Abstract
The placenta richly expresses nonheme and heme Fe transport proteins. To address the impact of maternal and neonatal Fe status and hepcidin on the regulation of these proteins, mRNA expression and protein abundance of nonheme and heme Fe transport proteins were evaluated in placental tissue from 154 adolescents. Regression analyses found maternal Fe status was significantly associated with multiple placental nonheme and heme transporters, whereas neonatal Fe status was related to only 3 heme transporters. Across statistical analyses, maternal Fe status was consistently associated with the placental nonheme Fe importer transferrin receptor 1 (TfR1). Protein abundance of TfR1 was related to midgestation maternal serum ferritin (SF) (β = −0.32; P = 0.005) and serum TfR (β = 0.25; P = 0.024). Protein abundance of the heme importer, proton-coupled folate transporter, was related to neonatal SF (β = 0.30; P = 0.016) and serum TfR (β = −0.46; P < 0.0001). Neonatal SF was also related to mRNA expression of the heme exporter feline leukemia virus subgroup C receptor 1 (β = −0.30; P = 0.004). In summary, maternal Fe insufficiency during pregnancy predicts increased expression of the placental nonheme Fe transporter TfR1. Associations between placental heme Fe transporters and neonatal Fe status require further study.—Best, C. M., Pressman, E. K., Cao, C., Cooper, E., Guillet, R., Yost, O. L., Galati, J., Kent, T. R., O’Brien, K. O. Maternal iron status during pregnancy compared with neonatal iron status better predicts placental iron transporter expression in humans.
Keywords: anemia, heme, TfR1 hepcidin, adolescents
A healthy singleton pregnancy requires a total of 1000 mg of Fe across gestation, of which approximately one third is present in the neonate at birth (1, 2). Human placenta meets this fetal Fe demand by actively transporting Fe across the syncytiotrophoblast into the fetal circulation. Maternal Fe deficiency or placental dysfunction may adversely limit fetal Fe accretion, which leads to irreversible metabolic and cognitive impairments in the offspring (3). Because Fe is critical to fetal development, yet is toxic in excess, placental transfer of Fe must be tightly controlled. Numerous Fe transport proteins are known to be expressed in human placenta, but regulation of these Fe trafficking proteins and the degree to which these can be altered in response to maternal or fetal signals has not been characterized.
Dietary Fe exists in 2 forms: nonheme Fe and heme Fe. Nonheme Fe is obtained from both plant and animal foods. In contrast, dietary heme Fe is obtained exclusively from animal sources, in the form of hemoglobin and myoglobin (4). Several studies have begun to characterize placental trafficking of nonheme Fe in humans (5–8). Whereas many nonheme Fe trafficking proteins in the placenta are the same as those used by the enterocyte, the placenta also uniquely expresses zyklopen, a placental-specific analog of hephaestin and ceruloplasmin (9). Likewise, the protein 6 transmembrane epithelial antigen of prostate, 4 is thought to play a role in Fe export from endosomes within placental tissue (10).
To date, little is known about the expression of heme trafficking proteins in the placenta and whether these proteins are responsive to fetal and/or maternal Fe status. Several recently identified heme transporters, including feline leukemia virus subgroup C receptor 1 (FLVCR1) (11), the proton-coupled folate transporter (PCFT), also known as heme carrier protein 1 (12), and the hemopexin-heme receptor (low density lipoprotein receptor–related protein 1 [LRP1]) (13), have been found to be highly expressed in human placenta, where their functions remain largely uncharacterized (14).
The hormone hepcidin is the principal regulator of systemic Fe homeostasis. Fe stores, inflammation, hypoxia, and erythropoietic activity impact production of hepcidin, which limits the export of Fe from cells (15). The placenta is exposed to both maternally and fetally derived hepcidin (16, 17). Data from animal studies have indicated that both maternal (18) and fetal hepcidin (18–20) may regulate expression of placental Fe transporters. In addition, hepcidin protein has been identified by immunostaining in the first trimester human gestational sac, which suggests the human placenta itself produces hepcidin early in gestation (17). Potential interactions between maternal and fetal concentrations of hepcidin, placental expression of hepcidin, and placental expression of Fe transport proteins have not been adequately characterized.
No animal or human studies to date have assessed the coordinate expression of nonheme and heme Fe transport proteins in human placental tissue in relation to maternal and neonatal Fe status. Toward this goal, we examined mRNA expression and protein abundance of a full complement of placental nonheme and heme Fe transport proteins in relation to serum indicators of maternal and neonatal Fe status and hepcidin in a population at increased risk for gestational Fe deficiency.
MATERIALS AND METHODS
Participants
Pregnant adolescents who received prenatal care at the Rochester Adolescent Maternity Program in Rochester, NY, USA, were recruited between 2006 and 2011. A total of 255 patients were enrolled in a larger longitudinal study of maternal and fetal bone health and/or maternal and neonatal Fe status across gestation. Of the 255 adolescents, 154 had placental tissue available for analysis of Fe trafficking proteins. Adolescents who were ≤18 yr old, carried a single fetus, were at 12–30 wk of gestation at entry into the study, and were otherwise healthy were eligible to participate. The institutional review boards at Cornell University and the University of Rochester approved all study procedures. Written informed consent was obtained from study participants who were 15–18 yr of age. Both assent and parental consent were obtained if participants were ≤14 yr of age. Characteristics of the larger study cohort, including maternal Fe status, have been described elsewhere (21–23), and data on placental expression of select proteins that are involved in Fe and vitamin D metabolism have been published (24–27).
Sample collection
Maternal nonfasting blood was obtained during pregnancy at 26 ± 4 wk gestation. At delivery, maternal blood, placental tissue, and umbilical cord blood were obtained. Fetal membranes were removed from the placenta, and tissue samples were collected from the middle of 4–5 different cotyledons and pooled. Tissue aliquots were either flash frozen for Western blot analyses or placed into RNAlater (Thermo Fisher Scientific, Waltham, MA, USA) and kept at −80°C until analysis. Placental tissue was not obtained from placentas that were sent to pathology for clinically indicated reasons.
Biochemical assessment
Maternal and neonatal Fe status indicators and Fe regulatory hormones were measured, including hemoglobin, serum ferritin (SF), serum transferrin receptor (sTfR), hepcidin, and erythropoietin. Biochemical assessment methods have been described previously in the maternal (23) and neonatal (28) cohorts. Maternal anemia was defined in our cohort by using the Centers for Disease Control and Prevention (Atlanta, GA, USA) trimester-specific definitions: hemoglobin < 11.0 g/dl in trimester 1 (<14.0 wk of gestation) and trimester 3 (≥28 wk of gestation) and hemoglobin < 10.5 g/dl in trimester 2 (14.0–27.9 wk of gestation) (29). We reduced the trimester-specific maternal anemia thresholds by 0.8 g/dl among African American participants as recommended by the Institute of Medicine (30). Neonatal anemia was defined as a cord blood hemoglobin concentration < 13 g/dl (31).
mRNA expression of placental Fe transporters
Expression of mRNA for placental Fe transporters and placental hepcidin was analyzed by quantitative RT-PCR. Total RNA was extracted from placental tissue by using the RNeasy Microarray Tissue Mini Kit (Qiagen, Valencia, CA, USA). RNA was quantified and verified for integrity by the Experion automated electrophoresis system (Bio-Rad, Hercules, CA, USA). A total of 1 μg of RNA per target was reverse-transcribed into cDNA by using a transcriptor cDNA synthesis kit (Roche, Basel, Switzerland) in a MyCycler personal thermal cycler (Bio-Rad). cDNA was then amplified in PCR reactions by using the LightCycler 480 Instrument (Roche) and SYBR Green I Master fluorescent technology (Roche). A total of 7 ng/µl of cDNA of each sample was pipetted into 384-well plates; samples were run in triplicate. A negative control of PCR-grade H2O and a positive control (human placental tissue) were used to correct for plate-to-plate variation. β-Actin was included on each plate as the reference gene. Reaction conditions have been described previously (25). The following primers were designed through Roche Applied Science Universal Probe Library using the National Center for Biotechnology Information (Bethesda, MD, USA) sequence ID and purchased from Integrated DNA Technologies (Coralville, IA, USA): DMT1 (divalent metal transporter): forward: 5′-CAC CGT CAG TAT CCC AAG GT-3′, reverse: 5′-CAT GTC TGA GCC GAT GAT AGC-3′; TfR1 (transferrin receptor 1): forward: 5′-ACC TGT CCA GAC AAT CTC CAG-3′, reverse: 5′-TGT TTT CCA GTC AGA GGG ACA-3′; ferroportin: forward: 5′-TTA CCA GAA AAC CCC AGC TC-3′, reverse: 5′-CAG GGG TTT TGG CTC AGT AT-3′; HEPHL1 (zyklopen): forward: 5′-ATT CCA AGT GCC CAT GAC A-3′, reverse: 5′-CCT GGA CCG GAT CTT TTA GG-3′; HAMP (hepcidin): forward: 5′- CTG TTT TCC CAC AAC AGA CG-3′, reverse: 5′-TTC GCC TCT GGA ACA TGG-3′; FLVCR1: forward: 5′-CCC AAA GAG GTG TCC ACA G-3′, reverse: 5′-GGT AGC AAA AAG CCA ACT GC-3′; FLVCR2 (feline leukemia virus subgroup C receptor 2): forward: 5′-AGC AAA CAA AGA AAC TCT TGA GAA C-3′, reverse: 5′-TTT GCT GGT GTT GCT CTC C-3′; SLC46A1 [solute carrier family 46 (folate transporter), member 1 or proton-coupled folate transporter]: forward: 5′-CAT CCC GGC TGT TCT GAT-3′, reverse: 5′-CTG CTG GAA CTC GAG GTG A-3′; and SLC48A1 [solute carrier family 48 (heme transporter) member 1 or heme-responsive gene 1 protein homolog (HRG1)]: forward: 5′-CTG CAC CTT CCT CGT GCT-3′, reverse: 5′-AAG GCC CAC TTG AAG GAA AT-3′. Primer sequences used for LRP1 and β-actin have been published (25). Plate-to-plate variation was controlled by normalizing gene expression to β-actin and a control placenta by using the ΔΔCt method: ΔCt = Ct (target) – Ct (β-actin); ΔΔCt = ΔCt (sample) – ΔCt (control placenta); fold change = 2– ΔΔCt.
Protein abundance of placental Fe transporters
Placental protein was isolated, homogenized, and quantified as previously reported (25, 32), and abundance of placental Fe transporters was measured by Western blot. Methods and materials used to measure ferroportin, FLVCR1, and LRP1 have been published (25, 32). For the remaining placental proteins, protein isolates were run on SDS-PAGE gels and transferred to PVDF fluorescence membranes (Millipore, Billerica, MA, USA). All membranes were blocked in Odyssey blocking buffer (Li-Cor Biosciences, Lincoln, NE, USA) for 1 h at room temperature and subsequently rinsed and probed for 1 h at room temperature with antibody specific to each Western experiment as follows: HRG1: rabbit anti-HRG1 (gift from Dr. Iqbal Hamza, University of Maryland, College Park, MD, USA) at dilution factor of 1:1000; PCFT: rabbit anti-PCFT (Abcam, Cambridge, MA, USA) at 1:1000; and TfR1: mouse anti-TfR (Thermo Fisher Scientific) at 1:3000. Blots were then incubated with fluorescence-coupled secondary antibodies (Li-Cor Biosciences) for 1 h, then washed in a 1:1 dilution of Odyssey blocking buffer to 1× PBS with 0.1% Tween. Membranes were scanned and quantified by using an Odyssey machine (Li-Cor Biosciences). β-Actin (Santa Cruz Biotechnology, Santa Cruz, CA, USA) was used as a loading control. Control human placenta lysate from our laboratory or human liver lysate (Abcam) was used as membrane control. Additional controls specific to each blot were as follows: HRG1: Hutu80 cells (gift from Dr. Iqbal Hamza) as positive control; and PCFT: human small intestine (Abcam) as positive control and human brain (Abcam) as negative control.
Statistical analyses
Statistical analyses were conducted with SAS 9.4 (SAS Institute, Cary, NC, USA), R version 3.1.1 (The R Foundation for Statistical Computing, Vienna, Austria), and JMP Pro 11 (SAS Institute). When necessary, variables that were not normally distributed were log transformed to meet the assumptions of the statistical model. The study population with placental tissue available (n = 154) was compared with the larger clinical cohort (n = 255) on select demographic and physical characteristics listed in Table 1. Student’s t test or Wilcoxon rank-sum test was used to compare the group means of continuous variables. Difference in proportions for each categorical variable was tested with the χ2 test.
TABLE 1.
Description of study participants
| Characteristic | n | Mean ± sd (%) |
|---|---|---|
| Maternal age at study enrollment (yr) | 154 | 17.1 ± 1.1 |
| Gestational age when entering prenatal care (wk) | 133 | 10.9 ± 5.4 |
| Gynecologic age at conception (yr) | 151 | 4.8 ± 1.9 |
| Prepregnancy BMI (kg/m2) | 153 | 24.9 ± 5.7 |
| Dietary Fe intake (mg/d) | 148 | 17.4 ± 7.7 |
| SF at 26 ± 4 wk gestation (midgestation) (μg/L) | 104 | 21.6 ± 17.9 |
| sTfR at 26 ± 4 wk gestation (midgestation) (mg/L) | 104 | 5.3 ± 4.7 |
| Hemoglobin at delivery (g/dl) | 145 | 11.5 ± 1.3 |
| Erythropoietin at delivery (mIU/ml) | 143 | 34.6 ± 29.4 |
| Gestational age at delivery (wk) | 154 | 39.8 ± 1.3 |
| Race | ||
| African American | 101 | (66) |
| White | 52 | (34) |
| American Indian | 1 | (<1) |
| Ethnicity | ||
| Hispanic | 42 | (27) |
| Non-Hispanic | 112 | (73) |
| Parity at study enrollment | ||
| 0 | 123 | (80) |
| ≥1 | 31 | (20) |
| Inadequate intake of dietary Fea | 112 | (76) |
| Anemic at deliveryb | 27 | (19) |
The study was conducted with archived samples from 154 placentas that were collected from adolescents who participated in a larger longitudinal study of adolescent pregnancy. BMI, body mass index. aInadequate intake = total daily dietary iron intake <23 mg/d (the estimated average requirement for pregnant females age 14–18 yr) (45). bAnemia defined as hemoglobin < 10.2 g/dl in African American adolescents and <11.0 g/dl in all other patients.
Pearson’s correlations were used to test associations between placental proteins. This analysis was not hypothesis-driven, so P values were adjusted for multiple comparisons by using the false discovery rate method, α = 0.05. Multiple linear regression models were then used to test the statistically significant associations with adjustment for potential confounders, such as placental weight. Separately, exploratory factor analysis with quartimin rotation was used to summarize the inter-relationships among placental protein variables, reduce them to fewer dimensions (factors), and assess whether the factors generated were consistent with known biologic roles of these proteins. Factors with an eigenvalue of >1 were retained because they reduce the number of variables in that they account for more of the variance than any single variable does (33). The degree to which each original variable correlated with a derived factor is referred to as the factor loading score. Factor analysis was undertaken among the subset of adolescents that had complete data for all 9 variables included.
We hypothesized that maternal and neonatal Fe status would predict placental expression of Fe transporters. We first screened for correlations between Fe status indicators and placental protein variables using P < 0.2 to determine significance. Correlations that achieved this level of statistical significance were then adjusted for potential confounders, which were defined as variables that were associated (P < 0.2) with Fe status indicators and placental protein variables. Potential confounders included gynecologic age at conception, maternal gestational weight gain, prepregnancy body mass index, maternal race, gestational age, birth weight, and placental weight. Gestational age on the date of blood draw was included as a covariate in models that included a measure of maternal Fe status at midgestation. In any model that included SF or serum hepcidin, we also ran the model with maternal or umbilical cord IL-6 concentration as an additional covariate to control for the possible effect of systemic inflammation. Partial correlations that were statistically significant at P < 0.05 are reported.
Neonatal Fe status depends on maternal Fe status, as insufficient maternal Fe supply may limit the amount of Fe that is available for placental transport. Because we previously reported a positive correlation between maternal Fe status and neonatal Fe status in the larger study cohort (28), we used mediation analyses to simultaneously model the effects of maternal and neonatal Fe status on placental Fe transporter mRNA expression and protein abundance. Each placental protein variable was modeled as a continuous outcome in a structural equation model that estimated the direct effects of maternal and neonatal Fe status and the mediation effect of neonatal Fe status, that is, the effect of maternal Fe status working through neonatal Fe status. In the structural equation models, maternal Fe status was represented by maternal SF or sTfR at midgestation. Neonatal Fe status was represented by cord SF or sTfR. We used the results of the preceding analyses—correlation between maternal Fe status indicator and neonatal Fe status indicator >0.2—and published results from the larger cohort (23) to identify which indicators to select. All structural equation models were performed with and without adjustment for the aforementioned potential confounders. For models that included SF, we included maternal or neonatal serum IL-6 to test whether adjustment for systemic inflammation impacted results.
RESULTS
Study population
Table 1 shows the demographics and Fe status of the 154 mother-neonate pairs that contributed data to this analysis. The 154 adolescents with placental protein data did not significantly differ from the larger cohort of 255 pregnant adolescents, except that mean gestational age was 39.8 wk in this study population and 39.3 wk in the larger cohort (P = 0.027). Three percent of teenagers delivered preterm (<37 wk of gestation), 20% delivered early term (37–39 wk), 55% delivered full term (39 to <41 wk), and 21% delivered late term (41 to <42 wk). One teen delivered post-term (≥42 wk). Placental Fe transporter expression was associated with several maternal and neonatal characteristics, including maternal gynecologic age, maternal gestational weight gain, maternal prepregnancy body mass index, maternal race, gestational age at birth, birth weight, and placental weight, all of which were included as covariates in the multiple regression and structural equation models.
Maternal and neonatal Fe status
Fe status in this study population did not significantly differ from that in the larger cohort (Table 1) (23). Prevalence of inadequate intake of dietary Fe (<23 mg/d) was 76%. Maternal anemia was evident in 19% of patients at delivery. Prevalence of neonatal anemia was 26%. Maternal IL-6 increased 65% on average from midgestation to delivery (P < 0.0001), and hepcidin increased 25%. Similar to findings in the larger cohort (28), as a result of delivery-associated inflammation, neonatal Fe status indicators were more strongly correlated with measures of maternal Fe status at midgestation than at delivery. Maternal sTfR at midgestation was positively associated with cord sTfR (r = 0.23; P = 0.02). Maternal SF at midgestation was positively associated with cord SF and hepcidin (r = 0.37; P < 0.001; and r = 0.21; P = 0.04, respectively). Similarly, maternal hepcidin at midgestation was positively associated with cord hepcidin and SF (r = 0.32; P < 0.01; and r = 0.21; P = 0.04, respectively).
Inter-relationships between placental nonheme and heme Fe transporters
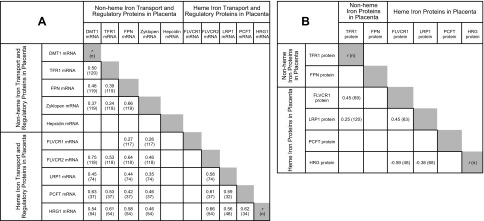
All transcripts and proteins targeted were detected in the human placental tissue analyzed. Supplemental Fig. 1 shows representative images of the Western blots. Figure 1A displays the correlations observed between the measures of mRNA expression. Only those correlations that remained significant after adjustment for false discovery and confounding are reported. Positive correlations were evident between all measures of mRNA expression for placental nonheme Fe transporters (r = 0.24–0.66), but none of these variables was significantly associated with placental hepcidin mRNA. Considering the heme transport proteins, all measures of mRNA expression, excluding FLVCR1 mRNA, were positively correlated (r = 0.56–0.66). Furthermore, mRNA expression of nonheme and heme Fe transport proteins seemed to be coordinated as evidenced by positive associations (r = 0.26–0.75) for 17 of 25 bivariate relationships tested. Analogous to the finding for nonheme transporters, placental hepcidin mRNA expression was unrelated to mRNA expression of the heme transport proteins.
Figure 1.
A) Correlations between measures of mRNA expression of placental nonheme and heme Fe transport proteins. B) Correlations between measures of protein abundance. Correlations were statistically significant at P < 0.05, adjusted for false discovery rate. These correlations remained statistically significant when tested in multiple linear regression models that adjusted for variables that can impact the functional status of the placenta, including placental weight, birth weight, gestational age (wk), maternal gynecologic age at conception (yr), maternal pregnancy weight gain, maternal prepregnancy body mass index, and maternal race. FPN, ferroportin; ZYK, zyklopen.
Figure 1B shows there were fewer statistically significant associations between the 6 measures of protein abundance. Heme transport proteins FLVCR1, LRP1, and HRG1 were associated, and FLVCR1 and LRP1 both correlated positively with the protein abundance of TfR1. PCFT protein abundance was unrelated to any of the other measures of protein abundance.
For all Fe transport proteins examined, mRNA expression was not statistically significantly associated with the corresponding downstream protein abundance. This was true before and after adjustment for confounding. There was a moderate positive correlation (r = 0.31; n = 78) between ferroportin mRNA and PCFT protein abundance that was still significant after adjustment.
As the 10 measures of mRNA expression were strongly correlated with one another, we reduced them to fewer dimensions by using exploratory factor analysis. The analysis was undertaken in a subset of adolescents (n = 48) that had mRNA data for all proteins evaluated. This subset of individuals did not significantly differ from the larger study population in any of the characteristics listed in Table 1. Missing mRNA expression data were missing primarily as a result of lack of tissue in the sample archive. Three factors adequately described 70% of the total variation among the measures of mRNA expression. Factor loadings are shown in Table 2. Placental nonheme Fe import proteins (TfR1 and DMT1) and putative heme Fe import proteins (FLVCR2, LRP1, and HRG1) were closely correlated and loaded primarily (r ≥ 0.54) on factor 1. Three proteins were implicated in Fe export (ferroportin, zyklopen, and FLVCR1) loaded predominantly on factor 2. Ferroportin and zyklopen were closely linked to factor 2 (r = 0.79 and 0.69, respectively), whereas only approximately one third of the variance in FLVCR1 was explained by factor 2. A third factor was characterized almost entirely by placental hepcidin mRNA expression (r = 0.73) and also explained approximately 17% of the variation in FLVCR1. Except for this weak connection to FLVCR1, placental hepcidin mRNA expression was essentially unrelated to expression of placental nonheme and heme Fe transporters examined. Several factor structures were tested, and the pattern described here persisted. The variation in FLVCR1 mRNA expression was not captured well by any of the tested factor structures. We had to exclude PCFT mRNA expression from the factor analysis because of the small number of observations for this transporter (n = 37).
TABLE 2.
Factor loadings from exploratory factor analysis
| mRNA expression of placental Fe transport proteins | Correlation with factor 1 | Correlation with factor 2 | Correlation with factor 3 |
|---|---|---|---|
| FLVCR2 | 0.88 | 0.08 | −0.05 |
| DMT1 | 0.86 | −0.10 | 0.19 |
| TfR1 | 0.62 | −0.03 | 0.00 |
| LRP1 | 0.57 | 0.10 | −0.15 |
| HRG1 | 0.54 | 0.25 | 0.06 |
| FPN | 0.19 | 0.79 | −0.13 |
| Zyklopen | 0.07 | 0.69 | −0.08 |
| FLVCR1 | −0.03 | 0.35 | 0.17 |
| Hepcidin | 0.06 | 0.02 | 0.73 |
Exploratory factor analysis with quartimin rotation was applied to measures of mRNA expression for placental Fe transporters, as these measures were shown to be highly interrelated. Three factors adequately described 70% of the variation among these variables. How much a variable correlated with a derived factor is represented by the factor loading score shown above (33). Factor loading scores >0.5 are italicized. Proteins known to facilitate nonheme Fe import loaded highly on factor 1 along with proteins thought to participate in cellular heme import. Ferroportin and zyklopen, the 2 proteins critical for nonheme Fe export, loaded predominantly on factor 2 with FLVCR1, which may be an intracellular or cellular heme exporter in placenta. Hepcidin loaded independently on factor 3. This analysis included a subset of adolescents that had mRNA expression data for all proteins evaluated (n = 48). This subset did not statistically differ from the larger study population for the characteristics listed in Table 1. FPN, ferroportin.
Maternal and neonatal Fe status and expression of placental Fe transporters
Tables 3 and 4 show associations of placental Fe transporter mRNA expression and protein abundance with indicators of maternal or neonatal Fe status. Maternal Fe status, rather than neonatal Fe status, was associated with multiple placental nonheme and heme Fe transport proteins (Table 3). Neonatal Fe status was only significantly associated with 3 putative heme transporters both before and after adjustment for confounders (Table 4). We also tested whether factors 1 and 2 derived from the factor analysis were associated with Fe status indicators, but found no statistically significant associations.
TABLE 3.
Associations between placental Fe transport proteins and indicators of maternal Fe status
| Maternal Fe status | Placental target | Correlation | P | Partial correlation | P | na |
|---|---|---|---|---|---|---|
| SF midgestation | TfR1 protein | −0.32 | <0.01 | −0.23 | <0.05 | 96 |
| sTfR midgestation | TfR1 protein | 0.23 | <0.05 | 0.09 | 0.39 | 96 |
| sTfR midgestation | TfR1 mRNA | 0.15 | 0.18 | 0.25 | <0.05 | 78 |
| sTfR delivery | 0.20 | <0.05 | 0.25 | <0.05 | 105 | |
| EPO delivery | 0.24 | <0.01 | 0.29 | <0.01 | 103 | |
| sTfR midgestation | FPN protein | 0.27 | <0.05 | 0.22 | 0.14 | 56 |
| sTfR delivery | −0.23 | 0.09 | −0.31 | <0.05 | 56 | |
| EPO midgestation | LRP1 mRNA | −0.26 | <0.05 | −0.28 | <0.05 | 61 |
| EPO midgestation | HRG1 protein | −0.27 | <0.05 | −0.33 | <0.01 | 71 |
| EPO delivery | −0.19 | 0.11 | −0.27 | <0.05 | 68 | |
| Hepcidin midgestation | HRG1 mRNA | −0.28 | <0.05 | −0.31b | <0.05b | 53 |
| Hepcidin midgestation | TfR1 protein | −0.27 | <0.01 | −0.16 | 0.15 | 95 |
Associations between maternal Fe status indicators and placental protein variables were tested with Pearson’s correlation and partial correlation tests. Raw and partial correlations and respective P values are shown. Partial correlation tests controlled for placental weight, birth weight, gestational age at birth (wk), maternal gynecologic age at conception (yr), maternal pregnancy weight gain, maternal prepregnancy body mass index, and maternal race. All measures of maternal Fe status at midgestation were adjusted for the gestational age on the date of blood collection. EPO, erythropoietin; FPN, ferroportin. aCorresponds to the partial correlation tests. bIn models that included SF or hepcidin, we assessed the potential impact of inflammation by including serum IL-6, and only this association was significantly affected. When maternal midgestation serum IL-6 was included, the partial correlation of midgestation hepcidin with HRG1 mRNA was −0.29; P = 0.056; n = 53.
TABLE 4.
Associations between placental Fe transport proteins and indicators of neonatal Fe status
| Neonatal Fe status | Placental target | Correlation | P | Partial correlation | P | na |
|---|---|---|---|---|---|---|
| Cord sTfR | PCFT protein | −0.36 | <0.001 | −0.37 | <0.001 | 95 |
| Cord SF | PCFT protein | 0.21 | <0.05 | 0.22 | <0.05 | 93 |
| Cord SF | FLVCR1 mRNA | −0.17 | 0.07 | −0.25 | <0.05 | 101 |
| Cord sTfR | TfR1 protein | 0.18 | <0.05 | 0.10 | 0.28 | 130 |
| Cord EPO | LRP1 protein | −0.25 | <0.05 | −0.22 | <0.05 | 100 |
Associations between neonatal Fe status indicators and placental protein variables were tested with Pearson’s correlation and partial correlation tests. Raw and partial correlations and respective P values are shown. Partial correlation tests controlled for placental weight, birth weight, gestational age at birth (wk), maternal gynecologic age at conception (yr), maternal pregnancy weight gain, maternal prepregnancy body mass index, and maternal race. EPO, erythropoietin. aCorresponds to the partial correlation tests.
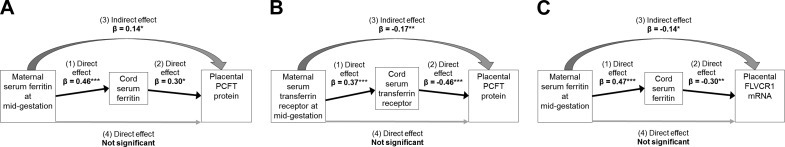
Because maternal and neonatal Fe status were significantly correlated with one another, mediation analyses allowed us to simultaneously model and thereby disentangle the effects of maternal and neonatal Fe status on placental Fe transporters. We detected direct, that is, independent, positive associations between maternal sTfR at midgestation and placental TfR1 protein abundance (β = 0.25; se = 0.11; P = 0.024; n = 93) and ferroportin protein abundance (β = 0.33; se = 0.13; P = 0.012; n = 56). The Effect of maternal Fe status on placental TfR1 was further supported by a direct, inverse association between maternal SF at midgestation and TfR1 protein abundance (β = −0.32; SE = 0.12; P = 0.005; n = 91). Maternal SF at midgestation was also associated with FLVCR1 mRNA expression and PCFT protein abundance, but mediation analyses revealed these were indirect effects and working through neonatal SF (Fig. 2). There were direct, that is, independent, associations between neonatal SF and FLVCR1 mRNA expression (β = −0.30; se = 0.10; P = 0.004; n = 75) and PCFT protein abundance (β = 0.30; SE = 0.12; P = 0.016; n = 85). Placental PCFT protein was also directly, inversely related to neonatal sTfR (β = −0.46; se = 0.11; P < 0.0001; n = 87). As with SF, neonatal sTfR mediated the indirect effect of maternal sTfR on PCFT protein (Fig. 2). We report the results of the adjusted mediation analyses, but the effects were consistent and statistically significant or borderline significant (P = 0.057 for the direct effect of maternal sTfR on TfR1; P = 0.068 for the indirect effect of maternal SF on FLVCR1 mRNA) in the unadjusted analyses as well (data not shown). Inclusion of IL-6 in the models had a negligible and statistically insignificant impact on these results.
Figure 2.
Direct and mediating effects of neonatal Fe status on placental expression of 2 heme transporters: Results from 3 structural equation models. A) Model 1: maternal SF at midgestation had an independent effect (1) on umbilical cord SF (β = 0.46; P < 0.001). Cord SF had an independent effect (2) (β = 0.30; P = 0.016) and mediated the indirect effect (3) of maternal SF (β = 0.14; P = 0.04) on the heme importer PCFT protein abundance. Maternal SF had no direct effect (4) on PCFT protein. B) Model 2: maternal sTfR at midgestation had an independent effect (1) on umbilical cord sTfR (β = 0.37; P < 0.001). Cord sTfR had an independent effect (2) (β = −0.46; P < 0.0001) and mediated the indirect effect (3) of maternal sTfR (β = −0.17; P = 0.006) on the heme importer PCFT protein abundance. Maternal sTfR had no direct effect (4) on PCFT protein. C) Model 3: maternal SF at midgestation had an independent effect (1) on umbilical cord SF (β = 0.47; P < 0.001). Cord SF had an independent effect (2) (β = −0.30; P = 0.004) and mediated the indirect effect (3) of maternal SF (β = −0.14; P = 0.02) on the heme exporter FLVCR1 mRNA expression. Maternal SF had no direct effect (4) on FLVCR1 mRNA. *P < 0.05; **P < 0.01; *** P < 0.001.
DISCUSSION
This is the first study, to our knowledge, to measure multiple nonheme and heme Fe transport proteins in human placental tissue and to evaluate their associations with maternal and neonatal Fe status and maternal and neonatal circulating hepcidin concentrations. Placental tissue was obtained from a group of pregnant adolescents who were at risk for Fe deficiency and anemia and participated in a longitudinal study of Fe status across gestation, which may increase the ability to detect coordinated regulation of Fe trafficking proteins. In this study population, highly significant positive correlations were evident between both the protein abundance and transcript expression of placental nonheme and heme Fe transport proteins, which suggests that these proteins may be coordinately regulated. Multiple maternal Fe status indicators were found to be significantly associated with protein abundance and transcript expression of both nonheme and heme Fe transporters. In contrast, neonatal Fe status was only significantly associated with 3 placental heme Fe transporters. The systemic Fe regulatory hormone, hepcidin, in maternal or neonatal circulation was not significantly associated with placental Fe transport proteins in this population at increased risk for anemia.
Protein abundance between samples was highly variable, ranging from a 35-fold change for FLVCR1 to only a 9-fold change for ferroportin. Similarly, transcript expression was highly variable and most variable for hepcidin, which differed by 130-fold. Whereas there was variable expression of individual proteins between individuals, nonheme and heme Fe transport proteins were highly correlated with one another at the protein and transcript level. The positive correlations between nonheme and heme Fe transport proteins, which ranged from r = 0.26 to 0.75, remained statistically significant after adjustment for multiple comparisons and for variables that can impact the functional status of the placenta, such as gestational age at birth, placental weight, and maternal anthropometry. To evaluate the overall pattern of expression, we used exploratory factor analysis. With no biologic assumptions imposed, cellular importers of nonheme or heme Fe were related to a common factor, whereas exporters of nonheme or heme Fe were related to another factor.
Several indicators of maternal Fe status were found to be significantly associated with the nonheme and heme Fe transporters evaluated. The most robust associations found were the inverse association between maternal SF and protein abundance of TfR1, and the positive association between maternal sTfR and protein abundance of TfR1. That these associations are opposite in direction is expected, as maternal SF decreases and sTfR increases in response to maternal Fe depletion. Our findings are consistent with data from a rat model of gestational Fe deficiency developed by Gambling and colleagues (34). In their study, a significant increase in placental TfR1 mRNA expression and protein abundance was evident in Fe-deficient compared with Fe-replete dams (34). Moreover, our group previously reported that maternal Fe status was inversely associated with placental TfR1 protein abundance in a group of 80 pregnant adolescents (24).
Hepcidin is known to regulate Fe export from the enterocyte, macrophage, and hepatic tissue (15). Of interest, placental nonheme Fe transporters were unrelated to maternal or neonatal hepcidin concentrations. There seemed to be an inverse association between maternal hepcidin at midgestation and placental TfR1 protein abundance, but this effect was no longer statistically significant after adjustment for potential confounding. Our results mirror those from a recent human study that found that placental TfR1 mRNA expression and protein abundance were inversely associated with maternal Fe status yet unrelated to maternal or cord serum hepcidin (35). The ability to detect associations between circulating hepcidin concentrations and placental Fe transporters may depend on the Fe status of the maternal population. A murine study that genetically knocked down hepcidin production found that, when dietary Fe was adequate, lack of maternal hepcidin led to increased Fe levels in the fetus and greater TfR1, DMT1, and ferroportin mRNA expression and ferroportin protein abundance in the placenta (18). In contrast, on an Fe-deficient diet, there were no significant differences between wild-type and knockout dams. Furthermore, fetal nonheme liver Fe and placental ferroportin mRNA and protein were increased in fetuses that lacked hepcidin compared with fetuses with hepcidin, but only when dams were fed a high-Fe diet, not the deficient or adequate diet. These results indicate that circulating hepcidin has a predominant role in limiting Fe transfer across the placenta when the maternal Fe supply is plentiful. Our present study found no associations between serum hepcidin concentrations and placental Fe transport proteins, which may be explained by the high prevalence of inadequate dietary Fe intake (76%) in this cohort and the fact that 19% of these adolescents and 26% of their neonates were anemic at delivery.
The placenta also expresses hepcidin, but the role of hepcidin in this tissue has not been fully characterized. Similar to the lack of significant findings for systemic hepcidin, hepcidin mRNA expression in human placenta was unrelated to any of the placental Fe transporters evaluated or to any indicator of maternal or neonatal Fe status. To our knowledge, no other study has assessed determinants of the expression of hepcidin mRNA in healthy term human placental tissue; however, our findings are similar to those from a study in rats in which placental hepcidin mRNA was unrelated to Fe content of the maternal diet during pregnancy and maternal or fetal liver Fe content at term (16). Hepcidin is also known to function as an antimicrobial peptide (36). The reported lack of association between placental hepcidin mRNA expression and Fe status is also consistent with a study of Plasmodium falciparum–infected placentas. Placental malarial infection was associated with placental hepcidin expression, but no significant associations were evident between maternal hemoglobin concentrations and placental hepcidin mRNA expression (37).
Early literature presented the human fetus as a perfect parasite that could accrue sufficient Fe stores across gestation irrespective of maternal Fe status. Whereas recent studies have addressed the issue, the degree to which placental Fe transporters can be regulated in response to maternal or fetal Fe status has yet to be explained. In the present study, 26% of neonates were anemic at birth as defined by using cord blood hemoglobin concentration. Despite significant neonatal anemia, neonatal Fe status was unrelated to placental nonheme Fe transporters both before and after adjustment for confounding. Neonatal Fe status, however, was significantly related to 3 heme transporters, PCFT (positive association), FLVCR1 (inverse association), and LRP1 (positive association). Mediation analyses were used to isolate the independent and mediation effects of neonatal Fe status on placental Fe transporters. With this approach, neonatal Fe status remained significantly related to PCFT protein abundance (positive) and FLVCR1 mRNA expression (inverse). PCFT is a folate transporter that also plays a role in intestinal heme absorption and may be regulated by Fe status (38–40). FLVCR1 is a key heme exporter that works to regulate intracellular heme content and is essential for erythroid cell development (41). Whereas maternal Fe status was also statistically significantly associated with placental PCFT and FLVCR1, mediation analyses indicated that these effects were indirect and mediated through neonatal Fe status. Because 60% of total body Fe exists within hemoglobin (42) and expression of the heme transporters PCFT, FLVCR1, and LRP1 is especially high in human placenta compared with other tissues (11–13), the role of these heme transporters in human placental tissue requires further study. Preferential placental use of heme Fe is known to occur in other carnivorous mammals that possess a placental hemophagous zone that obtains Fe via phagocytosis of maternal blood (43). A previous study from our group fed stable isotopes of nonheme Fe (57Fe) and intrinsically labeled porcine heme Fe (58Fe) to pregnant women (44). We found a significantly greater fraction of absorbed heme Fe was evident in the neonate at birth compared with the fraction of absorbed nonheme Fe in the neonate at birth. We also previously reported a statistically significant association between neonatal hepcidin and placental expression of the heme importer LRP1 (25). Together, our previous and present findings suggest that the placenta may use heme Fe to meet fetal Fe needs.
In the present study, we noted significant associations between neonatal Fe status and heme Fe transporters in placenta but could not confirm the direction of these effects. It may be that fetal Fe status regulates placental heme Fe transporter expression or that placental heme transporter expression may influence fetal Fe acquisition and thus neonatal Fe status at birth. To investigate the latter scenario, we revised the structural equation models to include the neonatal Fe status indicator as the dependent variable, the placental protein as the mediating factor, and the maternal Fe status indicator as an additional covariate of interest. These revised models detected no significant mediating effects, which supports the conclusion that fetal Fe status explains placental PCFT abundance and FLVCR1 mRNA expression and not vice versa. PCFT has been shown to import heme in the enterocyte, and placental PCFT was positively associated with neonatal Fe status. Additional research is required to identify the mechanism responsible for this association.
Our study was subject to limitations. We longitudinally assessed Fe status across gestation and in the neonate at birth in maternal-neonatal dyads, but such a design does not allow for determination of the causal direction of any of the associations noted. In addition, sample size varied across statistical tests as a result of missing data, often because a clinical sample was not collected or sample integrity was compromised. Protein abundance was only obtained for 6 of 10 placental transporters for which we have mRNA expression data. Our incomplete data set is an important limitation, but we chose to use all available data as opposed to discard incomplete cases and thereby significantly reduce the size of a unique data set from a large pregnancy cohort. Because missing data were missing as a result of a random process (a tissue sample was unavailable), we believe it does not systematically bias our results. Whether the result of each statistical analysis can be extrapolated to a larger cohort or to other study populations with variable risks for Fe deficiency and anemia is unknown.
In summary, in this group of pregnant adolescents who were at risk for Fe deficiency and anemia, placental nonheme and heme Fe transporters were interrelated and more frequently associated with maternal Fe status compared with neonatal Fe status. Our findings support other recent studies that found that the human placenta responds to maternal Fe status across gestation. Using a comprehensive set of Fe status indicators, neonatal Fe status was found to be unrelated to placental nonheme Fe transport proteins but was significantly associated with placental PCFT protein abundance and FLVCR1 mRNA expression. Future research is needed to elucidate the functions and regulation of the transporters PCFT and FLVCR1 in the human placental syncytiotrophoblast. Despite the capacity of the placenta to regulate key Fe trafficking proteins when maternal Fe supply is limited, this adaptation may not be sufficient to fully endow the neonate with Fe as evidenced by the high prevalence of anemia at birth in this population of term neonates.
ACKNOWLEDGMENTS
This work was supported by U.S. Department of Agriculture Grants 2005-35200 and 2008-01857, and by the U.S. National Institutes of Health (NIH) National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grant T32-DK007158. The authors thank Francoise Vermeylen and Dr. Erika Mudrak (Cornell University) for technical assistance with statistical analyses and Ann Lei for work to create Supplemental Fig. 1. The authors also thank Dr. Iqbal Hamza (University of Maryland, College Park, MD, USA) for the gift of HRG1 antibody, and Dr. Janis Abkowitz (University of Washington, Seattle, WA, USA) for the gift of the FLVCR1 antibody. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIDDK or the NIH. The authors declare no conflicts of interest.
Glossary
- DMT1
divalent metal transporter 1
- FLVCR1
feline leukemia virus subgroup C receptor 1
- FLVCR2
feline leukemia virus subgroup C receptor 2
- HRG1
heme-responsive gene 1 protein homolog [solute carrier family 48 (heme transporter) member 1]
- LRP1
low density lipoprotein receptor–related protein 1
- PCFT
proton-coupled folate transporter
- SF
serum ferritin
- sTfR
serum transferrin receptor
- TfR1
transferrin receptor 1
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
E. K. Pressman and K. O. O’Brien designed the study; E. K. Pressman, C. Cao, E. Cooper, R. Guillet, O. L. Yost, J. Galati, T. R. Kent and K. O. O’Brien acquired the data; C. M. Best analyzed the data; and C. M. Best, E. K. Pressman, C. Cao, E. Cooper, R. Guillet, O. L. Yost, J. Galati, T. R. Kent, and K. O. O’Brien wrote and revised the manuscript.
REFERENCES
- 1.Rao R., Georgieff M. K. (2007) Iron in fetal and neonatal nutrition. Semin. Fetal Neonatal Med. 12, 54–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Viteri F. E. (1994) The consequences of iron deficiency and anemia in pregnancy. Adv. Exp. Med. Biol. 352, 127–139 [DOI] [PubMed] [Google Scholar]
- 3.Georgieff M. K. (2007) Nutrition and the developing brain: nutrient priorities and measurement. Am. J. Clin. Nutr. 85, 614S–620S [DOI] [PubMed] [Google Scholar]
- 4.Hurrell R., Egli I. (2010) Iron bioavailability and dietary reference values. Am. J. Clin. Nutr. 91, 1461S–1467S [DOI] [PubMed] [Google Scholar]
- 5.Bradley J., Leibold E. A., Harris Z. L., Wobken J. D., Clarke S., Zumbrennen K. B., Eisenstein R. S., Georgieff M. K. (2004) Influence of gestational age and fetal iron status on IRP activity and iron transporter protein expression in third-trimester human placenta. Am. J. Physiol. Regul. Integr. Comp. Physiol. 287, R894–R901 [DOI] [PubMed] [Google Scholar]
- 6.Fuchs R., Ellinger I. (2004) Endocytic and transcytotic processes in villous syncytiotrophoblast: role in nutrient transport to the human fetus. Traffic 5, 725–738 [DOI] [PubMed] [Google Scholar]
- 7.Georgieff M. K., Wobken J. K., Welle J., Burdo J. R., Connor J. R. (2000) Identification and localization of divalent metal transporter-1 (DMT-1) in term human placenta. Placenta 21, 799–804 [DOI] [PubMed] [Google Scholar]
- 8.Bastin J., Drakesmith H., Rees M., Sargent I., Townsend A. (2006) Localisation of proteins of iron metabolism in the human placenta and liver. Br. J. Haematol. 134, 532–543 [DOI] [PubMed] [Google Scholar]
- 9.Chen H., Attieh Z. K., Syed B. A., Kuo Y. M., Stevens V., Fuqua B. K., Andersen H. S., Naylor C. E., Evans R. W., Gambling L., Danzeisen R., Bacouri-Haidar M., Usta J., Vulpe C. D., McArdle H. J. (2010) Identification of zyklopen, a new member of the vertebrate multicopper ferroxidase family, and characterization in rodents and human cells. J. Nutr. 140, 1728–1735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohgami R. S., Campagna D. R., McDonald A., Fleming M. D. (2006) The Steap proteins are metalloreductases. Blood 108, 1388–1394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keel S. B., Doty R. T., Yang Z., Quigley J. G., Chen J., Knoblaugh S., Kingsley P. D., De Domenico I., Vaughn M. B., Kaplan J., Palis J., Abkowitz J. L. (2008) A heme export protein is required for red blood cell differentiation and iron homeostasis. Science 319, 825–828 [DOI] [PubMed] [Google Scholar]
- 12.Qiu A., Jansen M., Sakaris A., Min S. H., Chattopadhyay S., Tsai E., Sandoval C., Zhao R., Akabas M. H., Goldman I. D. (2006) Identification of an intestinal folate transporter and the molecular basis for hereditary folate malabsorption. Cell 127, 917–928 [DOI] [PubMed] [Google Scholar]
- 13.Hvidberg V., Maniecki M. B., Jacobsen C., Højrup P., Møller H. J., Moestrup S. K. (2005) Identification of the receptor scavenging hemopexin-heme complexes. Blood 106, 2572–2579 [DOI] [PubMed] [Google Scholar]
- 14.Cao C., O’Brien K. O. (2013) Pregnancy and iron homeostasis: an update. Nutr. Rev. 71, 35–51 [DOI] [PubMed] [Google Scholar]
- 15.Nemeth E., Ganz T. (2006) Regulation of iron metabolism by hepcidin. Annu. Rev. Nutr. 26, 323–342 [DOI] [PubMed] [Google Scholar]
- 16.Gambling L., Czopek A., Andersen H. S., Holtrop G., Srai S. K. S., Krejpcio Z., McArdle H. J. (2009) Fetal iron status regulates maternal iron metabolism during pregnancy in the rat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 296, R1063–R1070 [DOI] [PubMed] [Google Scholar]
- 17.Evans P., Cindrova-Davies T., Muttukrishna S., Burton G. J., Porter J., Jauniaux E. (2011) Hepcidin and iron species distribution inside the first-trimester human gestational sac. Mol. Hum. Reprod. 17, 227–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Balesaria S., Hanif R., Salama M. F., Raja K., Bayele H. K., McArdle H., Srai S. K. (2012) Fetal iron levels are regulated by maternal and fetal Hfe genotype and dietary iron. Haematologica 97, 661–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin M. E., Nicolas G., Hetet G., Vaulont S., Grandchamp B., Beaumont C. (2004) Transferrin receptor 1 mRNA is downregulated in placenta of hepcidin transgenic embryos. FEBS Lett. 574, 187–191 [DOI] [PubMed] [Google Scholar]
- 20.Nicolas G., Bennoun M., Porteu A., Mativet S., Beaumont C., Grandchamp B., Sirito M., Sawadogo M., Kahn A., Vaulont S. (2002) Severe iron deficiency anemia in transgenic mice expressing liver hepcidin. Proc. Natl. Acad. Sci. USA 99, 4596–4601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee S., Young B. E., Cooper E. M., Pressman E., Queenan R. A., Olson C. M., Guillet R., O’Brien K. O. (2014) Nutrient inadequacy is prevalent in pregnant adolescents, and prenatal supplement use may not fully compensate for dietary deficiencies. Infant Child Adolesc. Nutr. 6, 152–159 [Google Scholar]
- 22.Whisner C. M., Young B. E., Witter F. R., Harris Z. L., Queenan R. A., Cooper E. M., O’Brien K. O. (2014) Reductions in heel bone quality across gestation are attenuated in pregnant adolescents with higher prepregnancy weight and greater increases in PTH across gestation. J. Bone Miner. Res. 29, 2109–2117 [DOI] [PubMed] [Google Scholar]
- 23.Lee S., Guillet R., Cooper E. M., Westerman M., Orlando M., Pressman E., O’Brien K. O. (2014) Maternal inflammation at delivery affects assessment of maternal iron status. J. Nutr. 144, 1524–1532 [DOI] [PubMed] [Google Scholar]
- 24.Young M. F., Pressman E., Foehr M. L., McNanley T., Cooper E., Guillet R., Orlando M., McIntyre A. W., Lafond J., O’Brien K. O. (2010) Impact of maternal and neonatal iron status on placental transferrin receptor expression in pregnant adolescents. Placenta 31, 1010–1014 [DOI] [PubMed] [Google Scholar]
- 25.Cao C., Pressman E. K., Cooper E. M., Guillet R., Westerman M., O’Brien K. O. (2014) Placental heme receptor LRP1 correlates with the heme exporter FLVCR1 and neonatal iron status. Reproduction 148, 295–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Young B. E., Cooper E. M., McIntyre A. W., Kent T., Witter F., Harris Z. L., O’Brien K. O. (2014) Placental vitamin D receptor (VDR) expression is related to neonatal vitamin D status, placental calcium transfer, and fetal bone length in pregnant adolescents. FASEB J. 28, 2029–2037 [DOI] [PubMed] [Google Scholar]
- 27.O’Brien K. O., Li S., Cao C., Kent T., Young B. V., Queenan R. A., Pressman E. K., Cooper E. M. (2014) Placental CYP27B1 and CYP24A1 expression in human placental tissue and their association with maternal and neonatal calcitropic hormones. J. Clin. Endocrinol. Metab. 99, 1348–1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee S., Guillet R., Cooper E. M., Westerman M., Orlando M., Kent T., Pressman E., O’Brien K. O. (2016) Prevalence of anemia and associations between neonatal iron status, hepcidin, and maternal iron status among neonates born to pregnant adolescents. Pediatr. Res. 79, 42–48 [DOI] [PubMed] [Google Scholar]
- 29.Centers for Disease Control and Prevention. (1998) Recommendations to prevent and control iron deficiency in the United States. MMWR. Recommendations and Reports, U.S. Department of Health and Human Services publication no. 47 (No. RR-3), pp. 1–29, Centers for Disease Control and Prevention, Atlanta, GA, USA [PubMed]
- 30.Committee on the Prevention, Detection, and Management of Iron Deficiency Anemia Among U.S. Children and Women of Childbearing Age (1993) Iron Deficiency Anemia: Recommended Guidelines for the Prevention, Detection, and Management Among U.S. Children and Women of Childbearing Age (Earl R., Woteki C. E., eds.), National Academies Press, Washington, DC, USA: [PubMed] [Google Scholar]
- 31.Orkin S. H., Fisher D. E., Look A. T., Lux S. E., Ginsburg D., Nathan D. G. (2009) Nathan and Oski’s Hematology of Infancy and Childhood, Saunders Elsevier, Philadelphia [Google Scholar]
- 32.Jaacks L. M., Young M. F., Essley B. V., McNanley T. J., Cooper E. M., Pressman E. K., McIntyre A. W., Orlando M. S., Abkowitz J. L., Guillet R., O’Brien K. O. (2011) Placental expression of the heme transporter, feline leukemia virus subgroup C receptor, is related to maternal iron status in pregnant adolescents. J. Nutr. 141, 1267–1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gleason P. M., Boushey C. J., Harris J. E., Zoellner J. (2015) Publishing nutrition research: a review of multivariate techniques--part 3: data reduction methods. J. Acad. Nutr. Diet. 115, 1072–1082 [DOI] [PubMed] [Google Scholar]
- 34.Gambling L., Danzeisen R., Gair S., Lea R. G., Charania Z., Solanky N., Joory K. D., Srai S. K., McArdle H. J. (2001) Effect of iron deficiency on placental transfer of iron and expression of iron transport proteins in vivo and in vitro. Biochem. J. 356, 883–889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garcia-Valdes L., Campoy C., Hayes H., Florido J., Rusanova I., Miranda M. T., McArdle H. J. (2015) The impact of maternal obesity on iron status, placental transferrin receptor expression and hepcidin expression in human pregnancy. Int. J. Obes. (London) 39, 571–578 [DOI] [PubMed] [Google Scholar]
- 36.Ganz T. (2003) Hepcidin, a key regulator of iron metabolism and mediator of anemia of inflammation. Blood 102, 783–788 [DOI] [PubMed] [Google Scholar]
- 37.Muehlenbachs A., Fried M., Lachowitzer J., Mutabingwa T. K., Duffy P. E. (2007) Genome-wide expression analysis of placental malaria reveals features of lymphoid neogenesis during chronic infection. J. Immunol. 179, 557–565 [DOI] [PubMed] [Google Scholar]
- 38.Shayeghi M., Latunde-Dada G. O., Oakhill J. S., Laftah A. H., Takeuchi K., Halliday N., Khan Y., Warley A., McCann F. E., Hider R. C., Frazer D. M., Anderson G. J., Vulpe C. D., Simpson R. J., McKie A. T. (2005) Identification of an intestinal heme transporter. Cell 122, 789–801 [DOI] [PubMed] [Google Scholar]
- 39.Latunde-Dada G. O., Takeuchi K., Simpson R. J., McKie A. T. (2006) Haem carrier protein 1 (HCP1): expression and functional studies in cultured cells. FEBS Lett. 580, 6865–6870 [DOI] [PubMed] [Google Scholar]
- 40.Le Blanc S., Garrick M. D., Arredondo M. (2012) Heme carrier protein 1 transports heme and is involved in heme-Fe metabolism. Am. J. Physiol. Cell Physiol. 302, C1780–C1785 [DOI] [PubMed] [Google Scholar]
- 41.Quigley J. G., Yang Z., Worthington M. T., Phillips J. D., Sabo K. M., Sabath D. E., Berg C. L., Sassa S., Wood B. L., Abkowitz J. L. (2004) Identification of a human heme exporter that is essential for erythropoiesis. Cell 118, 757–766 [DOI] [PubMed] [Google Scholar]
- 42.Carpenter C. E., Mahoney A. W. (1992) Contributions of heme and nonheme iron to human nutrition. Crit. Rev. Food Sci. Nutr. 31, 333–367 [DOI] [PubMed] [Google Scholar]
- 43.Carter A. M. (2012) Evolution of placental function in mammals: the molecular basis of gas and nutrient transfer, hormone secretion, and immune responses. Physiol. Rev. 92, 1543–1576 [DOI] [PubMed] [Google Scholar]
- 44.Young M. F., Griffin I., Pressman E., McIntyre A. W., Cooper E., McNanley T., Harris Z. L., Westerman M., O’Brien K. O. (2012) Maternal hepcidin is associated with placental transfer of iron derived from dietary heme and nonheme sources. J. Nutr. 142, 33–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Institute of Medicine Panel on Miconutrients (2001) Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc, National Academies Press, Washington, DC, USA: [PubMed] [Google Scholar]