Abstract
Purpose
Premenstrual dysphoric disorder (PMDD) is a psychiatric disorder that causes serious impairments in the functioning and quality of life of affected women. Until recently, research efforts were somewhat hampered by the lack of formal diagnostic criteria, which have now been codified as a category in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). Better characterization of deficits in socioemotional functioning caused by PMDD may aid in improving treatment efforts.
Methods
In this investigation, prospective symptom ratings, based on DSM-5 criteria, were used to measure PMDD symptoms in 36 women (18 with PMDD, 18 healthy controls). Two self-report inventories, the Emotion Regulation Questionnaire and the Difficulties in Emotion Regulation Scale, were used to measure ability to regulate emotions, and socioemotional functioning was measured by inventories of social connectedness, perceived stress, and affect. Potential relationships between ability to regulate emotion and PMDD symptom severity, as well as other measures of socioemotional functioning and affective state were tested.
Results
Women with PMDD reported significantly more behavioral impulsivity, and greater difficulties in regulating emotion and in socioemotional functioning.
Conclusions
Cognitive or behavioral strategies to improve these problems may benefit women with PMDD and help to alleviate distress caused by this disorder.
Keywords: PMDD, premenstrual dysphoric disorder, emotion regulation, affect
1. Introduction
Premenstrual dysphoric disorder (PMDD), a severe variant of premenstrual syndrome, affects 2–5% of women in their reproductive years, producing impairments in quality of life comparable to those observed in major depressive disorder (Epperson et al. 2012; Halbreich et al. 2003; Wittchen et al. 2002). PMDD is unique in that symptoms are most pronounced shortly before the onset of menses, and remit after the onset of menses. In 2013, PMDD was moved from the appendix of the DSM-IV-TR to be included as a category in the DSM-5. Diagnostic criteria for PMDD include the following types of emotional symptoms:
markedly depressed mood, feelings of hopelessness, or self-deprecating thoughts
marked anxiety, tension, feelings of being “keyed up” or “on edge”
marked affective lability (e.g., feeling suddenly sad or tearful or experiencing increased sensitivity to rejection)
persistent and marked anger or irritability or increased interpersonal conflicts
decreased interest in usual activities (e.g., work, school, friends, and hobbies)
a subjective sense of being overwhelmed or out of control
The ability to identify and influence which emotions a person feels, and how those emotions are experienced and expressed, is referred to as “emotion regulation” (for review see (Gross 1998). Because emotional problems constitute most PMDD symptoms, it seems likely that women with PMDD would experience greater difficulties with emotion regulation than healthy controls.
Deficits in emotion regulation have been linked to many other psychiatric disorders, including but not limited to affective disorders. Such problems are manifested by patients with generalized anxiety disorder (Roemer et al. 2009), attention-deficit/hyperactivity disorder (Seymour et al. 2012), major depression (Beblo et al. 2012; Brockmeyer et al. 2012; Cheavens and Heiy 2011; Svaldi et al. 2012), borderline personality disorder (Carpenter and Trull 2013; Cheavens and Heiy 2011; Svaldi et al. 2012), anorexia nervosa (Brockmeyer et al. 2012; Svaldi et al. 2012), bulimia nervosa, and binge eating disorder (Svaldi et al. 2012). However, potential deficits in emotion regulation in women with PMDD have not been previously investigated. We hypothesized that women with PMDD would experience significantly more difficulty regulating emotions, measured by the Emotion Regulation Questionnaire (ERQ; Gross and John, 2003) and Difficulties in Emotion Regulation Scale (DERS; Gratz and Roemer, 2004), compared with healthy control women.
Emotion regulation, a form of self-control, shares a common neural substrate with motor inhibitory control, consistent with the view that different forms of self-control are overlapping constructs that share some common cognitive features (Tabibnia et al. 2011). Therefore, if women with PMDD have a deficit in emotion regulation, this problem might be related to trait impulsivity. We therefore also assessed impulsivity using the Barratt Impulsiveness Scale (BIS-11; (Stanford et al. 2009)).
Finally, we also sought to determine whether difficulties with emotion regulation may be related to the severity of PMDD symptoms measured with prospective symptom rating scales throughout the cycle, as well as measurements of socioemotional functioning during the symptomatic and non-symptomatic phases of the cycle. Emotion regulation was measured with the DERS and ERQ; and socioemotional functioning was measured with the Perceived Stress Scale (PSS; Cohen, Kamarck, and Mermelstein, 1983), and Social Connectedness Scale, Revised (SCSR; Lee, Draper, and Lee, 2001). Additional information regarding affective state was collected with the Positive and Negative Affect Schedule (PANAS; Watson, Clark, and Tellegen, 1988).
2. Methods
2.1. Research Participants and Recruitment
Eighteen women with PMDD and 18 healthy controls were recruited for this study via Internet advertisements and flyers in the community, and all study procedures took place in research laboratories in the Semel Institute for Neuroscience and Human Behavior at UCLA. All procedures performed were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Data were collected from January 2014 until June 2015. An initial telephone screening included questions about demographics, contraceptive methods, premenstrual symptoms, physical and psychological health, and MRI safety requirements (results from the fMRI study will be published separately). Participants were required to meet all of the inclusion criteria and none of the exclusion criteria below.
Inclusion criteria
(1) 18–44 years of age, (2) English speaking, (3) self-reported history of PMDD (for PMDD group) or no premenstrual symptoms (for control group), (4) regular menstrual cycles between 24 and 32 days in length, (5) willingness to use acceptable non-hormonal contraceptive methods if sexually active, (6) generally healthy without cardiovascular, hepatic, renal, autoimmune diseases, diabetes, or cancer, (7) not currently seeking treatment for PMDD, (9) right-handed.
Exclusion criteria
(1) recent or ongoing psychiatric disease, (2) history of drug abuse, (3) currently taking medications or herbal products to treat PMDD and unwillingness to stop, (4) taking hormonal contraceptives 1 month prior to study, (5) taking medications that may affect cerebral perfusion or brain function, (6) central nervous system disease, (7) claustrophobia, (8) pregnancy, (9) presence of metal device in the body that could either interfere with the acquisition of the MRI scan of the brain or pose a potential risk to the subject.
Potentially eligible participants were then enrolled into an online data recording system to complete a Daily Record of Severity of Problems (DRSP), a well-validated and reliable diagnostic inventory of PMDD symptoms (Endicott et al. 2006). Informed consent was obtained electronically via the Internet prior to DRSP completion. After 1 month completion, research staff contacted the participants to verify eligibility to continue. Participants who met the DSM-IV criteria continued completing the DRSP for a 2nd month, while those who did not meet the criteria were disqualified. After the DRSP was completed for two months, participants who continue to meet the criteria were then invited to take part in additional in-person screening.
To be included in the PMDD group, the following criteria had to be met using the data reported on the DRSP:
During the follicular phase, defined here as days 8 to 12 of after the onset of menses, report average daily rating scores < 3 on all 14 DRSP items.
During the premenstrual phase, defined here as the 6 days before menses + day 1 of menses, report scores ≥ 4 for 2 or more days, and report scores ≥ 3 for 2 or more additional days on DRSP item 1, 2, 3, or 4.
During the premenstrual phase, on ≥ 5 of symptoms 1 through 11, report scores ≥ 4 for 2 or more days, and report scores ≥ 3 for 2 or more additional days.
During the premenstrual phase, on the impairment items 12, 13, and 14, report scores ≥ 4 for 2 or more days, and report scores ≥ 3 for 2 or more additional days.
Control participants were required to score ≤ 2 on each DRSP item during the follicular phase (days 8 to 12 after the onset of menstruation), and < 2 on all DRSP mood items during the late luteal phase (6 days before the onset of menstruation and day 1 of the next cycle).
During the in-person screening, written, informed consent was obtained voluntarily after thorough explanation of the protocol and prior to any procedures performed. Eligibility was determined through comprehensive medical history and physical assessments, psychiatric evaluation, and numerous questionnaires, such as a handedness inventory, MRI safety screener, and drug inventory. Psychiatric evaluation was performed using the Structured Clinical Interview for DSM-IV Axis I Disorders.
Participants who qualified after the in-person screening were invited to complete two testing sessions. One session occurred during the follicular phase, between days 8 – 12 of the menstrual cycle, when the participant was asymptomatic. The second testing session occurred during the late luteal phase, 10 – 14 days after ovulation, when PMDD symptoms were present. Each participant was given a Clearblue® Digital Ovulation kit (SPD Swiss Precision Diagnostics GmbH, Geneva), and was instructed on how to use and report the test results. Testing began a few days before the predicted ovulation as determined by the previous documented menstrual cycles from the DRSP. Participants were instructed to continue the ovulation tests daily until a positive result, indicated by a smiley face, was obtained. Positive results were then reported to the research staff, who then scheduled the second testing session.
The two testing sessions were identical except for the participant’s menstrual phase at the time of testing. A few days before the testing day, participants were instructed to refrain from any marijuana use for 48 h before testing, alcohol use 24 h before testing, and caffeine use 2 h before testing. On the test day, breath and urine were tested to determine abstinence from alcohol, tobacco, and marijuana. Non-compliant participants were excluded. Urine pregnancy test also was conducted to exclude pregnancy.
In total, 263 participants were enrolled to identify 18 eligible healthy controls and 18 women with PMDD. An additional 42 participants were enrolled and began the DRSP, but the study reached capacity before they had completed 2 months, and they were withdrawn by research staff. Reasons for exclusion, and the proportion excluded for each reason, are given in supplemental information.
Participants completed two experimental sessions, and menstrual cycle phase at session 1 was counterbalanced. Among healthy controls, 11 began in the follicular phase and 7 in the luteal phase; among PMDD participants, 8 began in the follicular phase and 10 in the luteal phase. Demographics of included participants are given in Table 1.
Table 1.
Average age, relationship status, ethnic background, and average years of education for each group are described.
| Controls | PMDD | |
|---|---|---|
| Age (mean ± SD) | 25.4 ± 7.0 | 29.2 ± 7.2 |
| Relationship status | ||
| Single | 56% | 56% |
| In relationship, living separately | 22% | 11% |
| In relationship, living together | 0% | 11% |
| Married | 17% | 17% |
| Divorced / Separated | 6% | 6% |
| Ethnicity | ||
| Asian | 17% | 17% |
| African-American | 11% | 6% |
| Hispanic | 33% | 0% |
| White | 22% | 78% |
| More than one | 17% | 0% |
| Years of education (mean ± SD) | 15.6 ± 2.7 | 16.7 ± 3.6 |
2.2 Self-report inventories
Measurements of emotion regulation were taken at a single time point without regard to menstrual cycle because of evidence that these are trait-like, rather than state-like measures (Gratz and Roemer 2004). Emotion regulation was measured with the ERQ and DERS. Impulsivity was measured using the Barratt Impulsiveness Scale (BIS-11). Total scores on the BIS-11 were calculated and subscales scores measuring cognitive and behavioral impulsivity were generated using a bifactor model (Reise et al. 2013).
Measurements of state-like constructs (SCSR, PSS, and PANAS) were taken once during the follicular phase and once during the luteal phase.
2.3 Serum hormone levels
Five mL blood samples were collected by venipuncture, once during the follicular phase (cycle days 8 to 12) and once during the late luteal phase (10 to 14 days after ovulation). Serum estradiol and progesterone levels were analyzed by the UCLA Clinical Laboratory and Pathological Services.
2.4 Statistical analysis
All statistical tests were performed in JMP® Pro 11.0.0. (SAS Institute Inc., Cary, NC). Statistical significance was thresholded at α < 0.05.
3. Results
3.1. DRSP Scores
DRSP scores differed significantly between groups and phases. These effects were tested in a 2 × 2 full factorial mixed-model ANOVA with group (PMDD or healthy control) as a between-subjects factor and menstrual cycle phase as a within-subjects factor. The main effects of cycle phase [F(1, 34) = 226.4, p < 0.0001] and group [F(1, 34) = 185.5, p < 0.0001] were significant, as was the group × phase interaction [F(1, 34) = 218.0, p < 0.0001].
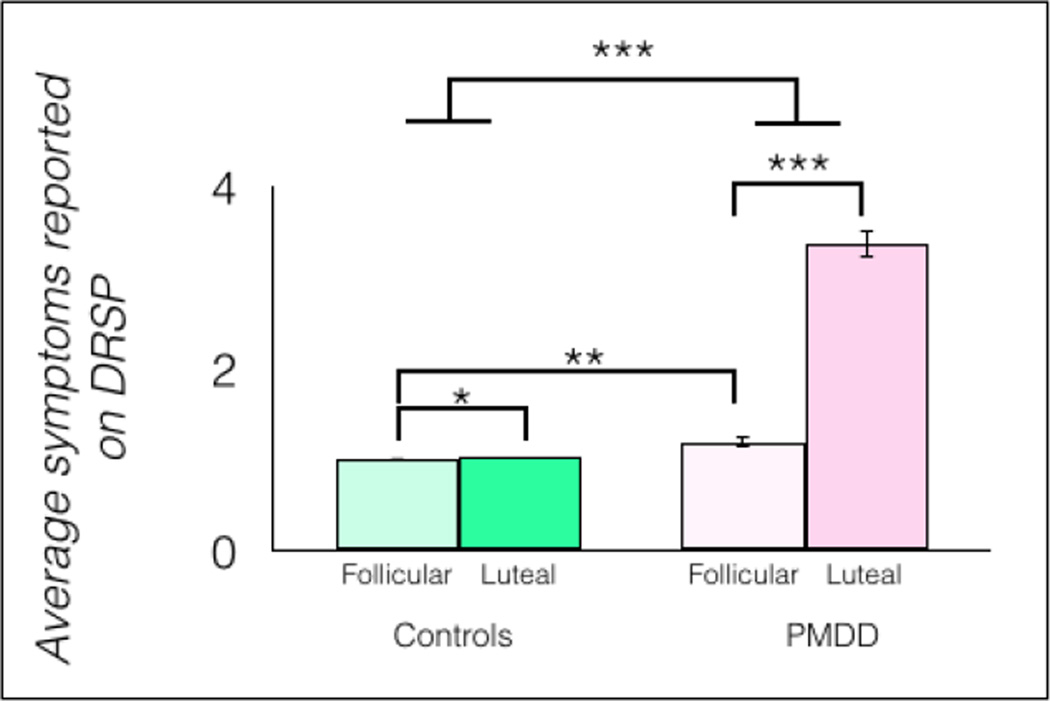
Post hoc t-tests showed that, in women with PMDD, DRSP scores were significantly higher during the luteal compared to the follicular phase [t(1, 34) = 21.1, p < 0.0001, η2 = 0.81]. Women with PMDD also reported significantly more symptoms than controls when each group was in the luteal phase, [t(1, 34) = 19.8, p < 0.0001, η2 = 0.87] (Figure 1). Total DRSP scores were also significantly higher in the follicular phase in women with PMDD compared with controls, [t(1, 34) = 2.69, p = 0.0114, η2 = 0.18].
Fig 1.
In the PMDD group (n = 18), total PMDD symptoms, measured by the DRSP, were significantly higher during the luteal phase than during the follicular phase, but also higher than in controls (n = 18) during the follicular phase and during the luteal phase, *p < 0.05, **p < 0.01, ***p < 0.001, error bars indicate ± 1 SEM.
3.2. Emotion Regulation
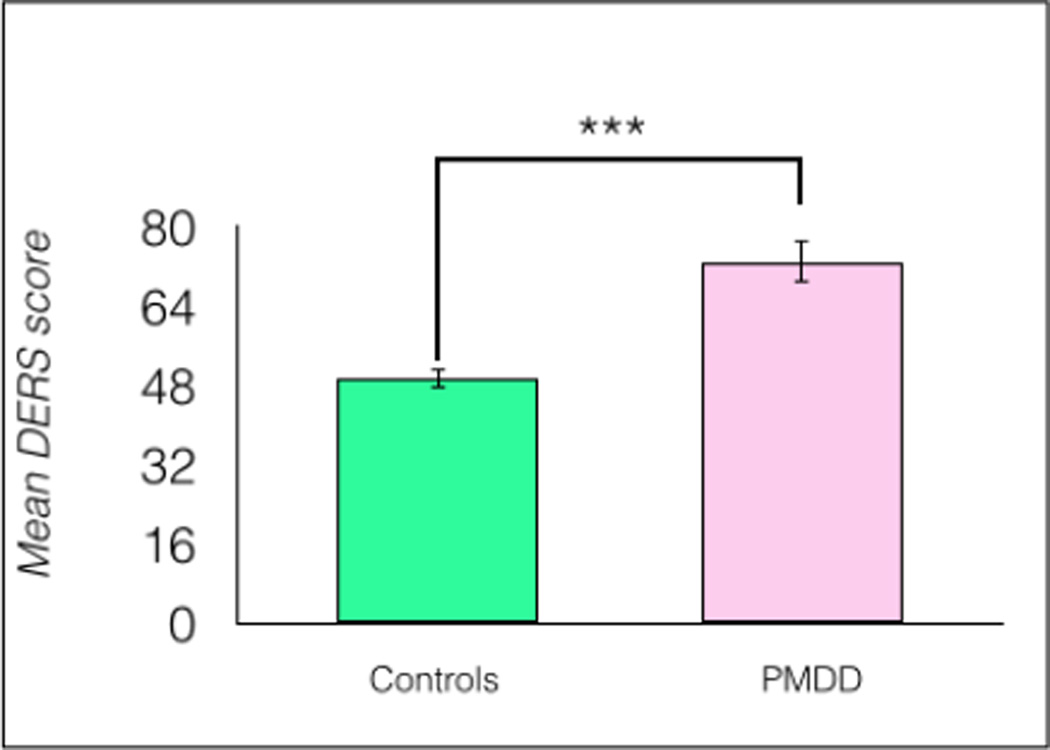
A comparison by Student’s t-test showed no significant differences between PMDD participants and healthy controls on ERQ scores, t(1, 34) = 1.44, p = 0.158. However, PMDD participants reported significantly higher DERS scores, t(1, 34) = 5.21, p < 0.0001, η2 = 0.44 (Figure 2).
Fig 2.
Women with PMDD reported significantly more difficulties regulating emotions compared to healthy controls (n = 18/group), ***p < 0.0001, error bars indicate ± 1 SEM.
In PMDD participants, average PMDD symptom severity was not correlated significantly with DERS scores, r(18) = −0.18, p = 0.477.
3.3. Social Connectedness
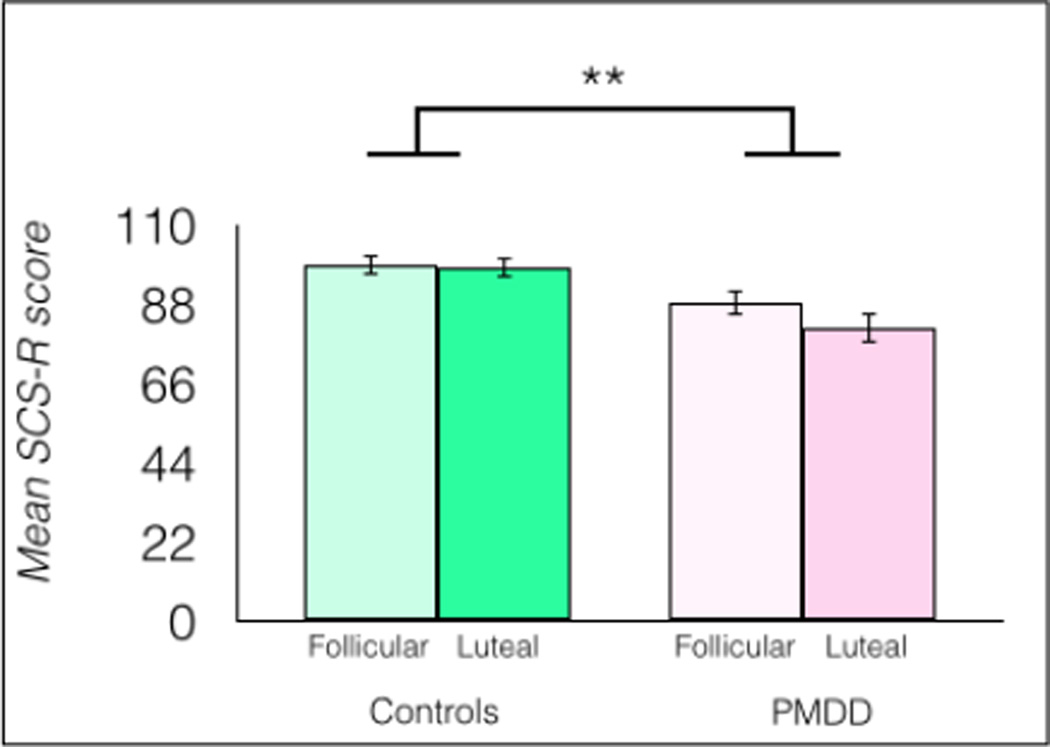
The effects of PMDD and menstrual cycle phase on SCS-R scores were tested in a 2 × 2 full factorial mixed-model ANOVA with group as a between-subjects factor and menstrual cycle phase as a within-subjects factor (Figure 3). The main effect of cycle phase was significant [F(1, 34) = 5.837, p = 0.021], with higher SCS-R scores reported during the follicular phase than the luteal phase. The main effect of group was significant [F(1, 34) = 9.910, p = 0.0034] with higher SCS-R scores reported by healthy controls compared to the PMDD group. A marginally significant interaction between group and menstrual phase was found, F(1, 34) = 3.978, p = 0.054. In PMDD participants, average symptom severity measured by the DRSP was not correlated significantly with luteal SCS-R scores, r(18) = 0.02, p = 0.9285.
Fig 3.
Women with PMDD report significantly lower social connectedness than healthy control women, as measured by the SCS-R, n = 18/group, **p < 0.01, error bars indicate ± 1 SEM.
Post hoc tests showed significantly lower SCS-R scores in follicular [t(1, 34) = 2.17, p = 0.037, η2 = 0.14] and luteal [t(1, 34) = 3.82, p = 0.0005, η2 = 0.27] women with PMDD compared to follicular healthy controls. Women with PMDD had significantly lower SCS-R scores during the luteal phase compared to the follicular phase [t(1, 34) = 3.12, p = 0.0037, η2 = 0.06], and women with PMDD had significantly lower SCS-R scores during the luteal phase compared to healthy controls during the luteal phase [t(1, 34) = 3.67, p = 0.0008, η2 = 0.25] (Figure 3).
3.4. Perceived Stress
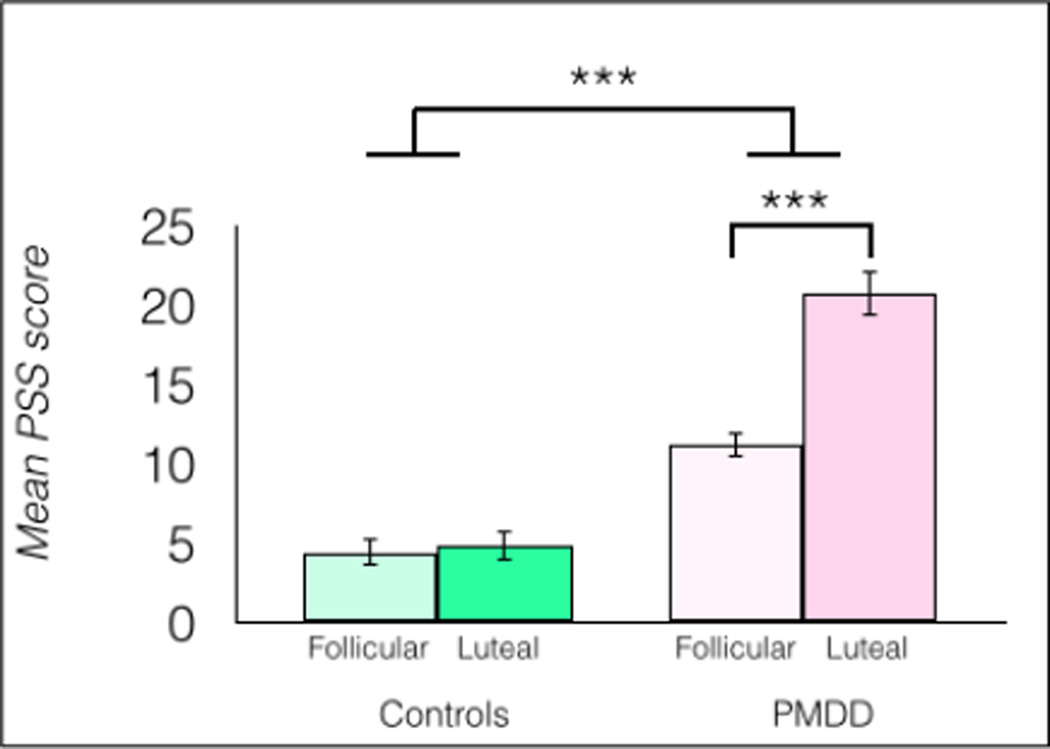
The effects of group and menstrual phase on PSS scores were tested in a 2 × 2 full factorial mixed-model ANOVA. This revealed a significant main effect of menstrual cycle phase, F(1, 34) = 31.3, p < 0.0001; a significant main effect of group, F(1, 34) = 90.4, p < 0.0001; and a group × phase interaction, F(1, 34) = 25.9, p < 0.0001. In PMDD participants, average symptom severity measured by the DRSP did not correlate significantly with luteal PSS scores, r(18) = −0.37, p = 0.1274.
Post hoc tests showed significantly higher perceived stress during the luteal phase in women with PMDD compared to women with PMDD in the follicular phase, η2 = 0.49; compared to luteal healthy controls, η2 = 0.72; and compared to follicular healthy controls, η2 = 0.73, all ps < 0.0001. Women with PMDD also reported more perceived stress during the follicular phase than healthy controls did during the follicular phase [t(1, 34) = 4.60, p < 0.0001, η2 = 0.48). Women with PMDD also reported more perceived stress during the follicular phase than healthy controls reported during the luteal phase, [t(1, 34) = 4.31, p = 0.0001, η2 = 0.42] (Figure 4).
Fig 4.
Women with PMDD reported significantly more perceived stress compared to healthy controls, n = 18/group, ***p < 0.0001, error bars indicate ± 1 SEM.
3.5. Impulsivity
The two groups (PMDD and healthy controls) did not differ significantly on total impulsivity scores, t(1, 34) = 1.53, p = 0.1348, or on cognitive impulsivity scores, t(1, 34) = 0.1597, p = 0.874. However, PMDD participants reported significantly higher behavioral impulsivity scores compared to healthy controls, t(1, 34) = 2.28, p = 0.0289, η2 = 0.13.
3.6. Positive and Negative Affect
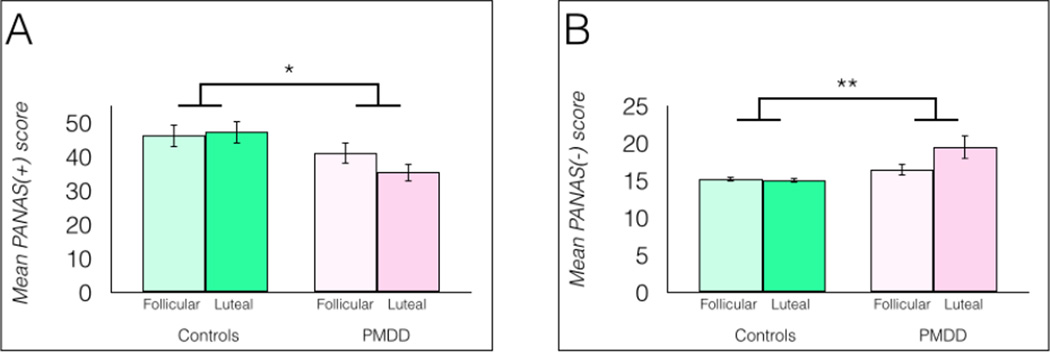
Positive and negative affect were measured using the Positive and Negative Affect Scale (PANAS). A 2 × 2 full factorial mixed-model ANOVA indicated a significant main effect of group on positive affect scores (PANAS+), F(1, 34) = 5.08, p = 0.0307 (Figure 5a). Cycle phase did not significantly influence positive affect scores [PANAS(+)], nor was a significant group × phase interaction found, ps > 0.05. Post hoc tests showed significantly lower PANAS(+) scores in luteal women with PMDD compared to luteal healthy controls [t(1, 34) = 2.70, p = 0.011, η2 = 0.19] and compared to follicular healthy controls [t(1, 34) = 2.48, p = 0.018, η2 = 0.16].
Fig 5.
a Women with PMDD reported significantly lower positive affect measured by the positive subscale of the PANAS, n = 18/group, *p < 0.05. 5b: Women with PMDD reported significantly higher negative affect measured by the negative subscale of the PANAS, n = 18/group, *p < 0.05. Both panels: error bars indicate ± 1 SEM.
The same statistical model yielded a significant main effect of group on negative affect scores (PANAS−), F(1, 34) = 7.79, p = 0.0086, but no significant main effect of menstrual cycle phase, nor a group × phase interaction, ps > 0.05, although the interaction approached significance, p = 0.0654 (Figure 5). Post hoc tests indicated that women with PMDD in the luteal phase differed significantly compared to their own scores during the follicular phase, p = 0.017, η2 = 0.08; compared to luteal healthy controls, p = 0.0019, η2 = 0.19; and compared to follicular healthy controls, p = 0.003, η2 = 0.16.
3.7. Tests of relationships between serum hormones and behavioral measures in women with PMDD
Progesterone levels were compared between groups and cycle phases using a 2 × 2 full factorial mixed-model ANOVA. No significant main effect of group was found, F(1, 34) = 0.785, p = 0.3819. A significant main effect of menstrual cycle phase was found, F(1, 34) = 36.58, p < 0.0001. No significant group × phase interaction was found, F(1, 34) = 0.5596, p = 0.4595. The change in progesterone levels from the follicular to luteal phase was similar between study groups (t(1,34) = 0.447, p = 0.66).
Estrogen and progesterone levels for each group are given in Table 2. Follicular phase estrogen levels did not differ significantly between groups, t(1, 22) = 0.038, p = 0.9703 (Note: data were missing for 6 PMDD participants and 6 healthy controls).
Table 2.
Estrogen and progesterone levels in healthy controls and women with PMDD were similar.
| Healthy controls | PMDD women | |||
|---|---|---|---|---|
| Follicular | Luteal | Follicular | Luteal | |
| Estrogen (pg/mL) ± 1 SD |
113.7 ± 132.6 | 115.5 ± 104.4 | ||
| Progesterone (ng/mL) ± 1 SD |
0.78 ± 0.75 | 5.96 ± 5.06 | 0.91 ± 0.77 | 7.21 ± 5.69 |
In PMDD participants, neither estrogen nor progesterone levels were correlated significantly with SCS-R, DERS, PSS, PANAS+, or PANAS− scores, all ps > 0.05.
3.8 Relationship between DERS and socioemotional functioning
DERS scores were not correlated significantly with severity of PMDD symptoms measured by the DRSP, or with the change in PSS, SCSR, or PANAS scores from the follicular phase to the luteal phase (all ps > 0.1).
4. Discussion
The list of symptoms in the DSM and prospective rating forms provide a limited view of the emotional functioning of women with PMDD. Using detailed, prospective symptom reporting, DSM-5 criteria to diagnose PMDD, and urinary luteinizing hormone detection tests and serum hormone measurements to identify menstrual cycle phases, we found that women with PMDD experience greater difficulties regulating emotions as measured by the DERS. Consistent with previous investigations, women with PMDD in this study also experienced more PMDD symptoms during the follicular phase of the cycle than healthy controls, and suffered from greater perceived stress, less social connectedness, greater negative affect, and lower positive affect than healthy controls. Notably, women with PMDD also self-reported greater behavioral impulsivity.
Despite positive findings using the DERS, no group differences in emotion regulation were observed using the ERQ. The ERQ and DERS differ in that the ERQ was designed specifically to assess whether and to what extent a person uses two emotion regulation strategies, cognitive reappraisal and expressive suppression (Gross and John 2003). The DERS, by contrast, measures emotion regulation strategies as well as acceptance of emotional responses, ability to perform goal-directed behavior, impulse control, emotional awareness, and emotional clarity. These differences in the inventories may explain the discrepancy in outcomes, and suggest that deficits in emotion regulation in women with PMDD may be more strongly related to problems in domains other than cognitive strategies to regulate emotion.
Previous reports have indicated that PMDD is associated with impairments in social functioning (Hylan et al. 1999). As measured by the Social Adjustment Scale (SAS), women with PMDD experience more problems with social adjustment during the luteal phase than the follicular phase, but they also experience more social adjustment problems than unaffected women during the follicular phase (Halbreich et al. 2003; Pearlstein et al. 2000). In a healthy sample, deficits in social connectedness have been significantly associated with psychological distress (Lee et al. 2001). Low social connectedness predicts the severity of depression symptomatology (Armstrong and Oomen-Early 2009; Williams and Galliher 2006), and is linked to higher trait anxiety (Lee and Robbins 1998). Our finding that women with PMDD experience deficits in social connectedness is generally consistent with previous evidence of impaired social functioning in this population, and may serve to identify social connectedness as a potential therapeutic target to improve socioemotional functioning in women with PMDD.
Perceived stress is another potentially relevant target for PMDD therapies. Previous evidence linked premenstrual symptoms to perceived stress using the same inventory employed in this investigation. Hoyer et al. (2013) reported significantly higher ratings on the PSS in women with PMS during the luteal phase compared to healthy controls during the luteal phase. Similarly, in a community sample of 830 women contacted by telephone through random digit dialing, perceived stress measured by the PSS was found to be the strongest predictor of premenstrual syndrome (Deuster et al. 1999). Here, we replicated the finding that women who met DSM-5 criteria for PMDD experience greater perceived stress than healthy controls, suggesting that strategies to ameliorate perceived stress may also be a useful therapeutic target in behavioral interventions to treat PMDD.
Despite group differences in social connectedness, emotion regulation, and perceived stress, PMDD symptoms were not correlated with scores measuring these domains. Further, neither estradiol nor progesterone levels in serum were correlated with PMDD symptom severity or with scores on any inventories administered. This latter finding is generally consistent with previous literature indicating that ovarian hormone levels, and those of many of their metabolites, do not differ in women with PMDD compared to healthy controls: Both lower (Thys-Jacobs et al. 2008) and higher (Wang et al. 1996) estradiol levels have been observed in women with menstrual-related mood disorders. Lower levels of progesterone have also been reported in women with PMS (Wang et al. 1996), a finding not replicated by several previous and subsequent studies (Backstrom et al. 1983; Eriksson et al. 1992; Facchinetti et al. 1993; Rubinow et al. 1988; Thys-Jacobs et al. 2008; Rapkin et al. 1997). Different (Facchinetti et al. 1993) and similar (Reame et al. 1992) patterns of luteinizing hormone release have been reported in women with PMS compared to controls. Our finding that estrogen and progesterone levels did not relate to PMDD symptom severity adds to the body of literature suggesting that PMDD is not linearly related to ovarian hormone levels.
This study had both strengths and limitations. Its strengths included the use of prospective, daily ratings of PMDD symptoms in both PMDD participants and controls; using luteinizing hormone to confirm ovulation and allow precise premenstrual phase window identification; and measuring the same women in each menstrual cycle phase. One limitation was the administration of the DERS at only a single time point; however, test-retest reliability has shown a correlation of 0.88 (Gratz and Roemer 2004), suggesting that ability to regulate emotion is a stable trait. Further, the interpretation of these data is limited by the finding that scores on the inventories used were not correlated with symptom severity. It is unclear why PMDD would produce a dichotomous rather than continuous effect on DERS, SCSR, PSS, BIS, and PANAS scores. One potential explanation may be that women with PMDD might have limited insight into their own emotional state. Emotional awareness is one construct measured by the DERS. That women with PMDD reported higher DERS scores suggests a lack of emotional awareness, which may confound self-report measures. Investigations of insight in women with PMDD, and investigations of emotion regulation using objective biomarkers rather than self-report, may clarify the relationship between PMDD severity, emotion regulation, and socioemotional functioning. It is possible that this study was underpowered to detect statistically significant correlations between symptom severity and these scales.
In conclusion, these data suggest that women with PMDD struggle with emotion regulation. While not directly related to emotion regulation, these women also exhibit evidence of behavioral impulsivity, impaired social connectedness, and elevated perceived stress. Coping strategies to improve these domains may benefit women with PMDD and help to alleviate distress caused by this disorder. Emotion Regulation Therapy has previously been proposed as a method to treat Generalized Anxiety Disorder (Mennin, 2004), and constructs from this practice may be adapted to treat PMDD. Mindfulness-based therapies have previously been proposed as a therapeutic approach that can lead to improved emotion regulation (Chambers et al., 2009); we propose that on the basis of these findings, such therapies may benefit women with PMDD.
Supplementary Material
Acknowledgments
This work was supported by R21MH098668 to E.D.L and A.J.R., and endowments from the Thomas P. and Katherine K. Pike Chair in Addiction Studies, and the Marjorie M. Greene Trust (E.D.L). The funding sources were not involved in the study design, collection, analysis/interpretation of data, nor were they involved with the decision to submit the article for publication.
We wish to thank Ms. Aqsah Choudhary and Ms. Chelsea Cox for assistance collecting and managing data. We also thank Dr. Steven Berman for his guidance designing this project.
Footnotes
Author Disclosures:
The authors report no conflicts of interest.
References
- Armstrong S, Oomen-Early J. Social connectedness, self-esteem, and depression symptomatology among collegiate athletes versus nonathletes. J Am Coll Health. 2009;57(5):521–526. doi: 10.3200/JACH.57.5.521-526. [DOI] [PubMed] [Google Scholar]
- Backstrom T, Sanders D, Leask R, Davidson D, Warner P, Bancroft J. Mood, sexuality, hormones, and the menstrual cycle. II. Hormone levels and their relationship to the premenstrual syndrome. Psychosom Med. 1983;45(6):503–507. doi: 10.1097/00006842-198312000-00004. [DOI] [PubMed] [Google Scholar]
- Beblo T, Fernando S, Klocke S, Griepenstroh J, Aschenbrenner S, Driessen M. Increased suppression of negative and positive emotions in major depression. J Affect Disord. 2012;141(2–3):474–479. doi: 10.1016/j.jad.2012.03.019. [DOI] [PubMed] [Google Scholar]
- Bloch M, Schmidt PJ, Su TP, Tobin MB, Rubinow DR. Pituitary-adrenal hormones and testosterone across the menstrual cycle in women with premenstrual syndrome and controls. Biol Psychiatry. 1998;43(12):897–903. doi: 10.1016/s0006-3223(98)00403-x. [DOI] [PubMed] [Google Scholar]
- Brockmeyer T, Bents H, Holtforth MG, Pfeiffer N, Herzog W, Friederich HC. Specific emotion regulation impairments in major depression and anorexia nervosa. Psychiatry Res. 2012;200(2–3):550–553. doi: 10.1016/j.psychres.2012.07.009. [DOI] [PubMed] [Google Scholar]
- Carpenter RW, Trull TJ. Components of emotion dysregulation in borderline personality disorder: a review. Curr Psychiatry Rep. 2013;15(1):335. doi: 10.1007/s11920-012-0335-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers R, Gullone E, Allen NB. Mindful emotion regulation: An integrative review. Clinical Psychology Review. 2009;29(6):560–572. doi: 10.1016/j.cpr.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Cheavens JS, Heiy J. The Differential Roles of Affect and Avoidance in Major Depressive and Borderline Personality Disorder Symptoms. Journal of Social and Clinical Psychology. 2011;30(5):441–457. [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A Global Measure of Perceived Stress. Journal of Health and Social Behavior. 1983;24(4):385–396. [PubMed] [Google Scholar]
- Deuster PA, Adera T, South-Paul J. Biological, social, and behavioral factors associated with premenstrual syndrome. Arch Fam Med. 1999;8(2):122–128. doi: 10.1001/archfami.8.2.122. [DOI] [PubMed] [Google Scholar]
- Endicott J, Nee J, Harrison W. Daily Record of Severity of Problems (DRSP): reliability and validity. Arch Womens Ment Health. 2006;9(1):41–49. doi: 10.1007/s00737-005-0103-y. [DOI] [PubMed] [Google Scholar]
- Epperson CN, Steiner M, Hartlage SA, Eriksson E, Schmidt PJ, Jones I, Yonkers KA. Premenstrual dysphoric disorder: evidence for a new category for DSM-5. Am J Psychiatry. 2012;169(5):465–475. doi: 10.1176/appi.ajp.2012.11081302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson E, Sundblad C, Lisjo P, Modigh K, Andersch B. Serum Levels of Androgens Are Higher in Women with Premenstrual Irritability and Dysphoria Than in Controls. Psychoneuroendocrinology. 1992;17(2–3):195–204. doi: 10.1016/0306-4530(92)90058-f. [DOI] [PubMed] [Google Scholar]
- Facchinetti F, Genazzani AD, Martignoni E, Fioroni L, Nappi G, Genazzani AR. Neuroendocrine Changes in Luteal Function in Patients with Premenstrual-Syndrome. Journal of Clinical Endocrinology & Metabolism. 1993;76(5):1123–1127. doi: 10.1210/jcem.76.5.8496301. [DOI] [PubMed] [Google Scholar]
- Gratz KL, Roemer L. Multidimensional assessment of emotion regulation and dysregulation: Development, factor structure, and initial validation of the difficulties in emotion regulation scale. Journal of Psychopathology and Behavioral Assessment. 2004;26(1):41–54. [Google Scholar]
- Gross JJ. The Emerging Field of Emotion Regulation: An Integrative Review. Review of General Psychology. 1998;2(3):271–299. [Google Scholar]
- Gross JJ, John OP. Individual differences in two emotion regulation processes: implications for affect, relationships, and well-being. J Pers Soc Psychol. 2003;85(2):348–362. doi: 10.1037/0022-3514.85.2.348. [DOI] [PubMed] [Google Scholar]
- Halbreich U, Borenstein J, Pearlstein T, Kahn LS. The prevalence, impairment, impact, and burden of premenstrual dysphoric disorder (PMS/PMDD) Psychoneuroendocrinology. 2003;28(Suppl 3):1–23. doi: 10.1016/s0306-4530(03)00098-2. [DOI] [PubMed] [Google Scholar]
- Hoyer J, Burman I, Kieseler M, Vollrath F, Hellrung L, Arelin K, Roggenhofer E, Villringer A, Sacher J. Menstrual Cycle Phase Modulates Emotional Conflict Processing in Women with and without Premenstrual Syndrome (PMS) – A Pilot Study. PLoS ONE. 2013;8(4):e59780. doi: 10.1371/journal.pone.0059780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hylan TR, Sundell K, Judge R. The impact of premenstrual symptomatology on functioning and treatment-seeking behavior: experience from the United States, United Kingdom, and France. J Womens Health Gend Based Med. 1999;8(8):1043–1052. doi: 10.1089/jwh.1.1999.8.1043. [DOI] [PubMed] [Google Scholar]
- Lee RM, Draper M, Lee S. Social connectedness, dysfunctional interpersonal behaviors, and psychological distress: Testing a mediator model. Journal of Counseling Psychology. 2001;48(3):310–318. [Google Scholar]
- Lee RM, Robbins SB. The relationship between social connectedness and anxiety, self-esteem, and social identity. Journal of Counseling Psychology. 1998;45(3):338–345. [Google Scholar]
- Mennin DS. Emotion regulation therapy for generalized anxiety disorder. Clinical Psychology & Psychotherapy. 2004;11(1):17–29. [Google Scholar]
- Pearlstein TB, Halbreich U, Batzar ED, Brown CS, Endicott J, Frank E, Freeman EW, Harrison WM, Haskett RF, Stout AL, Yonkers KA. Psychosocial functioning in women with premenstrual dysphoric disorder before and after treatment with sertraline or placebo. J Clin Psychiatry. 2000;61(2):101–109. doi: 10.4088/jcp.v61n0205. [DOI] [PubMed] [Google Scholar]
- Rapkin AJ, Morgan M, Goldman L, Brann DW, Simone D, Mahesh VB. Progesterone metabolite allopregnanolone in women with premenstrual syndrome. Obstetrics and Gynecology. 1997;90(5):709–714. doi: 10.1016/S0029-7844(97)00417-1. [DOI] [PubMed] [Google Scholar]
- Reame NE, Marshall JC, Kelch RP. Pulsatile Lh-Secretion in Women with Premenstrual-Syndrome (Pms) - Evidence for Normal Neuroregulation of the Menstrual-Cycle. Psychoneuroendocrinology. 1992;17(2–3):205–213. doi: 10.1016/0306-4530(92)90059-g. [DOI] [PubMed] [Google Scholar]
- Reise SP, Moore TM, Sabb FW, Brown AK, London ED. The Barratt Impulsiveness Scale-11: reassessment of its structure in a community sample. Psychol Assess. 2013;25(2):631–642. doi: 10.1037/a0032161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roemer L, Lee JK, Salters-Pedneault K, Erisman SM, Orsillo SM, Mennin DS. Mindfulness and emotion regulation difficulties in generalized anxiety disorder: preliminary evidence for independent and overlapping contributions. Behav Ther. 2009;40(2):142–154. doi: 10.1016/j.beth.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinow DR, Hoban MC, Grover GN, Galloway DS, Roybyrne P, Andersen R, Merriam GR. Changes in Plasma Hormones across the Menstrual-Cycle in Patients with Menstrually Related Mood Disorder and in Control Subjects. American Journal of Obstetrics and Gynecology. 1988;158(1):5–11. doi: 10.1016/0002-9378(88)90765-x. [DOI] [PubMed] [Google Scholar]
- Seymour KE, Chronis-Tuscano A, Halldorsdottir T, Stupica B, Owens K, Sacks T. Emotion regulation mediates the relationship between ADHD and depressive symptoms in youth. J Abnorm Child Psychol. 2012;40(4):595–606. doi: 10.1007/s10802-011-9593-4. [DOI] [PubMed] [Google Scholar]
- Stanford MS, Mathias CW, Dougherty DM, Lake SL, Anderson NE, Patton JH. Fifty years of the Barratt Impulsiveness Scale: An update and review. Personality and Individual Differences. 2009;47(5):385–395. [Google Scholar]
- Svaldi J, Griepenstroh J, Tuschen-Caffier B, Ehring T. Emotion regulation deficits in eating disorders: A marker of eating pathology or general psychopathology? Psychiatry Research. 2012;197(1–2):103–111. doi: 10.1016/j.psychres.2011.11.009. [DOI] [PubMed] [Google Scholar]
- Tabibnia G, Monterosso JR, Baicy K, Aron AR, Poldrack RA, Chakrapani S, Lee B, London ED. Different forms of self-control share a neurocognitive substrate. J Neurosci. 2011;31(13):4805–4810. doi: 10.1523/JNEUROSCI.2859-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thys-Jacobs S, McMahon D, Bilezikian JP. Differences in free estradiol and sex hormone-binding globulin in women with and without premenstrual dysphoric disorder. Journal of Clinical Endocrinology & Metabolism. 2008;93(1):96–102. doi: 10.1210/jc.2007-1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang MD, Seippel L, Purdy RH, Backstrom T. Relationship between symptom severity and steroid variation in women with premenstrual syndrome: Study on serum pregnenolone, pregnenolone sulfate, 5 alpha-pregnane-3,20-dione and 3 alpha-hydroxy-5 alpha-pregnan-20-one. Journal of Clinical Endocrinology & Metabolism. 1996;81(3):1076–1082. doi: 10.1210/jcem.81.3.8772579. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54(6):1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Williams KL, Galliher RV. Predicting depression and self-esteem from social connectedness, support, and competence. Journal of Social and Clinical Psychology. 2006;25(8):855–874. [Google Scholar]
- Wittchen HU, Becker E, Lieb R, Krause P. Prevalence, incidence and stability of premenstrual dysphoric disorder in the community. Psychol Med. 2002;32(1):119–132. doi: 10.1017/s0033291701004925. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.