Abstract
Previous studies have demonstrated that cleaved high-molecular-weight kininogen (HKa) induces endothelial apoptosis and inhibits angiogenesis and have suggested that this occurs through inhibition of Src family kinases. This study assessed the role of tyrosine–protein kinase Lck (p56/Lck) in this pathway. We analyzed early events leading to apoptosis of human endothelial cells exposed to HKa. The role of p56/Lck was investigated using short interfering (si) RNA knockdown and lentivirus expression in assays of endothelial tube formation, sprouting of neovessels from murine aorta, and angiogenesis in Matrigel plugs. HKa stimulated expression and phosphorylation of p56/Lck. siRNA knockdown of p56/Lck promoted endothelial proliferation and blocked HKa-induced apoptosis and activation of p53, Bax, and Bak. Lentivirus expression of p56/Lck in endothelial cells induced apoptosis and blocked tube formation. Expression of p56/Lck in murine aortic rings blocked sprouting angiogenesis. Lentivirus expressing p56/Lck blocked angiogenesis in Matrigel plugs, while p56/Lck short hairpin RNA inhibited the antiangiogenic effect of HKa. Scrambled siRNAs and empty lentiviral vectors were used in all experiments. Apoptosis of proliferating endothelial cells and inhibition of angiogenesis by HKa requires p56/Lck. This suggests a novel role for p56/Lck in regulation of endothelial cell survival and angiogenesis.—Betapudi, V., Shukla, M., Alluri, R., Merkulov, S., McCrae, K. R. Novel role for p56/Lck in regulation of endothelial cell survival and angiogenesis.
Keywords: apoptosis, kininogen, p53, tyrosine protein kinase Lck
Angiogenesis is tightly regulated by the balance between pro- and antiangiogenic mediators. The latter include polypeptides derived from proteolysis of a parental precursor, often a coagulation or extracellular matrix protein (1–4). One such polypeptide is cleaved high-molecular-weight kininogen (HKa). We and others have shown that HKa induces apoptosis of proliferating endothelial cells at low nanomolar concentrations (5–7), and inhibits angiogenesis in vitro and in vivo (5). Detection of circulating high-molecular-weight kininogen (HK) fragments in patients with angiogenic disorders such as cancer (8) suggests the biological and clinical importance of these activities. However, the mechanisms by which HKa and other antiangiogenic polypeptides regulate endothelial cell function and inhibit angiogenesis are not well understood.
Angiogenesis is stimulated through a variety of pathways that are context dependent (9, 10). Receptor tyrosine kinases, such as the VEGF receptor type 2, play a critical role in mediating the endothelial cell response to proangiogenic growth factors (11). However, the somewhat disappointing results of studies targeting such receptors in patients with malignancy highlights the need to further define and understand the roles of specific signaling nodes and resistance mechanisms in regulation of endothelial cell survival and apoptosis (12).
Nonreceptor tyrosine kinases, particularly Src family kinases (SFKs), play a critical role in many processes, including angiogenesis (13). Members of this multikinase family are expressed in a cell-specific manner, with individual members regulating diverse cellular activities such as migration, proliferation, and survival (14). The function of one SFK member, tyrosine–protein kinase Lck (p56/Lck), has been investigated almost exclusively in T cells, in which it plays a central role in cellular activation downstream of the T-cell receptor (15–17). T-cell receptor engagement leads to activation of 2 SFKs, p56/Lck and Fyn, which phosphorylate immunoreceptor tyrosine-based motifs in the T-cell receptor (15). Phosphorylation of these motifs promotes assembly of a signaling complex that includes ZAP-70, endowing the T-cell receptor with kinase function and leading to activation of MAPK, phospholipase C, and other signaling proteins (18).
A role for p56/Lck in T cells is its ability to regulate cell survival. p56/Lck is essential for induction of T-cell apoptosis by several mediators, including chemotherapeutic agents (19), ceramide (20), sphingosine (21), galectin-1 (22), and radiation (23). Some studies suggest that p56/Lck mediates apoptosis in response to these agonists through the mitochondrial pathway (23).
A role for p56/Lck in regulation of endothelial function has not been described. Here we report that p56/Lck plays an essential role in mediating apoptosis of endothelial cells in response to HKa. p56/Lck is required for phosphorylation of p53, loss of mitochondrial membrane potential with release of cytochrome c, and increased expression and activation of proapoptotic Bax and Bak after addition of HKa to proliferating umbilical vein or dermal microvascular endothelial cells. Inhibition of p56/Lck expression in endothelial cells stimulated cell proliferation and conferred resistance to HKa-induced apoptosis. Moreover, lentivirus-mediated expression of p56/Lck impaired the ability of endothelial cells to form tubes in Matrigel, prevented vessel outgrowth from murine aortic rings, and blocked angiogenesis in Matrigel plugs implanted in mice. These studies suggest an unappreciated role for p56/Lck in regulation of endothelial cell viability, proliferation, and angiogenesis.
MATERIALS AND METHODS
Materials
Medium 199 was obtained from Cellgro (Mediatech, Manassas, VA, USA) and bovine calf serum (Cosmic Calf serum; CCS) from Thermo Scientific–HyClone (Logan, UT, USA). Endothelial cell growth supplement was from Biomedical Technologies (Stoughton, MA, USA). Gelatin was from Thermo Fisher Scientific (Waltham, MA, USA), and basic fibroblast growth factor (bFGF) and VEGF were from BD Biosciences (San Jose, CA, USA). HKa was from Enzyme Research Laboratories (South Bend, IN, USA). Antibodies to caspase-3 (#9661), SFK (#9320), p53 (#2527), phospho-p53 (#9281), and β-actin (#4967) were from Cell Signaling Technology (Danvers, MA, USA). Antibody to cytochrome c (#556433) was obtained from BD Biosciences. Anti–urokinase receptor (uPAR) antibodies (#CA1344) were from Cell Applications (San Diego, CA, USA). Human p56/Lck cDNA (accession number BC013200) in pCS6 was from TransOMIC Technologies (Huntsville, AL, USA). MitoTracker Orange, pENTR/D-TOPO entry vector, Gateway LR Clonase enzyme mix, and BLOCK-iT U6 RNAi Entry Vector Kit were from Thermo Fisher Scientific. The GP2-293 packaging cell line, Lenti-X Concentrator, Lenti-X quantitative real-time PCR Titration Kit, and Lenti-X GoStix kit were from Clontech Laboratories (Mountain View, CA, USA). Smartpool human short interfering (si) RNAs specific for C-terminal Src kinase (Csk), p56/Lck, and uPAR were from Dharmacon–Thermo Scientific (Waltham, MA, USA). Protease inhibitors were from Roche Life Science (Branford, CT, USA). Tris-glycine precast polyacrylamide gels were from Novex (Life Technologies, Carlsbad, CA, USA). All other chemicals and reagents were of the best quality available and were obtained from Sigma-Aldrich (St. Louis, MO, USA).
Cell culture
Human umbilical vein endothelial cells (HUVECs) were isolated and propagated as previously described (24) and maintained in medium 199 containing 10% CCS, 1% penicillin–streptomycin, and endothelial cell growth supplement. Human dermal microvascular endothelial cells were obtained from Cell Applications and cultured under identical conditions. Experiments were performed using cells of passage 3 or lower.
An MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium]-based cell proliferation assay was performed to measure relative numbers of viable endothelial cells after exposure to HKa or other test reagents (5). In brief, 100 µl of cell suspension (3 × 104/ml) was plated in individual wells of 96-well microplates precoated with 1% gelatin. Cells were allowed to attach and spread before addition of fresh medium containing 2% CCS, 20 ng/ml bFGF, 15 µM ZnCl2, and 15 nM HKa. Relative numbers of viable cells remaining in each well after incubation for 48 h were determined using the Cell Proliferation Assay kit (Promega, Madison, WI, USA). Results are presented as the percentage inhibition of bFGF-stimulated endothelial proliferation, as previously defined (5).
Immunoblotting and immunoprecipitation
For immunoblotting, cell lysates were mixed with an equal volume of 2× SDS sample buffer containing 2-mercaptoethanol and heated at 95°C for 2 min. Samples were resolved on 10% polyacrylamide gels and transferred to PVDF. Membranes were probed with primary antibodies followed by horseradish peroxidase–conjugated secondary antibodies, and proteins were detected using SuperSignal West Femto reagent (Pierce, Rockford, IL, USA). Immunoprecipitation was performed as described previously (25). In brief, cell lysates were sonicated and centrifuged for 10 min at 10,000 g. Supernatants were subjected to immunoprecipitation, and proteins within immunoprecipitates were separated using 10% SDS-PAGE, transferred to PVDF, and analyzed by immunoblotting.
Reverse transcription-PCR
Quantitative reverse transcription (RT)-PCR was performed to assess levels of mRNA encoding endothelial cell SFK family members, and RT-PCR was used to confirm the presence of the lentiviral vector containing the p56/Lck open reading frame (ORF) in endothelial cells. For each experiment, total RNA was isolated using TRIzol, and cDNA was prepared using the RT2 First Strand kit (Qiagen, Germantown, MD, USA). Quantitative PCR was performed using the StepOnePlus PCR System (Thermo Fisher Scientific) with the following parameters: denaturation at 94°C for 10 min, 40 cycles at 95°C for 15 s each, and a final cycle at 62°C for 60 s. Amplification of GAPDH cDNA was used as an internal control. Fold induction after normalization to GAPDH was calculated using the ΔΔCt method. Primers are listed Supplemental Table 1.
Measurement of caspase-3/7 activity
Caspase-3/7 activities were measured using the Apo3 HTS assay kit (Cell Technology, Mountain View, CA, USA). In brief, 100 µl of cell suspension (2.5 × 104 cells/ml) was seeded in individual wells of black 96-well microplates and treated as described for cell proliferation and viability assays. After 3 h, 100 µl of 2× caspase-3/7 detection reagent containing a (z-DEVD)2-R110 peptide was added. Fluorescence was then measured using a BioTek Synergy HT plate reader (488 nm excitation, 530 nm emission; BioTek Instruments, Winooski, VT, USA).
Immunofluorescence staining
Endothelial cells cultured on gelatin-coated Lab-Tek II–chambered slides were loaded with MitoTracker Orange, then incubated in the absence or presence of 15 nM HKa for 1 h and fixed in PBS containing 2 mM MgCl2, 2 mM EGTA, and 4% paraformaldehyde. Cells were then permeabilized using PBS containing 2 mM MgCl2, 2 mM EGTA, and 0.5% Triton X-100 and blocked by incubation in PBS containing 0.1% Tween 20 and 1% bovine serum albumin before incubation with anti-cytochrome c antibodies. Cells were incubated with Alexa Fluor 488–conjugated secondary antibodies and DAPI before imaging using a Leica DMRXE confocal microscope and a ×63 objective (Leica, Wetzlar, Germany).
Lentivirus production
p56/Lck cDNA-specific primers (forward: 5′-CACCATGGGCTGTGGCTGCAGCTC-3′; reverse: 5′-TCAAGGCTGAGGCTGGTAC-3′) were used to amplify the ORF from Lck cDNA and the product cloned into the pENTR/D-TOPO entry vector. Clones positive for p56/Lck were recombined with pLenti CMV Puro DEST using the Gateway LR Clonase enzyme mix. Recombined clones were confirmed by sequencing, and positive clones were used in lentivirus production.
A complementary short hairpin (sh) RNA for human p56/Lck was designed using Thermo Fisher Scientific BLOCK-iT RNAi Designer software (Lck_shRNA-2_top strand: 5′-CACCGCATCAAGTTGACCATCAACACGAATGTTGATGGTCAACTTGATGC-3′; Lck_shRNA-2_bottom strand: 5′-AAAAGCATCAAGTTGACCATCAACATTCGTGTTGATGGTCAACTTGATGC-3′). The 2 oligonucleotides were annealed and cloned into pENTR/U6 using the BLOCK-iT U6 RNAi Entry Vector kit. Clones containing the p56/Lck shRNA sequence were recombined with pLenti CMV Puro DEST using Gateway LR Clonase Enzyme mix. p56/Lck shRNA positive clones were used for virus production.
Lentivirus was produced using the Lentiviral Gateway Expression kit (Life Technologies). Twelve million GP2-293 (HEK) cells were grown in Opti-MEM with reduced serum, and cells were cotransfected with 9 μg each of pLP1, pLP2, pVSVG, and pDEST-Lck plasmid DNA using 150 μl of Lipofectamine 2000 (Thermo Fisher Scientific). Three days later, supernatant was removed and concentrated using a Lenti-X concentrator (Clontech Laboratories) and the lentivirus pellet titered by serial dilution against GP2-293 cells.
Assessment of the role of uPAR in endothelial cell apoptosis and inhibition of angiogenesis by HKa
We used several approaches to determine the contribution of uPAR to the antiendothelial and antiangiogenic properties of HKa. First, we assessed the effects of HKa on proliferation of endothelial cells pretreated with scrambled (control) or uPAR siRNA. Second, because it has been proposed that the mechanism of HKa-induced endothelial cell apoptosis involves dissociation of uPAR from a putative signaling partner, integrin αVβ3 (26, 27), we used coimmunoprecipitation to determine whether dissociation of uPAR and integrin αVβ3 was specific for HKa-induced apoptosis or rather a nonspecific consequence of apoptosis by determining whether dissociation occurred in endothelial cells treated with HKa or other apoptosis inducing agents, specifically staurosporine or TNF-α. Third, we compared the ability of HKa to inhibit angiogenesis in Matrigel plugs implanted in wild-type or uPAR−/− mice.
Endothelial tube formation
Wells of 48-well tissue culture plates were coated with growth factor–reduced Matrigel before addition of 50 × 103 endothelial cells in the absence or presence of empty or p56/Lck-expressing lentivirus (multiplicity of infection ∼10). Tube formation was examined after 24 h at 37°C and fixation with 4% paraformaldehyde. In selected studies, cells were stained with phycoerythrin–annexin V and images collected using a Leica DMRXE microscope and a ×20 objective. All assays were performed in triplicate. To confirm transduction of cells with lenti-p56/Lck, endothelial cells were collected from selected wells after digestion of Matrigel with dispase and analyzed for p56/Lck by immunoblotting, and for lenti-p56/Lck by RT-PCR using primers that spanned sequences of pLenti CMV Puro DEST and the p56/Lck ORF (Supplemental Table 1).
Aortic ring angiogenesis assay
Aortic ring angiogenesis assays were performed as described by Baker et al. (28). Briefly, 2 mm aortic rings were obtained from thoracic aortas of wild-type C57/Bl6 mice and incubated for 24 h with control (empty) or p56/Lck-expressing lentivirus at 100 multiplicity of infection in Opti-MEM medium. Rings were then washed in fresh medium and embedded in Matrigel. Vessel growth was examined by bright-field microscopy using a ×4 objective after 5 d of culture. To assess transduction of the rings with lenti-p56/Lck, Matrigel was digested with dispase and cells isolated by centrifugation. Cell extracts were analyzed by immunoblotting for p56/Lck, and RNA was analyzed by RT-PCR using the same primers described for tube formation assays.
Matrigel plug assays
Matrigel plug assays were used to assess growth factor–stimulated angiogenesis, as previously described (5). In brief, growth factor–reduced Matrigel was mixed with 50 µg/ml heparin, 800 ng/ml bFGF, and 300 ng/ml VEGF. In selected studies, either HKa (10 µg), lenti-p56/Lck, lenti-p56/Lck shRNA, or HKa plus lenti-p56/Lck shRNA was incorporated into Matrigel plugs to determine their effect on angiogenesis. The final mixture (0.25 ml) was injected subcutaneously, with one Matrigel injection placed in the right and left flank of each mouse. Nine days after injection, mice were humanely killed and the plugs gently removed using blunt dissection to avoid bleeding. Plugs were placed in optimal cutting temperature compound and flash-frozen in liquid nitrogen or homogenized for determination of hemoglobin content using a modification of the Drabkin assay (29). The hemoglobin content was expressed as milligrams of hemoglobin per deciliter per gram of Matrigel.
To assess the role of uPAR in inhibition of angiogenesis by HKa, we used Matrigel plug studies to assess the ability of HKa to inhibit angiogenesis in Matrigel implanted in wild-type and uPAR deficient (uPAR−/−) mice (30).
Statistical analysis
Data are expressed as means ± sd. All experimental points were determined in triplicate, and all assays were repeated at least 3 times. Differences between control and experimental conditions were assessed by Student’s 2-tailed t test for unpaired samples. P < 0.05 was considered significant.
RESULTS
Effects of HKa on p56/Lck expression and activation
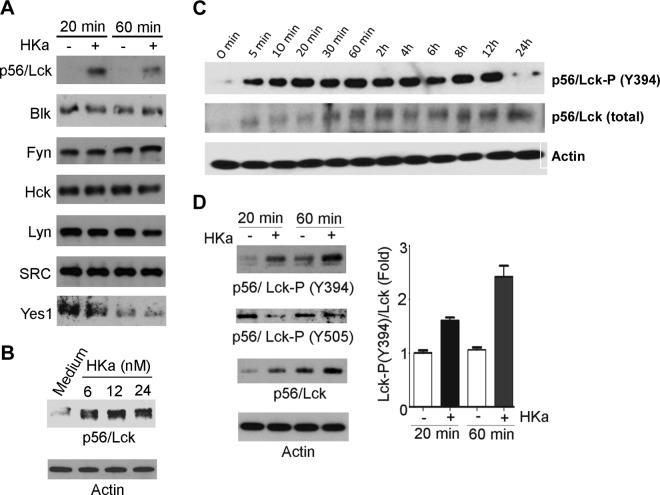
On the basis of previous studies demonstrating that HKa caused endothelial cell apoptosis (5, 7) through inhibition of SFK (27), we assessed the effects of HKa on expression of Src and other endothelial SFK members. A specific and marked increase in expression of a single SFK, p56/Lck, was observed after exposure of cells to HKa concentrations as low as 6 nM (Fig. 1A, B). In contrast, no HKa-specific changes were observed in expression of other endothelial SFKs, including Src, Blk, Fyn, Hck, Lyn, or Yes (Fig. 1A). Increased expression of p56/Lck was accompanied by a significant, ∼1.9-fold increase in p56/Lck mRNA (Supplemental Fig. 1A). Examination of the time course of p56/Lck expression and phosphorylation demonstrated that phosphorylation occurred within 5 min of exposure of cells to HKa and increased for at least 6 h. In contrast, increased p56/Lck protein was noted 30 to 60 min after exposure to HKa (Fig. 1C). The enhanced phosphorylation of p56/Lck was due to phosphorylation of Y394, which is analogous to Src Y416 and associated with the open, active conformation of p56/Lck (31). In contrast, phosphorylation of Y505, which is analogous to Src Y527 and is phosphorylated in the closed inactive form of p56/Lck, decreased by 85% (Fig. 1D).
Figure 1.
HKa selectively stimulates p56/Lck expression and phosphorylation in endothelial cells. Proliferating HUVECs were cultured in absence or presence of 15 nM HKa for indicated times, and cell extracts were prepared and subjected to immunoblotting. A) HKa specifically induces expression of p56/Lck but does not affect other SFK members. B) Concentration-dependent stimulation of p56/Lck expression by HKa. C) Time course of p56/Lck and p56/Lck phosphorylation on Y394 (associated with active form of p56/Lck) after exposure of endothelial cells to 15 nM HKa. D) Analysis of p56/Lck Y394 and Y505 phosphorylation after exposure to HKa.
Inhibition of p56/Lck expression blocks HKa-induced apoptosis and stimulates endothelial cell proliferation
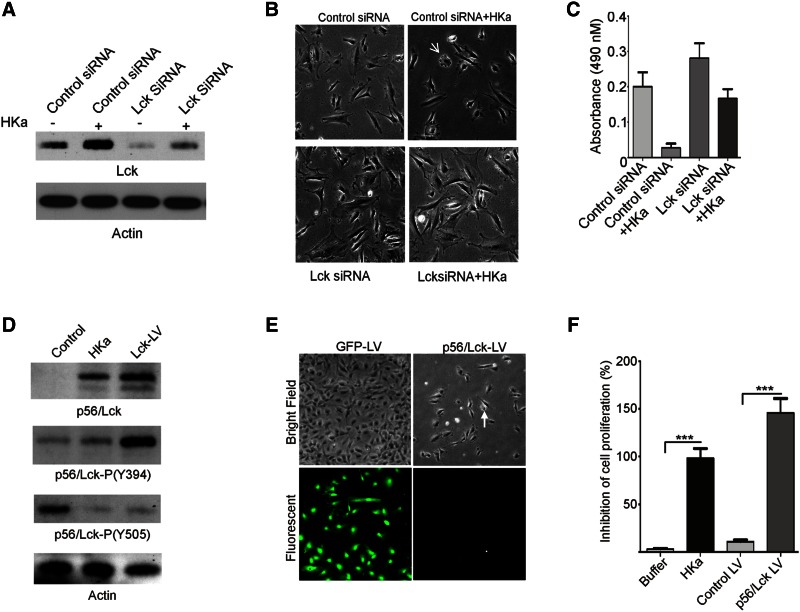
To define the consequence of increased p56/Lck expression and phosphorylation, we treated cells with p56/Lck siRNA before exposure to HKa. p56/Lck siRNA blocked the increased expression of p56/Lck observed in response to HKa (Fig. 2A). Moreover, endothelial cells treated with p56/Lck siRNA exhibited elongated morphology (Fig. 2B) and demonstrated a 40% increase in proliferation in response to bFGF compared to cells treated with control siRNA (Fig. 2B, C). Cells treated with p56/Lck siRNA were also resistant to HKa-induced apoptosis (Fig. 2C).
Figure 2.
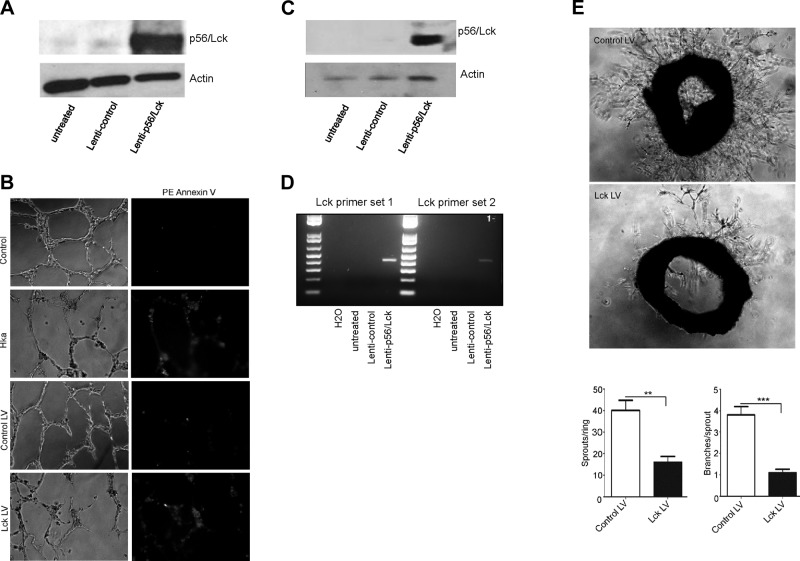
p56/Lck depletion stimulates endothelial cell proliferation and confers resistance to HKa-induced apoptosis. Endothelial cells were transfected with p56/Lck siRNA or lentivirus encoding GFP or p56/Lck. A) Reduction in p56/Lck after transfection of cells with control or p56/Lck siRNA. B) Bright-field images of control and p56/Lck siRNA-transfected endothelial cells 90 min after exposure to 15 nM HKa, demonstrating that HKa induces characteristic changes of apoptosis (arrow) only in cells transfected with control siRNA. C) p56/Lck siRNA induces proliferation of endothelial cells and protects from HKa-induced apoptosis. D) Expression and phosphorylation of p56/Lck in endothelial cells transduced with lentivirus containing p56/Lck cDNA. E) Endothelial cells photographed 48 h after transduction with lenti-GFP or lenti-p56/Lck. Proliferation of cells transduced with lentivirus p56/Lck is markedly impaired compared to cells transduced with control GFP. Cellular rounding (arrowhead) is consistent with apoptosis. F) Lentiviral transduction with p56/Lck inhibits endothelial cell proliferation and viability, as determined by MTS assay (***P < 0.0001).
Lentivirus-induced expression of p56/Lck causes endothelial cell apoptosis
Cells transduced with lenti-p56/Lck demonstrated increased expression of p56/Lck and phosphorylation of p56/Lck Y394 and Y505 that was comparable to that of cells exposed to HKa (Fig. 2D). Moreover, in comparison to control cells transduced with lentivirus green fluorescent protein (GFP), cells transduced with p56/Lck did not proliferate and demonstrated morphologic changes consistent with apoptosis (Fig. 2E). Assessment of the effect of p56/Lck expression in endothelial cells using an MTS assay confirmed that p56/Lck significantly decreased numbers of viable cells (Fig. 2F).
p56/Lck is required for p53-, Bax-, and Bak-dependent endothelial cell apoptosis induced by HKa
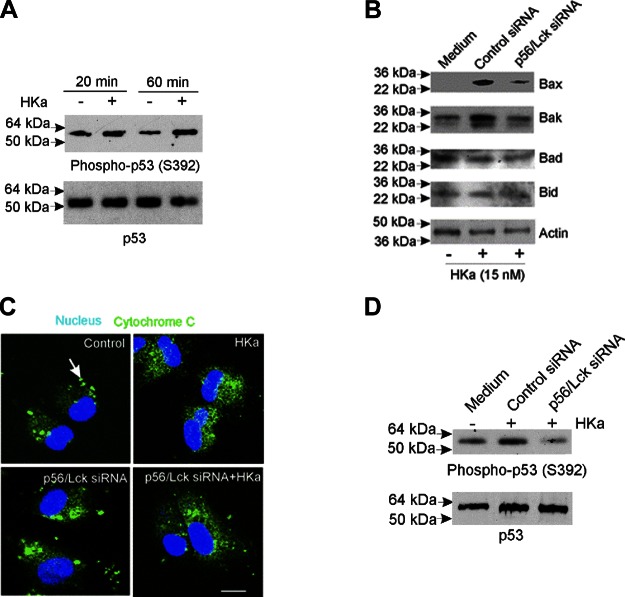
HKa stimulated phosphorylation of p53 on S392, which is associated with p53 activation (32) by 2.3-fold (Fig. 3A); in parallel, we observed a 4.5-fold and 2.7-fold increase in the expression of the proapoptotic mediators Bax and Bak (Fig. 3B). No changes in the expression of the antiapoptotic Bcl-2 family members Bid and Bad were detected. Concordant with these changes, HKa caused the release of mitochondrial cytochrome c (Fig. 3C and Supplemental Fig. 1B).
Figure 3.
p56/Lck siRNA blocks p53 phosphorylation, increased Bax and Bak phosphorylation, and cytochrome c release in response to HKa. Proliferating endothelial cells pretreated with control or p56/Lck siRNA (cell extracts from experiment depicted in Fig. 2 were used in these studies) were incubated in absence or presence of HKa and cell extracts subjected to immunoblotting. A) HKa (15 nM) caused increased phosphorylation of p53 on S392, corresponding to active conformation. B) HKa (15 nM) induced expression of proapoptotic mediators Bax and Bak in endothelial cells incubated in medium alone or with control siRNA, but not in cells pretreated with p56/Lck siRNA. Expression of Bad and Bid was unaffected. C) Release of cytochrome c from mitochondria was inhibited by p56/Lck siRNA. In untreated cells, cytochrome c is present in mitochondria (arrows, punctate staining in upper left). HKa induced release of cytochrome c (upper right). Release of cytochrome c was unaffected by p56/Lck siRNA alone (lower left), but p56/Lck siRNA blocked cytochrome c release in response to HKa (lower right). D) p56/Lck, but not control siRNA, blocked p53 S392 phosphorylation in response to HKa. Scale bar = 100 µM.
To examine whether p56/Lck was required for activation of a p53-dependent apoptosis pathway, we examined p53 phosphorylation in endothelial cells treated with p56/Lck siRNA before exposure to HKa. As demonstrated in Fig. 3B, D, depletion of p56/Lck prevented phosphorylation of p53 as well as increased expression of Bax and Bak. In fact, phosphorylation of p53 was suppressed by >25% below basal levels in cells treated with p56/Lck siRNA.
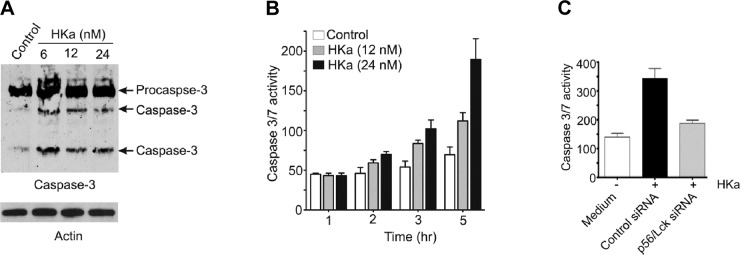
Because Bcl-2 family members regulate mitochondrial integrity and caspase activation (33–35), we determined the dependence of caspase activation on p56/Lck. Active caspase-3 increased markedly after exposure of cells to HKa (Fig. 4A). Caspase activity in HKa-treated cells also increased in a time and concentration-dependent manner (Fig. 4B), increasing 4-fold in cells exposed to HKa for 5 h. Caspase activation was inhibited by 90% in cells pretreated with p56/Lck siRNA (Fig. 4C; P < 0.001).
Figure 4.
p56/Lck is required for HKa-induced caspase activation. Proliferating endothelial cells were incubated in absence or presence of HKa, and cell extracts were prepared and subjected to immunoblotting. A) Incubation of proliferating endothelial cells with HKa increased expression and activation of pro-caspase-3. B) Caspase-3/7 activity in extracts of endothelial cells incubated with HKa demonstrates time- and concentration-dependent increase. C) Pretreatment of endothelial cells with p56/Lck siRNA blocked caspase activation.
Our previous work demonstrated endonucleolytic DNA cleavage as a terminal component of HKa-induced endothelial cell apoptosis (24). DNA cleavage is mediated through the activity of DFF40, which is inhibited through formation of a complex with DFF45 and DFF35. However, in HKa-treated endothelial cells, levels of DFF45 and DFF35 diminished, whereas levels of free DFF40 increased 6-fold (Supplemental Fig. 2). Pretreatment of cells with p56/Lck siRNA before incubation with HKa caused sustained expression of DFF45 and DFF35 and blocked DNA fragmentation.
Expression of p56/Lck in endothelial cells blocks tube formation in Matrigel and prevents vessel outgrowth from murine aortic rings
To investigate the role of endothelial cell p56/Lck in angiogenesis, we assessed the effects of lentivirus-mediated p56/Lck expression on the ability of endothelial cells to form vascular tubes in Matrigel. Transduction of endothelial cells by lenti-p56/Lck, but not control lentivirus, caused markedly increased expression of p56/Lck (Fig. 5A). As depicted in Fig. 5B, both nontransduced endothelial cells, as well as those transduced with empty (control) lentivirus, formed tubes when placed on a Matrigel substrate. In contrast, tube formation was blocked by ∼60%, as determined from measurement of mean total vessel length per high-power field, after exposure of cells to HKa or transduction of cells with lenti-p56/Lck. Both HKa and lenti-p56/Lck transduction caused endothelial cell apoptosis, as demonstrated by staining of nascent tubes with annexin A5.
Figure 5.
p56/Lck inhibits endothelial tube formation and sprouting angiogenesis from aortic rings. Endothelial cells were transduced with empty lentivirus (control) or lenti-p56/Lck before assessing their ability to form vascular tubes in Matrigel. A) p56/Lck expression in cells transduced with control or p56/Lck-expressing lentivirus. B) Endothelial tubes formed on Matrigel were stained with phycoerythrin–annexin V before collection of bright-field and fluorescent images using DMRXE microscope and ×20 objective. C) Expression of p56/Lck in murine aortic rings transduced with lenti-p56/Lck. D) RT-PCR-amplified nucleotide sequence of lenti-p56/Lck using primers that span vector sequence and p56/Lck ORF. E) Murine aortic rings transduced with empty lentivirus or lenti-p56/Lck were embedded in Matrigel for assessment of vessel sprouting. Bright-field images of neovessels 7 d after placement of rings in Matrigel. Below) Graphs represent quantification of vessel sprouting from control lentivirus or lenti-p56/Lck transduced aortic rings. Lentiviral p56/Lck expression significantly reduced both total number of new vessel sprouts and branches per sprout, respectively (P < 0.0024 and 0.0002, respectively).
We also assessed whether lentiviral expression of p56/Lck blocked outgrowth of new vessels from murine aortic rings embedded in Matrigel. Transduction of aortic rings with lenti-p56/Lck led to significantly increased expression of p56/Lck, while control lentivirus had no effect (Fig. 5C); immunoblotting and the use of specific primers to amplify a PCR product spanning the junction between the lentivirus vector sequence and the p56/Lck ORF (Fig. 5D) confirmed specific transduction of the aortic rings (Fig. 5D). Although the formation of vascular sprouts was unaffected by control lentivirus, lenti-p56/Lck inhibited vessel formation by 80% (Fig. 5E; P < 0.001).
Effect of p56/Lck on angiogenesis in vivo
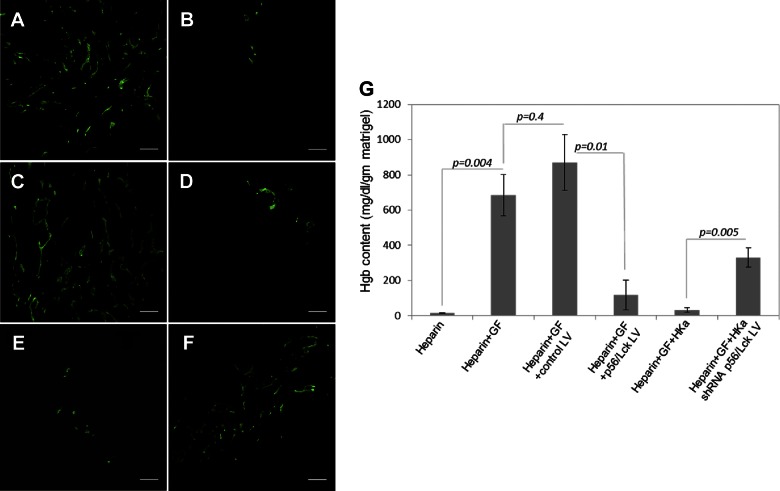
To define the effect of p56/Lck on angiogenesis in vivo, we performed Matrigel plug assays. We have previously demonstrated that angiogenesis is increased in Matrigel plugs placed in mKng1−/− mice relative to wild-type littermates, and the former were used for these experiments. Robust angiogenesis was observed in Matrigel plugs containing bFGF and VEGF after implantation in the flanks of these mice (Fig. 6A), while Matrigel without growth factors stimulated angiogenesis (Fig. 6B). Inclusion of an empty lentivirus vector did not affect angiogenesis in response to growth factors (Fig. 6C). However, a lentiviral vector containing the p56/Lck cDNA potently blocked angiogenesis (Fig. 6D), as did HKa (Fig. 6E). However, the effects of HKa were significantly inhibited by a lentiviral construct expressing p56/Lck shRNA (Fig. 6F). Corresponding hemoglobin content of Matrigel plugs removed from these animals was consistent with immunofluorescent staining (Fig. 6G).
Figure 6.
Effect of p56/Lck on angiogenesis in vivo. Matrigel plugs were prepared in wild-type mice as described in Materials and Methods. Angiogenesis in plugs was quantified 9 d after placement by immunofluorescence staining and determination of hemoglobin content. A–F) Representative fields are shown from Matrigel plugs containing growth factors (bFGF and VEGF) and heparin (A) heparin alone (B), growth factors plus heparin plus empty lentivirus (used as control) (C), growth factors plus heparin plus lenti-p56/Lck (D), growth factors plus heparin plus HKa (E), and growth factors plus heparin plus HKa plus lentivirus expressing p56/Lck shRNA (F). G) Hemoglobin content of plugs, prepared as described. GF, growth factor; LV, lentivirus; WT, wild type. Data represent mean ± sem of 10 plugs derived from 5 mice each receiving 2 plugs. Scale bars = 100 µM.
Role of the uPAR in the antiangiogenic effect of HKa
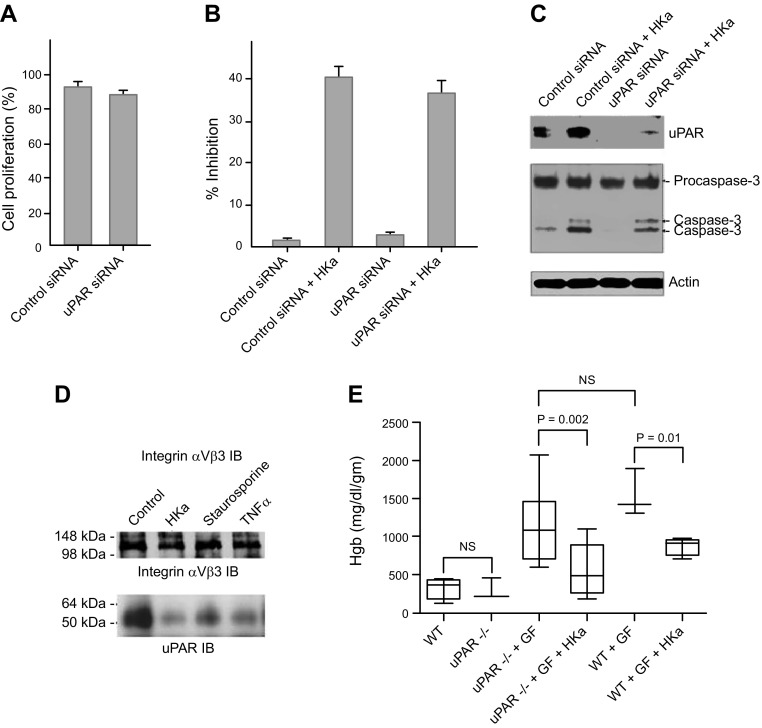
Previous studies from our laboratory (36) and from others demonstrate that HKa binds to uPAR (37, 38) and other cellular receptors (39–42). In some systems, HKa inhibits the adhesion of endothelial and other cells to vitronectin via such interactions (43). However, the role of uPAR in mediating the antiangiogenic activity of HKa has not been definitively assessed. To address this issue, we determined the effect of HKa on proliferating endothelial cells pretreated with control or uPAR siRNA. Endothelial cells treated with uPAR siRNA proliferated normally in response to growth factors (Fig. 7A). HKa was equally potent in reducing the viability of proliferating endothelial cells pretreated with control or uPAR siRNA (Fig. 7B). Moreover, inhibition of uPAR expression did not prevent generation of active caspase-3 in response to HKa (Fig. 7C).
Figure 7.
uPAR is not essential for antiendothelial cell activity of HKa. A) Proliferation of endothelial cells pretreated with control or uPAR siRNA. Cell proliferation was determined by counting of cells and comparison to cells treated with growth factors in the absence of siRNA. B) Inhibition of proliferation of endothelial cells pretreated with control or uPAR siRNA by HKa (15 nM). C) HKa-induced caspase activation in endothelial cells pretreated with control or uPAR siRNA. D) Immunoblots of uPAR immunoprecipitate from control endothelial cells incubated in medium alone, or endothelial cells induced to undergo apoptosis by HKa, staurosporine, or TNF-α, using antibody to integrin αVβ3. Coimmunoprecipitation of uPAR and integrin αVβ3 was reduced in response to all 3 agonists. E) Inhibition of angiogenesis by HKa in wild-type and uPAR−/− mice. Matrigel plugs assays were performed as described in Materials and Methods. Figure depicts hemoglobin content in individual Matrigel plugs 9 d after plug placement. Each data point represents mean ± sem of 10 Matrigel plugs (2 plugs each placed into 5 mice per group). HKa inhibited angiogenesis equally well in wild-type and uPAR−/− mice.
The dissociation of uPAR from integrin αVβ3 after exposure of proliferating endothelial cells to HKa has been proposed as evidence that HKa mediates its antiendothelial cell activity by disrupting the uPAR–integrin association (26, 27). However, we hypothesized that dissociation of these receptors might be a nonspecific effect of apoptosis. To test this hypothesis, apoptosis of proliferating endothelial cells was induced using 3 different agonists, HKa, staurosporine, and TNF-α. Apoptosis in response to each of these was confirmed by DNA fragmentation analyses. After induction of apoptosis, coimmunoprecipitation of endothelial cell uPAR with integrin αVβ3 was decreased in response to all agonists (Fig. 7D). These results suggest that dissociation of the receptors is unlikely to be a specific mechanism accounting for the effects of HKa.
To further explore the requirement for uPAR in inhibition of angiogenesis mediated by HKa, we assessed the effect of HK on angiogenesis in Matrigel plugs in wild-type and uPAR-deficient mice. Consistent with in vitro studies using uPAR siRNA, the growth of new vessels into HKa-containing Matrigel plugs was inhibited equally well in wild-type and uPAR-deficient mice (Fig. 7E), demonstrating that uPAR is not essential for the antiangiogenic activity of HKa in vivo.
DISCUSSION
Our studies demonstrate for the first time a critical role for p56/Lck in regulation of endothelial cell proliferation, survival, tube formation, and sprouting angiogenesis. Evidence for such a role is provided by the following: 1) the ability of p56/Lck siRNA to stimulate endothelial cell proliferation and inhibit apoptosis while preventing increased expression and phosphorylation of p53, Bax, and Bak in response to HKa; 2) the ability of lenti-p56/Lck to cause endothelial apoptosis; and 3) the inhibition of endothelial cell tube formation and sprouting of new vessels from murine aortic rings by lenti-p56/Lck. Finally, lenti-p56/Lck blocked angiogenesis in Matrigel plugs in vivo, and lenti-p56/Lck shRNA inhibited the in vivo antiangiogenic effects of HKa. These results suggest that p56/Lck plays a central role in regulating the transition of endothelial cells from a static to a proliferative, migratory phenotype necessary for angiogenesis (44), and they also suggest that p56/Lck may be a target for modulation of angiogenesis and vascular remodeling.
An important role for p56/Lck in T-cell function has been demonstrated (45). In T cells, engagement of the T-cell receptor stimulates a pathway through which p56/Lck is activated in a manner dependent on UNC119, leading to T-cell proliferation and maturation (46). Mutations in UNC119 have been associated with T-cell lymphopenia (47), while inhibitors of p56/Lck have been sought for treatment of autoimmune disorders (48, 49). Increased expression and/or activation of p56/Lck may occur in lymphoid malignancies, suggesting that in transformed lymphoid cells, p56/Lck may contribute to apoptosis resistance (50). In support of this, p56/Lck mediates resistance to dexamethasone-induced T-cell apoptosis, and inhibition of p56/Lck by dasatinib reverses this resistance (51). Moreover, a constitutively active p56/Lck mutant blocked apoptosis of BaF3 cells in response to IL-3 withdrawal through a pathway involving STAT5b (50). In contrast, other studies demonstrate that p56/Lck is essential for sphingosine-induced activation of Bak, leading to apoptotic death of Jurkat T-lymphoma cells (21). Proteasome inhibitor-induced death of these cells is also potentiated by p56/Lck (52), and p56/Lck is required for apoptosis of JCaM 1.6 cells caused by doxorubicin, paclitaxel, and 5-fluorouracil (19). Thus, in T cells, the effects of p56/Lck on cell survival or death depends on the nature of the stimulus, and additional work is needed to define the molecular mechanisms underlying these outcomes.
There is no information available concerning a role of p56/Lck in regulation of endothelial cell function. Although the parental Src promotes angiogenesis (53), our studies demonstrate that p56/Lck is the only endothelial SFK member affected by HKa and that it plays a critical role in mediating apoptosis through activation and increased expression of p53, Bax, and Bak. This unexpected finding was confirmed through several approaches, including siRNA knockdown of p56/Lck and lentivirus-mediated p56/Lck expression in vitro and in vivo. Although the role of p56/Lck was discovered by dissecting the events that occur after exposure of endothelial cells to HKa, the effects of p56/Lck on endothelial proliferation even in the absence of HKa suggest that it may regulate responses to other agents that influence angiogenesis.
In T cells, p56/Lck activity is regulated through phosphorylation and the protein tyrosine phosphatase CD45 (54). CD45 is thought to preferentially dephosphorylate p56/Lck Y505, thus promoting activation of the kinase, although it may also act on p56/Lck Y394 to inhibit p56/Lck activity (54). However, endothelial cells do not express CD45 (55), and other phosphatases are likely to regulate the p56/Lck phosphorylation status, with their effects on p56/Lck activity depending on their specificity for p56/Lck Y394 vs. Y505 phosphorylation sites. Our studies suggest that blocking p56/Lck Y394 dephosphorylation in endothelial cells may perpetuate p56/Lck activity and present a strategy for inhibiting angiogenesis. In theory, such a strategy might inhibit angiogenesis and stimulate T-cell function.
Previous studies have demonstrated binding of HKa to uPAR (36, 37). HKa-uPAR interactions inhibit uPAR-mediated cellular adhesion, particularly to vitronectin (43). However, HKa binds to endothelial cells through several different binding sites, including the receptor for the globular head of complement C1q (gC1qR) (40, 42), cytokeratin (39, 40), and heparan sulfate (41). The relevance of these interactions to the antiendothelial cell activity of HKa is uncertain. Although several studies have implicated uPAR in mediating these effects (26, 27), this issue has not been directly addressed through siRNA knockdown and/or mice genetically deficient in uPAR. Moreover, in a previous report, we were unable to inhibit the antiendothelial cell activity of HKa using an anti-uPAR antibody that blocks binding of HKa to uPAR (5). Consistent with this, we now demonstrate that uPAR are not essential for HKa-induced endothelial cell apoptosis in vitro or for inhibition of angiogenesis by HKa in vivo. Hence, the antiangiogenic receptor for HKa requires further definition.
The upstream pathways that regulate the expression and phosphorylation of p56/Lck in endothelial cells remain undefined. Our preliminary studies suggest that HKa causes a rapid decrease in endothelial Csk (Supplemental Fig. 3), which normally suppresses expression of SFKs. Moreover, Csk knockdown induces endothelial cell apoptosis (Supplemental Fig. 3). However, these results conflict with a previous report in which HKa was associated with increased expression of Csk, which was proposed to cause inhibition of Src expression and endothelial proliferation (27, 56). These differences may be accounted for by several facts. First, we assessed signaling responses occurring immediately after exposure of cells to HKa rather than at later times, when cells had initiated apoptosis (5, 57). Second, we used substantially lower concentrations of HKa, consistent with those detected in patients with malignancy, to avoid off-target effects (8). Third, we assessed the effects of HKa on individual SFK members through the use of specific antibodies rather than a pan-Src antibody used in previous studies. Finally, we used specific strategies to block or augment expression of p56/Lck and assessed their effects in primary endothelial cells as well as in functional models of angiogenesis in vivo.
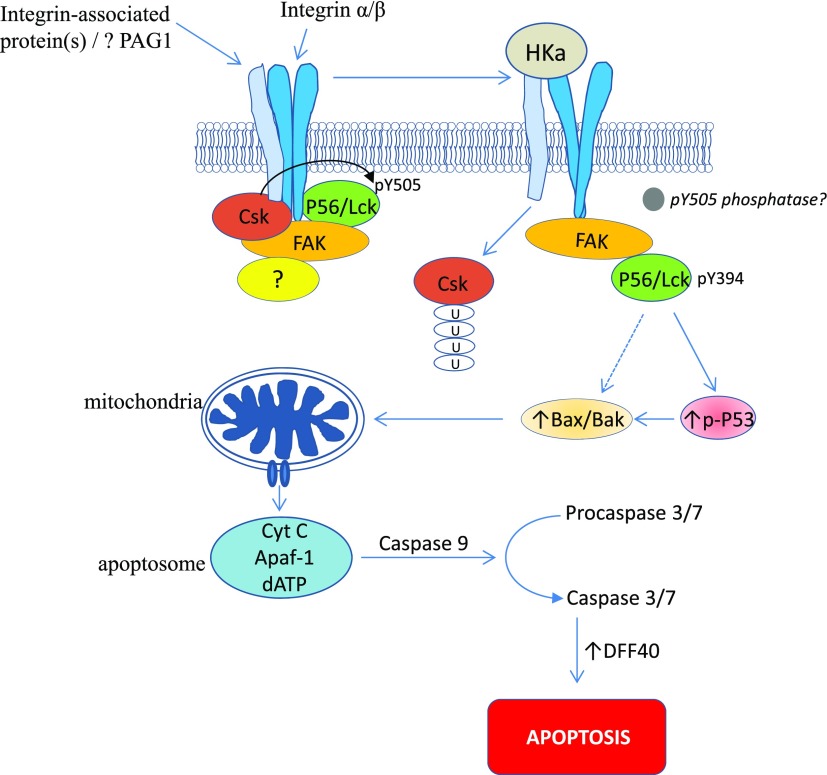
Our studies suggest a new paradigm for the regulation of endothelial cell proliferation and angiogenesis by kininogen, though additional work is needed to better define the mechanisms that initiate this pathway. Because endothelial cells lack a T-cell receptor, we presume that the mechanism of HKa-induced p56/Lck activation utilizes an upstream pathway that differs from that in T cells. Yet certain components of this pathway that regulate Csk-p56/Lck interactions in T cells are also present in endothelial cells. For example, focal adhesion kinase (58, 59), phosphoprotein associated with glycosphingolipid-enriched domains (60), and cytoskeleton-associated proteins such as paxillin or filamin (58, 61, 62) may be shared and contribute to regulation of p56/Lck activity in both cell types. A working model outlining a potential pathway of HKa-induced endothelial cell apoptosis mediated through p56/Lck is depicted in Fig. 8.
Figure 8.
Potential mechanism for HKa-induced endothelial apoptosis and inhibition of angiogenesis. We speculate that HKa interrupts integrin-dependent signaling needed for maintenance of endothelial cell viability; although HKa antiangiogenic receptor has not been defined, our studies demonstrate that it is not uPAR. We propose that through binding to integrin or integrin-associated protein, HKa alters integrin structure, leading to conformational change in integrin cytoplasmic domain. Focal adhesion kinase (FAK) associates with integrin cytoplasmic tail, binds Csk and p56/Lck, and may function as scaffold to enhance interaction between these 2 SFKs in nonperturbed state, leading to inactivation of p56/Lck by Csk-mediated phosphorylation of p56/Lck Y416. After HKa binding, association of FAK with integrin may change, leading to Csk dissociation and degradation, possibly by ubiquitination. In turn, p56/Lck undergoes dephosphorylation on Y505 and phosphorylation on Y394, becoming activated and able to phosphorylate p53 and stimulate expression of Bax and Bak. Bax and Bak induce mitochondrial depolarization, release of cytochrome c, and mitochondrial pathway of apoptosis.
Although a dual role for kininogen in the regulation of angiogenesis has been proposed (63), with bradykinin stimulating and HKa inhibiting angiogenesis, the observation that mice deficient in HK display increased angiogenesis and tumor growth (64) is consistent with a central role for HKa as an endogenous angiogenesis inhibitor. Our findings suggest that a novel p56/Lck-dependent pathway may be a target for manipulating angiogenesis in vivo.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported in part by the U.S. National Institutes of Health National Heart, Lung, and Blood Institute Grant R01-HL098796 (to K.R.M.).
Glossary
- bFGF
basic fibroblast growth factor
- CCS
cosmic calf serum
- Csk
C-terminal Src kinase
- GFP
green fluorescent protein
- HK
high-molecular-weight kininogen
- HKa
cleaved high-molecular-weight kininogen
- HUVEC
human umbilical vein endothelial cell
- lenti-p56/Lck
lentivirus expressing p56/Lck
- MTS
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
- ORF
open reading frame
- p56/Lck
tyrosine–protein kinase Lck
- RT-PCR
reverse transcriptase PCR
- SFK
Src family kinase
- shRNA
short hairpin RNA
- siRNA
short interfering RNA
- uPAR
urokinase receptor
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
K. R. McCrae, V. Betapudi, and M. Shukla designed the experiments. V. Betapudi, M. Shukla, R. Alluri, and S. Merkulov performed the experiments. K. R. McCrae and V. Betapudi wrote the manuscript.
REFERENCES
- 1.Adamis A. P., Shima D. T., Yeo K. T., Yeo T. K., Brown L. F., Berse B., D’Amore P. A., Folkman J. (1993) Synthesis and secretion of vascular permeability factor/vascular endothelial growth factor by human retinal pigment epithelial cells. Biochem. Biophys. Res. Commun. 193, 631–638 [DOI] [PubMed] [Google Scholar]
- 2.Folkman J. (2004) Endogenous angiogenesis inhibitors. APMIS 112, 496–507 [DOI] [PubMed] [Google Scholar]
- 3.Folkman J. (2006) Angiogenesis. Annu. Rev. Med. 57, 1–18 [DOI] [PubMed] [Google Scholar]
- 4.O’Reilly M. S., Holmgren L., Shing Y., Chen C., Rosenthal R. A., Moses M., Lane W. S., Cao Y., Sage E. H., Folkman J. (1994) Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell 79, 315–328 [DOI] [PubMed] [Google Scholar]
- 5. Zhang J.-C., Claffey K., Sakthivel R., Darzynkiewicz Z., Shaw D. E., Leal J., Wang Y.-C., Lu F. M., McCrae K. R. (2000) Two-chain high molecular weight kininogen induces endothelial cell apoptosis and inhibits angiogenesis: partial activity within domain 5. FASEB J. 14, 2589–2600 [DOI] [PubMed] [Google Scholar]
- 6.Zhang J. C., Qi X., Juarez J., Plunkett M., Donaté F., Sakthivel R., Mazar A. P., McCrae K. R. (2002) Inhibition of angiogenesis by two-chain high molecular weight kininogen (HKa) and kininogen-derived polypeptides. Can. J. Physiol. Pharmacol. 80, 85–90 [DOI] [PubMed] [Google Scholar]
- 7.Colman R. W., Jameson B. A., Lin Y., Johnson D., Mousa S. A. (2000) Domain 5 of high molecular weight kininogen (kininostatin) down-regulates endothelial cell proliferation and migration and inhibits angiogenesis. Blood 95, 543–550 [PubMed] [Google Scholar]
- 8.Umemura H., Togawa A., Sogawa K., Satoh M., Mogushi K., Nishimura M., Matsushita K., Tanaka H., Takizawa H., Kodera Y., Nomura F. (2011) Identification of a high molecular weight kininogen fragment as a marker for early gastric cancer by serum proteome analysis. J. Gastroenterol. 46, 577–585 [DOI] [PubMed] [Google Scholar]
- 9.Weis S. M., Cheresh D. A. (2011) Tumor angiogenesis: molecular pathways and therapeutic targets. Nat. Med. 17, 1359–1370 [DOI] [PubMed] [Google Scholar]
- 10.Prager G. W., Poettler M. (2012) Angiogenesis in cancer. Basic mechanisms and therapeutic advances. Hamostaseologie 32, 105–114 [DOI] [PubMed] [Google Scholar]
- 11.Shibuya M. (2013) VEGFR and type-V RTK activation and signaling. Cold Spring Harb. Perspect. Biol. 5, a009092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bottsford-Miller J. N., Coleman R. L., Sood A. K. (2012) Resistance and escape from antiangiogenesis therapy: clinical implications and future strategies. J. Clin. Oncol. 30, 4026–4034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park S. I., Shah A. N., Zhang J., Gallick G. E. (2007) Regulation of angiogenesis and vascular permeability by Src family kinases: opportunities for therapeutic treatment of solid tumors. Expert Opin. Ther. Targets 11, 1207–1217 [DOI] [PubMed] [Google Scholar]
- 14.Aleshin A., Finn R. S. (2010) SRC: a century of science brought to the clinic. Neoplasia 12, 599–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salmond R. J., Filby A., Qureshi I., Caserta S., Zamoyska R. (2009) T-cell receptor proximal signaling via the Src-family kinases, Lck and Fyn, influences T-cell activation, differentiation, and tolerance. Immunol. Rev. 228, 9–22 [DOI] [PubMed] [Google Scholar]
- 16.Salmond R. J., Huyer G., Kotsoni A., Clements L., Alexander D. R. (2005) The src homology 2 domain-containing tyrosine phosphatase 2 regulates primary T-dependent immune responses and Th cell differentiation. J. Immunol. 175, 6498–6508 [DOI] [PubMed] [Google Scholar]
- 17.Papatriantafyllou M. (2013) Signal transduction: LCK regulation is hidden in details. Nat. Rev. Immunol. 13, 222–223 [DOI] [PubMed] [Google Scholar]
- 18.Kane L. P., Lin J., Weiss A. (2000) Signal transduction by the TCR for antigen. Curr. Opin. Immunol. 12, 242–249 [DOI] [PubMed] [Google Scholar]
- 19.Gruber C., Henkel M., Budach W., Belka C., Jendrossek V. (2004) Involvement of tyrosine kinase p56/Lck in apoptosis induction by anticancer drugs. Biochem. Pharmacol. 67, 1859–1872 [DOI] [PubMed] [Google Scholar]
- 20.Ion G., Fajka-Boja R., Kovács F., Szebeni G., Gombos I., Czibula A., Matkó J., Monostori E. (2006) Acid sphingomyelinase mediated release of ceramide is essential to trigger the mitochondrial pathway of apoptosis by galectin-1. Cell. Signal. 18, 1887–1896 [DOI] [PubMed] [Google Scholar]
- 21.Kim M. J., Park M. T., Yoon C. H., Byun J. Y., Lee S. J. (2008) Activation of Lck is critically required for sphingosine-induced conformational activation of Bak and mitochondrial cell death. Biochem. Biophys. Res. Commun. 370, 353–358 [DOI] [PubMed] [Google Scholar]
- 22.Novák J., Kriston-Pál É., Czibula Á., Deák M., Kovács L., Monostori É., Fajka-Boja R. (2014) GM1 controlled lateral segregation of tyrosine kinase Lck predispose T-cells to cell-derived galectin-1-induced apoptosis. Mol. Immunol. 57, 302–309 [DOI] [PubMed] [Google Scholar]
- 23.Belka C., Gruber C., Jendrossek V., Wesselborg S., Budach W. (2003) The tyrosine kinase Lck is involved in regulation of mitochondrial apoptosis pathways. Oncogene 22, 176–185 [DOI] [PubMed] [Google Scholar]
- 24.Ma K., Simantov R., Zhang J. C., Silverstein R., Hajjar K. A., McCrae K. R. (2000) High affinity binding of beta 2-glycoprotein I to human endothelial cells is mediated by annexin II. J. Biol. Chem. 275, 15541–15548 [DOI] [PubMed] [Google Scholar]
- 25.Allen K. L., Fonseca F. V., Betapudi V., Willard B., Zhang J., McCrae K. R. (2012) A novel pathway for human endothelial cell activation by antiphospholipid/anti-β2 glycoprotein I antibodies. Blood 119, 884–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cao D. J., Guo Y. L., Colman R. W. (2004) Urokinase-type plasminogen activator receptor is involved in mediating the apoptotic effect of cleaved high molecular weight kininogen in human endothelial cells. Circ. Res. 94, 1227–1234 [DOI] [PubMed] [Google Scholar]
- 27.Liu Y., Sainz I. M., Wu Y., Pixley R., Espinola R. G., Hassan S., Khan M. M., Colman R. W. (2008) The inhibition of tube formation in a collagen-fibrinogen, three-dimensional gel by cleaved kininogen (HKa) and HK domain 5 (D5) is dependent on Src family kinases. Exp. Cell Res. 314, 774–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baker M., Robinson S. D., Lechertier T., Barber P. R., Tavora B., D’Amico G., Jones D. T., Vojnovic B., Hodivala-Dilke K. (2011) Use of the mouse aortic ring assay to study angiogenesis. Nat. Protoc. 7, 89–104 [DOI] [PubMed] [Google Scholar]
- 29.Moore G. L., Ledford M. E., Merydith A. (1981) A micromodification of the Drabkin hemoglobin assay for measuring plasma hemoglobin in the range of 5 to 2000 mg/dl. Biochem. Med. 26, 167–173 [DOI] [PubMed] [Google Scholar]
- 30.Bugge T. H., Suh T. T., Flick M. J., Daugherty C. C., Rømer J., Solberg H., Ellis V., Danø K., Degen J. L. (1995) The receptor for urokinase-type plasminogen activator is not essential for mouse development or fertility. J. Biol. Chem. 270, 16886–16894 [DOI] [PubMed] [Google Scholar]
- 31.Laham L. E., Mukhopadhyay N., Roberts T. M. (2000) The activation loop in Lck regulates oncogenic potential by inhibiting basal kinase activity and restricting substrate specificity. Oncogene 19, 3961–3970 [DOI] [PubMed] [Google Scholar]
- 32.Sen B., Johnson F. M. (2011) Regulation of SRC family kinases in human cancers. J. Signal Transduct. 2011, 865819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hardwick J. M., Soane L. (2013) Multiple functions of BCL-2 family proteins. Cold Spring Harb. Perspect. Biol. 5, 5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martinou J. C., Youle R. J. (2011) Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev. Cell 21, 92–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kapoor M., Hamm R., Yan W., Taya Y., Lozano G. (2000) Cooperative phosphorylation at multiple sites is required to activate p53 in response to UV radiation. Oncogene 19, 358–364 [DOI] [PubMed] [Google Scholar]
- 36.Colman R. W., Pixley R. A., Najamunnisa S., Yan W., Wang J., Mazar A., McCrae K. R. (1997) Binding of high molecular weight kininogen to human endothelial cells is mediated via a site within domains 2 and 3 of the urokinase receptor. J. Clin. Invest. 100, 1481–1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mahdi F., Shariat-Madar Z., Kuo A., Carinato M., Cines D. B., Schmaier A. H. (2004) Mapping the interaction between high molecular mass kininogen and the urokinase plasminogen activator receptor. J. Biol. Chem. 279, 16621–16628 [DOI] [PubMed] [Google Scholar]
- 38.Pixley R. A., Espinola R. G., Ghebrehiwet B., Joseph K., Kao A., Bdeir K., Cines D. B., Colman R. W. (2011) Interaction of high-molecular-weight kininogen with endothelial cell binding proteins suPAR, gC1qR and cytokeratin 1 determined by surface plasmon resonance (BiaCore). Thromb. Haemost. 105, 1053–1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hasan A. A. K., Zisman T., Schmaier A. H. (1998) Identification of cytokeratin 1 as a binding protein and presentation receptor for kininogens on endothelial cells. Proc. Natl. Acad. Sci. USA 95, 3615–3620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Joseph K., Ghebrehiwet B., Kaplan A. P. (1999) Cytokeratin 1 and gC1qR mediate high molecular weight kininogen binding to endothelial cells. Clin. Immunol. 92, 246–255 [DOI] [PubMed] [Google Scholar]
- 41.Renné T., Dedio J., David G., Müller-Esterl W. (2000) High molecular weight kininogen utilizes heparan sulfate proteoglycans for accumulation on endothelial cells. J. Biol. Chem. 275, 33688–33696 [DOI] [PubMed] [Google Scholar]
- 42.Herwald H., Dedio J., Kellner R., Loos M., Müller-Esterl W. (1996) Isolation and characterization of the kininogen-binding protein p33 from endothelial cells. Identity with the gC1q receptor. J. Biol. Chem. 271, 13040–13047 [DOI] [PubMed] [Google Scholar]
- 43.Chavakis T., Kanse S. M., Lupu F., Hammes H. P., Müller-Esterl W., Pixley R. A., Colman R. W., Preissner K. T. (2000) Different mechanisms define the antiadhesive function of high molecular weight kininogen in integrin- and urokinase receptor–dependent interactions. Blood 96, 514–522 [PubMed] [Google Scholar]
- 44.Boehm T., Folkman J., Browder T., O’Reilly M. S. (1997) Antiangiogenic therapy of experimental cancer does not induce acquired drug resistance. Nature 390, 404–407 [DOI] [PubMed] [Google Scholar]
- 45.Cooper J. C., Shi M., Chueh F. Y., Venkitachalam S., Yu C. L. (2010) Enforced SOCS1 and SOCS3 expression attenuates Lck-mediated cellular transformation. Int. J. Oncol. 36, 1201–1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gorska M. M., Stafford S. J., Cen O., Sur S., Alam R. (2004) Unc119, a novel activator of Lck/Fyn, is essential for T cell activation. J. Exp. Med. 199, 369–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gorska M. M., Alam R. (2012) A mutation in the human Uncoordinated 119 gene impairs TCR signaling and is associated with CD4 lymphopenia. Blood 119, 1399–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martin M. W., Machacek M. R. (2010) Update on lymphocyte specific kinase inhibitors: a patent survey. Expert Opin. Ther. Pat. 20, 1573–1593 [DOI] [PubMed] [Google Scholar]
- 49.Bhagwat S. S. (2009) Kinase inhibitors for the treatment of inflammatory and autoimmune disorders. Purinergic Signal. 5, 107–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shi M., Cooper J. C., Yu C. L. (2006) A constitutively active Lck kinase promotes cell proliferation and resistance to apoptosis through signal transducer and activator of transcription 5b activation. Mol. Cancer Res. 4, 39–45 [DOI] [PubMed] [Google Scholar]
- 51.Harr M. W., Caimi P. F., McColl K. S., Zhong F., Patel S. N., Barr P. M., Distelhorst C. W. (2010) Inhibition of Lck enhances glucocorticoid sensitivity and apoptosis in lymphoid cell lines and in chronic lymphocytic leukemia. Cell Death Differ. 17, 1381–1391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Park H. S., Jun Y., Han C. R., Woo H. J., Kim Y. H. (2011) Proteasome inhibitor MG132-induced apoptosis via ER stress-mediated apoptotic pathway and its potentiation by protein tyrosine kinase p56lck in human Jurkat T cells. Biochem. Pharmacol. 82, 1110–1125 [DOI] [PubMed] [Google Scholar]
- 53.Creedon H., Brunton V. G. (2012) Src kinase inhibitors: promising cancer therapeutics? Crit. Rev. Oncog. 17, 145–159 [DOI] [PubMed] [Google Scholar]
- 54.Saunders A. E., Johnson P. (2010) Modulation of immune cell signalling by the leukocyte common tyrosine phosphatase, CD45. Cell. Signal. 22, 339–348 [DOI] [PubMed] [Google Scholar]
- 55.Mancuso P., Calleri A., Gregato G., Labanca V., Quarna J., Antoniotti P., Cuppini L., Finocchiaro G., Eoli M., Rosti V., Bertolini F. (2014) A subpopulation of circulating endothelial cells express CD109 and is enriched in the blood of cancer patients. PLoS One 9, e114713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu Y., Cao D. J., Sainz I. M., Guo Y. L., Colman R. W. (2008) The inhibitory effect of HKa in endothelial cell tube formation is mediated by disrupting the uPA-uPAR complex and inhibiting its signaling and internalization. Am. J. Physiol. Cell Physiol. 295, C257–C267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sun D., McCrae K. R. (2006) Endothelial-cell apoptosis induced by cleaved high-molecular-weight kininogen (HKa) is matrix dependent and requires the generation of reactive oxygen species. Blood 107, 4714–4720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Berg N. N., Ostergaard H. L. (1997) T cell receptor engagement induces tyrosine phosphorylation of FAK and Pyk2 and their association with Lck. J. Immunol. 159, 1753–1757 [PubMed] [Google Scholar]
- 59.Chapman N. M., Connolly S. F., Reinl E. L., Houtman J. C. (2013) Focal adhesion kinase negatively regulates Lck function downstream of the T cell antigen receptor. J. Immunol. 191, 6208–6221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Svec A. (2008) Phosphoprotein associated with glycosphingolipid-enriched microdomains/Csk-binding protein: a protein that matters. Pathol. Res. Pract. 204, 785–792 [DOI] [PubMed] [Google Scholar]
- 61.Goldmann W. H. (2002) p56(lck) Controls phosphorylation of filamin (ABP-280) and regulates focal adhesion kinase (pp125(FAK)). Cell Biol. Int. 26, 567–571 [DOI] [PubMed] [Google Scholar]
- 62.Ostergaard H. L., Lou O., Arendt C. W., Berg N. N. (1998) Paxillin phosphorylation and association with Lck and Pyk2 in anti-CD3- or anti-CD45-stimulated T cells. J. Biol. Chem. 273, 5692–5696 [DOI] [PubMed] [Google Scholar]
- 63.Guo Y. L., Colman R. W. (2005) Two faces of high-molecular-weight kininogen (HK) in angiogenesis: bradykinin turns it on and cleaved HK (HKa) turns it off. J. Thromb. Haemost. 3, 670–676 [DOI] [PubMed] [Google Scholar]
- 64.Zhang W. M., Hsi L., McCrae K. R. (2011) Angiogenesis and tumor growth are increased in kininogen deficient mice. Blood 118, abstract 371 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.