Abstract
Bioactive lipids derived from the metabolism of polyunsaturated fatty acids are important mediators of the inflammatory response. Labor per se is considered a sterile inflammatory process. Intra-amniotic inflammation (IAI) due to microorganisms (i.e., intra-amniotic infection) or danger signals (i.e., sterile IAI) has been implicated in the pathogenesis of preterm labor and clinical chorioamnionitis at term. Early and accurate diagnosis of microbial invasion of the amniotic cavity (MIAC) requires analysis of amniotic fluid (AF). It is possible that IAI caused by microorganisms is associated with a stereotypic lipidomic profile, and that analysis of AF may help in the identification of patients with this condition. To test this hypothesis, we analyzed the fatty acyl lipidome of AF by liquid chromatography—mass spectrometry from patients in spontaneous labor at term and preterm gestations. We report that the AF concentrations of proinflammatory lipid mediators of the 5-lipoxygenase pathway are significantly higher in MIAC than in cases of sterile IAI. These results suggest that the concentrations of 5-lipoxygenase metabolites of arachidonic acid, 5-hydroxyeicosatetraenoic acid, and leukotriene B4 in particular could serve as potential biomarkers of MIAC. This finding could have important implications for the rapid identification of patients who may benefit from anti-microbial treatment.—Maddipati, K. R., Romero, R., Chaiworapongsa ,T., Chaemsaithong, P., Zhou, S.-L., Xu, Z., Tarca, A. L., Kusanovic, J. P., Gomez, R., Chaiyasit, N., Honn, K. V. Lipidomic analysis of patients with microbial invasion of the amniotic cavity reveals up-regulation of leukotriene B4.
Keywords: infection, inflammation, clinical chorioamnionitis, amniotic fluid, 5-lipoxygenase
Intra-amniotic inflammation (IAI) caused by microorganisms (i.e., intra-amniotic infection) or by danger signals [i.e., sterile IAI (SI)] is often subclinical (1–3) and is a major cause of maternal and neonatal morbidity (4–7). The differential diagnosis of these 2 conditions requires analysis of the amniotic fluid (AF) and has clinical implications, because treatment of infection requires the administration of antimicrobial agents. Early treatment has beneficial effects for both mother and neonate (8–10).
Bioactive lipids derived from the metabolism of polyunsaturated fatty acids (PUFAs), such as arachidonic acid, play a dual role in promoting and dampening the inflammatory response to injury because of microorganisms, such as bacteria, viruses, or sterile insults (11–19). Prostaglandins (20–33) and arachidonate lipoxygenase products, such as leukotriene B4 (LTB4) (34–36), are also involved in parturition at term.
The objective of this study was to determine whether the fatty acyl lipidome of AF analyzed by an unbiased liquid chromatography–mass spectometry (LC-MS) approach differs among patients with spontaneous labor at term (TLB), clinical chorioamnionitis at term (TCC), and preterm labor (PTL), as well as among subgroups of patients stratified according to the status of AF microbiology and inflammation.
MATERIALS AND METHODS
Study design and population
A retrospective cross-sectional study was conducted by searching the clinical database and Bank of Biologic Samples of the Detroit Medical Center, Wayne State University, and the Perinatology Research Branch of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services (NICHD/NIH/DHHS; Bethesda, MD, USA). The inclusion criteria were: 1) singleton gestation; 2) transabdominal amniocentesis performed between 20 and 42 wk of gestation, before the rupture of the chorioamniotic membranes; 3) absence of chromosomal or structural fetal anomalies; and 4) sufficient AF available for lipidomic studies. Women from the following groups with singleton pregnancies who had AF samples obtained by transabdominal amniocentesis were included: 1) women at term gestation with TLB (TLB; n = 35); 2) women with PTL and IAI [PTL-IAI; n = 25; divided into 2 subgroups: those without detectable microbial invasion of the amniotic cavity (MIAC) (PTL-IAI-noMIAC; n = 15) and those with demonstrable MIAC (PTL-IAI-MIAC; or intra-amniotic infection or microbe-associated IAI; n = 10)]; 3) women with a sonographic short cervix (SCX; n = 28); and 4) women with clinical chorioamnionitis at term (TCC; n = 24). Patients with TCC were further subdivided into those with IAI (TCC-IAI-MIAC; n = 12), those with SI (TCC-SI; n = 8), and those with no AIA (NI) (TCC-NI; n = 4). The microbial status of the amniotic cavity was determined by both broad-range PCR and cultivation techniques in patients with TCC, whereas only the cultivation approach was used in patients with PTL and SCX. The fatty acyl lipidome of human AF was analyzed by LC-MS.
All patients provided written informed consent before the collection of AF samples. The collection and utilization of the samples were approved by the Human Investigation Committee of the participating institutions and the Institutional Review Boards of the participating institutions where the patients received care. Many of these samples had been used in previous studies of the biology of cytokines and inflammatory mediators in intra-amniotic infection/inflammation, PTL (5, 37), and TCC (38–43).
Clinical definitions
Spontaneous term labor was defined as the presence of regular uterine contractions with a frequency of at least 1 every 10 min and cervical changes after 37 wk of gestation leading to delivery of a term neonate. Clinical chorioamnionitis was diagnosed by the presence of maternal fever (temperature, >37.8°C) accompanied by 2 or more of the following criteria: 1) maternal tachycardia (heart rate >100 beats/min); 2) uterine tenderness; 3) foul-smelling AF; 4) fetal tachycardia (heart rate >160 beats/min); and 5) maternal leukocytosis (leukocyte count >15,000 cells/mm3) (40). PTL was diagnosed by the presence of at least 2 regular uterine contractions every 10 min associated with cervical changes in patients with a gestational age between 20 and 36/37 wk. An SCX was defined as a cervical length ≤25 mm between 16 and 32 wk of gestation. IAI was diagnosed when the AF IL-6 concentration was ≥2.6 ng/ml, determined by ELISA (37, 44, 45). MIAC was defined according to the results of AF culture and PCR coupled with electrospray ionization mass spectrometry (PCR/ESI-MS; Ibis Technology; Athogen, Carlsbad, CA, USA). Based on the results of AF cultures and/or PCR/ESI-MS, as well as AF concentrations of IL-6, patients were classified as having NI (IL-6 < 2.6 ng/ml), intra-amniotic infection (combination of MIAC and IAI), or SI (IAI without detectable bacteria; an elevated AF IL-6 concentration without evidence of bacteria detected by either cultivation or molecular microbiologic methods).
Sonographic assessment of the cervix
Transvaginal ultrasound examinations were performed with commercially available ultrasound systems (Acuson Sequoia; Siemens Medical Systems, Mountain View, CA, USA; Voluson 730 Expert or Voluson E8, GE Healthcare, Milwaukee, WI, USA) equipped with endovaginal transducers with frequency ranges of 5–7.5 and 5–9 MHz, respectively. Sonographic examinations of the cervix were performed according to a published technique (46, 47).
Sample collection
AF samples were obtained by transabdominal amniocentesis performed for evaluation of the microbial and inflammatory status of the amniotic cavity in patients presenting with PTL or TCC, whereas patients approaching term underwent an amniocentesis for assessment of fetal lung maturity. The results of microbiology and inflammatory status of the AF were used by obstetricians and neonatologists in the management of mothers and neonates in terms of treatment with antibiotics. Women at term in labor consisted of those who were admitted for suspected PTL because of uncertain dates and who had an amniocentesis for the assessment of fetal lung maturity. The criteria for considering whether these patients were at term in labor were derived retrospectively as: 1) spontaneous labor; 2) delivery within 24 h of amniocentesis; 3) analysis of AF consistent with fetal lung maturity; 4) birth weight >2500 g; 5) absence of respiratory distress syndrome or other complications of prematurity; and 6) findings in physical examination of the newborn by a pediatrician consistent with a term neonate. Samples of AF were transported to the laboratory in a sterile capped syringe and cultured for aerobic/anaerobic bacteria and genital mycoplasmas. White blood cell (WBC) count (48), glucose concentration (49), and Gram stain (50) were also performed shortly after collection. The results of these tests were used for clinical management, in contrast to the results of AF IL-6 concentration, which were used only for research purposes. AF that was not needed for clinical assessment was centrifuged at 1300 g for 10 min at 4°C, and the supernatant was stored at −70°C.
Detection of microorganisms with molecular methods
In addition to standard cultivation techniques that included aerobic and anaerobic bacteria, as well as genital mycoplasmas, the AF of patients with clinical chorioamnionitis was analyzed by using broad-range real-time PCR/ESI-MS (Ibis Technology; Athogen) (37). In brief, DNA was extracted from 300 µl of AF by using a method that combines bead-beating cell lysis with a magnetic bead-based extraction method (51, 52). The extracted DNA was amplified by the previously described broad bacteria and Candida (BAC) detection assay according to the manufacturer’s instructions. PCR/ESI-MS can identify 3400 bacteria and 40 Candida spp., which are represented in the platform’s signature database (53, 54).
After PCR amplification, 30 µl aliquots of each PCR product were desalted and analyzed via ESI-MS. The presence of microorganisms was determined by signal processing and triangulation analysis of all base composition signatures obtained from each sample and compared to a database. The sensitivity (limit of detection) of the assay for the detection of bacteria in blood is, on average, 100 CFU/ml (95% CI, 6–600 CFU/ml). A comparison of detection limits between blood and AF showed that the assays have comparable detection limits (100 CFU/ml).
Determination of IL-6 in AF
The concentrations of IL-6 in AF were determined by sensitive and specific enzyme immunoassays obtained from R&D Systems (Minneapolis, MN, USA). The initial assay validation was performed in our laboratory before this study was conducted. The immunoassay uses the quantitative sandwich enzyme immunoassay technique, and the concentrations were determined by interpolation from the standard curves. The inter- and intra-assay coefficients of variation for IL-6 were 8.7 and 4.6%, respectively. The sensitivity of the assay for IL-6 was 0.09 pg/ml.
Sample preparation and LC-MS analysis
Fatty acyl lipids from AF samples were extracted and analyzed by LC-MS essentially as described earlier (55, 56). However, the LC-MS analysis was focused on the 5-lipoxygenase pathway metabolites of arachidonic acid that included 5-hydroxyeicosatetraenoic acid (5-HETE), 5-oxoeicosatetraenoic acid (5-oxoETE), leukotriene B4 (LTB4), 5(S),12(S)-dihydroxyeicosatetraenoic acid [5(S),12(S)-diHETE], and lipoxin B4 (LXB4). Octadeuterated-15(S)-hydroxyeicosatetraenoic acid (15-HETE-d8) and tetradeuterated leukotriene B4 (LTB4-d4) were used as internal standards to monitor recovery and for quantitation. Under standardized conditions of LC-MS quantitation, the detection limits for the eicosanoids are 1–2 pg on the column and the limit of quantitation is 5 pg at a signal-to-noise ratio of 3. Because the sample volume used was 200 µl, these conditions translate to an assay sensitivity of 0.03 nM for an average molecular weight of 330 of the detected eicosanoids.
Statistical analysis
For demographics data analysis, the Kolmogorov-Smirnov test was used to test whether the distribution of continuous variables was normal. Comparisons of continuous data were analyzed by using the Kruskal-Wallis and Mann-Whitney U tests. Statistical analysis was performed with SPSS software (version 19; IBM Corp., Armonk, NY, USA). A value of P < 0.05 indicated statistical significance.
For any detectable lipid analyte in a subject group, a zero value observed in any sample was replaced with one-half the average detection limit of the LC-MS method used for the eicosanoids (i.e., 0.015 nM). This method ensured that information from all samples was used in the statistical analysis and that the fold change between groups was finite for each analyte. A Wilcoxon test, which does not rely on any distributional assumptions about the data, was used for all pair-wise group comparisons. The significance of the P value of the Wilcoxon test is independent of the choice of the threshold concentration used to replace the values below the quantitation limits of the assay.
A parametric alternative to the Wilcoxon test was also applied by using a Student's t test for analytes with concentrations above the sensitivity of the assay in all samples, or using censored regression otherwise. In the presence of a perfect separation between groups, the maximum likelihood estimation involved in censored regression could not be applied; hence, a Student’s t test was used instead.
To account for multiple testing, the P values obtained for all analytes in a given group comparison were adjusted to control the false-discovery rate (FDR) (57). A threshold of 10% on the FDR was used to infer significance. All analyses were performed in the R statistical language and environment, version 3.0 (http://www.r-project.org), with the censReg R package for the censored regression analysis (58).
RESULTS
Clinical characteristics of the study populations
The demographic and clinical characteristics of patients in this study are displayed in Table 1. Designation of IAI was based on an AF IL-6 concentration ≥2.6 ng/ml. All patients with TCC, with the exception of those without intra-amniotic infection/inflammation (TCC-NI group) had a median AF IL-6 concentration ≥2.6 ng/ml.
TABLE 1.
Clinical and obstetric characteristics of women with TLB, TCC, SCX, and PTL-IAI
| Characteristic | TLB (n = 35) | TCC (n = 24) | Pa | SCX (n = 28) | PTL-IAI (n = 25) | Pb |
|---|---|---|---|---|---|---|
| Gestational age at amniocentesis (wk) | 39 (38–40.2) | 40.0 (39.2–40.6) | 0.075 | 26.6 (25.0–28.6) | 31.1 (28.5–33.4) | <0.001 |
| AF IL-6 (ng/ml) | 1.1 (0.8–1.7)c | 10.4 (3.0–18.3) | <0.001 | ND | 21.5 (6.0–140.1) | ND |
| AF glucose (mg/dl) | 10.0 (8.5–12.0)d | 9 (9–9) | 0.068 | 30.0 (26–35)e | 15.0 (5.0–32.5) | 0.002 |
| AF WBC (cell/mm3) | 7.0 (5.0–25.0) | 22.5 (5.0–656.3) | 0.048 | 0 (0–2) | 6.0 (2.5–257.5) | <0.001 |
| Gestational age at delivery (wk) | 39.0 (38.0–40.2) | 40.0 (39.2–40.6) | 0.067 | 39.3 (38.8–40.1) | 32.1 (29.9–34.0) | <0.001 |
| Birth weight (g) | 3250 (3100–3730) | 3625 (3385–3790) | 0.100 | 3148 (2986–3510) | 2000 (1300–2575) | <0.001 |
Values are expressed as median and interquartile range (in parentheses). ND, not determined.
Represents TLB vs. TCC.
Represents SCX vs. PTL-IAI.
Detectable in only 11 of the 35 samples.
Data not available in 6 cases.
Data not available in 1 case.
Patients with an SCX (a control group for patients in PTL) had a significantly shorter gestational age at amniocentesis and lower AF inflammatory response (higher AF glucose and lower AF WBC counts) than those who had PTL and IAI (PTL-IAI group). All patients with an SCX delivered at term.
Table 2 displays the clinical characteristics of the patients with TCC and PTL. There was no significant difference in gestational age at amniocentesis and delivery among the subgroups of patients with TCC. The median AF WBC count and IL-6 concentrations were significantly higher in patients with TCC who had IAI (TCC-SI and TCC-IAI-MIAC) than in those without intra-amniotic infection/inflammation (TCC-NI). The most common microorganism isolated from the amniotic cavity in patients at term with clinical chorioamnionitis and intra-amniotic infection (TCC-IAI-MIAC group) was Ureaplasma urealyticum (4/12 cases). Patients with PTL-IAI-MIAC had a significantly higher median AF IL-6 concentration and WBC count, but lower AF glucose and gestational age at delivery than those with IAI and no MIAC (PTL-IAI-noMIAC). The most common microorganism identified in AF of patients with PTL-IAI-MIAC was Ureaplasma urealyticum (5/10 cases; Table 2).
TABLE 2.
Clinical and obstetric characteristics of the subgroups of women with TCC and PTL-IAI
| Characteristic | TCC-NI (n = 4) | TCC-SI (n = 8) | TCC-IAI-MIAC (n = 12) | P* | PTL-IAI-noMIAC (n = 15) | PTL-IAI-MIAC (n = 10) | Pb |
|---|---|---|---|---|---|---|---|
| Gestational age at amniocentesis (wk) | 39.0 (37.8–40.4) | 39.5 (39.2–40.1) | 40.4 (39.6–40.7) | 0.183 | 32.6 (28.7–33.7) | 30.2 (26.9–31.6) | 0.113 |
| AF IL-6 concentration (ng/ml) | 1.5 (0.9–2.0) | 10.7 (3.1–18.0) | 13.9 (4.9–29.2) | 0.007 | 8.4 (4.2–23.7) | 96 (40.7–219.5) | 0.008 |
| AF glucose (mg/dl) | 11 (9.3–34.5) | 9 (9–9) | 9.0 (6.0–9.0) | 0.053 | 24.0 (15.0–37.0) | 7.0 (2.5–10.0) | 0.014 |
| AF WBC (cell/mm3) | 1.5 (0–15.8) | 22.5 (5–546.3) | 350.0 (18.5–775.0) | 0.014 | 3.0 (0–10.0) | 156.0 (8.8–1123.8) | 0.012 |
| Gestational age at delivery (wk) | 39.1 (38.0–40.4) | 39.5 (39.2–40.1) | 40.4 (39.6–40.7) | 0.174 | 33.1 (31.1–35.3) | 30.4 (26.9–31.7) | 0.013 |
| Birth weight (g) | 3415 (2845–3685) | 3550 (2833–3808) | 3710 (3555–3805) | 0.198 | 2100 (1950–2610) | 1455 (875–2338) | 0.059 |
| Microorganisms in the AF | Ureaplasma urealyticum 33% (n = 4) | Ureaplasma urealyticum 50% (n = 5) | |||||
| Streptococcus agalactiae 25% (n = 3) | Mycoplasma hominis 20% (n = 2) | ||||||
| Gardnerella vaginalis 25% (n = 3) | Haemophilus influenza 10% (n = 1) | ||||||
| Mycoplasma hominis 25% (n = 3) | Staphylococcus epidermidis 10% (n = 1) | ||||||
| Lactobacillus spp. 25% (n = 3) | Candida sp. 10% (n = 1) |
Values are expressed as median and interquartile range (in parentheses).
Represents TCC-NI-SI vs. TCC-IAI-MIAC.
Represents PTL-IAI-no-MIAC vs. PTL-IAI-MIAC.
LC-MS analysis of arachidonic acid metabolites in human AF
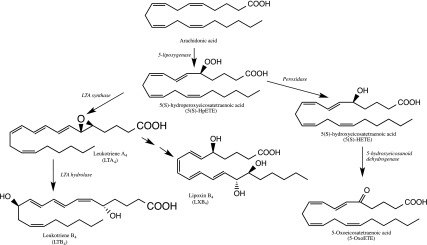
Metabolism of arachidonic acid by the 5-lipoxygenase pathway results in several inflammatory and resolution mediators (Fig. 1). The LC-MS method included multiple reaction monitoring (MRM) transitions for the 5-lipoxygenase metabolites of linoleic acid, eicosapentaenoic acid, and docosahexaenoic acid, in addition to arachidonic acid. Each detected fatty acyl lipid was positively identified by a combination of matching HPLC retention time with the authentic standard and the unique MRM transition (56). Although hydroxy fatty acids derived from all of the above-listed PUFAs were detectable, only the concentrations of arachidonic acid metabolites including 5-HETE, 5-oxoETE, LTB4, 5(S),12(S)-diHETE, and LXB4 were significantly different between patient groups (P < 0.05 and FDR < 0.1) and are shown in the tables and figures. Eicosanoids derived from the cyclooxygenase pathway were also detected in these samples by LC-MS. However, there was no statistically significant difference in the concentrations of these metabolites between any of the study groups (Supplemental Table S1).
Figure 1.
Metabolites of arachidonic acid in the 5-lipoxygenase pathway that showed significant concentration differences in human AF in the patient groups of the study.
Inflammatory lipid mediators in patients with TCC
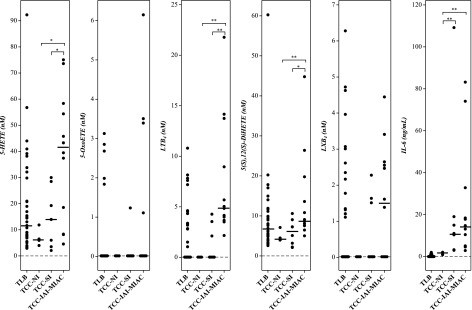
AF inflammatory lipid mediators derived from the metabolism of arachidonic acid by 5-lipoxygenase pathway had significantly higher concentrations in patients with TCC-IAI-MIAC than in those with TCC-SI or no TCC-NI (Fig. 2 and Table 3). This difference was more marked for 5-HETE and LTB4 (Table 4). The concentrations of AF IL-6 were not significantly different between TCC-IAI-MIAC and TCC-SI. LTB4 concentrations alone could separate patients belonging to these 2 groups (P < 1.6 × 10−3; Table 4). As expected, both 5-HETE and LTB4 are significantly higher in patients with TCC with TCC-IAI-MIAC, compared to those at TLB. However, such differences were not observed between patients in the TCC-SI group and those with spontaneous TLB (Fig. 2 and Tables 3 and 4).
Figure 2.
Arachidonate 5-lipoxygenase metabolites in human AF at term in spontaneous labor. Concentrations of the lipid mediators from patients at term in labor without clinical chorioamnionitis (TLB) compared to those with clinical chorioamnionitis but no intra-amniotic infection or inflammation (TCC-NI), SI (TCC-SI), and MIAC as well as IAI (TCC-IAI-MIAC). Results of assessments are shown. Cross bars: median concentration. *P < 0.05; **P < 0.005.
TABLE 3.
Concentrations of arachidonate 5-lipoxygenase pathway metabolites and IL-6 detected in AF of patients groups described in Table 1
| Group | 5-HETE | 5-OxoETE | LTB4 | 5(S),12(S)-diHETE | LXB4 | IL-6 |
|---|---|---|---|---|---|---|
| TLB (n = 35) | 11.6 (7.10–25.4) [35] | 0.02 (0.02–0.02) [5] | 0.02 (0.02–3.35) [17] | 6.84 (4.92–11.4) [35] | 0.02 (0.02–1.97) [15] | 1.08 (0.83–1.57) [11] |
| TCC-NI (n = 4) | 6.19 (5.43–7.80) [4] | 0.02 (0.02–0.02) [0] | 0.02 (0.02–0.02) [0] | 4.22 (3.96–5.13) [4] | 0.02 (0.02–0.02) [0] | 1.48 (0.97–2.00) [4] |
| TCC-SI (n = 8) | 13.9 (5.37–21.6) [8] | 0.02 (0.02–0.02) [1] | 0.02 (0.02–2.47) [3] | 6.17 (2.93–7.72) [8] | 0.02 (0.02–1.54) [3] | 10.7 (3.22–16.0) [8] |
| TCC-IAI-MIAC (n = 12) | 41.4 (16.0–55.4) [12] | 0.02 (0.02–1.68) [4] | 4.93 (3.91–10.2) [12] | 8.85 (7.58–15.2) [12] | 1.50 (0.02–2.58) [7] | 13.95 (5.02–22.01) [12] |
| SCX (n = 28) | 0.11 (0.07–0.15) [27] | 0.02 (0.02–0.02) [0] | 0.26 (0.16–0.35) [28] | 0.93 (0.58–1.36) [28] | 0.02 (0.02–0.02) [0] | ND |
| PTL-IAI-noMIAC (n = 15) | 2.55 (1.31–8.21) [12] | 0.02 (0.02–0.02) [0] | 0.02 (0.02–0.02) [1] | 2.16 (0.02–3.78) [8] | 0.02 (0.02–0.02) [1] | 8.39 (4.55–22.3) [15] |
| PLT-IAI-MIAC (n = 10) | 12.8 (6.21 – 53.7) [10] | 0.02 (0.02 – 0.02) [2] | 3.19 (0.02 – 7.22) [6] | 4.61 (2.92 – 6.39) [8] | 0.02 (0.02 – 3.20) [3] | 96.0 (49.3 – 193.8) [10] |
| IAI-noMIAC (n = 27)a | 5.92 (2.25–12.9) [24] | 0.02 (0.02–0.02) [1] | 0.02 (0.02–0.02) [4] | 3.69 (1.06–5.07) [20] | 0.02 (0.02–0.02) [4] | 5.98 (3.03–18.2) [27] |
| IAI-MIAC (n = 22)b | 38.4 (8.2–54.8) [22] | 0.02 (0.02–0.83) [6] | 4.49 (3.14–7.96) [18] | 7.57 (4.72–11.7) [20] | 0.02 (0.02–2.62) [10] | 27.1 (12.7–80.9) [22] |
Median and interquartile range (in parentheses) data are presented in nanomolar concentration for lipids and nanograms/milliliter for IL-6. Values in brackets denote the number of samples with detectable levels. ND, not determined.
Cumulative data from TCC-NI, TCC-SI, and PTL-IAI-noMIAC samples.
Cumulative data from TCC-IAI-MIAC and PTL-IAI-MIAC samples.
TABLE 4.
Statistical significance of the difference in 5-lipoxygenase metabolites of arachidonic acid and IL-6 between patient groups as determined by the Wilcoxon test
| Group | 5-HETE | 5-OxoETE | LTB4 | 5(S), 12(S)-diHETE | LXB4 | IL-6 |
|---|---|---|---|---|---|---|
| TCC-NI vs. TCC-IAI-MIAC | 0.02* | 0.23 | 4.1 × 10−3# | 4.4 × 10−3# | 0.07 | 1.1 × 10−3# |
| TCC-SI vs. TCC-IAI-MIAC | 0.03* | 0.29 | 1.6 × 10−3# | 0.02* | 0.22 | 0.57 |
| TCC-NI vs. TCC-SI | 0.37 | NA | 0.22 | 0.68 | 0.22 | 4.0 × 10−3# |
| PTL-IAI-noMIAC vs. PTL-IAI-MIAC | 6.9 × 10−3* | 0.15 | 4.0 × 10−3# | 0.08 | NA | 0.04 |
| IAI-noMIAC vs. IAI-MIAC | 1.9 × 10−4# | 0.02* | 1.9 × 10−5# | 7.9 × 10−4# | 0.05 | 5.5 × 10−3* |
*P < 0.05, #P < 0.005. NA, not applicable.
AF inflammatory lipid mediators in PTL-IAI
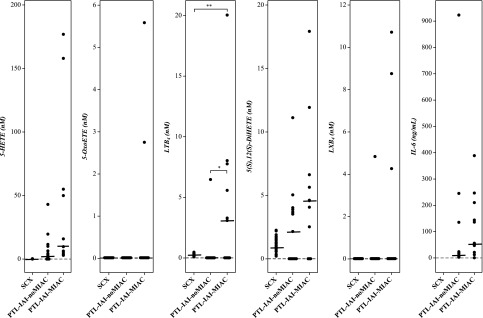
Arachidonate 5-lipoxygenase metabolites were also detectable in the AF of patients with PTL-IAI, regardless of the presence of microorganisms in the amniotic cavity (PTL-IAI-noMIAC or PTL-IAI-MIAC) (Fig. 3 and Table 3). To account for any specific differences related to gestational age, AF samples from patients with SCX who delivered at term were used as controls. Except for LTB4, all other 5-lipoxygenase metabolites, when detectable, were present at a higher concentration in patients with PTL in the PTL-IAI-noMIAC group than in those with a gestational age–matched SCX (Table 3). Similar to observations at term, concentrations of both 5-HETE and LTB4 were significantly higher in the subset of patients with PTL-IAI-MIAC than in those with PTL-IAI-noMIAC (P < 6.9 × 10−3 and 4.0 × 10−3, respectively; Table 4). Whereas LXB4 was virtually undetectable in the PTL-IAI-noMIAC group, 3 of the 10 samples in the PTL-IAI-MIAC group showed the metabolite; however, the difference was not statistically significant (Fig. 3). AF IL-6 concentration was significantly higher in the PTL-IAI-MIAC group than in the PTL-IAI-MIAC group (P < 0.04; Fig. 3 and Table 4).
Figure 3.
Arachidonate 5-lipoxygenase metabolites in human AF of patients in the PTL-IAI group. Lipid mediator concentrations of AF with similar gestational age, but without IAI (SCX), compared with those in the PTL-IAI group, but without microbial infection (PTL-IAI-noMIAC) or with microbial invasion of the amniotic cavity (PTL-IAI-MIAC). Results of assessments are shown. Cross bars: median concentration. *P < 0.05, **P < 0.005.
AF inflammatory lipid mediators and MIAC
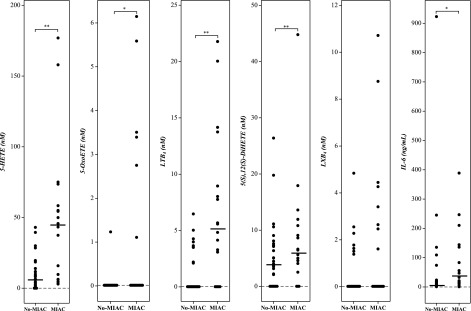
Regardless of gestational age (term or preterm), 5-lipoxygenase metabolites of arachidonic acid, especially 5-HETE and LTB4, had higher concentrations in the AF of patients in the IAI-MIAC group than in those in the IAI-noMIAC group (P < 1.9 × 10−4 and 1.9 × 10−5, respectively) (Fig. 4 and Tables 3 and 4). The concentration of the double lipoxygenase metabolite, 5(S),12(S)-diHETE was also significantly higher in patients in the IAI-MIAC group than in those in the IAI-noMIAC group (P < 7.9 × 10−4). 5-OxoETE, on the other hand, although showing a statistically significant difference between IAI-MIAC and IAI-noMIAC, was not detectable in most samples, and there was no difference in the median concentration.
Figure 4.
Comparison of arachidonate 5-lipoxygenase metabolite concentrations in human AF with respect to MIAC, irrespective of gestational age. noMIAC: combined data from the TCC-NI, TCC-SI, and PTL-IAI-noMIAC groups. MIAC: combined data from TCC-IAI-MIAC and PTL-IAI-MIAC groups. Cross bars: median concentration. *P < 0.05; **P < 0.005.
DISCUSSION
Principal findings of the study
First, the AF concentrations of metabolites of arachidonic acid by the 5-lipoxygenase pathway were significantly higher in the IAI-MIAC group than in those in the IAI-noMIAC group; second, although the inflammatory cytokine IL-6 in AF is a biomarker of IAI (irrespective of the cause; i.e., SI or IAI–MIAC), LTB4 and 5-HETE were increased in the subset in which microorganisms were detected by cultivation or molecular methods; therefore, LTB4 and 5-HETE could serve as biomarkers for the presence of microorganisms in the amniotic cavity. This finding is important because the detection of microorganisms takes time (days when cultivation techniques are used), which delays the clinical decision-making process, whereas, a streamlined LC-MS method for the analysis of these lipid mediators can be accomplished in less than 1 hour. Chemical methods can also be developed for the rapid detection of these metabolites at the bedside.
AF lipid mediators of IAI without demonstrable microorganisms in the amniotic cavity
Spontaneous parturition at term has been likened to an inflammatory process by virtue of leukocyte infiltration of gestational tissues (59–62), as well as elevated concentrations of inflammatory cytokines, such as IL-1β (63, 64), IL-6 (60, 65, 66), TNF-α (67, 68), IL-8 (69, 70), and monocyte chemotactic protein (MCP)-1 (72, 73). Induction of cyclooxygenase (COX)-2 expression in placental membranes in response to elevated cytokines also results in enhanced biosynthesis of proinflammatory prostaglandins, such as PGE2 and PGF2α in AF, even in the absence of IAI, and precedes the onset of labor (21, 23, 24, 26, 27, 31, 32, 73, 74). Our recent MS-based lipidomic analysis of human AF at term confirmed that significantly higher concentrations of prostaglandins were observed in patients with spontaneous TLB than in those not in labor (55). TCC is an intra-amniotic inflammatory state that can present with clinical signs such as maternal fever and foul-smelling AF (40).
Similarly, PTL is associated with IAI. Biochemically, IAI is characterized by a high concentration of IL-6 (≥2.6 ng/ml) in the AF (5, 6, 37, 44, 75). As expected, the median concentration of AF IL-6 in patients in all groups (TCC-SI, TCC-IAI-MIAC, PTL-IAI-noMiac, and PTL-IAI-MIAC), other than in women in TLB and those with TCC without IAI, was significantly higher (median: 8.4–96 ng/ml; Table 3).
Despite the demonstrable IAI process (neutrophil infiltration and elevated cytokines in AF) in patients with TCC (including TCC-SI and TCC-IAI-MIAC), the concentrations of inflammatory prostaglandins such as PGE2 were unexpectedly not significantly different among the TCC-IAI-MIAC, TCC-SI, and TLB groups (Supplemental Table S1). LTB4, the chemotactic leukotriene of the 5-lipoxygenase pathway, was virtually undetectable in the PTL-IAI-noMIAC, TCC-SI, or TCC-NI groups and similar to the TLB group (Fig. 2 and Tables 3 and 4). Therefore, SI associated with clinical chorioamnionitis is not reflected in higher concentrations of prostaglandins and other inflammatory lipid mediators normally increased in AF during spontaneous labor, suggesting that PTL in the absence of intra-amniotic infection/inflammation may not be mediated by prostaglandins.
AF lipid mediators of IAI with demonstrable microorganisms in the amniotic cavity
In contrast to the comparable concentrations of most of the eicosanoids between TLB and TCC without detectable microorganisms in the amniotic cavity (TCC-NI and TCC-SI) (unpublished results), higher concentrations of inflammatory lipid mediators derived from the 5-lipoxygenase pathway were observed in the AF of patients with microbial-associated IAI (TCC-IAI-MIAC and PTL-IAI-MIAC) compared to those without IAI or with IAI but without demonstrable microorganisms (TCC-NI, TCC-SI, and PTL-IAI-noMIAC) (Table 3). Of these, 5-HETE, 5-oxoETE, and LTB4 are well-known lipid mediators involved in host defense against infection (76–80).
The neutrophil chemotactic lipid mediator LTB4 can elicit an innate immune response against microbial and fungal pathogens (81) and is detected at significantly higher concentrations in TCC-IAI-MIAC and PTL-IAI-MIAC, compared to TCC-NI, TCC-SI, and PTL-IAI-noMIAC (undetectable) (Table 3). It is noteworthy that the levels of LTB4 in MIAC (median concentration 4.5 nM; Table 3) are well above the saturating concentrations for the high-affinity LTB4 receptor BLT1 [KD = 0.15 nM (82)], suggesting that our findings have functional consequences.
In addition to LTB4, another neutrophil chemotactic lipid mediator of the 5-lipoxygenase pathway detected primarily in TCC-IAI-MIAC is 5-oxoETE (Fig. 3 and Table 3,). 5-OxoETE induces receptor-mediated inflammatory response in eosinophils and neutrophils in concert with inflammatory cytokines, such as granulocyte macrophage–colony-stimulating factor and TNF-α (80, 83, 84). 5-HETE is the precursor of 5-oxoETE, and the reaction is catalyzed by 5-hydroxyeicosanoid dehydrogenase, an enzyme present in a wide variety of cells, such as monocytes, dendritic cells, platelets, endothelial cells, epithelial cells, and airway smooth muscle cells in addition to neutrophils (80, 85). Despite the significant concentrations of 5-HETE in AF of TCC-NI, TCC-SI, and TLB groups (median: 6–14 nM), 5-oxoETE was not detectable in these groups compared to one-third of TCC-IAI-MIAC samples with detectable levels (Table 3), suggesting an alternate route of inflammatory lipid mediator generation in response to microbial infection. It should be noted that 5-oxoETE is highly reactive and further metabolized by leukotriene C4 (LTC4) synthase to 5-oxo-7-glutathionyl-8,11,14-eicosatrienoic acid (FOG)7, an analog and isobar of LTC4 (86, 87). (FOG7), also exhibits neutrophil and eosinophil chemotaxis, but without an increase in intracellular calcium levels, thereby extending the biologic activity of 5-oxoETE (86). It is possible that the low levels of 5-oxoETE detected are due to the results of its further metabolism or other chemical modifications that arise from its inherent reactivity. Further analysis of AF for FOG7 will be helpful in extending the understanding of the role of the 5-lipoxygenase pathway in MIAC.
LXB4 is an anti-inflammatory/proresolution lipid mediator that follows acute inflammatory events and prevents further recruitment of leukocytes (88–91). AF LXB4 concentration appeared to be higher in patients with chorioamnionitis at term and microbial-associated IAI than in those with no intra-amniotic infection/inflammation or SI (median: 1.5 nM for TCC-IAI-MIAC vs. undetectable in TCC-NI and TCC-SI; P < 0.07 and 0.22, respectively). LXB4 was present in some PTL-IAI-MIAC cases (3/10) but was undetectable in most of the PTL-IAI-noMIAC cases (14/15). Therefore, the 5-lipoxygenase pathway is functional in each of the 5 patient groups (term gestation with spontaneous labor, TCC, and PTL, with or without MIAC in the latter 2); however, only the AF of patients with microbial-associated IAI has detectable inflammatory lipid mediators, LTB4 and 5-oxoETE, as well as the resolution mediator, LXB4. Since biosynthesis of LXB4 involves the intermediacy of LTA4 in the 5-lipoxygenase pathway, its absence is also reflected in the absence of microbe-driven inflammation.
The detection of bacteria in the amniotic cavity is typically determined by traditional culture methods. Although the culture method remains the gold standard, the time needed for such diagnosis (days) and the fact that many microorganisms responsible for intra-amniotic infection are nonculturable presents a formidable clinical challenge and can delay treatment. However, recently developed molecular methods based on PCR combined with MS that reduce the detection time to ∼8 h offer great promise (37, 53). The near-exclusive detection of LTB4 in AF with MIAC, regardless of gestational age (Fig. 4), offers yet another molecular technique for the rapid detection of intra-amniotic infection. The MS-based assay for LTB4 can be very fast (with the use of ion mobility spectrometry, for example), significantly reducing the time to diagnosis of intra-amniotic infection. Since LTB4 represents a host inflammatory response to microbial infection, this biomarker could reflect the presence of bacteria in patients with IAI associated with sustained bacterial or viral infection or both, and not to the results of any potential sample contamination of AF from the vaginal microbiome. Concerns of contamination in transvaginal retrieval make transabdominal amniocentesis the gold standard to collect AF for the detection of MIAC. However, measurement of LTB4 and 5-HETE in transvaginally retrieved AF has the potential to alleviate such concerns and obviate the need for significantly more invasive procedures for the detection of MIAC.
CONCLUSIONS
Human AF contains significantly higher concentrations of the inflammatory lipid mediator LTB4 as well as 5-HETE, in cases with microbial-associated IAI compared to SI. IL-6 concentrations in AF cannot distinguish between IAI, with and without detectable microorganisms in the amniotic cavity (IAI-noMIAC vs. IAI-MIAC), whereas LTB4 measurements have the potential to serve as a biomarker of microbial presence. LC-MS quantification of these metabolites offers a rapid, efficient, and definitive method for the identification of intra-amniotic infection.
ACKNOWLEDGMENTS
This research was supported, in part, by the Perinatology Research Branch, Division of Intramural Research Branch of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, U.S. National Institutes of Health, U.S. Department of Health and Human Services (NICHD/NIH/DHHS); and, in part, with Federal funds from NICHD/NIH/DHHS Contract No. HHSN275201300006C; and, in part, by the National Center for Research Resources Grant S10RR027926 and the Perinatal Virtual Discovery Grant from Wayne State University (to K.R.M.). The authors declare no conflicts of interest.
Glossary
- 5-oxoETE
5-oxoeicosatetraenoic acid; 5(S),12(S)-diHETE, 5(S),12(S)-dihydroxyeicosatetraenoic acid
- AF
amniotic fluid
- FDR
false-discovery rate
- FOG7
5-oxo-7-glutathionyl-8,11,14-eicosatrienoic acid
- HETE
hydroxyeicosatetraenoic acid
- IAI
intra-amniotic inflammation
- LC-MS
liquid chromatography–mass spectrometry
- LTB4
leukotriene B4
- LXB4
lipoxin B4
- MIAC
microbial invasion of the amniotic cavity
- MRM
multiple reaction monitoring
- NI
no intra-amniotic inflammation
- PCR/ESI-MS
polymerase chain reaction coupled with electrospray ionization mass spectrometry
- PG
prostaglandin
- PTL
preterm labor
- PUFA
polyunsaturated fatty acid
- SCX
sonographic short cervix
- SI
sterile intra-amniotic inflammation
- TCC
clinical chorioamnionitis at term
- TLB
spontaneous labor at term
- WBC
white blood cell
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
K. R. Maddipati performed lipidomic analysis, interpreted the data, and wrote the paper; R. Romero designed the study, collected and selected the samples, conceived the article, and wrote the paper; T. Chaiworapongsa selected the samples and conceived of the article; P. Chaemsaithong and N. Chaiyasit conceived of the article and analyzed demographic data; S.-L. Zhou prepared the samples for LC-MS analysis; Z. Xu and A. L. Tarca performed the statistical analysis; J. P. Kusanovic and R. Gomez collected the samples; and K. V. Honn interpreted the data and wrote the paper.
REFERENCES
- 1.Romero R., Dey S. K., Fisher S. J. (2014) Preterm labor: one syndrome, many causes. Science 345, 760–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gravett M. G., Hummel D., Eschenbach D. A., Holmes K. K. (1986) Preterm labor associated with subclinical amniotic fluid infection and with bacterial vaginosis. Obstet. Gynecol. 67, 229–237 [DOI] [PubMed] [Google Scholar]
- 3.Gibbs R. S., Romero R., Hillier S. L., Eschenbach D. A., Sweet R. L. (1992) A review of premature birth and subclinical infection. Am. J. Obstet. Gynecol. 166, 1515–1528 [DOI] [PubMed] [Google Scholar]
- 4.Yoon B. H., Romero R., Moon J. B., Shim S. S., Kim M., Kim G., Jun J. K. (2001) Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. Am. J. Obstet. Gynecol. 185, 1130–1136 [DOI] [PubMed] [Google Scholar]
- 5.Romero R., Miranda J., Chaiworapongsa T., Korzeniewski S. J., Chaemsaithong P., Gotsch F., Dong Z., Ahmed A. I., Yoon B. H., Hassan S. S., Kim C. J., Yeo L. (2014) Prevalence and clinical significance of sterile intra-amniotic inflammation in patients with preterm labor and intact membranes. Am. J. Reprod. Immunol. 72, 458–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Combs C. A., Gravett M., Garite T. J., Hickok D. E., Lapidus J., Porreco R., Rael J., Grove T., Morgan T. K., Clewell W., Miller H., Luthy D., Pereira L., Nageotte M., Robilio P. A., Fortunato S., Simhan H., Baxter J. K., Amon E., Franco A., Trofatter K., Heyborne K. (2014) Amniotic fluid infection, inflammation, and colonization in preterm labor with intact membranes. Am. J. Obstet. Gynecol. 210, 125.e121–125.e115 [DOI] [PubMed] [Google Scholar]
- 7.Cobo T., Kacerovsky M., Jacobsson B. (2014) Amniotic fluid infection, inflammation, and colonization in preterm labor with intact membranes. Am. J. Obstet. Gynecol. 211, 708 [DOI] [PubMed] [Google Scholar]
- 8.Gibbs R. S., Dinsmoor M. J., Newton E. R., Ramamurthy R. S. (1988) A randomized trial of intrapartum versus immediate postpartum treatment of women with intra-amniotic infection. Obstet. Gynecol. 72, 823–828 [DOI] [PubMed] [Google Scholar]
- 9.National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network (1997) Antibiotic therapy for reduction of infant morbidity after preterm premature rupture of the membranes. A randomized controlled trial. JAMA 278, 989–995 [PubMed] [Google Scholar]
- 10.Lee J., Romero R., Kim S. M., Chaemsaithong P., Park C. W., Park J. S., Jun J. K., Yoon B. H. (2016) A new antibiotic regimen treats and prevents intra-amniotic inflammation/infection in patients with preterm PROM. J. Matern. Fetal Neonatal Med. 29, 707–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Funk C. D. (2001) Prostaglandins and leukotrienes: advances in eicosanoid biology. Science 294, 1871–1875 [DOI] [PubMed] [Google Scholar]
- 12.Harris S. G., Padilla J., Koumas L., Ray D., Phipps R. P. (2002) Prostaglandins as modulators of immunity. Trends Immunol. 23, 144–150 [DOI] [PubMed] [Google Scholar]
- 13.Serhan C. N., Savill J. (2005) Resolution of inflammation: the beginning programs the end. Nat. Immunol. 6, 1191–1197 [DOI] [PubMed] [Google Scholar]
- 14.Serhan C. N., Chiang N., Van Dyke T. E. (2008) Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol. 8, 349–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Serhan C. N. (2008) Systems approach with inflammatory exudates uncovers novel anti-inflammatory and pro-resolving mediators. Prostaglandins Leukot. Essent. Fatty Acids 79, 157–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shimizu T. (2009) Lipid mediators in health and disease: enzymes and receptors as therapeutic targets for the regulation of immunity and inflammation. Annu. Rev. Pharmacol. Toxicol. 49, 123–150 [DOI] [PubMed] [Google Scholar]
- 17.Ricciotti E., FitzGerald G. A. (2011) Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 31, 986–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chiang N., Fredman G., Bäckhed F., Oh S. F., Vickery T., Schmidt B. A., Serhan C. N. (2012) Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature 484, 524–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buckley C. D., Gilroy D. W., Serhan C. N. (2014) Proresolving lipid mediators and mechanisms in the resolution of acute inflammation. Immunity 40, 315–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karim S. M. (1971) The role of prostaglandins in human parturition. Proc. R. Soc. Med. 64, 10–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitchell M. D. (1981) Prostaglandins during pregnancy and the perinatal period. J. Reprod. Fertil. 62, 305–315 [DOI] [PubMed] [Google Scholar]
- 22.Nieder J., Augustin W. (1983) Increase of prostaglandin E and F equivalents in amniotic fluid during late pregnancy and rapid PG F elevation after cervical dilatation. Prostaglandins Leukot. Med. 12, 289–297 [DOI] [PubMed] [Google Scholar]
- 23.Berryman G. K., Strickland D. M., Hankins G. D., Mitchell M. D. (1987) Amniotic fluid prostaglandin D2 in spontaneous and augmented labor. Life Sci. 41, 1611–1614 [DOI] [PubMed] [Google Scholar]
- 24.Romero R., Gonzalez R., Baumann P., Behnke E., Rittenhouse L., Barberio D., Cotton D. B., Mitchell M. D. (1994) Topographic differences in amniotic fluid concentrations of prostanoids in women in spontaneous labor at term. Prostaglandins Leukot. Essent. Fatty Acids 50, 97–104 [DOI] [PubMed] [Google Scholar]
- 25.Romero R., Baumann P., Gonzalez R., Gomez R., Rittenhouse L., Behnke E., Mitchell M. D. (1994) Amniotic fluid prostanoid concentrations increase early during the course of spontaneous labor at term. Am. J. Obstet. Gynecol. 171, 1613–1620 [DOI] [PubMed] [Google Scholar]
- 26.Mitchell M. D., Romero R. J., Edwin S. S., Trautman M. S. (1995) Prostaglandins and parturition. Reprod. Fertil. Dev. 7, 623–632 [DOI] [PubMed] [Google Scholar]
- 27.Romero R., Munoz H., Gomez R., Parra M., Polanco M., Valverde V., Hasbun J., Garrido J., Ghezzi F., Mazor M., Tolosa J. E., Mitchell M. D. (1996) Increase in prostaglandin bioavailability precedes the onset of human parturition. Prostaglandins Leukot. Essent. Fatty Acids 54, 187–191 [DOI] [PubMed] [Google Scholar]
- 28.Gibb W. (1998) The role of prostaglandins in human parturition. Ann. Med. 30, 235–241 [DOI] [PubMed] [Google Scholar]
- 29.Keelan, J. A., Blumenstein, M., Helliwell, R. J., Sato, T. A., Marvin, K. W., and Mitchell, M. D. (2003) Cytokines, prostaglandins and parturition: a review. Placenta 24(Suppl A), S33–S46 doi:10.1053/plac.2002.0948 [DOI] [PubMed]
- 30.Olson D. M. (2003) The role of prostaglandins in the initiation of parturition. Best Pract. Res. Clin. Obstet. Gynaecol. 17, 717–730 [DOI] [PubMed] [Google Scholar]
- 31.Mitchell M. D., Chang M. C., Chaiworapongsa T., Lan H. Y., Helliwell R. J., Romero R., Sato T. A. (2005) Identification of 9alpha,11beta-prostaglandin F2 in human amniotic fluid and characterization of its production by human gestational tissues. J. Clin. Endocrinol. Metab. 90, 4244–4248 [DOI] [PubMed] [Google Scholar]
- 32.Lee S. E., Romero R., Park I. S., Seong H. S., Park C. W., Yoon B. H. (2008) Amniotic fluid prostaglandin concentrations increase before the onset of spontaneous labor at term. J. Matern. Fetal Neonatal Med. 21, 89–94 [DOI] [PubMed] [Google Scholar]
- 33.Menon R., Fortunato S. J., Milne G. L., Brou L., Carnevale C., Sanchez S. C., Hubbard L., Lappas M., Drobek C. O., Taylor R. N. (2011) Amniotic fluid eicosanoids in preterm and term births: effects of risk factors for spontaneous preterm labor. Obstet. Gynecol. 118, 121–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Romero R., Quintero R., Emamian M., Wan M., Grzyboski C., Hobbins J. C., Mitchell M. D. (1987) Arachidonate lipoxygenase metabolites in amniotic fluid of women with intra-amniotic infection and preterm labor. Am. J. Obstet. Gynecol. 157, 1454–1460 [DOI] [PubMed] [Google Scholar]
- 35.Van der Elst C. W., Lòpez Bernal A., Sinclair-Smith C. C. (1991) The role of chorioamnionitis and prostaglandins in preterm labor. Obstet. Gynecol. 77, 672–676 [PubMed] [Google Scholar]
- 36.Hsu C. D., Meaddough E., Aversa K., Hong S. F., Lee I. S., Bahodo-Singh R. O., Lu L. C., Copel J. A. (1998) Dual roles of amniotic fluid nitric oxide and prostaglandin E2 in preterm labor with intra-amniotic infection. Am. J. Perinatol. 15, 683–687 [DOI] [PubMed] [Google Scholar]
- 37.Romero R., Miranda J., Chaiworapongsa T., Chaemsaithong P., Gotsch F., Dong Z., Ahmed A. I., Yoon B. H., Hassan S. S., Kim C. J., Korzeniewski S. J., Yeo L. (2014) A novel molecular microbiologic technique for the rapid diagnosis of microbial invasion of the amniotic cavity and intra-amniotic infection in preterm labor with intact membranes. Am. J. Reprod. Immunol. 71, 330–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Romero R., Miranda J., Kusanovic J. P., Chaiworapongsa T., Chaemsaithong P., Martinez A., Gotsch F., Dong Z., Ahmed A. I., Shaman M., Lannaman K., Yoon B. H., Hassan S. S., Kim C. J., Korzeniewski S. J., Yeo L., Kim Y. M. (2015) Clinical chorioamnionitis at term I: microbiology of the amniotic cavity using cultivation and molecular techniques. J. Perinat. Med. 43, 19–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Romero R., Chaemsaithong P., Korzeniewski S. J., Tarca A. L., Bhatti G., Xu Z., Kusanovic J. P., Dong Z., Docheva N., Martinez-Varea A., Yoon B. H., Hassan S. S., Chaiworapongsa T., Yeo L. (2016) Clinical chorioamnionitis at term II: the intra-amniotic inflammatory response. J. Perinat. Med. 44, 5–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Romero R., Chaemsaithong P., Korzeniewski S. J., Kusanovic J. P., Docheva N., Martinez-Varea A., Ahmed A. I., Yoon B. H., Hassan S. S., Chaiworapongsa T., Yeo L. (2016) Clinical chorioamnionitis at term III: how well do clinical criteria perform in the identification of proven intra-amniotic infection? J. Perinat. Med. 44, 23–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Romero R., Chaemsaithong P., Docheva N., Korzeniewski S. J., Tarca A. L., Bhatti G., Xu Z., Kusanovic J. P., Dong Z., Chaiyasit N., Ahmed A. I., Yoon B. H., Hassan S. S., Chaiworapongsa T., Yeo L. (2016) Clinical chorioamnionitis at term IV: the maternal plasma cytokine profile. J. Perinat. Med. 44, 77–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Romero R., Chaemsaithong P., Docheva N., Korzeniewski S. J., Tarca A. L., Bhatti G., Xu Z., Kusanovic J. P., Chaiyasit N., Dong Z., Yoon B. H., Hassan S. S., Chaiworapongsa T., Yeo L., Kim Y. M. (2016) Clinical chorioamnionitis at term V: umbilical cord plasma cytokine profile in the context of a systemic maternal inflammatory response. J. Perinat. Med. 44, 53–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Romero R., Chaemsaithong P., Docheva N., Korzeniewski S. J., Kusanovic J. P., Yoon B. H., Kim J. S., Chaiyasit N., Ahmed A. I., Qureshi F., Jacques S. M., Kim C. J., Hassan S. S., Chaiworapongsa T., Yeo L., Kim Y. M. (2016) Clinical chorioamnionitis at term VI: acute chorioamnionitis and funisitis according to the presence or absence of microorganisms and inflammation in the amniotic cavity. J. Perinat. Med. 44, 33–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gervasi M. T., Romero R., Bracalente G., Erez O., Dong Z., Hassan S. S., Yeo L., Yoon B. H., Chaiworapongsa T. (2012) Midtrimester amniotic fluid concentrations of interleukin-6 and interferon-gamma-inducible protein-10: evidence for heterogeneity of intra-amniotic inflammation and associations with spontaneous early (<32 weeks) and late (>32 weeks) preterm delivery. J. Perinat. Med. 40, 329–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chaemsaithong P., Romero R., Korzeniewski S. J., Dong Z., Yeo L., Hassan S. S., Kim Y. M., Yoon B. H., Chaiworapongsa T. (2015) A point of care test for the determination of amniotic fluid interleukin-6 and the chemokine CXCL-10/IP-10. J. Matern. Fetal Neonatal Med. 28, 1510–1519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Andersen H. F., Nugent C. E., Wanty S. D., Hayashi R. H. (1990) Prediction of risk for preterm delivery by ultrasonographic measurement of cervical length. Am. J. Obstet. Gynecol. 163, 859–867 [DOI] [PubMed] [Google Scholar]
- 47.National Institute of Child Health and Human Development Maternal Fetal Medicine Unit Network (1996) The length of the cervix and the risk of spontaneous premature delivery. N. Engl. J. Med. 334, 567–573 [DOI] [PubMed] [Google Scholar]
- 48.Romero R., Quintero R., Nores J., Avila C., Mazor M., Hanaoka S., Hagay Z., Merchant L., Hobbins J. C. (1991) Amniotic fluid white blood cell count: a rapid and simple test to diagnose microbial invasion of the amniotic cavity and predict preterm delivery. Am. J. Obstet. Gynecol. 165, 821–830 [DOI] [PubMed] [Google Scholar]
- 49.Romero R., Jimenez C., Lohda A. K., Nores J., Hanaoka S., Avila C., Callahan R., Mazor M., Hobbins J. C., Diamond M. P. (1990) Amniotic fluid glucose concentration: a rapid and simple method for the detection of intraamniotic infection in preterm labor. Am. J. Obstet. Gynecol. 163, 968–974 [DOI] [PubMed] [Google Scholar]
- 50.Romero R., Emamian M., Quintero R., Wan M., Hobbins J. C., Mazor M., Edberg S. (1988) The value and limitations of the Gram stain examination in the diagnosis of intraamniotic infection. Am. J. Obstet. Gynecol. 159, 114–119 [DOI] [PubMed] [Google Scholar]
- 51.Eshoo M. W., Crowder C. C., Rebman A. W., Rounds M. A., Matthews H. E., Picuri J. M., Soloski M. J., Ecker D. J., Schutzer S. E., Aucott J. N. (2012) Direct molecular detection and genotyping of Borrelia burgdorferi from whole blood of patients with early Lyme disease. PLoS One 7, e36825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shin J. H., Ranken R., Sefers S. E., Lovari R., Quinn C. D., Meng S., Carolan H. E., Toleno D., Li H., Lee J. N., Stratton C. W., Massire C., Tang Y. W. (2013) Detection, identification, and distribution of fungi in bronchoalveolar lavage specimens by use of multilocus PCR coupled with electrospray ionization/mass spectrometry. J. Clin. Microbiol. 51, 136–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ecker D. J., Sampath R., Li H., Massire C., Matthews H. E., Toleno D., Hall T. A., Blyn L. B., Eshoo M. W., Ranken R., Hofstadler S. A., Tang Y. W. (2010) New technology for rapid molecular diagnosis of bloodstream infections. Expert Rev. Mol. Diagn. 10, 399–415 [DOI] [PubMed] [Google Scholar]
- 54.Metzgar D., Frinder M., Lovari R., Toleno D., Massire C., Blyn L. B., Ranken R., Carolan H. E., Hall T. A., Moore D., Hansen C. J., Sampath R., Ecker D. J. (2013) Broad-spectrum biosensor capable of detecting and identifying diverse bacterial and Candida species in blood. J. Clin. Microbiol. 51, 2670–2678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maddipati K. R., Zhou S. L. (2011) Stability and analysis of eicosanoids and docosanoids in tissue culture media. Prostaglandins Other Lipid Mediat. 94, 59–72 [DOI] [PubMed] [Google Scholar]
- 56.Maddipati K. R., Romero R., Chaiworapongsa T., Zhou S. L., Xu Z., Tarca A. L., Kusanovic J. P., Munoz H., Honn K. V. (2014) Eicosanomic profiling reveals dominance of the epoxygenase pathway in human amniotic fluid at term in spontaneous labor. FASEB J. 28, 4835–4846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Benjamini Y., Hochberg Y. (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. B 57, 289–300 [Google Scholar]
- 58.Henningsen, A. (2011) censReg: Censored Regression (Tobit) Models. pp. R package, Version 0.5, http://CRAN.R-project.org/package=censReg
- 59.Liggins G. (1981) Cervical ripening as an inflammatory reaction. In The Cervix in Pregnancy and Labor: Clinical and Biochemical Investigations (Ellwood E., Anderson A., eds), pp. 1–9, Churchill Livingstone, Edinburgh, UK [Google Scholar]
- 60.Osman I., Young A., Ledingham M. A., Thomson A. J., Jordan F., Greer I. A., Norman J. E. (2003) Leukocyte density and pro-inflammatory cytokine expression in human fetal membranes, decidua, cervix and myometrium before and during labour at term. Mol. Hum. Reprod. 9, 41–45 [DOI] [PubMed] [Google Scholar]
- 61.Gomez-Lopez N., Estrada-Gutierrez G., Jimenez-Zamudio L., Vega-Sanchez R., Vadillo-Ortega F. (2009) Fetal membranes exhibit selective leukocyte chemotaxic activity during human labor. J. Reprod. Immunol. 80, 122–131 [DOI] [PubMed] [Google Scholar]
- 62.Gomez-Lopez N., Vadillo-Perez L., Nessim S., Olson D. M., Vadillo-Ortega F. (2011) Choriodecidua and amnion exhibit selective leukocyte chemotaxis during term human labor. Am. J. Obstet. Gynecol. 204, 364.e369–364.e316 [DOI] [PubMed] [Google Scholar]
- 63.Romero R., Brody D. T., Oyarzun E., Mazor M., Wu Y. K., Hobbins J. C., Durum S. K. (1989) Infection and labor: III, interleukin-1: a signal for the onset of parturition. Am. J. Obstet. Gynecol. 160, 1117–1123 [DOI] [PubMed] [Google Scholar]
- 64.Romero R., Mazor M., Brandt F., Sepulveda W., Avila C., Cotton D. B., Dinarello C. A. (1992) Interleukin-1 alpha and interleukin-1 beta in preterm and term human parturition. Am. J. Reprod. Immunol. 27, 117–123 [DOI] [PubMed] [Google Scholar]
- 65.Romero, R., Sepulveda, W., Kenney, J. S., Archer, L. E., Allison, and A. C., Sehgal, P. B. (1992) Interleukin 6 determination in the detection of microbial invasion of the amniotic cavity. Ciba Found. Symp. 167, 205–220; discussion 220–203 [DOI] [PubMed]
- 66.Andrews W. W., Hauth J. C., Goldenberg R. L., Gomez R., Romero R., Cassell G. H. (1995) Amniotic fluid interleukin-6: correlation with upper genital tract microbial colonization and gestational age in women delivered after spontaneous labor versus indicated delivery. Am. J. Obstet. Gynecol. 173, 606–612 [DOI] [PubMed] [Google Scholar]
- 67.Romero R., Mazor M., Sepulveda W., Avila C., Copeland D., Williams J. (1992) Tumor necrosis factor in preterm and term labor. Am. J. Obstet. Gynecol. 166, 1576–1587 [DOI] [PubMed] [Google Scholar]
- 68.Maymon E., Ghezzi F., Edwin S. S., Mazor M., Yoon B. H., Gomez R., Romero R. (1999) The tumor necrosis factor alpha and its soluble receptor profile in term and preterm parturition. Am. J. Obstet. Gynecol. 181, 1142–1148 [DOI] [PubMed] [Google Scholar]
- 69.Romero R., Ceska M., Avila C., Mazor M., Behnke E., Lindley I. (1991) Neutrophil attractant/activating peptide-1/interleukin-8 in term and preterm parturition. Am. J. Obstet. Gynecol. 165, 813–820 [DOI] [PubMed] [Google Scholar]
- 70.Saito S., Kasahara T., Kato Y., Ishihara Y., Ichijo M. (1993) Elevation of amniotic fluid interleukin 6 (IL-6), IL-8 and granulocyte colony stimulating factor (G-CSF) in term and preterm parturition. Cytokine 5, 81–88 7683506 [Google Scholar]
- 71.Esplin M. S., Romero R., Chaiworapongsa T., Kim Y. M., Edwin S., Gomez R., Gonzalez R., Adashi E. Y. (2003) Amniotic fluid levels of immunoreactive monocyte chemotactic protein-1 increase during term parturition. J. Matern. Fetal Neonatal Med. 14, 51–56 [DOI] [PubMed] [Google Scholar]
- 72.Shynlova O., Tsui P., Dorogin A., Lye S. J. (2008) Monocyte chemoattractant protein-1 (CCL-2) integrates mechanical and endocrine signals that mediate term and preterm labor. J. Immunol. 181, 1470–1479 [DOI] [PubMed] [Google Scholar]
- 73.Strickland D. M., Saeed S. A., Casey M. L., Mitchell M. D. (1983) Stimulation of prostaglandin biosynthesis by urine of the human fetus may serve as a trigger for parturition. Science 220, 521–522 [DOI] [PubMed] [Google Scholar]
- 74.Lee S. E., Park I. S., Romero R., Yoon B. H. (2009) Amniotic fluid prostaglandin F2 increases even in sterile amniotic fluid and is an independent predictor of impending delivery in preterm premature rupture of membranes. J. Matern. Fetal Neonatal Med. 22, 880–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Romero R., Miranda J., Chaiworapongsa T., Chaemsaithong P., Gotsch F., Dong Z., Ahmed A. I., Yoon B. H., Hassan S. S., Kim C. J., Korzeniewski S. J., Yeo L., Kim Y. M. (2015) Sterile intra-amniotic inflammation in asymptomatic patients with a sonographic short cervix: prevalence and clinical significance. J. Matern. Fetal Neonatal Med. 28, 1394–1409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Samuelsson B. (1983) Leukotrienes: mediators of immediate hypersensitivity reactions and inflammation. Science 220, 568–575 [DOI] [PubMed] [Google Scholar]
- 77.Kumlin M., Falck J. R., Raud J., Harada Y., Dahlén S. E., Granström E. (1990) Identification and biological activity of dihydroleukotriene B4: a prominent metabolite of leukotriene B4 in the human lung. Biochem. Biophys. Res. Commun. 170, 23–29 [DOI] [PubMed] [Google Scholar]
- 78.Ford-Hutchinson A. W. (1990) Leukotriene B4 in inflammation. Crit. Rev. Immunol. 10, 1–12 [PubMed] [Google Scholar]
- 79.Zarini S., Gijón M. A., Ransome A. E., Murphy R. C., Sala A. (2009) Transcellular biosynthesis of cysteinyl leukotrienes in vivo during mouse peritoneal inflammation. Proc. Natl. Acad. Sci. USA 106, 8296–8301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Grant G. E., Rokach J., Powell W. S. (2009) 5-Oxo-ETE and the OXE receptor. Prostaglandins Other Lipid Mediat. 89, 98–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Peters-Golden M., Canetti C., Mancuso P., Coffey M. J. (2005) Leukotrienes: underappreciated mediators of innate immune responses. J. Immunol. 174, 589–594 [DOI] [PubMed] [Google Scholar]
- 82.Nakamura M., Shimizu T. (2011) Leukotriene receptors. Chem. Rev. 111, 6231–6298 [DOI] [PubMed] [Google Scholar]
- 83.O’Flaherty J. T., Taylor J. S., Thomas M. J. (1998) Receptors for the 5-oxo class of eicosanoids in neutrophils. J. Biol. Chem. 273, 32535–32541 [DOI] [PubMed] [Google Scholar]
- 84.Jones C. E., Holden S., Tenaillon L., Bhatia U., Seuwen K., Tranter P., Turner J., Kettle R., Bouhelal R., Charlton S., Nirmala N. R., Jarai G., Finan P. (2003) Expression and characterization of a 5-oxo-6E,8Z,11Z,14Z-eicosatetraenoic acid receptor highly expressed on human eosinophils and neutrophils. Mol. Pharmacol. 63, 471–477 [DOI] [PubMed] [Google Scholar]
- 85.Patel P., Cossette C., Anumolu J. R., Erlemann K.-R., Grant G. E., Rokach J., Powell W. S. (2009) Substrate selectivity of 5-hydroxyeicosanoid dehydrogenase and its inhibition by 5-hydroxy-Δ6-long-chain fatty acids. J. Pharmacol. Exp. Ther. 329, 335–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bowers R. C., Hevko J., Henson P. M., Murphy R. C. (2000) A novel glutathione containing eicosanoid (FOG7) chemotactic for human granulocytes. J. Biol. Chem. 275, 29931–29934 [DOI] [PubMed] [Google Scholar]
- 87.Hevko J. M., Murphy R. C. (2002) Formation of murine macrophage-derived 5-oxo-7-glutathionyl-8,11,14-eicosatrienoic acid (FOG7) is catalyzed by leukotriene C4 synthase. J. Biol. Chem. 277, 7037–7043 [DOI] [PubMed] [Google Scholar]
- 88.Lee T. H., Horton C. E., Kyan-Aung U., Haskard D., Crea A. E., Spur B. W. (1989) Lipoxin A4 and lipoxin B4 inhibit chemotactic responses of human neutrophils stimulated by leukotriene B4 and N-formyl-L-methionyl-L-leucyl-L-phenylalanine. Clin. Sci. 77, 195–203 [DOI] [PubMed] [Google Scholar]
- 89.Serhan C. N. (1994) Lipoxin biosynthesis and its impact in inflammatory and vascular events. Biochim. Biophys. Acta 1212, 1–25 [DOI] [PubMed] [Google Scholar]
- 90.Papayianni A., Serhan C. N., Brady H. R. (1996) Lipoxin A4 and B4 inhibit leukotriene-stimulated interactions of human neutrophils and endothelial cells. J. Immunol. 156, 2264–2272 [PubMed] [Google Scholar]
- 91.Serhan C. N. (2005) Lipoxins and aspirin-triggered 15-epi-lipoxins are the first lipid mediators of endogenous anti-inflammation and resolution. Prostaglandins Leukot. Essent. Fatty Acids 73, 141–162 [DOI] [PubMed] [Google Scholar]