Abstract
Obesity is a major public health problem. An in-depth knowledge of the molecular mechanisms of oro-sensory detection of dietary lipids may help fight it. Humans and rodents can detect fatty acids via lipido-receptors, such as CD36 and GPR120. We studied the implication of the MAPK pathways, in particular, ERK1/2, in the gustatory detection of fatty acids. Linoleic acid, a dietary fatty acid, induced via CD36 the phosphorylation of MEK1/2-ERK1/2-ETS-like transcription factor-1 cascade, which requires Fyn-Src kinase and lipid rafts in human taste bud cells (TBCs). ERK1/2 cascade was activated by Ca2+ signaling via opening of the calcium-homeostasis modulator-1 (CALHM1) channel. Furthermore, fatty acid–evoked Ca2+ signaling and ERK1/2 phosphorylation were decreased in both human TBCs after small interfering RNA knockdown of CALHM1 channel and in TBCs from Calhm1−/− mice. Targeted knockdown of ERK1/2 by small interfering RNA or PD0325901 (MEK1/2 inhibitor) in the tongue and genetic ablation of Erk1 or Calhm1 genes impaired preference for dietary fat in mice. Lingual inhibition of ERK1/2 in healthy volunteers also decreased orogustatory sensitivity for linoleic acid. Our data demonstrate that ERK1/2-MAPK cascade is regulated by the opening of CALHM1 Ca2+ channel in TBCs to modulate orogustatory detection of dietary lipids in mice and humans.—Subramaniam, S., Ozdener, M. H., Abdoul-Azize, S., Saito, K., Malik, B., Maquart, G., Hashimoto, T., Marambaud, P., Aribi, M., Tordoff, M. G., Besnard, P., Khan, N. A. ERK1/2 activation in human taste bud cells regulates fatty acid signaling and gustatory perception of fat in mice and humans.
Keywords: MAPK, CALHM1, lipids
In the Western diet, approximately 40% of daily energy intake is composed of lipids. This fat supply contributes to obesity and associated diseases, such as type 2 diabetes, atherosclerosis, and hypertension (1). The obese have stronger preferences for dietary lipids than do the lean (2), which suggests that inappropriate orogustatory perception of lipids influences feeding behavior; however, how fat is detected is unclear. Early work emphasized textural, olfactory, and postingestive cues (3), but recent research has revealed that there is also a gustatory component for the detection of long-chain fatty acids (LCFAs) (3, 4). Humans can detect LCFAs even when olfaction is eliminated and texture is masked (5, 6). Initial transduction of lipids in taste bud cells (TBCs) seems to involve several mechanisms, including inhibition of delayed rectifying K+ channels (7), fatty acid–responsive proteins, such as CD36 (8–13), and/or several GPCRs, including GPR120 (14–17).
Despite the identification of some of these fatty acid–responsive proteins, underlying mechanisms for fat taste transduction in human taste bud cells (hTBCs) are still unknown. Intracellular Ca2+ ([Ca2+]i) signaling seems to be involved during LCFA-induced TBC activation (18, 19). We have shown that fatty acids, acting via lingual CD36, trigger increases in free [Ca2+]i concentrations by inducing production of inositol-tris-phosphate (IP3) and opening of multiple store-operated Ca2+ (SOC) channels (19). Hence, stromal interaction motif-1 (STIM1), an endoplasmic protein, was found to regulate fatty acid–induced opening of SOC channels (13). Of interest, Stim1−/− mice lost the spontaneous preference for fat that was observed in wild-type animals. It is noteworthy that membrane ion channels that replenish cellular Ca2+ after endoplasmic reticulum Ca2+ depletion are regulated by phosphorylation and mediated by Src family kinases, in particular, Fyn kinase (20–22), which is known to directly interact with CD36 (23, 24). Src kinases also regulate activation of MAPKs (25–27); however, no study is available on the role of these second messengers in regulation of fat taste.
The present investigation was primarily conducted on hTBCs to assess coupling of fatty acid–mediated gustatory cell activation by ERK1/2 signaling and, in some experiments, by other MAPKs, such as p38 and JNK. We assessed the upstream and downstream targets of the ERK1/2-MAPK cascade. We also investigated the role of calcium-homeostasis modulator-1 (CALHM1), a calcium channel, in regulation of ERK1/2-MAPK activation and serotonin release in human and mouse TBCs. Finally, the importance of CALHM1 channel and ERK1/2 signaling in spontaneous fat preference was analyzed in vivo by conducting 2-bottle choice tests in Calhm1−/− and Erk1−/− mice. In human volunteers, we determined the thresholds for orosensorial detection of a fatty acid after the lingual applications of PD-0325901, an inhibitor of ERK1/2-MAPK cascade.
MATERIALS AND METHODS
Materials
Culture media (Iscove’s modified Dulbecco’s medium and RPMI 1640) and l-glutamine were purchased from Lonza (Basel, Switzerland). Fura- 2/AM was procured from Thermo Fisher Scientific Life Sciences (Waltham, MA, USA). All other chemicals, including PD0325901, MCDB153 medium, SU6656, 2-aminoethyl diphenylborinate (2-APB), ruthenium red (RR), SP600125, SB202190, U0126, and anti-actin antibody (A1978) were from Sigma-Aldrich (St. Louis, MO, USA). The antibody against CD36 (AF 2519) was from R&D Systems (Madison, WI, USA) and against phosphotyrosine kinase (OBT 3179) from Bio-Rad (Bio-Rad, Hercules, CA, USA). GPR120 (ab75313) and CALHM1 (ab75313) antibodies were from Abcam (Cambridge, MA, USA). Antibody for caveolin 1 was from BD Biosciences (Le Pont de Claix, France). Antibodies from Cell Signaling Technology (Danvers, MA, USA) were used for p-c-Raf (9427), c-Raf (9422), p-MEK1/2 (9154), MEK1/2 (9126), p-ERK1/2 (4370), ERK1/2 (4695), p-p90ribosomal S6 kinase (RSK) (9335), p-mitogen- and stress-activated protein kinase (MSK1) (9595), p-ETS-like transcription factor-1 (ELK1; 9181), p-p38 (4511), p38 (9212), p-JNK (4668), JNK (9258), p-Src (6943), Src (2123), and FYN (4023). Accell small interfering RNA (siRNA) ERK1 (E-040126-00-0005), Accell siRNA ERK2 (E-040613-00-0005), Accell Green control (D-001910-01-05), On-Target plus SmartPool (L-032306-02), and CALHM1 siRNA were purchased from Thermo Fisher Scientific Life Sciences.
C57BL/6J mice were obtained from Janvier Labs (Saint Berthevin Cedex, France). Knockout mice for Erk1 (Erk1−/−) gene, generated from the C57BL/6J genetic background as previously described by Pages et al. (28), were kindly given by Prof. Jacques Pouyssegur (Institute for Research on Cancer and Aging, Nice, France). Calhm1−/− mice in C57BL/6J genetic background were generated as described previously (29). Animals were euthanized under food-withholding conditions.
Culture of human fungiform TBCs
Human TBCs were prepared and maintained as previously reported (30). CD36+ TBCs were isolated by using a positive selection technique, as described elsewhere (19).
Measurement of Ca2+ signaling
CD36+ TBCs were suspended in fresh Iscove’s modified Dulbecco’s medium that contained 10% fetal bovine serum and seeded (2 × 105/well) onto Willico-Dish wells (13). Changes in [Ca2+]i (F340/F380) were monitored by using a Nikon microscope (TiU; Nikon, Tokyo, Japan) equipped with EM-CCD (Luca S; Andor, Tokyo, Japan) camera for real time recording of 16-bit digital images and an S-fluor ×40 oil immersion objective. Planes were taken at Z intervals of 0.3 μm and software (NIS Elements; Nikon) was used to analyze the images. Changes in [Ca2+]i were calculated as the difference between peak F340/F380 ratio. Data were summarized from a large number of individual cells (20–40 cells in a single run, with 3–9 identical experiments that included at least 3 cell preparations). For experiments in Ca2+-free medium, CaCl2 was replaced by EGTA (2 mM).
siRNA knockdown of CALHM1
Human fungiform TBCs were transfected with CALHM1 siRNA On-Target plus SmartPool (L-032306-02-0005; 25 nM) or nontargeting siRNA as a control (Dharmacon, Lafayette, CO, USA) using the DharmaFect protocol (Dharmacon). Target sequences for CALHM1 siRNA were: 5′-AUGGACAAGUUCCGGAUGA-3′ (forward); 5′-GAGAGGUGGCCGUGCGUUA-3′ (reverse); 5′-GCACAAAUGCAAACCGCCU-3′ (forward); and 5′-GGGCAAGCGUGUCGUAGGU-3′ (reverse). Twenty-four hours before siRNA transfection, cells were placed in culture medium without antibiotics. After transfection (24 h), medium was replaced with fresh medium without siRNA for 48 h before performing experiments.
Application of ERK1/2 siRNA and PD0325901 on tongue
ERK1/2 siRNA was commercially designed and processed for in vivo use (Thermo Fisher Scientific Life Sciences). A nontargeting sequence (siGenome Non-Targeting siRNA; Dharmacon) was used as control. Accel siRNAs were prepared as suggested by the manufacturer. For application of siRNA, each mouse was anesthetized with isoflurane (1.5–3% in oxygen). All 3 regions of taste tissue, circumvallate papillae, fungiform papillae, and foliate papillae were targeted for ERK1/2 siRNA application. To this end, 5 μl siRNA was slowly pipetted in a circular pattern onto the most caudal 1/4 region of the tongue and 5 μl onto the rostral 3/4 of the tongue. The mouth was held open for 3–5 min to allow for absorption of siRNA. Then, the mouse was returned to its home cage. This process was repeated for 3 consecutive days at the same time each day. Beginning 72 h after treatment, mice either received 2-bottle choice tests or were euthanized and the circumvallate and fungiform papillae were removed for physiologic experiments.
PD0325901 was dissolved in DMSO as a 10 mM stock solution and stored at −20°C. For in vivo experiments, PD0325901 was diluted in 80 mM citric buffer (pH 7) and was applied on the tongue (50 to 100 µl per application with help of a brush) for 1 wk consecutively. Control mice were administered the same volume of citrate buffer (80 mM, pH 7). After the week, mice received 2-bottle choice tests or were euthanized for physiologic experiments.
CD36+ mouse TBCs were isolated as described previously (13, 19). CD36+ cells were subjected to detection of fluorescent siRNA by confocal microscopy. Cells were fixed in 95% ethanol and rehydrated in 0.1 M PBS, pH 7.4. After 3 washings with PBS, a drop of Aqua Poly-Mount mounting medium was added on the slide for analysis under fluorescent microscope (Zeiss, Jena, Germany) with an excitation filter of 445–490 nm and a long-pass emission filter (515 nm).
Lipid raft isolation
Lipid rafts were isolated by sucrose density gradient centrifugation (17). In brief, control and linoleic acid (LA)–treated (10 min) hTBCs (2.5 × 107) were washed with PBS, lysed on ice in 2 ml 1% Triton X-100 in 25 mM morpholineethanesulfonic acid, 150 mM NaCl (pH 6.5) buffer with a protease inhibitor cocktail, and homogenized (Dounce homogenizer). Homogenates equalized on the basis of protein concentrations, mixed with 2 ml 80% sucrose in 1% Triton X-100, 25 mM morpholineethanesulfonic acid, 150 mM NaCl (pH 6.5) buffer, were placed in a centrifuge tube, overlaid with 4 ml 30% sucrose and 4 ml 5% sucrose, and centrifuged at 175,000 g for 20 h at 4°C (SW41 rotor Beckman Ultracentrifuge; Beckman Coulter, Brea, CA, USA). Eleven fractions (1.1 ml each) were collected from the top of the gradient and fractions 2–6 were precipitated with 2 volumes of cold acetone for immunoblotting. Caveolin was the positive control for lipid raft detection and β-actin was used for Triton-soluble fractions.
Western blots
TBCs were serum starved for 6 h and then stimulated by LA and lysed in 50 μl buffer (20 mM HEPES pH 7.3, 1 mM EDTA, 1 mM EGTA, 0.15 mM NaCl, 1% Triton X-100, 10% glycerol, 1 mM phenylmethylsulfonyl fluoride, 2 mM sodium orthovanadate, and 2 μl/ml anti-protease cocktail) and centrifuged (13,000 g × 10 min). Denatured proteins (30 μg) were separated by SDS-PAGE (10%), transferred to PVDF membranes, and probed with primary antibodies from Cell Signaling Technology (1:2000) as well as with anti-CD36 (1:500 dilution) and anti-GPR120 (1:500 dilution) antibodies. Immunoblots were then incubated with a specific horseradish peroxidase–conjugated secondary antibody and developed by using ECL method and reagents according to manufacturer protocol (PerkinElmer, Waltham, MA, USA).
Immunoprecipitation
Cells were lysed in immunoprecipitation buffer [50 mM HEPES pH 7.4, 140 mM NaCl, 5 mM EDTA, 0.2% NP-40, protease inhibitor cocktail (Sigma-Aldrich)] for 30 min on ice, followed by centrifugation at 12,000 g for 10 min at 4°C. Protein concentration was determined (bicinchoninic acid method). Total protein (500 µg) of each lysate was precleared with 40 µl of sepharose beads for 45 min and incubated with 3 µg of antibody for phosphotyrosine kinase with constant agitation at 4°C. Then, immunocomplexes were precipitated with 25 µl of protein G-Sepharose (GE Healthcare, Pittsburgh, PA, USA) and beads were washed 4 times in 20 mM HEPES, pH 7.4, EDTA 5 mM, NaCl 150 mM, 0.2% NP-40, and resuspended in Laemmli buffer. The fraction of cell lysate used in the inputs was 30 µg.
Licking and 2-bottle preference tests
Erk1−/− and CALHM1−/− mice were used in behavioral experiments. Two different tests, which consisted of licking test or 2-bottle preference test, have been used.
The licking test consists of subjecting a mouse to control or experimental solution successively to determine the number of licks given on each bottle by using contact lickometer (Med Associates, St. Albans, VT, USA). Mice were deprived of food and water for 6 h before the test, which took place 6 h after beginning the dark period. After a training period, which was required to learn the procedure, mice were randomly subjected to a bottle that contained control solution (mineral oil; Cooper, Melun, France) or a bottle that contained an experimental solution (mineral oil + 2 or 0.2% LA) for 15 min. Mice were then offered the other bottle for an additional 15 min session. In this experiment, data were analyzed for 1 min from first lick to exclude postingestive signals.
Acceptability of lipid-enriched solutions was assessed by using 12-h 2-bottle choice tests (13, 17). Individually caged Calhm1−/−, ERK1−/−, and wild-type (WT) control mice could choose between 0.2% canola oil emulsified in 0.3% xanthan gum (w/v) in water and water with 0.3% xanthan gum (w/v). In some experiments, 0.2% LA was used in place of canola oil. All solutions were served at room temperature. Mice had ad libitum access to a standard diet and drinking solutions. Canola oil contained as percent the following fatty acids: 61%, oleic acid (18:1 n-9), 25%, linoleic acid (18:2 n-6), and 10%, rumelenic acid (18:3 n-3) (31). Intake was determined by weighing the bottles.
Orosensorial detection of LA in humans
We recruited healthy, age-matched, student volunteers (n = 19; female = 17, male = 2; age 22.2 ± 1.8 yr; body mass index, 20.53 ± 1.4) of Arabic origin in the Laboratory of Applied Molecular Biology and Immunology, Abou Bekr Belkaid University (Tlemcen, Algeria) in January 2015. Physicians and nurses of the University Health Care Center routinely performed the medical check-up of the volunteers. Volunteers had no history of a chronic condition, such as cardiovascular disease, diabetes, liver, or kidney disease. Written consent was obtained and volunteers were assured about the confidentiality of the project. Volunteers were informed about the purpose, protocol, and potential risks of the study. All personal data were erased from the database.
Women were asked to come for testing sessions while they were in their first week of menstruation. LA (18:2) orosensory test was performed as previously described by Sayed et al. (32). We prepared emulsions that contained food-grade (Sigma-Aldrich) LA at various concentrations in deionized water. Acacia gum (0.01%) was used as control to mimic textural properties of fatty acid in control solution, similarly prepared. Volunteers came in a fasting state and underwent the taste preference test at different ascending concentrations of LA (0.018, 0.18, 0.37, 0.75, 1.5, 3, 6, and 12 mM), according to the 3 alternative-forced choice (3-AFC) method, as we have described in detail previously (32). In brief, participants were asked to detect an odd solution that contained LA in the ascending order. They were presented with 2 more sets of samples of the same concentration of LA. Hence, if the response was correct, the concentration was considered as the LA detection threshold. We confirmed it by presenting another solution that contained lower LA concentration, and after the negative response, we increased concentrations up to the correctly detected fatty acid concentration. Participants were asked to rinse their mouth between each new set of samples. To avoid visual and olfactory cues, fatty acid testing sessions were conducted under red light and participants were asked to wear nose clips.
PD-0325901 was applied at 5 mg/ml with the help of a painting brush onto the upper tongue epithelium twice with an interval of 20 min. Participants were asked to keep the mouth slightly open and to not swallow the saliva. Saliva secreted during the test was quickly aspirated off. After 10 min of the second application of PD-0325901 (5 mg/ml), volunteers were asked to spit out and rinse the mouth with distilled water. Then, the second round of orogustatory detection of LA was performed as per 3-AFC protocol.
Statistical analysis
Statistical analysis of data was carried out by using Statistica (version 4.1; Statsoft, Paris, France). Data are presented as means ± sem. The significance of the differences between means was determined by 1-way ANOVA, followed by a least significant difference test. For all the tests, significance level chosen was P < 0.05.
Study approval
All study protocols on mice, including ERK1−/−, were conducted as per the Declaration of Helsinki and European ethical guidelines, and protocols were approved by the Regional Ethical Committee (Dijon, France). All studies on hTBCs adhered to protocols approved by the Schulman Associates Institutional Review Board (Cincinnati, OH, USA). Behavioral studies with Calhm1−/− mice were approved by the Institutional Animal Care and Use Committee of the Monell Chemical Senses Center (Philadelphia, PA, USA).
RESULTS
LA induces MAPK activation in hTBCs
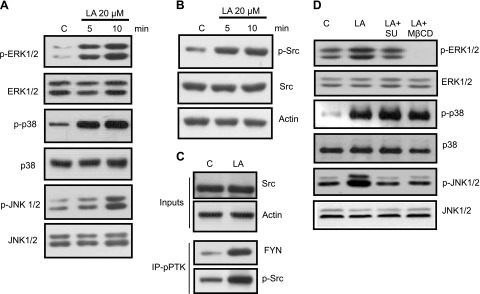
To assess the effects of fatty acids on phosphorylation of MAPKs, hTBCs were exposed to 20 µM LA for 5 or 10 min. LA induced phosphorylation of ERK1/2, p38, and JNK 1/2 (Fig. 1A).
Figure 1.
Effects of LA exposure on MAPK and Src kinase activation. Cultured hTBCs were exposed to 20 µM LA for 5 or 10 min. A) Western blot analysis of cell lysates for phosphorylated (p) or total ERK1/2, p38, and JNK1/2 proteins are shown, as mentioned in Materials and Methods. C, control. B) Western blot analysis of phosphorylated Src (Tyr416) and total Src protein levels in control (c) or LA-treated samples. C) LA-treated (5 min) hTBC lysate was subjected to immunoprecipitation with phosphotyrosine kinase (pPTK) antibody and immunoblotting for FYN and p-Src. Inputs were analyzed for total Src and actin. D) hTBCs were pretreated for 30 min with either PTK inhibitor SU6656 (SU) at 5 µM or lipid raft disruptor M-βCD at 2.5 mM and then exposed to LA for 10 min. Data are representative of 3–4 independent experiments.
Src-kinases and raft integrity are involved in LA-induced ERK1/2 activation in hTBCs
LA induced phosphorylation of Src kinase (Fig. 1B), as reported previously in mouse TBCs (19). Immunoprecipitation with anti-phosphotyrosine kinase antibody showed that the kinase belonged to the Fyn family (Fig. 1C). No increase in phosphorylated Lyn or Yes could be detected in these immunoblots (data not shown).
To shed light on the involvement of Src-kinase in ERK1/2 activation, we used SU6656, a selective inhibitor of Src family kinases. As illustrated in Fig. 1D, pretreatment of hTBCs with SU6656 inhibited phosphorylation of ERK1/2 in response to LA. LA-induced phosphorylation of ERK1/2 was also inhibited by pretreatment of hTBCs with PP2 (another Src family kinase inhibitor; data not shown); however, SU6656 pretreatment did not influence LA-induced phosphorylation of p38, though JNK phosphorylation was diminished in hTBCs (Fig. 1D).
CD36 and GPR120 are localized in plasma membrane lipid rafts in hTBCs (17). To assess the importance of lipid rafts in fatty acid–induced signal transduction, we employed methyl-β-cyclodextrin (M-βCD), a lipid raft disruptor. Pretreatment of hTBCs with M-βCD inhibited LA-induced phosphorylation of both ERK1/2 and JNK1/2 without exerting any effect on p38 phosphorylation in these cells (Fig. 1D). These results suggest that lipid raft–associated CD36 or GPR120 receptors might be involved in ERK1/2 activation by LA in hTBCs. To strengthen the implication of lipid raft in LA-induced TBC activation, we isolated raft and Triton soluble fractions in the TBCs that were treated with LA. We observed that LA treatment of TBCs recruited Fyn contents toward the raft fraction, which suggests that LA treatment did not influence CD36 raft levels but up-regulated Fyn-raft localization that might be involved in signaling mechanisms (Supplemental Fig. 1). Of interest, CD36 and Fyn contents were not altered in the Triton-soluble fractions.
LA induces ERK1/2 phosphorylation via CD36 in hTBCs
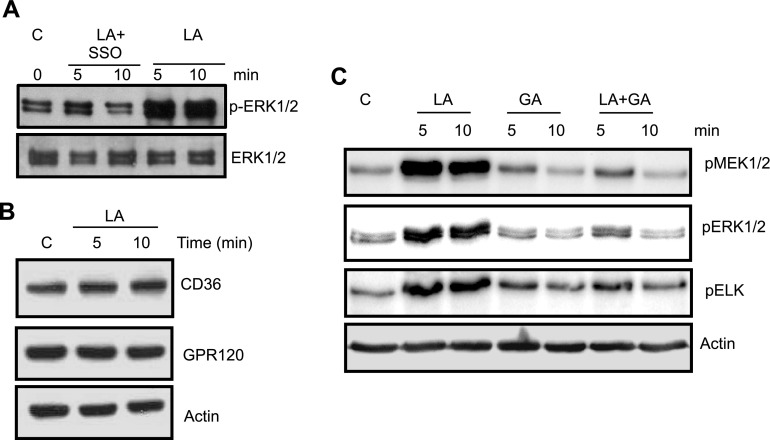
To investigate whether LA induces ERK1/2 phosphorylation via CD36, we used sulfo-N-succinimidyl derivative of oleate (SSO), which is known to bind specifically to CD36. SSO curtailed LA-induced ERK1/2 phosphorylation (Fig. 2A). SSO treatment exerted no alterations in CD36 levels and no cytotoxic effect as assessed by trypan blue exclusion assay (not shown). Of interest, LA did not induce any down- or up-regulation of CD36 during incubation periods (Fig. 2B).
Figure 2.
Role of CD36 and GPR120 in LA-mediated MAPK activation in hTBCs. A) hTBCs were pretreated with SSO (20 µM, 30 min) before addition of LA for 5 or 10 min. B) CD36 and GPR120 levels in control (c; solvent treated) or LA (20 µM)-treated TBC lysates. C) Western blot analysis of phosphorylated (p) MEK1/2, ERK1/2 and ELK in control (c) or cells treated with 20 µM LA, 20 µM GA, or both 20 µM LA and 20 µM GA. Data are representative of 3–5 independent experiments.
We also tested grifolic acid (GA), a known agonist of GPR120 (17). Surprisingly, the GPR120 agonist did not induce phosphorylation of the ERK1/2-MEK1/2 cascade in hTBCs (Fig. 2C). Similarly, this agonist did not induce phosphorylation of p38 and JNK1/2 in these cells. On the contrary, GA curtailed LA-induced phosphorylation of ERK1/2 and MEK1/2, which showed that GPR120 signaling is different from that triggered by CD36 regarding its coupling to downstream cascade. GA did not affect expression of CD36 in these cells (not shown). These results suggest that LA evoked MAPK activation via CD36 but not via GPR120 in hTBCs. LA did not affect GPR120 protein contents during incubation periods (Fig. 2B).
Because the ERK1/2-MEK1/2-ELK-1 cascade was specifically activated by LA, we conducted subsequent experiments on only this MAPK.
CALHM1 channels are upstream regulators of LA-induced ERK1/2 phosphorylation
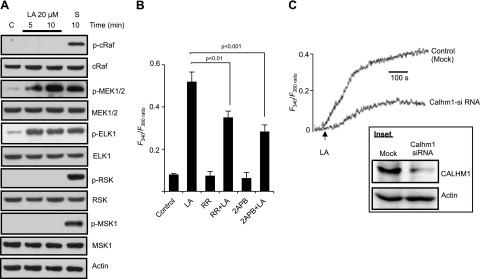
We investigated upstream and downstream regulators of the MAPK cascade in hTBCs. As expected, MEK1/2 was the upstream regulator of ERK1/2 activation (Fig. 3A); however, unexpectedly, phosphorylation of c-Raf in hTBCs was not induced by LA (Fig. 3A). Similarly, we did not observe phosphorylation of RSK and MSK1, the downstream targets of the MAPK cascade, though serum-stimulated cells exhibited the phosphorylation of c-Raf, MEK1/2, RSK, and MSK1 proteins (Fig. 3A). ELK-1, the downstream transcriptional factor, was phosphorylated by both by serum and LA in these cells (Fig. 3A).
Figure 3.
Involvement of CALHM1 channels in hTBC Ca2+ signaling. A) Control (c) and LA-treated hTBC samples were analyzed for activation of upstream and downstream pathway components of the ERK1/2 cascade. Serum–starved (S; 6 h) hTBCs were exposed for 10 min to serum that contained medium. B) Cultured hTBCs (2 × 105 cells/assay) were loaded with Fura-2/AM and changes in [Ca2+]i (F340/F380) were monitored in response to 20 µM LA. hTBC, preincubated (30 min) with: RR (20 µM) or 2-APB (30 µM), were exposed to 20 µM LA. Experiments were performed in Ca2+-containing medium. C) Changes in [Ca2+]i in mock- or Calhm1 siRNA-transfected hTBCs exposed to 20 µM LA. CALHM1 levels in mock- or Calhm1 siRNA-transfected hTBCs (inset). Actin served as loading control. Data are means ± sem (n = 7) conducted in triplicate. Data are representative of 3–5 independent experiments.
In many cell types, including neurons, ERK1/2 is phosphorylated by increases in [Ca2+]i. CALHM1 is a highly conserved N-glycosylated transmembrane protein that acts as a Ca2+ channel (33, 34). A recent study has shown that ERK1/2 phosphorylation was regulated upstream by Ca2+ influx through CALHM1 channel in neuronal cells (35). Of interest, hTBCs expressed this protein (Fig. 3C, inset). Pharmacologic inhibition of CALHM1 by RR or 2-APB attenuated increases in [Ca2+]i in hTBCs (Fig. 3B). We also silenced expression of CALHM1 in hTBCs (Fig. 3C, inset), which also attenuated the increases in [Ca2+]i in hTBCs (Fig. 3C). It is noteworthy that agents such as RR and 2-ABP alone did not trigger an increase in [Ca2+]i in hTBCs (Fig. 3B).
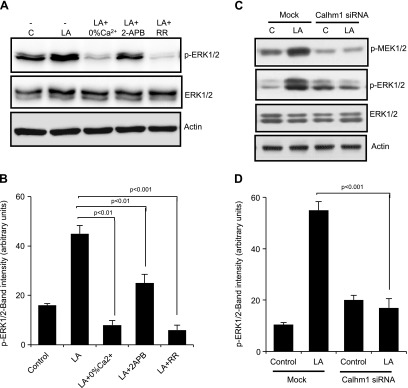
Pretreatment of hTBCs with RR or 2-APB also decreased LA-mediated phosphorylation of ERK1/2 without influencing that of p38 or JNK 1/2 (not shown). Removing Ca2+ from the extracellular medium also blocked LA-induced phosphorylation of ERK1/2 (Fig. 4A, B), but exerted no effect on p38 and JNK1/2 activation (not shown). Of interest, silencing of CALHM1 curtailed phosphorylation of ERK1/2 in hTBCs (Fig. 4C, D). These results suggest that the ERK1/2 cascade is modulated by the opening of CALHM1 channel and the presence of Ca2+ in the extracellular environment.
Figure 4.
LA-mediated ERK1/2 activation is regulated by Ca2+ and CALHM1 channels. Western blot analysis of phosphorylated (p) or total proteins of the MAPK pathway. A) hTBCs were preincubated (30 min) with: RR (20 µM) or 2-APB (30 µM) in 100% Ca2+ buffer and exposed to 20 µM LA for 5 min. Cell lysates were subjected to Western blot analysis for phosphorylated or total proteins of ERK1/2. C) Effect of selective down-regulation of CALHM1 by siRNA on LA-induced activation of MEK and ERK1/2 in hTBCs. B, D) Histograms show the relative band intensity (arbitrary units) of p-ERK1/2 measured by densitometry of protein content. Data were normalized with respect to band intensity of total ERK1/2, measured under similar conditions. Data in panel B are derived from panel A, whereas those in panel D are derived from panel C (n = 5). C, control; Mock, nontargeting siRNA. Data are representative of 4–5 independent experiments.
LA-induced Ca2+ signaling and ERK1/2 phosphorylation are impaired in Calhm1−/− TBCs
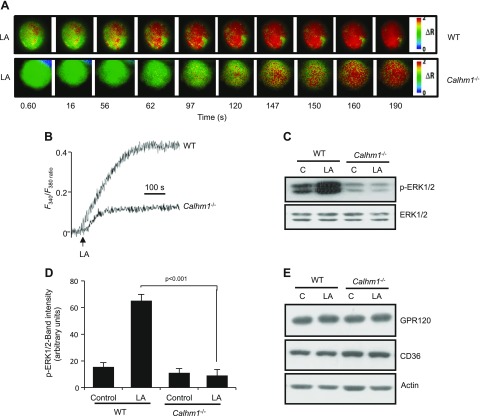
To validate our observations based in hTBC, we isolated fungiform TBCs from WT and Calhm1−/− mice. LA-induced increases in [Ca2+]i were significantly decreased in TBCs that were isolated from Calhm1−/− mice compared with those from WT mice (Fig. 5A, B). LA-induced phosphorylation of ERK1/2, but not of p38 or JNK1/2 (not shown), was impaired in TBCs that were isolated from Calhm1−/− mice (Fig. 5C, D). Integrity of CD36 and GPR120 lipido-receptors was not altered in TBCs of Calhm1−/− mice (Fig. 5E).
Figure 5.
Effects of CALHM1 deficiency on LA-evoked Ca2+ signaling and MAPK activation in mouse TBCs. Ca2+ imaging studies and MAPK activation assay were performed on fungiform TBCs from WT or Calhm1−/− mice. A, B) Colored time-lapse images (A) and graphical representation (B) show changes in [Ca2+]i evoked by LA 20 µM. Changes in [Ca2+]i (F340/F380) were monitored as for Fig. 4. C) LA (20 µM)-induced activation of ERK1/2 in WT and Calhm1−/− mouse fungiform TBCs. D) Histograms, derived from panel C, show the relative band intensity (arbitrary units) of p-ERK1/2 measured by densitometry of protein content. Data were normalized with respect to band intensity of total ERK1/2, measured under similar conditions (n = 5). E) CD36 and GPR120 levels in WT and Calhm1−/− mouse TBCs. C, control. Data are representative of experiments reproduced 3 times independently.
Fat preference is decreased by down-regulation of ERK1/2 in mice and humans
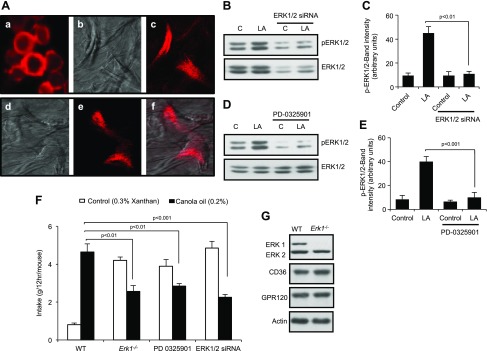
To investigate the physiologic relevance of these findings, we used RNA interference to decrease the expression of ERK1/2 in the tongues of WT mice. Exogenous siRNA coupled to a fluorescent probe was incorporated into freshly isolated mouse CD36+ TBCs (Fig. 6Aa). Figure 6Ab, d shows confocal microscopic photographs of cultured cells taken without filters. Figure 6Ac, e, f shows cultured TBCs that were isolated from WT mice after siRNA was applied to their papillae. These mice, treated with ERK1/2 siRNA, exhibited reduced LA-induced ERK1/2 phosphorylation (Fig. 6B, C) without any effect on p38 or JNK1/2 phosphorylation (not shown). Application of PD0325901, a chemical MEK1/2 inhibitor, on the tongues of WT mice also decreased ERK1/2 (Fig. 6D, E).
Figure 6.
Effects of ERK1 deficiency or inhibition of ERK1/2 activation in WT mice on fat preference. A) Images of nontarget siRNA. Accell fluorescence was detected by confocal microscopy with an excitation filter of 445–490 nm and a long-pass emission filter of 515 nm. a) Freshly isolated CD36+ cells. b, d) Mouse TBCs, cultured for 3 d. c) Same cells as in b, but through color filters. e) Image of cells as in d, but only through color filters. f) Same image as in d and f, but superimposed. B, D) Effect of selective down-regulation of ERK1/2 by siRNA (B) or inhibition of ERK activation by PD-0325901 (D) on 20 µM LA-induced activation of ERK1/2 in mouse fungiform TBCs. C, E) Histograms, derived from panels B and D, respectively, show the relative band intensity (arbitrary units) of p-ERK1/2 measured by densitometry of protein content. Data were normalized with respect to band intensity of total ERK1/2, measured under similar conditions (n = 5). F) Preference for 0.2% canola oil in Erk1−/− or WT mice subjected to siRNA or PD-0325901 application onto the tongue. Control solution was 0.3% xanthan gum. Values are means ± sem (n = 5). G) ERK1/2, CD36, and GPR120 levels in fungiform TBCs from WT and Erk1−/− mice. C, control. Data are representative of 5 independent experiments. Original magnification, ×10.
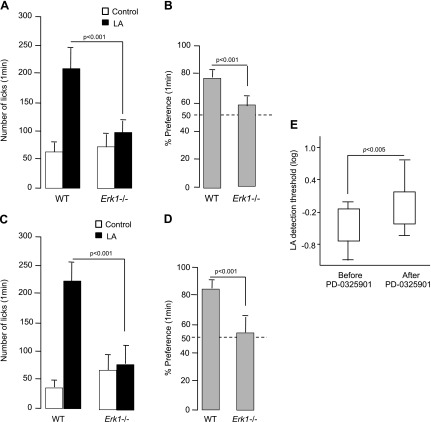
Down-regulation of ERK1/2 phosphorylation by application of either siRNA or PD0325901 onto the tongue significantly decreased the preferences of mice for 0.2% canola oil relative to control treatments (Fig. 6F). Likewise, Erk1−/− mice also exhibited decreased preferences for canola oil relative to WT mice (Fig. 6F). Of note, expression of CD36 and GPR120 was not compromised in TBCs of Erk1−/− mice (Fig. 6G). To confirm that the decrease in preference for fat observed in Erk1−/− mice was related to a dysfunction of the LA-mediated signaling cascade in TBCs, Erk1−/− mice were next subjected to a brief access procedure (1 min) to minimize postingestive effects by using computer-controlled lickometers. As shown in Fig. 7A–D, Erk1−/− mice were unable to detect and prefer LA solutions (0.2 and 2% w/v) compared with WT animals.
Figure 7.
Implication of MAPK inhibition on orosensorial detection of fatty acids in mice and humans. Animals (WT and ERK1−/−) were subjected successively in a randomized manner to a control solution (mineral oil) or to a solution that contained a fatty acid. A, B) LA was used at 0.2% (w/v). C, D) LA was used at 2% (w/v). Licking results are shown in panels A and C, whereas panels B and D show percentage of preference for LA. Dotted line represents absence of taste preference. Values are means ± sem (n = 10/group). E) Log plot of oral detection thresholds of LA in humans before and after lingual applications of PD-0325901. Values are median ± percentile. Values are significantly different according to 1-way ANOVA (n = 19).
In 3-AFC tests, we observed that the thresholds for oro-sensorial detection of LA were significantly increased after 2 applications of PD-0325901 on the tongues of human volunteers (Fig. 7E).
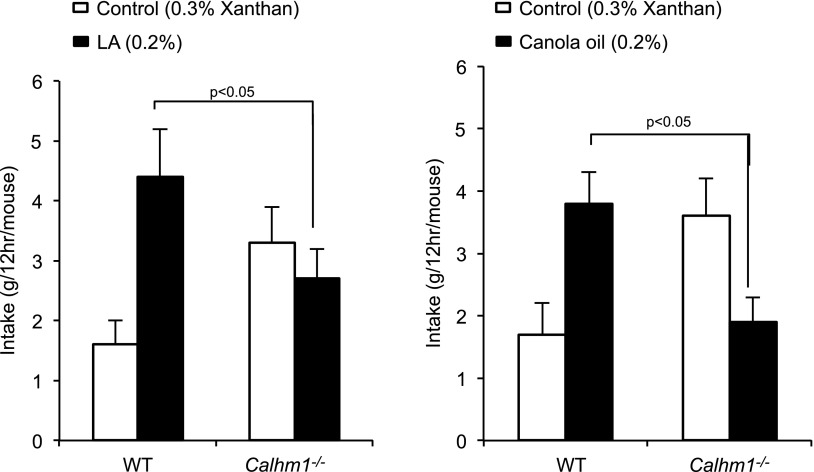
Preference for fat is abolished in Calhm 1−/− mice
The findings described above led us to investigate whether CALHM1 influenced the orosensory perception of lipids. We found that relative to WT littermates, Calhm1−/− mice had diminished preferences for solutions that contained 0.2% LA or 0.2% canola oil in 2-bottle choice tests (Fig. 8). Numbers of animals used in all experimental groups are mentioned in figure legends.
Figure 8.
Spontaneous fat preference in WT and Calhm1−/− mice. WT and Calhm1−/− mice received 12-h 2-bottle preference tests with a choice between control solution (0.3% xanthan gum) and LA (0.2%; left panel) or canola oil (0.2%; right panel) diluted in 0.3% xanthan gum. Values are means ± sem (n = 8/group).
DISCUSSION
Lipids in the human diet are predominantly in the form of triglycerides. Lingual lipases act on triglycerides to release free fatty acids, which are detected by lingual gustatory TBCs (36). Kulkarni and Mattes (37) observed that mastication of fatty foods increased free fatty acids in the saliva up to concentrations that were sufficient to initiate gustatory signaling. Both human (17) and mouse (38) TBCs express lipido-receptors CD36 and GPR120, which are involved in regulation of [Ca2+]i signaling via IP3 pathway (13, 17–19). The present study implicates the MAPK pathway and CALHM1 channels as additional molecular participants required for orogustatory perception of LCFAs.
LA-induced phosphorylation of all 3 MAPKs—ERK1/2, p38, and JNK1/2—in hTBCs. It is noteworthy that LA acts via CD36, but not GPR120, to trigger MAPK phosphorylation. Indeed, the GPR120 agonist, GA, diminished LA-induced phosphorylation of the 3 MAPKs. The inhibitory effect of GA on MAPK phosphorylation corroborates the findings of Hara et al. (39), who observed an inhibitory action of this agonist on ERK1/2 activation in T-REx human embryonic kidney 293 cells that stably expressed GPR120. Furthermore, LA-induced phosphorylation of MAPK was coupled to phosphorylation of Fyn Src kinase in hTBCs. These observations closely agree with our previous report, showing that fatty acid–induced Ca2+ signaling requires activation of Fyn kinase in CD36+ mouse TBC (19). C-terminal cytoplasmic domain of CD36 is involved in assembling a signaling complex that includes Src family kinases and MAPKs (25). CD36-dependent activation of MAPKs is mediated by upstream activation of Src family kinases in other cell types (25, 40).
CD36 is localized in both the raft- and Triton-soluble fractions of hTBCs (17). In the present study, LA-induced activation of ERK and JNK, but not of p38, was inhibited by a lipid raft disruptor, M-βCD, which suggests that lipid raft integrity is essential for fatty acid–mediated hTBC signaling. Furthermore, LA-treatment of hTBCs up-regulated Fyn contents without influencing those of CD36 in the raft fractions. Previously, we have reported that LA triggered a decrease in CD36 raft fraction in hTBCs (17), and this contradiction may be a result of the fact that, in the previous study (17), we incubated hTBCs for 20 min, whereas in the present study, incubation period is 10 min. Nonetheless, localization of CD36 and Fyn up-regulation into raft suggest that CD36-associated Fyn kinase is implicated in signaling mechanisms (20).
LA failed to trigger phosphorylation of c-raf, which is a known upstream regulator, and RSK and MSK, which are downstream regulators of the ERK1/2 cascade, although ELK-1, a downstream transcriptional factor, was phosphorylated. Becuase LA triggers increases in [Ca2+]i in mouse TBCs (19), and this phenomenon is found to regulate ERK1/2 activation in neurons (33), we assessed the role of Ca2+ signaling in ERK1/2 activation.
We found that extracellular Ca2+ was required for LA to influence ERK1/2 phosphorylation and that CALHM1 is most likely the channel that links these events. CALHM1 channels are expressed in mouse (29) and primate (41) TBCs. Ca2+ influx via CALHM1 channel has been reported to evoke ERK1/2 phosphorylation in HT-22 cells (35). We observed that blocking of CALHM1 channel by chemical agents or siRNA resulted in decreased fatty acid–mediated Ca2+ signaling and ERK1/2 phosphorylation without affecting p38 and JNK activation in hTBCs. Moreover, fatty acid–induced increases in [Ca2+]i and ERK1/2 activation were nearly abolished in TBCs that were isolated from Calhm1−/− mice. Our observations on CALHM1 channel, which is involved in Ca2+ gating, corroborate the findings of Dreses-Werringloer et al. (33), who have shown that CALHM1 induces a novel plasma membrane Ca2+ permeability, which thus contributes to increases in [Ca2+]i. A recent study has proposed that CALHM1 is required for perception of sweet, bitter, and umami taste (29). Contrary to the effect of fatty acid, increases in [Ca2+]i, triggered by sweet and bitter compounds, was reported to be identical in TBCs from both WT and Calhm1−/− mice (29). The authors proposed that CALHM1 did not act by controlling calcium permeability but by releasing ATP (29), a required neurotransmitter for activation of afferent neural gustatory pathways. This difference in the role played by CALHM1 in TBC signaling is not well understood; however, we can state that sweet and bitter compounds act on receptors present on type II TBCs, whereas fatty acid seems to act on a subtype of type II cells that express the markers of type II and also of type III cells (Khan, unpublished observations; see also ref. 4). It remains to be ascertained how voltage-gated CALHM1 Ca2+ channels are opened in these different populations of TBCs. It is possible that LA might specifically trigger the opening of CALHM1 Ca2+ channels. Gilbertson et al. (7) have shown that dietary fatty acids via delayed-rectifying K+ channel evoke depolarization and opening of voltage-gated Ca2+ channels in mice TBCs. It is still unknown how [Ca2+]i modulates MEK1/2 phosphorylation. We can rule out the implication of Ca2+/calmodulin complex as a calmodulin inhibitor did not affect phosphorylation of MEK1/2-ERK1/2 in mouse and hTBCs (not shown).
The fact that the fatty acid–evoked rise in [Ca2+]i in Calhm1−/− mice is decreased, as mentioned above, but not completely suppressed up to the basal level, suggests that several Ca2+ signaling cascades are operational during hTBC activation, such as opening of Orai1 and Orai3 Ca2+ channels under the control of STIM1 (13). Whether the opening of CALHM1 channels is orchestrated by STIM1 remains to be studied.
Because Ca2+ signaling via CALHM1 channels induced phosphorylation of MEK1/2-ERK1/2 cascade, we assessed their involvement in fat ingestion. In our study, both siRNA-mediated down-regulation of ERK1/2 expression and PD0325901-mediated inhibition of ERK1/2 activation in mouse TBCs significantly decreased preference for canola oil (0.2%) in WT mice. Moreover, Erk1−/− mice show reduced intakes of LA in 2-bottle choice tests compared with WT mice. Reduced licking of a solution that contained LA by Erk1−/− mice further confirms involvement of ERK1/2 cascade in fat preference in mice. In human volunteers, application of PD0325901 resulted in a decrease in oro-sensorial detection of LA in a 3-AFC test. Two-bottle preference tests revealed that Calhm1−/− mice had diminished preferences for both LA and canola oil. These results suggest that ERK1/2 cascade and CALHM1 channels are mandatory for orogustatory detection of dietary lipids.
Overall, our results suggest that dietary fatty acids via CALHM1, a Ca2+-permeable ion channel, regulate TBCs activation. Dietary fatty acids, by binding to CD36 and localized in the lipid rafts (17), induce phosphorylation of Fyn-src kinase that evokes the hydrolysis of phosphatidyl-inositol-bisphosphate, which thus gives rise to IP3 (19). IP3-triggered Ca2+ release from endoplasmic reticulum results in Ca2+ efflux, followed by a massive Ca2+ influx via the opening of SOC channels, such as Orai1 and Orai3 (not shown) (13). It is possible that SOC channels might also be involved in MEK1/2-ERK1/2 cascade because 2-APB blocks not only CALHM1 channels but also SOC channels (42). The opening of voltage-gated CALHM1 channels, probably by delayed-rectifying K+ channel current, would trigger Ca2+ influx, leading to phosphorylation of MEK1/2-ERK1/2-ELK-1 cascade, which releases 5-HT into the extracellular environment, perhaps via action of ERK1/2 on nuclear ELK-1. ELK-1 may also influence other signaling mechanisms in these cells.
Our study sheds light on novel cellular Ca2+ signaling mechanisms, mediated by CALHM1 channels in TBCs, as an essential mechanism of fat taste perception. It is possible that the ERK1/2 cascade and CALHM1 channels play a key role in health and disease. Indeed, altered TBC Ca2+ signaling has been reported in diet-induced obese mice (17, 43, 44). Whether the activity of CALHM1 channels and ERK1/2 phosphorylation are altered in obesity remains to be determined in future.
ACKNOWLEDGMENTS
This work was supported by grants from the French Ministry of Higher Education and Research, Région Bourgogne “Innovation PARI” (Post Doctoral; to S.S.), ANR SensoFat-2 (ANR-12-BSV1-0027-01; to P.B.), and U.S. National Institutes of Health (NIH) National Institute of Diabetes and Digestive and Kidney Diseases Grant R01-DK46791 (to M.G.T.) and NIH National Institute on Aging Grant R01-AG042508 (to P.M.). The authors thank Dr. Gilles Pagès and Dr. Jacques Pouyssegur (Institute for Research on Cancer and Aging, Nice, France) for providing Erk−/− mice. The authors also sincerely thank Dr. A. Hichami (INSERM U866, Dijon, France), and N. Remla, Z. Tarhda, and Dr. Ghezzaz (Université Aboubekr Belkaid, Tlemcen, Algeria) for helping with orosensorial detection of fatty acids in human volunteers. The authors declare no conflicts of interest.
Glossary
- 2-APB
2-aminoethyl diphenylborinate
- 3-AFC
3 alternative-forced choice
- [Ca2+]i
intracellular Ca2+
- CALHM1
calcium-homeostasis modulator-1
- ELK-1
ETS-like transcription factor-1
- GA
grifolic acid
- hTBC
human taste bud cell
- IP3
inositol-tris-phosphate
- LA
linoleic acid
- LCFA
long-chain fatty acid
- M-βCD
methyl-β-cyclodextrin
- MSK1
mitogen- and stress-activated protein kinase
- RR
ruthenium red
- RSK
ribosomal S6 kinase
- siRNA
small interfering RNA
- SOC
store-operated Ca2+
- SSO
sulfo-N-succinimidyl derivative of oleate
- STIM1
stromal interaction motif-1
- TBC
taste bud cell
- WT
wild-type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
S. Subramaniam, M. H. Ozdener, and N. A. Khan developed the study concept and design; S. Subramaniam, S. Abdoul-Azize, K. Saito, B. Malik, G. Maquart, and M. G. Tordoff participated in acquisition of data; S. Subramaniam and N. A. Khan performed analysis and interpretation of data; M. Aribi and N. A. Khan performed human oral detection of oleic acid studies; S. Subramaniam and N. A. Khan drafted the manuscript; T. Hashimoto, P. Marambaud, M. G. Tordoff, and P. Besnard performed critical review; and N. A. Khan supervised the study.
REFERENCES
- 1.Gurevich-Panigrahi T., Panigrahi S., Wiechec E., Los M. (2009) Obesity: pathophysiology and clinical management. Curr. Med. Chem. 16, 506–521 [DOI] [PubMed] [Google Scholar]
- 2.Mela D. J., Sacchetti D. A. (1991) Sensory preferences for fats: relationships with diet and body composition. Am. J. Clin. Nutr. 53, 908–915 [DOI] [PubMed] [Google Scholar]
- 3.Besnard P., Passilly-Degrace P., Khan N. A. (2016) Taste of fat: a sixth taste modality? Physiol. Rev. 96, 151–176 [DOI] [PubMed] [Google Scholar]
- 4.Gilbertson T. A., Khan N. A. (2014) Cell signaling mechanisms of oro-gustatory detection of dietary fat: advances and challenges. Prog. Lipid Res. 53, 82–92 [DOI] [PubMed] [Google Scholar]
- 5.Chalé-Rush A., Burgess J. R., Mattes R. D. (2007) Evidence for human orosensory (taste?) sensitivity to free fatty acids. Chem. Senses 32, 423–431 [DOI] [PubMed] [Google Scholar]
- 6.Mattes R. D. (2009) Oral thresholds and suprathreshold intensity ratings for free fatty acids on 3 tongue sites in humans: implications for transduction mechanisms. Chem. Senses 34, 415–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gilbertson T. A., Fontenot D. T., Liu L., Zhang H., Monroe W. T. (1997) Fatty acid modulation of K+ channels in taste receptor cells: gustatory cues for dietary fat. Am. J. Physiol. 272, C1203–C1210 [DOI] [PubMed] [Google Scholar]
- 8.Abumrad N. A. (2005) CD36 may determine our desire for dietary fats. J. Clin. Invest. 115, 2965–2967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baillie A. G., Coburn C. T., Abumrad N. A. (1996) Reversible binding of long-chain fatty acids to purified FAT, the adipose CD36 homolog. J. Membr. Biol. 153, 75–81 [DOI] [PubMed] [Google Scholar]
- 10.Fukuwatari T., Kawada T., Tsuruta M., Hiraoka T., Iwanaga T., Sugimoto E., Fushiki T. (1997) Expression of the putative membrane fatty acid transporter (FAT) in taste buds of the circumvallate papillae in rats. FEBS Lett. 414, 461–464 [DOI] [PubMed] [Google Scholar]
- 11.Laugerette F., Passilly-Degrace P., Patris B., Niot I., Febbraio M., Montmayeur J. P., Besnard P. (2005) CD36 involvement in orosensory detection of dietary lipids, spontaneous fat preference, and digestive secretions. J. Clin. Invest. 115, 3177–3184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sclafani A., Ackroff K., Abumrad N. A. (2007) CD36 gene deletion reduces fat preference and intake but not post-oral fat conditioning in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 293, R1823–R1832 [DOI] [PubMed] [Google Scholar]
- 13.Dramane G., Abdoul-Azize S., Hichami A., Vögtle T., Akpona S., Chouabe C., Sadou H., Nieswandt B., Besnard P., Khan N. A. (2012) STIM1 regulates calcium signaling in taste bud cells and preference for fat in mice. J. Clin. Invest. 122, 2267–2282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsumura S., Eguchi A., Mizushige T., Kitabayashi N., Tsuzuki S., Inoue K., Fushiki T. (2009) Colocalization of GPR120 with phospholipase-Cbeta2 and α-gustducin in the taste bud cells in mice. Neurosci. Lett. 450, 186–190 [DOI] [PubMed] [Google Scholar]
- 15.Hirasawa A., Hara T., Katsuma S., Adachi T., Tsujimoto G. (2008) Free fatty acid receptors and drug discovery. Biol. Pharm. Bull. 31, 1847–1851 [DOI] [PubMed] [Google Scholar]
- 16.Cartoni C., Yasumatsu K., Ohkuri T., Shigemura N., Yoshida R., Godinot N., le Coutre J., Ninomiya Y., Damak S. (2010) Taste preference for fatty acids is mediated by GPR40 and GPR120. J. Neurosci. 30, 8376–8382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ozdener M. H., Subramaniam S., Sundaresan S., Sery O., Hashimoto T., Asakawa Y., Besnard P., Abumrad N. A., Khan N. A. (2014) CD36- and GPR120-mediated Ca²⁺ signaling in human taste bud cells mediates differential responses to fatty acids and is altered in obese mice. Gastroenterology 146, 995–1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gaillard D., Laugerette F., Darcel N., El-Yassimi A., Passilly-Degrace P., Hichami A., Khan N. A., Montmayeur J. P., Besnard P. (2008) The gustatory pathway is involved in CD36-mediated orosensory perception of long-chain fatty acids in the mouse. FASEB J. 22, 1458–1468 [DOI] [PubMed] [Google Scholar]
- 19.El-Yassimi A., Hichami A., Besnard P., Khan N. A. (2008) Linoleic acid induces calcium signaling, Src kinase phosphorylation, and neurotransmitter release in mouse CD36-positive gustatory cells. J. Biol. Chem. 283, 12949–12959 [DOI] [PubMed] [Google Scholar]
- 20.Hisatsune C., Kuroda Y., Nakamura K., Inoue T., Nakamura T., Michikawa T., Mizutani A., Mikoshiba K. (2004) Regulation of TRPC6 channel activity by tyrosine phosphorylation. J. Biol. Chem. 279, 18887–18894 [DOI] [PubMed] [Google Scholar]
- 21.Vazquez G., Wedel B. J., Kawasaki B. T., Bird G. S., Putney J. W. Jr (2004) Obligatory role of Src kinase in the signaling mechanism for TRPC3 cation channels. J. Biol. Chem. 279, 40521–40528 [DOI] [PubMed] [Google Scholar]
- 22.Babnigg G., Bowersox S. R., Villereal M. L. (1997) The role of pp60c-src in the regulation of calcium entry via store-operated calcium channels. J. Biol. Chem. 272, 29434–29437 [DOI] [PubMed] [Google Scholar]
- 23.Huang M. M., Bolen J. B., Barnwell J. W., Shattil S. J., Brugge J. S. (1991) Membrane glycoprotein IV (CD36) is physically associated with the Fyn, Lyn, and Yes protein-tyrosine kinases in human platelets. Proc. Natl. Acad. Sci. USA 88, 7844–7848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bull H. A., Brickell P. M., Dowd P. M. (1994) Src-related protein tyrosine kinases are physically associated with the surface antigen CD36 in human dermal microvascular endothelial cells. FEBS Lett. 351, 41–44 [DOI] [PubMed] [Google Scholar]
- 25.Rahaman S. O., Lennon D. J., Febbraio M., Podrez E. A., Hazen S. L., Silverstein R. L. (2006) A CD36-dependent signaling cascade is necessary for macrophage foam cell formation. Cell Metab. 4, 211–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maulon L., Mari B., Bertolotto C., Ricci J. E., Luciano F., Belhacene N., Deckert M., Baier G., Auberger P. (2001) Differential requirements for ERK1/2 and P38 MAPK activation by thrombin in T cells. Role of P59Fyn and PKCepsilon. Oncogene 20, 1964–1972 [DOI] [PubMed] [Google Scholar]
- 27.Zhu X., Jacobs B., Boetticher E., Myou S., Meliton A., Sano H., Lambertino A. T., Muñoz N. M., Leff A. R. (2002) IL-5-induced integrin adhesion of human eosinophils caused by ERK1/2-mediated activation of cPLA2. J. Leukoc. Biol. 72, 1046–1053 [PubMed] [Google Scholar]
- 28.Pagès G., Guérin S., Grall D., Bonino F., Smith A., Anjuere F., Auberger P., Pouysségur J. (1999) Defective thymocyte maturation in p44 MAP kinase (Erk 1) knockout mice. Science 286, 1374–1377 [DOI] [PubMed] [Google Scholar]
- 29.Taruno A., Vingtdeux V., Ohmoto M., Ma Z., Dvoryanchikov G., Li A., Adrien L., Zhao H., Leung S., Abernethy M., Koppel J., Davies P., Civan M. M., Chaudhari N., Matsumoto I., Hellekant G., Tordoff M. G., Marambaud P., Foskett J. K. (2013) CALHM1 ion channel mediates purinergic neurotransmission of sweet, bitter and umami tastes. Nature 495, 223–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ozdener M. H., Brand J. G., Spielman A. I., Lischka F. W., Teeter J. H., Breslin P. A., Rawson N. E. (2011) Characterization of human fungiform papillae cells in culture. Chem. Senses 36, 601–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ayorinde F. O., Garvin K., Saeed K. (2000) Determination of the fatty acid composition of saponified vegetable oils using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 14, 608–615 [DOI] [PubMed] [Google Scholar]
- 32.Sayed A., Šerý O., Plesnik J., Daoudi H., Rouabah A., Rouabah L., Khan N. A. (2015) CD36 AA genotype is associated with decreased lipid taste perception in young obese, but not lean, children. Int. J. Obes. 39, 920–924 [DOI] [PubMed] [Google Scholar]
- 33.Dreses-Werringloer U., Lambert J. C., Vingtdeux V., Zhao H., Vais H., Siebert A., Jain A., Koppel J., Rovelet-Lecrux A., Hannequin D., Pasquier F., Galimberti D., Scarpini E., Mann D., Lendon C., Campion D., Amouyel P., Davies P., Foskett J. K., Campagne F., Marambaud P. (2008) A polymorphism in CALHM1 influences Ca2+ homeostasis, Abeta levels, and Alzheimer’s disease risk. Cell 133, 1149–1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ma Z., Siebert A. P., Cheung K. H., Lee R. J., Johnson B., Cohen A. S., Vingtdeux V., Marambaud P., Foskett J. K. (2012) Calcium homeostasis modulator 1 (CALHM1) is the pore-forming subunit of an ion channel that mediates extracellular Ca2+ regulation of neuronal excitability. Proc. Natl. Acad. Sci. USA 109, E1963–E1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dreses-Werringloer U., Vingtdeux V., Zhao H., Chandakkar P., Davies P., Marambaud P. (2013) CALHM1 controls the Ca²⁺-dependent MEK, ERK, RSK and MSK signaling cascade in neurons. J. Cell Sci. 126, 1199–1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pepino M. Y., Love-Gregory L., Klein S., Abumrad N. A. (2012) The fatty acid translocase gene CD36 and lingual lipase influence oral sensitivity to fat in obese subjects. J. Lipid Res. 53, 561–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kulkarni B., Mattes R. (2013) Evidence for presence of nonesterified fatty acids as potential gustatory signaling molecules in humans. Chem. Senses 38, 119–127 [DOI] [PubMed] [Google Scholar]
- 38.Martin C., Passilly-Degrace P., Gaillard D., Merlin J. F., Chevrot M., Besnard P. (2011) The lipid-sensor candidates CD36 and GPR120 are differentially regulated by dietary lipids in mouse taste buds: impact on spontaneous fat preference. PLoS One 6, e24014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hara T., Hirasawa A., Sun Q., Sadakane K., Itsubo C., Iga T., Adachi T., Koshimizu T. A., Hashimoto T., Asakawa Y., Tsujimoto G. (2009) Novel selective ligands for free fatty acid receptors GPR120 and GPR40. Naunyn Schmiedebergs Arch. Pharmacol. 380, 247–255 [DOI] [PubMed] [Google Scholar]
- 40.Chen K., Febbraio M., Li W., Silverstein R. L. (2008) A specific CD36-dependent signaling pathway is required for platelet activation by oxidized low-density lipoprotein. Circ. Res. 102, 1512–1519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moyer B. D., Hevezi P., Gao N., Lu M., Kalabat D., Soto H., Echeverri F., Laita B., Yeh S. A., Zoller M., Zlotnik A. (2009) Expression of genes encoding multi-transmembrane proteins in specific primate taste cell populations. PLoS One 4, e7682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goto J., Suzuki A. Z., Ozaki S., Matsumoto N., Nakamura T., Ebisui E., Fleig A., Penner R., Mikoshiba K. (2010) Two novel 2-aminoethyl diphenylborinate (2-APB) analogues differentially activate and inhibit store-operated Ca2+ entry via STIM proteins. Cell Calcium 47, 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chevrot M., Bernard A., Ancel D., Buttet M., Martin C., Abdoul-Azize S., Merlin J. F., Poirier H., Niot I., Khan N. A., Passilly-Degrace P., Besnard P. (2013) Obesity alters the gustatory perception of lipids in the mouse: plausible involvement of lingual CD36. J. Lipid Res. 54, 2485–2494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gilbertson T. A., Liu L., Kim I., Burks C. A., Hansen D. R. (2005) Fatty acid responses in taste cells from obesity-prone and -resistant rats. Physiol. Behav. 86, 681–690 [DOI] [PubMed] [Google Scholar]