | 0.1 | 2.29 ± 0.09 | 2.03 ± 0.10 | 0.05* |
Values are least-squares means ± se. Betaine-d9/PC-d9 values are ratios, choline → betaine values are in micromolar betaine/study period, and betaine → methionine values are in micromolar methionine/study period. Cho, choline intake level. P values represent the highest order interaction or main effect; symbols denote significance at the indicated α-cutoff. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001.
Secondly, genotype interacted with reproductive state and choline intake to influence the flux of choline → betaine (P = 0.008; Fig. 3 and Table 4). Genotypic differences were not observed in the lower choline intake group. However, within the higher choline intake group, NP nonvariant women exhibited a lower flux of choline → betaine than NP variant women (50.5 ± 5 vs. 94 ± 9 µM betaine/study period; P = 0.0008; Fig. 3 and Table 4). Furthermore, different responses to increased choline intake were detected between variant and nonvariant women. Whereas NP nonvariant women did not display differences in choline → betaine turnover as a function of choline intake, NP variant women used more dietary choline for betaine synthesis in the higher intake group compared with the lower intake group (94 ± 9 vs. 23 ± 7 µM betaine/study period; P = 5.8 × 10−7; Table 5). Like the MTHFR risk genotype, NP women without the MTR variant in the higher choline intake (930 mg/d) exhibited betaine-d9/PC-d9 enrichment ratios reminiscent of MTR variant women in the lower (recommended) intake group (P > 0.99; Table 3).
TABLE 5.
Comparison of choline → betaine and betaine → methionine turnover between choline intake groups among MTR rs1805087 variant and nonvariant women
| MTR rs1805087 group | 480 mg/d choline | 930 mg/d choline | P |
|---|---|---|---|
| Choline → betaine | |||
| NP WT | 33 ± 6 | 50 ± 5 | >0.99 |
| NP variant | 23 ± 7 | 94 ± 9 | <0.001*** |
| Betaine → methionine | |||
| WT | 1.5 ± 0.06 | 1.8 ± 0.06 | <0.001*** |
| Variant | 1.7 ± 0.08 | 1.6 ± 0.08 | 0.6 |
Values are least-squares means ± se in millimolar product per study period; symbols denote significance at the indicated α-cutoff. ***P ≤ 0.001.
Finally, genotype interacted with choline intake to influence the metabolic flux of betaine → methionine (P = 0.001). MTR nonvariant women and MTR variant women again displayed different responses to increased choline intake. Nonvariant women in the higher intake group exhibited a greater turnover of betaine → methionine (1.8 ± 0.06 µM methionine/study period) compared with MTR nonvariant women in the lower intake group (1.5 ± 0.06 µM methionine/study period; P = 0.0008), as well as compared with variant women in the higher intake group (1.6 ± 0.08 µM methionine/study period; P = 0.05; Fig. 3 and Tables 4 and 5). Conversely, variant women did not display differences in betaine → methionine turnover as a function of choline intake (P = 0.6).
MTRR rs1801394 (c.66 A > G; p.Ile22Met; RefSeq NG_008856.1)
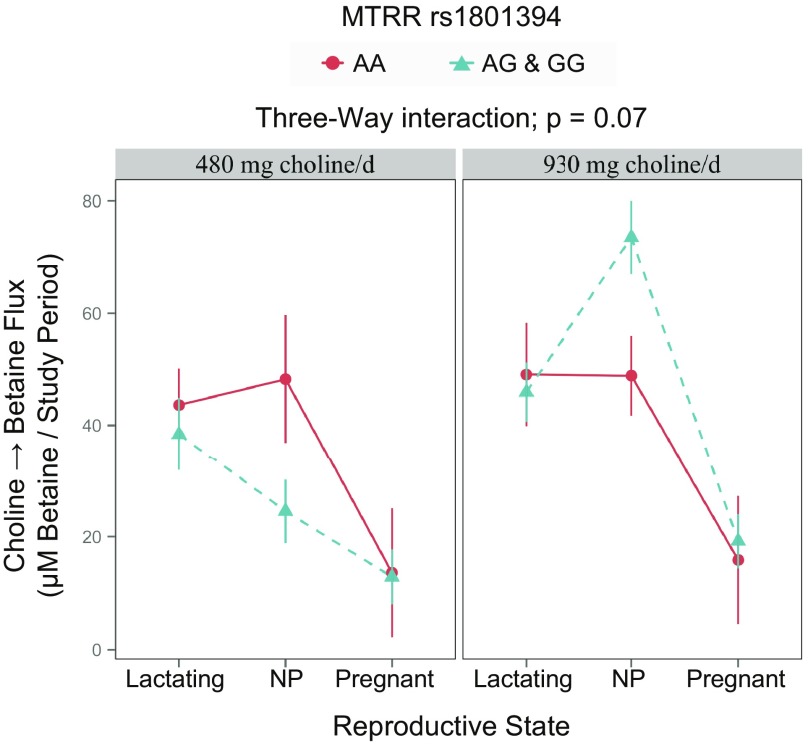
MTRR genotype interacted with reproductive state and choline intake to influence the metabolic flux of choline → betaine (P = 0.07; Fig. 4 and Table 6). Whereas genotypic differences were not detected within choline intake groups (P > 0.16), a differential response to choline intake was observed among NP women, with and without the variant, similar to MTR risk genotype carriers. Specifically, whereas differences in choline → betaine were not observed as a function of choline intake among those without the variant (P > 0.99), those with the variant had a greater turnover of choline → betaine in the higher intake group compared with the lower intake group (73 ± 6 vs. 49 ± 7; P = 6 × 10−6; Tables 6 and 7).
Figure 4.
Effect of MTRR (dbSNP: rs1801394; c.66 A > G; p.Ile22Met; RefSeq NG_008856.1) genotype on the metabolic flux of plasma choline metabolites.
TABLE 6.
The effect of MTRR rs1801394 genotype on the metabolic flux of choline → betaine
| MTRR rs18011394 group | 480 mg/d choline |
930 mg/d choline |
||||
|---|---|---|---|---|---|---|
| Nonvariant | Variant | P | Nonvariant | Variant | P | |
| Choline → betaine | ||||||
| 3-Way interaction; P = 0.07 | ||||||
| Lactating | 44 ± 7 | 38 ± 7 | >0.99 | 49 ± 9 | 46 ± 5 | >0.99 |
| NP | 48 ± 11 | 25 ± 6 | 0.8 | 49 ± 7 | 73 ± 7 | 0.2 |
| Pregnant | 14 ± 11 | 13 ± 5 | >0.99 | 16 ± 11 | 19 ± 5 | >0.99 |
Values are ratios as least-squares means ± se. P values represent the highest order interaction or main effect.
TABLE 7.
Comparison of betaine → methionine turnover between choline intake groups among MTRR rs1801394 variant and nonvariant women
| MTRR rs1801394 choline→ betaine | 480 mg/d choline | 930 mg/d choline | P |
|---|---|---|---|
| Variant | |||
| Lactating | 38 ± 7 | 46 ± 5 | >0.99 |
| NP | 25 ± 6 | 73 ± 7 | <0.001 |
| Pregnant | 13 ± 5 | 19 ± 5 | >0.99 |
| Nonvariant | |||
| Lactating | 44 ± 7 | 49 ± 9 | >0.99 |
| NP | 48 ± 11 | 49 ± 7 | >0.99 |
| Pregnant | 14 ± 11 | 16 ± 11 | >0.99 |
Values are least-squares means ± se in ratios.
MTHFD1 rs2236225 (c.1958 G > A; p.Arg653Gln; RefSeq NT_026437.13)
Much like women with the MTHFR and MTR risk genotypes, the MTHFD1 variant influenced the partitioning of dietary choline between the CDP-choline pathway and betaine synthesis.
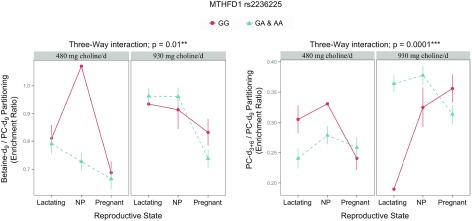
Genotype interacted with reproductive state and choline intake to influence enrichment of betaine-d9/PC-d9 (P = 0.01; Fig. 5 and Table 8). Among NP women in the lower intake group, variant women had a betaine-d9/PC-d9 enrichment ratio well below that of the representative nonvariant individual (variant least-squares mean: 0.73, nonvariant least-squares mean: 1.07); importantly, the variant’s 95% CI (0.66–0.79) did not include the nonvariant (1.07; Table 8). Genotypic differences were not observed within the higher choline intake group (P > 0.99; Fig. 5 and Table 8). However, NP and lactating variant women exhibited higher (P < 0.003) betaine-d9/PC-d9 enrichment ratios in the higher intake group (0.96 ± 0.03 and 0.96 ± 0.03, respectively) than in the lower intake group (0.73 ± 0.03 and 0.79 ± 0.03, respectively; Table 9).
Figure 5.
Effect of MTHFD1 (dbSNP: rs2236225; c.1958 G > A; p.Arg653Gln; RefSeq NT_026437.13) genotype on the metabolic partitioning of plasma choline metabolites.
TABLE 8.
MTHFD1 rs2236225 genotype alters plasma choline metabolite partitioning
| MTHFD1 rs2236225 group | 480 mg/d choline |
930 mg/d choline |
||||
|---|---|---|---|---|---|---|
| Nonvariant | Variant | P | Nonvariant | Variant | P | |
| Betaine-d9/PC-d9 | ||||||
| 3-Way interaction; P = 0.01** | ||||||
| Lactating | 0.81 ± 0.05 | 0.79 ± 0.03 | >0.99 | 0.93a | 0.96 ± 0.03 | in CI |
| NP | 1.07a | 0.73 ± 0.03 | ɸ | 0.91 ± 0.07 | 0.96 ± 0.03 | >0.99 |
| Pregnant | 0.69 ± 0.04 | 0.67 ± 0.04 | >0.99 | 0.83 ± 0.05 | 0.74 ± 0.03 | >0.99 |
| PC-d3 + 6/PC-d9 | ||||||
| 3-Way interaction; P = 0.0001*** | ||||||
| Lactating | 0.31 ± 0.02 | 0.24 ± 0.02 | 0.2 | 0.19a | 0.36 ± 0.01 | ɸ |
| NP | 0.33a | 0.28 ± 0.02 | ɸ | 0.32 ± 0.03 | 0.38 ± 0.02 | >0.99 |
| Pregnant | 0.24 ± 0.02 | 0.26 ± 0.02 | >0.99 | 0.36 ± 0.02 | 0.31 ± 0.02 | >0.99 |
Values are ratios as least-squares means ± se. ɸ, the least-squares mean of the representative individual falls outside of the 95% CI of the comparing group. P values represent the highest order interaction or main effect; symbols denote significance at the indicated α-cutoff.
Not available, single participant.
**P ≤ 0.01, ***P ≤ 0.001.
TABLE 9.
Comparison of betaine→methionine turnover between choline intake groups among MTHFD1 rs2236225 variant and nonvariant women
| MTHFD1 rs2236225 variant | 480 mg/d choline | 930 mg/d choline | P |
|---|---|---|---|
| Betaine-d9/PC-d9 | |||
| Lactating | 0.79 ± 0.03 | 0.96 ± 0.03 | 0.003** |
| NP | 0.73 ± 0.03 | 0.96 ± 0.03 | <0.001*** |
| Pregnant | 0.67 ± 0.04 | 0.74 ± 0.03 | >0.99 |
| PC-d3 + 6/PC-d9 | |||
| Lactating | 0.24 ± 0.02 | 0.36 ± 0.01 | <0.001*** |
| NP | 0.28 ± 0.02 | 0.38 ± 0.02 | <0.001*** |
| Pregnant | 0.26 ± 0.02 | 0.31 ± 0.02 | 0.2 |
Values are least-squares means ± se in ratios. Symbols denote significance at the indicated α-cutoff. **P ≤ 0.01, ***P ≤ 0.001.
Furthermore, within the higher intake group, both NP and lactating women with the variant exhibited a greater betaine-d9/PC-d9 enrichment ratio (both 0.96 ± 0.03) than variant pregnant women (0.74 ± 0.03; P < 0.001), although NP and lactating women with the variant (both 0.96 ± 0.03) did not differ (P = 0.2) from nonvariant NP women (0.83 ± 0.05) or the representative nonvariant woman (0.93).
Genotype also interacted with reproductive state and choline intake to influence the partitioning of dietary choline between PEMT-PC (PC-d3 + 6) and CDP-PC (PC-d9; P = 0.0001; Fig. 5 and Table 8). Among NP women consuming the lower choline intake, variants had a PC-d3 + 6/PC-d9 enrichment ratio below that of the nonvariant individual (variant least-squares mean: 00.24 ± 0.02, nonvariant least-squares mean: 0.31); importantly, the variant’s 95% CI (0.21–0.27) did not include the nonvariant (0.19; Fig. 5). Among lactating women consuming the higher choline intake, variants had a PC-d3 + 6/PC-d9 enrichment ratio nearly double that of the nonvariant individual (00.36 ± 0.01 vs. 0.19); importantly, the variant’s 95% CI (0.34–0.39) did not include the nonvariant (0.19; Fig. 5 and Table 8). In addition, NP and lactating women with the variant and nonvariant pregnant women exhibited increased PC-d3 + 6/PC-d9 as a function of choline intake, displaying a greater PC-d3 + 6/PC-d9 enrichment ratio at the higher choline intake compared with the lower intake (NP variant: 0.38 ± 0.02 vs. 0.28 ± 0.02; P = 0.0002; lactating variant: 0.36 ± 0.01 vs. 0.24 ± 0.02; P = 3 × 10−6; pregnant nonvariant: 0.36 ± 0.02 vs. 0.24 ± 0.02; P = 0.002; Table 9). Pregnant women with the variant also exhibited a greater PC-d3 + 6/PC-d9 enrichment ratio in the higher choline intake group compared with the lower intake group, but this difference was no longer significant after adjusting for multiple comparisons (0.31 ± 0.02 vs. 0.26 ± 0.02; P = 0.2; Table 9).
DISCUSSION
The present study is one of few to explore gene by nutrient interactions using stable isotope methodology, particularly within the context of a controlled feeding study. The use of an isotope label allows for the detection of dynamic changes in metabolite flux, providing an advantage over commonly used static measures, which do not reflect kinetic changes (24). In fact, Zeisel and colleagues (25) found that plasma concentrations of choline, betaine, and PC did not correlate with clinical signs of choline deficiency in humans deprived of choline. Conversely, although our post hoc design allowed us the benefit of a controlled environment, the original study did not consider genotype during study enrollment, leading to unequal genotype distribution that lessened our ability to evaluate pair-wise comparisons within some higher-order interactions. To address this limitation and avoid overinterpretation of any single comparison with a small sample size, we focused on the identification of broad patterns across genes, metabolites, and tissues that arose from many separate comparisons. This approach also mitigates the effect of myriad unexamined genetic factors that may be unequally distributed within the study population. Overall, the following several themes common to risk genotypes emerged from our data.
Variants impairing folate enzymes increase the use of dietary choline for PC biosynthesis
Adequate PC is critical for the structural integrity of cell membranes and cell survival. Both the CDP-choline pathway, which uses choline as a substrate, and the PEMT-PC pathway, which uses SAM as a methyl donor, have a high metabolic priority. Indeed, one of the primary uses of SAM is for PEMT-PC-synthesis (26). Given that PEMT-PC comprises a substantial portion of cellular PC pools (∼30% based on data from rat liver extracts) (27), impairments in folate-dependent SAM synthesis may decrease the contribution of folate-derived methyl groups for cellular PC production and increase the metabolic burden on choline-dependent PC synthesis. Our results, described below, support this notion by demonstrating that women with common genetic variants (MTHFR rs1801133, MTR rs1805087 WT, and MTHFD1 rs2236225) impairing folate-dependent enzymes display increased use of dietary choline for PC synthesis, primarily through the CDP-choline pathway.
MTHFR rs1801133
The MTHFR rs1801133 variant encodes a thermolabile enzyme with reduced capacity to bind its cofactor, flavin adenine dinucleotide (28). This translates to impaired production of 5-methyl THF, the substrate for the folate-dependent remethylation of homocysteine to methionine. Under conditions of folate deprivation, the variant is associated with decreased folate status and increased use of the choline derivative betaine for methyl donation (17). Our data extend these findings to include women consuming a high folate diet.
Women with the rs1801133 variant used more betaine as a methyl donor, as indicated by a greater turnover of betaine → DMG within the study period. Notably, despite the increased turnover of betaine, NP variant women preferentially partitioned choline to the CDP-choline pathway at the expense of betaine synthesis, as indicated by decreased turnover of choline → betaine and a lower betaine-d9/PC-d9 enrichment ratio (Fig. 2 and Table 2). The enhanced partitioning of choline to the CDP-choline pathway, despite greater use of betaine as a methyl donor, may be a response to impaired PC synthesis via the PEMT pathway, which is dependent on folate-derived SAM. Although choline-derived methyl groups can also be used for SAM synthesis, SAM is used for many different reactions in the cell, whereas the CDP-choline pathway is a more direct method of PC production. Among NP variant women, higher choline intakes (930 mg/d) restored the rate of conversion of choline → betaine and the partitioning between betaine and the CDP-choline pathway to levels observed among nonvariant women in the 480 mg/d choline group (Fig. 2 and Table 3). Given the low presence of homozygous variants within our sample, these findings largely reflect the effect of 1 copy of the variant, and the burden on dietary choline for PC synthesis may be compounded further in women with 2 copies of the variant.
MTR rs1805087
MTR catalyzes the folate-dependent conversion of homocysteine to methionine (29). The MTR rs1805087 variant encodes an aspartate-to-glycine amino acid change located in the region of the enzyme that binds accessory proteins to facilitate the regeneration of the active enzyme when oxidized (30). The variant has been associated with decreased plasma homocysteine concentration, indicating that it may provide a gain of function (i.e., an increased use of folate-derived methyl groups for the remethylation of homocysteine to methionine) (31, 32). Thus, although the variant has been associated with disease risk (33, 34), within the context of choline requirement, the WT genotype may constitute a risk genotype given the comparatively reduced efficiency of folate-dependent homocysteine remethylation.
Indeed, our findings for the MTR WT genotype support this notion and parallel those observed with the MTHFR rs1801133 risk genotype, indicating a prioritization of dietary choline for PC production when folate-dependent SAM synthesis is impaired. Specifically, within the higher choline intake group, NP women with the MTR WT risk genotype partitioned more choline to the CDP-choline pathway at the expense of betaine synthesis, as indicated by a decreased turnover of choline → betaine, and a lower betaine-d9/PC-d9 enrichment ratio, compared with MTR variant women (Fig. 3 and Table 4). Even under conditions of folate adequacy, NP MTR WT women displayed an increased use of choline as a methyl donor in response to increased dietary choline, as indicated by a greater turnover of betaine → methionine (Fig. 3 and Table 5). In contrast, although NP MTR variant women exhibited an increased flux of choline → betaine with increased choline intake, they did not increase their use of betaine as a methyl donor, instead accumulating plasma betaine, which manifested as larger plasma betaine pools among variant women compared with WT women in the higher choline intake group (53 ± 5 vs. 35 ± 3 µM betaine; P = 0.003). This supports the notion that NP women with the variant may have a decreased need for dietary choline compared with WT women in conditions of folate adequacy. In addition, women with the MTR WT risk genotype may benefit from choline intakes exceeding the current recommendation, given the restoration of choline partitioning among WT women in the higher choline intake group to levels observed in the MTR variant women in the lower intake group (Fig. 3 and Table 3).
MTRR rs1801394
MTRR is a diflavin enzyme that participates in methionine biosynthesis by regenerating the oxidized MTR-Co(II) to active enzyme via a reductive methylation with SAM (35). Some, but not all, studies have identified moderate associations between the MTRR rs1801394 variant and neural tube defects (36) and elevated homocysteine concentrations, particularly in the presence of the MTHFR rs1801133 variant and low vitamin B12 status (37). As a result of power limitations, we did not consider vitamin B12 status or MTHFR rs1801133 genotype and therefore, may not have fully captured its metabolic consequences. We did, however, detect a different response among variant individuals to increased choline intake (Fig. 4 and Table 7). Although nonvariants did not use additional choline for betaine synthesis, variants exhibited increased turnover of choline → betaine in the higher choline intake group, suggesting that variants may have a greater need for choline-derived methyl groups.
MTHFD1 rs2236225
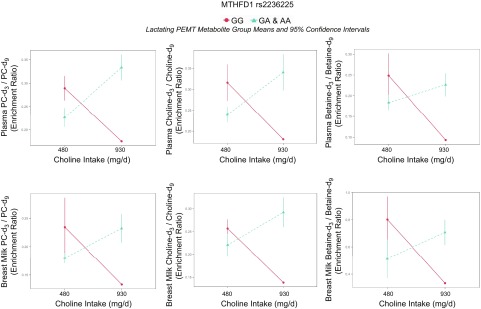
The MTHFD1 rs2236225 polymorphism encodes a thermolabile enzyme with an ∼50% reduction in enzymatic activity (38) and appears to favor thymidylate biosynthesis at the expense of folate-dependent homocysteine remethylation. The variant also increases susceptibility to choline deficiency among premenopausal women deprived of choline (7). Our findings suggest that the MTHFD1 rs2236225 variant may increase susceptibility to choline and PC inadequacy, even at choline intakes that align with current recommendations. NP variant women consuming 480 mg/d choline preferentially partitioned choline toward the CDP-choline pathway at the expense of betaine synthesis (i.e., a lower betaine-d9/PC-d9 enrichment ratio was observed among variant women compared with the representative nonvariant woman), which signifies a strain on cellular PC production (Fig. 5). In addition, pregnant and lactating women exhibited increased CDP-PC synthesis across genotypes at the lower intake level, indicating a greater need for choline-dependent PC production during these reproductive states (Fig. 5).
Nonetheless, our data showed favorable metabolic changes among NP and lactating women with the MTHFD1 rs2236225 variant when choline intake exceeded current recommendations. Both NP and lactating variant women displayed greater betaine-d9/PC-d9, suggesting that increased dietary choline among variant carriers may decrease strain on PC production capacity (Fig. 5 and Table 8). Notably, at the higher choline intake level, nonvariant women, at all 3 reproductive states, as well as NP and lactating women with the variant, exhibited similar partitioning between betaine and the CDP-choline pathway (Fig. 5). This suggests that intakes of 930 mg/d are sufficient to restore partitioning among pregnant and lactating women without the variant to levels observed in NP women, as well as partitioning among NP and lactating women with the variant to levels observed among nonvariants. Variant pregnant women, on the other hand, exhibited preferential partitioning to the CDP-choline pathway, even at the higher choline intake level, indicating that pregnant women with the variant may have a comparatively greater requirement for dietary choline to support PC production.
A second favorable metabolic change observed among variant NP and lactating women consuming higher choline intakes was the greater relative presence of PEMT-derived metabolites in circulation. Both NP and lactating women with the variant exhibited greater PC-d3 + 6/PC-d9, indicating that a greater portion of the dietary choline converted to PC was synthesized through the PEMT-PC pathway (Fig. 5 and Tables 8 and 9). In addition, among lactating women, higher choline consumption yielded greater enrichments of favorable PEMT-derived metabolites in plasma and breast milk compared with the representative nonvariant lactating woman in the higher intake group, as well as compared with lactating variant women in the lower intake group (Fig. 6 and Tables 10 and 11). PEMT-PC and its lipid derivatives are enriched with long-chain polyunsaturated fatty acids, such as DHA (39), which beneficially influence brain development (40). Improved plasma and breast-milk PEMT metabolite composition may therefore provide an evolutionary basis for the high prevalence of this SNP in populations consuming high choline diets, despite its association with increased disease risk and evidence for negative selection in low choline environments (41).
Figure 6.
Effect of MTHFD1 (dbSNP: rs2236225; c.1958 G > A; p.Arg653Gln; RefSeq NT_026437.13) genotype on enrichment ratios of PEMT-derived d3 choline metabolites to dietary choline-derived d9 choline metabolites.
TABLE 10.
MTHFD1 rs2236225 genotype alters plasma and breast-milk choline metabolite partitioning among lactating women
| MTHFD1 rs2236225 group | 480 mg/d choline |
930 mg/d choline |
||||
|---|---|---|---|---|---|---|
| Nonvariant | Variant | In CI? | Nonvariant | Variant | In CI? | |
| Plasma | ||||||
| PC-d3/PC-d9 | 0.29 (0.26–0.32) | 0.23 (0.21–0.25) | No | 0.18a | 0.33 (0.31–0.36) | No |
| Choline-d3/choline-d9 | 0.32 (0.27–0.36) | 0.24 (0.22–0.26) | No | 0.18a | 0.34 (0.30–0.36) | ɸ |
| Betaine-d3/betaine-d9 | 0.25 (0.20–0.30) | 0.18 (0.17–0.20) | No | 0.09a | 0.23 (0.20–0.25) | No |
| Breast milk | ||||||
| PC-d3/PC-d9 | 0.23 (0.18–0.29) | 0.18 (0.17–0.19) | Yesb | 0.13a | 0.23 (0.21–0.26) | No |
| Choline-d3/choline-d9 | 0.26 (0.24–0.28) | 0.22 (0.20–0.25) | Yes* | 0.14a | 0.29 (0.26–0.34) | No |
| Betaine-d3/betaine-d9 | 0.80 (0.63–0.97) | 0.51 (0.37–0.66) | Yes* | 0.33a | 0.71 (0.61–0.80) | No |
Values are group means with 95% CIs in parentheses. Although nonoverlapping CIs indicate a significant difference, overlapping CIs do not necessarily exclude a significant difference. In cases where CIs were overlapping, a Student’s t test was performed using the Welch–Satterthwaite equation to calculate degrees of freedom.
Not available, single participant.
Student’s t test failed to reject the null hypothesis.
P ≤ 0.05 by Student’s t test.
TABLE 11.
Comparison of d3/d9 metabolite partitioning between choline intake groups among MTHFD1 rs2236225 lactating women consuming 480 or 930 mg/d choline
| MTHFD1 rs2236225 variant | 480 mg/d choline | 930 mg/d choline | In CI? |
|---|---|---|---|
| Plasma | |||
| PC-d3/PC-d9 | 0.23 (0.21–0.25) | 0.33 (0.31–0.36) | No |
| Choline-d3/choline-d9 | 0.24 (0.22–0.26) | 0.34 (0.30–0.36) | No |
| Betaine-d3/betaine-d9 | 0.18 (0.17–0.20) | 0.23 (0.20–0.25) | No |
| Breast milk | |||
| PC-d3/PC-d9 | 0.18 (0.17–0.19) | 0.23 (0.21–0.26) | No |
| Choline-d3/choline-d9 | 0.22 (0.20–0.25) | 0.29 (0.26–0.34) | No |
| Betaine-d3/betaine-d9 | 0.51 (0.37–0.66) | 0.71 (0.61–0.80) | Yes* |
Values are group means of ratios with 95% CIs in parentheses. In cases where CIs were overlapping, a Student’s t test was performed using the Welch–Satterthwaite equation to calculate degrees of freedom.
P ≤ 0.05 by Student's t test.
Pregnant women exhibit few alterations in choline metabolism as a function of SNPs impairing folate enzymes
Given that the demand for choline is higher during pregnancy and lactation and that genotypic effects are often more pronounced under conditions of nutrient inadequacy, we expected pregnant and lactating women to exhibit greater genotype-specific differences in choline partitioning than their NP counterparts. However, the metabolic flux of choline among pregnant women (and in some cases, lactating women) was similar across genotypes, perhaps because the risk genotypes altered choline dynamics in ways that mirror changes observed during pregnancy. Thus, the physiologic changes that alter choline metabolism in pregnancy (and to a lesser extent, lactation) seemingly outweigh the effect of genotype in many of the examined polymorphisms and indicate a greater choline requirement for this reproductive state as a whole.
Consideration of genotype in defining choline requirements
Our results indicate that genotype influences choline dynamics, even at and above the choline adequate intake (AI). Women with SNPs that impair folate-mediated methionine biosynthesis (i.e., MTHFR rs1801133, MTR rs1805087 WT, and MTHFD1 rs2236225) displayed a pattern of preferential partitioning of dietary choline to the CDP-choline pathway, despite using more betaine for methionine biosynthesis (i.e., MTHFR rs1801133, MTR rs1805087 WT). This indicates a greater burden on dietary choline for PC synthesis, perhaps as a result of impaired folate-dependent PC production through the PEMT pathway. Notably, across these risk genotypes, increased choline intake appeared to restore dietary choline partitioning between betaine and the CDP-choline pathway to levels observed in those without the risk allele (Table 3). These findings collectively suggest that even in conditions of folate adequacy, women of reproductive age with SNPs impairing folate metabolism may benefit from choline intakes exceeding current recommendations.
CONCLUSIONS
In sum, these data provide compelling evidence that common SNPs in folate-metabolizing enzymes modulate choline dynamics in women of reproductive age consuming choline and folate intakes relevant to the population at large. Carriers of SNPs that alter cellular methylation capacity may rely more on choline-dependent PC synthesis, as observed among MTR rs1805087 WT, MTHFR rs1801133, MTHFD1 rs2236225, and possibly MTRR rs1801394 variant women consuming 480 mg/d choline. Choline partitioning was restored with higher choline intakes (930 mg/d). The metabolic strain on choline for PC synthesis may be particularly pronounced in the general population, as American women consume, on average, 260 mg/d choline, well below the lower study intake level and choline AI (42). The present findings also suggest that the partitioning of dietary choline between betaine and the CDP-choline pathway is a sensitive indicator of subclinical differences in choline requirements. These metabolic differences, although not severe enough to present as acute muscle or liver pathologies, may have long-term consequences on human health and chronic disease. Further studies with greater sample size are needed to confirm these findings and identify whether such metabolic differences have clinical implications.
Supplementary Material
ACKNOWLEDGMENTS
A.B.G. thanks Leslie Ruff and LeClairRyan (New York, New York, USA) for generously sharing office space during the writing of the manuscript. This work was supported by the U.S. National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases (T32-DK007158), and by the U.S. Department of Agriculture, National Institute of Food and Agriculture (Hatch Project 231646). The content is the responsibility of the authors.
Glossary
- AI
adequate intake
- CDP
cytidine diphosphate
- CI
confidence interval
- dbSNP
Single Nucleotide Polymorphism Database
- DHA
docosahexaenoic acid
- DMG
dimethylglycine
- LC
liquid chromatography
- MS
mass spectrometry
- MTHFD1
methylenetetrahydrofolate dehydrogenase-methenyltetrahydrofolate cyclohydrolase-formyltetrahydrofolate synthetase
- MTHFR
methylenetetrahydrofolate reductase
- MTR
methionine synthase
- MTRR
methionine synthase reductase
- NCBI
U.S. National Center for Biotechnology Information
- NP
nonpregnant
- OMIM
Online Mendelian Inheritance in Man
- PC
phosphatidylcholine
- PEMT
phosphatidylethanolamine N-methyltransferase
- QE
Q Exactive
- RefSeq
U.S. Reference Sequence Database
- SAM
S-adenosylmethionine
- SNP
single nucleotide polymorphism
- THF
tetrahydrofolate
- WT
wild-type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
A. B. Ganz and M. A. Caudill designed research; A. B. Ganz, K. Shields, V. G. Fomin, Y. S. Lopez, S. Mohan, J. Lovesky, J. C. Chuang, A. Ganti, B. Carrier, J. Yan, S. Taeswuan, V. V. Cohen, C. C. Swersky, J. A. Stover, G. A. Vitiello, and O. V. Malysheva performed research; A. B. Ganz analyzed data; E. Mudrak consulted on a statistical approach; and A. B. Ganz and M. A. Caudill wrote the paper.
REFERENCES
- 1.Da Costa K. A., Kozyreva O. G., Song J., Galanko J. A., Fischer L. M., Zeisel S. H. (2006) Common genetic polymorphisms affect the human requirement for the nutrient choline. FASEB J. 20, 1336–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guinotte C. L., Burns M. G., Axume J. A., Hata H., Urrutia T. F., Alamilla A., McCabe D., Singgih A., Cogger E. A., Caudill M. A. (2003) Methylenetetrahydrofolate reductase 677C → T variant modulates folate status response to controlled folate intakes in young women. J. Nutr. 133, 1272–1280 [DOI] [PubMed] [Google Scholar]
- 3.Glunde K., Bhujwalla Z. M., Ronen S. M. (2011) Choline metabolism in malignant transformation. Nat. Rev. Cancer 11, 835–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.MRC Vitamin Study Research Group (1991) Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. Lancet 338, 131–137 [PubMed] [Google Scholar]
- 5.Pitkin R. M. (2007) Folate and neural tube defects. Am. J. Clin. Nutr. 85, 285S–288S [DOI] [PubMed] [Google Scholar]
- 6.Shaw G. M., Lu W., Zhu H., Yang W., Briggs F. B. S., Carmichael S. L., Barcellos L. F., Lammer E. J., Finnell R. H. (2009) 118 SNPs of folate-related genes and risks of spina bifida and conotruncal heart defects. BMC Med. Genet. 10, 49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Da Costa K. A., Corbin K. D., Niculescu M. D., Galanko J. A., Zeisel S. H. (2014) Identification of new genetic polymorphisms that alter the dietary requirement for choline and vary in their distribution across ethnic and racial groups. FASEB J. 28, 2970–2978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caudill M. A., Gregory J. F. III, Miller J., Shane B. (2012) Folate, choline, vitamin B-12 and vitamin B-6. In Biochemical, Physiological and Molecular Aspects of Human Nutrition, 3rd ed. (Stipanuk M. H., Caudill M. A., eds.), pp. 565–608, Elsevier Saunders, St. Louis, MO [Google Scholar]
- 9.Niculescu M. D., Zeisel S. H. (2002) Diet, methyl donors and DNA methylation: interactions between dietary folate, methionine and choline. J. Nutr. 132(8 Suppl), 2333S–2335S [DOI] [PubMed] [Google Scholar]
- 10.Fox J. T., Stover P. J. (2008) Folate-mediated one-carbon metabolism. Vitam. Horm. 79, 1–44 [DOI] [PubMed] [Google Scholar]
- 11.Shaw G. M., Carmichael S. L., Yang W., Selvin S., Schaffer D. M. (2004) Periconceptional dietary intake of choline and betaine and neural tube defects in offspring. Am. J. Epidemiol. 160, 102–109 [DOI] [PubMed] [Google Scholar]
- 12.Yan J., Jiang X., West A. A., Perry C. A., Malysheva O. V., Brenna J. T., Stabler S. P., Allen R. H., Gregory J. F. III, Caudill M. A. (2013) Pregnancy alters choline dynamics: results of a randomized trial using stable isotope methodology in pregnant and nonpregnant women. Am. J. Clin. Nutr. 98, 1459–1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davenport C., Yan J., Taesuwan S., Shields K., West A. A., Jiang X., Perry C. A., Malysheva O. V., Stabler S. P., Allen R. H., Caudill M. A. (2015) Choline intakes exceeding recommendations during human lactation improve breast milk choline content by increasing PEMT pathway metabolites. J. Nutr. Biochem. 26, 903–911 [DOI] [PubMed] [Google Scholar]
- 14.Jiang X., West A. A., Caudill M. A. (2014) Maternal choline supplementation: a nutritional approach for improving offspring health? Trends Endocrinol. Metab. 25, 263–273 [DOI] [PubMed] [Google Scholar]
- 15.Jiang X., Jones S., Andrew B. Y., Ganti A., Malysheva O. V., Giallourou N., Brannon P. M., Roberson M. S., Caudill M. A. (2014) Choline inadequacy impairs trophoblast function and vascularization in cultured human placental trophoblasts. J. Cell. Physiol. 229, 1016–1027 [DOI] [PubMed] [Google Scholar]
- 16.Jiang X., Yan J., West A. A., Perry C. A., Malysheva O. V., Devapatla S., Pressman E., Vermeylen F., Caudill M. A. (2012) Maternal choline intake alters the epigenetic state of fetal cortisol-regulating genes in humans. FASEB J. 26, 3563–3574 [DOI] [PubMed] [Google Scholar]
- 17.Yan J., Wang W., Gregory J. F. III, Malysheva O., Brenna J. T., Stabler S. P., Allen R. H., Caudill M. A. (2011) MTHFR C677T genotype influences the isotopic enrichment of one-carbon metabolites in folate-compromised men consuming d9-choline. Am. J. Clin. Nutr. 93, 348–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yan J., Jiang X., West A. A., Perry C. A., Malysheva O. V., Devapatla S., Pressman E., Vermeylen F., Stabler S. P., Allen R. H., Caudill M. A. (2012) Maternal choline intake modulates maternal and fetal biomarkers of choline metabolism in humans. Am. J. Clin. Nutr. 95, 1060–1071 [DOI] [PubMed] [Google Scholar]
- 19.West A. A., Yan J., Jiang X., Perry C. A., Innis S. M., Caudill M. A. (2013) Choline intake influences phosphatidylcholine DHA enrichment in nonpregnant women but not in pregnant women in the third trimester. Am. J. Clin. Nutr. 97, 718–727 [DOI] [PubMed] [Google Scholar]
- 20.Hol F. A., van der Put N. M. J., Geurds M. P. A., Heil S. G., Trijbels F. J. M., Hamel B. C. J., Mariman E. C. M., Blom H. J. (1998) Molecular genetic analysis of the gene encoding the trifunctional enzyme MTHFD (methylenetetrahydrofolate-dehydrogenase, methenyltetrahydrofolate-cyclohydrolase, formyltetrahydrofolate synthetase) in patients with neural tube defects. Clin. Genet. 53, 119–125 [DOI] [PubMed] [Google Scholar]
- 21.Frosst P., Blom H. J., Milos R., Goyette P., Sheppard C. A., Matthews R. G., Boers G. J., den Heijer M., Kluijtmans L. A., van den Heuvel L. P., Rozen R. (1995) A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat. Genet. 10, 111–113 [DOI] [PubMed] [Google Scholar]
- 22.Kim J. K., Harada K., Bamba T., Fukusaki E., Kobayashi A. (2005) Stable isotope dilution-based accurate comparative quantification of nitrogen-containing metabolites in Arabidopsis thaliana T87 cells using in vivo (15)N-isotope enrichment. Biosci. Biotechnol. Biochem. 69, 1331–1340 [DOI] [PubMed] [Google Scholar]
- 23.R Core Team. (2014) R: A Language and Eenvironment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. Available at: http://www.R-project.org/
- 24.Chew T. W., Jiang X., Yan J., Wang W., Lusa A. L., Carrier B. J., West A. A., Malysheva O. V., Brenna J. T., Gregory J. F. III, Caudill M. A. (2011) Folate intake, MTHFR genotype, and sex modulate choline metabolism in mice. J. Nutr. 141, 1475–1481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fischer L. M., daCosta K. A., Kwock L., Stewart P. W., Lu T. S., Stabler S. P., Allen R. H., Zeisel S. H. (2007) Sex and menopausal status influence human dietary requirements for the nutrient choline. Am. J. Clin. Nutr. 85, 1275–1285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jacobs R. L., Stead L. M., Devlin C., Tabas I., Brosnan M. E., Brosnan J. T., Vance D. E. (2005) Physiological regulation of phospholipid methylation alters plasma homocysteine in mice. J. Biol. Chem. 280, 28299–28305 [DOI] [PubMed] [Google Scholar]
- 27.Reo N. V., Adinehzadeh M., Foy B. D (2002) Kinetic analyses of liver phosphatidylcholine and phosphatidylethanolamine biosynthesis using 13C NMR spectroscopy. Biochim. Biophys. Acta 1580, 171–188 [DOI] [PubMed] [Google Scholar]
- 28.Kang S. S., Wong P. W., Susmano A., Sora J., Norusis M., Ruggie N. (1991) Thermolabile methylenetetrahydrofolate reductase: an inherited risk factor for coronary artery disease. Am. J. Hum. Genet. 48, 536–545 [PMC free article] [PubMed] [Google Scholar]
- 29.Leclerc D., Campeau E., Goyette P., Adjalla C. E., Christensen B., Ross M., Eydoux P., Rosenblatt D. S., Rozen R., Gravel R. A. (1996) Human methionine synthase: cDNA cloning and identification of mutations in patients of the cblG complementation group of folate/cobalamin disorders. Hum. Mol. Genet. 5, 1867–1874 [DOI] [PubMed] [Google Scholar]
- 30.Chen L. H., Liu M. L., Hwang H. Y., Chen L. S., Korenberg J., Shane B. (1997) Human methionine synthase. cDNA cloning, gene localization, and expression. J. Biol. Chem. 272, 3628–3634 [PubMed] [Google Scholar]
- 31.Harmon D. L., Shields D. C., Woodside J. V., McMaster D., Yarnell J. W. G., Young I. S., Peng K., Shane B., Evans A. E., Whitehead A. S. (1999) Methionine synthase D919G polymorphism is a significant but modest determinant of circulating homocysteine concentrations. Genet. Epidemiol. 17, 298–309 [DOI] [PubMed] [Google Scholar]
- 32.Silaste M. L., Rantala M., Sämpi M., Alfthan G., Aro A., Kesäniemi Y. A. (2001) Polymorphisms of key enzymes in homocysteine metabolism affect diet responsiveness of plasma homocysteine in healthy women. J. Nutr. 131, 2643–2647 [DOI] [PubMed] [Google Scholar]
- 33.Galbiatti, A. L., Ruiz, M. T., Raposo, L. S., and Maniglia, J. V. (2010) 5-Methyltetrahydrofolate-homocysteine methyltransferase gene polymorphism (MTR) and risk of head and neck cancer. Braz. J. Med. Biol. Res. 43, 445–450 [DOI] [PubMed]
- 34.Akbari M. T., Naderi A., Saremi L., Sayad A., Irani S., Ahani A. (2015) Methionine synthase A2756G variation is associated with the risk of retinoblastoma in Iranian children. Cancer Epidemiol. 39, 1023–1025 [DOI] [PubMed] [Google Scholar]
- 35.Olteanu H., Munson T., Banerjee R. (2002) Differences in the efficiency of reductive activation of methionine synthase and exogenous electron acceptors between the common polymorphic variants of human methionine synthase reductase. Biochemistry 41, 13378–13385 [DOI] [PubMed] [Google Scholar]
- 36.NTD Collaborative Group (2006) Neural tube defects and folate pathway genes: family-based association tests of gene-gene and gene-environment interactions. Environ. Health Perspect. 114, 1547–1552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilson A., Platt R., Wu Q., Leclerc D., Christensen B., Yang H., Gravel R. A., Rozen R. (1999) A common variant in methionine synthase reductase combined with low cobalamin (vitamin B12) increases risk for spina bifida. Mol. Genet. Metab. 67, 317–323 [DOI] [PubMed] [Google Scholar]
- 38.Christensen K. E., Rohlicek C. V., Andelfinger G. U., Michaud J., Bigras J. L., Richter A., Mackenzie R. E., Rozen R. (2009) The MTHFD1 p.Arg653Gln variant alters enzyme function and increases risk for congenital heart defects. Hum. Mutat. 30, 212–220 [DOI] [PubMed] [Google Scholar]
- 39.Pynn C. J., Henderson N. G., Clark H., Koster G., Bernhard W., Postle A. D. (2011) Specificity and rate of human and mouse liver and plasma phosphatidylcholine synthesis analyzed in vivo. J. Lipid Res. 52, 399–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Innis S. M. (2008) Dietary omega 3 fatty acids and the developing brain. Brain Res. 1237, 35–43 [DOI] [PubMed] [Google Scholar]
- 41.Silver M. J., Corbin K. D., Hellenthal G., da Costa K. A., Dominguez-Salas P., Moore S. E., Owen J., Prentice A. M., Hennig B. J., Zeisel S. H. (2015) Evidence for negative selection of gene variants that increase dependence on dietary choline in a Gambian cohort. FASEB J. 29, 3426–3435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chester, D. N., Goldman, J. D., Ahuja, J. K., and Moshfegh, A. J. (2011) Dietary Intakes of Choline: What We Eat in America, National Health and Nutrition Examination Survey (NHANES) 2007–2008. Food Surveys Research Group Dietary Data Brief No. 9, U.S. Department of Agriculture, Agricultural Research Service, Washington, DC. Available at: http://ars.usda.gov/Services/docs.htm?docid=19476
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.