Abstract
Background:
Increased interest in using platelet-rich plasma (PRP) as an augment to rotator cuff repair warrants further investigation, particularly in smaller rotator cuff tears.
Purpose:
To examine the effectiveness of PRP application in improving perioperative pain and function and promoting healing at 6 months after arthroscopic repair of small- or medium-sized rotator cuff tears.
Study Design:
Randomized controlled trial; Level of evidence, 1.
Methods:
This was a double-blinded randomized controlled trial of patients undergoing arthroscopic repair of partial- or full-thickness rotator cuff tears of up to 3 cm who were observed for 6 months. Patients were randomized to either repair and PRP application (study group) or repair only (control group) groups. The patient-oriented outcome measures utilized were the visual analog scale (VAS), the Short Western Ontario Rotator Cuff Index (ShortWORC), the American Shoulder and Elbow Surgeons (ASES) form, and the Constant-Murley Score (CMS). Range of motion (ROM) and inflammatory and coagulation markers were measured before and after surgery. Magnetic resonance imaging was used at 6 months to assess retear and fatty infiltration rate.
Results:
Eighty-two patients (41 males) with a mean age of 59 ± 8 years were enrolled; 41 patients were included in each group. Both the PRP and control groups showed a significant improvement in their pain level based on the VAS within the first 30 days (P < .0001), with the PRP group reporting less pain than the control group (P = .012), which was clinically significantly different from days 8 through 11. The PRP group reported taking less painkillers (P = .026) than the control group within the first 30 days. All outcome measure scores and ROM improved significantly after surgery (P < .0001), with no between-group differences. No differences were observed between groups in inflammatory or coagulation marker test results (P > .05), retear (14% vs 18% full retear; P = .44), or fatty infiltration rate (P = .08).
Conclusion:
The PRP biological augmentation for repair of small- to medium-sized rotator cuff tears has a short-term effect on perioperative pain without any significant impact on patient-oriented outcome measures or structural integrity of the repair compared with control group.
Keywords: platelet-rich plasma, healing, rotator cuff
Advances in rotator cuff (RC) surgery, such as improved implants and sutures to secure the repair, have enhanced the initial strength of repairs done arthroscopically. However, despite reduced pain and improved function, a significant incidence of residual or recurrent cuff tears ranging from 11% to 40% after surgery remains a concern.14,16,18,33 Although failure of the repair may be attributed to age, tear size, tendon quality, and repair techniques, intrinsic factors that affect healing are of interest to clinicians. Recent interest in using biologic augmentation to improve the healing process of the repaired RC tendons has led to an explosion of data on the use of platelet-rich plasma (PRP).2,15,19,25,39 The healing potential of PRP has been attributed to the release of multiple growth factors that have been identified as playing key roles in tendon healing at the repair site.10,23 These biologically active growth factors work by stimulating angiogenesis, epithelialization, cell differentiation-replication-proliferation, and the formation of extracellular matrix and fibrovascular callus and have the potential to improve tendon regeneration.21,23 In addition, platelets influence the early phases of the healing process, which allows mechanical stimulation to start driving neotendon development at an earlier time point.38 This may have an impact on tendon quality, reducing retear size and increasing the likelihood of a more successful repair.
Recent systematic reviews have concluded that using PRP at the time of arthroscopic RC repair does not universally improve retear rates or affect clinical outcome scores.3,32,40,41 However, patients with small- or medium-sized RC tears appear to show better outcomes with PRP than without it.3,37 Further investigation of the PRP efficacy and impact on perioperative pain, functional outcome, and structural integrity of the RC repair is therefore warranted.
The purpose of this study was to examine the effectiveness of PRP application in improving perioperative pain and function and promoting healing after arthroscopic repair of small- and medium-sized RC tears through a randomized controlled study. Group differences in inflammatory and coagulation markers and adverse events were also examined. We hypothesized that patients who receive PRP would have less perioperative pain with better range of motion and fewer retears detected by magnetic resonance imaging (MRI) at 6 months postoperatively compared with a control group.
Methods
Patient Population
This was a prospective, double-blinded randomized controlled trial. The target population was patients undergoing arthroscopic RC repair of partial- or full-thickness tears of up to 3 cm from March 2011 to September 2014. Considering lack of difference in outcomes reported by previous investigators,2,19,39 patients were observed for 6 months, and the rate of revision was monitored for 1 year after surgery.
In addition to informed consent, the inclusion criteria included age ≥18 years and a diagnosis of partial- (>50% cuff thickness) or full-thickness RC tear of ≥3 cm diagnosed by MRI and confirmed on arthroscopic assessment. Exclusion criteria were inability to speak or read English, previous surgery of the affected shoulder, evidence of infection, underlying metabolic or inflammatory disease, avascular necrosis, adhesive capsulitis, concurrent pathology of subscapularis, superior labral anterior and posterior (SLAP) or Bankart lesions requiring repair, bone marrow pathology, abnormal platelet count, serum hemoglobin concentration <11 g/dL or hematocrit <34%, use of systemic steroids, current use of anticoagulants, use of an investigational drug and/or blood donation within the previous 3 months prior to surgery, and psychiatric illness that precluded informed consent. Approval for use of human subjects was obtained from the Research Ethics Board of the Sunnybrook Health Sciences Centre. The clinical trials.gov identifier was NCT01000935.
Clinical and Laboratory Tests
Patient history, including mechanism of injury and symptom characteristics, was documented at approximately 2 to 3 weeks prior to surgery. Objective clinical examination included range of motion (ROM) prior to surgery and at 6 weeks and 3 and 6 months after surgery. ROM involved active and passive flexion, abduction, external rotation in neutral, and internal rotation at 90° of abduction. Active ROM was not collected at 6 weeks to avoid stressing the repair. Preoperative level of comorbidity (0-52) was calculated as continuous data based on a validated score, the Cumulative Illness Rating Scale,7 which examines the overall health. In this scale, 0 represents no impairment and 52 represent the highest level of possible impairment.
Clinical laboratory tests examined inflammatory and coagulation markers pre- and postoperatively and included white blood cell count (WBC), hemoglobin (Hb), hematocrit (HCT), platelet count, hemoglobin A1c (HbA1c), and proinflammatory markers: C-reactive protein (CRP) and partial thromboplastin time (PTT). These tests were conducted preoperatively and at 6 weeks. Patients were monitored for any adverse effect (eg, infection, stiffness) or laboratory abnormalities. The Data and Safety Monitoring Board (DSMB) monitored adverse events and provided evaluations of the safety-related events throughout the study for occurrence of any undesirable events (ie, significant changes in laboratory values and clinical, surgical, or rehabilitation results).
Patient-Oriented Outcome Measures
Preoperative medication consumption related to acetaminophen, nonsteroidal anti-inflammatory drugs (NSAIDS), and narcotics was documented as yes or no. Postoperatively, patients were asked to complete a pain diary using a visual analog scale (VAS)11,36 and a medication diary every day from day 1 to day 30 at a specific time. Postoperatively, type and number of pills taken was documented on the medication diary.
Shoulder-related measures consisted of the Short Western Ontario Rotator Cuff (WORC) Index,28 the American Shoulder and Elbow Surgeons (ASES) form,29 and the Constant-Murley Score (CMS).6 The ASES and ShortWORC were collected preoperatively and again at 6 weeks and 3 and 6 months after surgery. The CMS was not collected at 6 weeks as it was not feasible to perform the strength component at that time. The strength component of the CMS was measured using a simple tensiometer with the shoulder at 90° of elevation in the plane of the scapula and the elbow extended while the clinician pulled down on the tensiometer. The maximum pain-free force that the patient could resist for 5 seconds as the examiner pulled down on the device was measured. All measures have established reliability and validity for patients with RC disease.22,26–28,30
Randomization
Randomization was done through a central system, and groups were balanced for sex using a random permuted block. The operating surgeon who administered the PRP treatment was aware of the group allocation. Patients who met the exclusion criteria (eg, larger tears, concomitant pathology) were excluded after arthroscopic examination. Patients and assessors who performed the outcome scores, ROM, and strength measures were blinded to group allocation.
Surgical Procedures
This multicenter trial involved 3 orthopaedic surgeons. Arthroscopic inspection of the shoulder using standard portals was performed to assess intra-articular structures with the patient in either the lateral decubitus or beach-chair position according to the surgeon’s preference. The full-thickness tears were classified as small (<1 cm) and moderate (1-3 cm) based on the largest dimension.8 The tear edges and the bone at the lateral articular margin were debrided. Both articular- and bursal-sided tears of >50% tendon thickness were converted to full-thickness and repaired. Standard tendon-to-bone repair techniques were used depending on the tear morphology and mobility. Single-row repairs were performed in the majority of cases. Anterior acromioplasty was performed routinely, with resection of the lateral end of the clavicle if there was evidence of moderate or severe degenerative changes (Collin grades 3 and 4) in the acromioclavicular (AC) joint.
Application of the Platelet-Rich Plasma (PRP)
This study used the SmartPReP 2 system (Harvest Technologies Corp). The patient’s blood was drawn prior to the start of the procedure. The procedure pack was designed to process 54 mL of whole blood, which produced 7 mL of concentrated PRP. The processing disposable (PD) size of 60 mL was used following manufacturers’ instructions. Eight milliliters of anticoagulant citrate dextrose solution, solution A (ACD-A), was drawn into a 60-mL syringe, and after transferring 2 mL of this solution into the white port of the PD, 54 mL of venous blood was then drawn into the syringe. The total syringe volume was transferred into the red port of the PD. Anticoagulant solution (0.1 mL) was transferred into a process tube in addition to 1.7 mL of the processing solution. After 60 minutes of incubation, the PD was put into the SmartPReP 2 System and was spun in the centrifuge for 14 minutes, separating the blood into 3 fractions. The concentrated plasma was then resuspended with platelets and kept in a sterile red cup. The autologous thrombin solution of the process tube was poured into a clear cup. An applicator system was used to deliver the platelet concentrate and thrombin solution. Seven milliliters of the autologous platelet concentrate (APC) was drawn with a 10-mL syringe and 2.5 mL of the thrombin solution was drawn with a 3-mL syringe. Both syringes were then attached to the applicator tip. A spinal needle was used to access the surgical site.
Using the lateral portal, the applicator for the PRP was positioned in between the bone and the repaired RC. The inflow was then closed and the subacromial space was drained via the outflow cannula. A fine suction tip set at very low pressure was used to carefully drain the subacromial space and clear the site of the cuff repair of blood and fluid. The PRP required approximately 60 seconds in the connector to transform into a gel before injected to the site of repair. The PRP was then slowly injected to build a thick layer of adherent clot filling the tendon-bone interface beneath the repaired tendon guided by direct vision. Perioperative pain management included an interscalene nerve block for the first 12 to 18 hours followed by oxycodone/acetaminophen (1-2 tablets every 4 hours) as needed. Other medications taken as needed were documented in the diary.
Postoperative Rehabilitation
A shoulder immobilizer, removed for shower and exercise, was applied for 4 weeks. Early active-assisted forward flexion and pendulum motions were initiated on day 1 postoperatively. At 4 weeks, external rotation was added. Internal rotation and submaximal isometric exercises started at 6 weeks. Strength exercises against resistance were delayed until 12 weeks postsurgery. A standardized rehabilitation program was given to patients to be performed under supervision of their physical therapist.
Healing and Retear Status
For assessment of tendon healing, anatomic evaluation of the cuff repair was conducted with use of MRI prior to and at 6 months after surgery by a senior musculoskeletal-trained radiologist who was blinded to group allocation. Patients were imaged in the supine position with their arm rested in neutral. Postoperative MRI was performed on a 1.5-T system (General Electric Medical Systems) using a 15-platform and dedicated GE shoulder surface coil. Field of view was 13 cm with T1-weighted sagittal images (600/min), sagittal proton density fast spin-echo with fat saturation (2000/22.7), axial proton density fast spin-echo with fat saturation (2000/22.7), coronal oblique T2-weighted fast spin-echo (2800/83.2), and coronal oblique proton density fast spin-echo (2000/34) (slice thickness, 3.0-4.0 mm with 0.5- to 1-mm gap; matrix, 288-320 × 192-244). Flow compensation and spatial presaturation were routinely employed.
The extent of any retear was determined in the coronal plane using a system described by Thomazeau et al.35 The postsurgical tear status was categorized as (1) none, (2) partial retear, (3) stage I retear (tear edge was lying over the greater tuberosity [usually <1 cm in greatest diameter]), (4) stage II retear (tear exposed the humeral head but did not retract to the glenoid articular surface [1-3 cm in greatest diameter]), and (5) stage III (3-5 cm in greatest diameter). Biceps tendon status was categorized as normal, tendonitis, subluxated, dislocated, partial tear, or full tear.
Fatty infiltration was classified on the T1 sagittal image using the Goutallier fatty index on the most lateral oblique image in which the spine is seen in contact with the scapular body (Y-shaped view).12 According to this classification, stage 0 indicates a muscle with no fat, stage 1 indicates a muscle containing some fatty streaks, stage 2 indicates more muscle than fat, stage 3 indicates as much fat as muscle, and stage 4 indicates more fat than muscle.
Statistical Analysis
The sample size for a comparative longitudinal study design at 2 time points9 was based on the VAS pain score:
where N is the number of subjects in each of 2 groups, σ is the pooled standard deviation, effect size4 = (μ1 − μ2)/σ = 0.2, 0.5, and 0.8 for “small,” “medium,” and “large” effects, μ1 − μ2 is the difference in means of the 2 groups, n is the number of time points, and ρ is the correlation of the repeated measures. Previously collected data of patients with RC tear who had undergone arthroscopic repair had a correlation of 0.12 between preoperative and 6-month postoperative pain scores, and with a medium effect size of 0.5,4 35 subjects were required in each group. This value was inflated by 15% to compensate for patients who were lost to follow-up. Therefore, a minimum of 80 patients were required to answer the primary research question.
Group differences in preoperative characteristics (side, mechanism of injury, and symptom characteristics) and MRI findings were explored using the Fisher exact or chi-square tests for categorical data as appropriate. The Cumulative Illness Rating Scale was examined with independent t tests. SAS Proc Mixed repeated-measures analysis of variance (ANOVA) was used to examine between-group differences and overall change in pain and medication use for 30 consecutive days and in disability scores and ROM for 6 months. The pain scores were further examined for clinically meaningful difference if they exceeded 1.4 points, considered as the minimal clinically important difference (MCID) reported for patients with RC disease by Tashjian et al.34 Preoperative and 6-week postoperative laboratory findings were examined with independent t tests. Statistical analysis was performed using SAS version 9.1.3 (SAS Institute). Statistical results are reported using 2-tailed P values, with significance set at P < .05.
Results
A total of 92 patients met the eligibility criteria, of whom 10 patients were excluded intraoperatively (2 had a large RC tear, 5 had no RC tear, 2 had a subscapularis repair, 1 had intubation difficulty that led to cancelation of surgery). Therefore, 82 patients were included (41 females, 41 males; mean age ± SD, 59 ± 8 years; range, 38-76 years), of whom 41 patients received PRP. Seventy-three patients had a full-thickness tear and 9 patients (4 PRP, 5 controls) had a high-grade partial-thickness tear that required a repair. Figure 1 shows the progress of the subjects through the phases of the study. Of these 82 patients, all completed their 6-week assessment. One study patient (72-year-old woman) sustained a displaced 3-part fracture of the proximal humerus of the involved side as a result of an unrelated fall after the 6-week appointment and was excluded from further assessment.
Figure 1.
Flowchart of participants. FTRCT, full-thickness rotator cuff tear; MRI, magnetic resonance imaging; PRP, platelet-rich plasma.
Table 1 shows the subgroup analysis of pre- and intraoperative characteristics. There were no statistically significant differences between groups in terms of age (PRP, 59 years; control, 59 years), sex (PRP, 21 females/20 males; control, 20 females/21 males), symptom duration (25 vs 29 months), medication use, type of tear (full- vs partial-thickness), or medical comorbidity (2.62 vs 3.02). Preoperative pain and disability scores (ASES, ShortWORC, and CMS) were also comparable. Of 3 patients with active compensation claims related to the shoulder, 2 received PRP and 1 did not. Although the PRP group had 4 patients with stage 1 supraspinatus fatty infiltration compared with only 1 patient in the control group, this was not statistically significantly different.
TABLE 1.
Subject Demographicsa
| Variable | PRP Group (n = 41) | Control Group (n = 41) |
|---|---|---|
| Age, y, mean ± SD | 59 ± 8 | 59 ± 8 |
| Sex, female/male, n | 21/20 | 20/21 |
| Symptom duration, mo, mean ± SD | 25 ± 18 | 29 ± 37 |
| Affected side, right/left, n | 26/15 | 25/16 |
| Comorbidity (0-52), mean ± SDb | 2.7 ± 2 | 3.0 ± 2 |
| Medication use | ||
| Painkillers | 12 (29) | 15 (36) |
| NSAIDs | 12 (29) | 9 (22) |
| Narcotics | 4 (10) | 4 (10) |
| Symptoms characteristics | ||
| Pain on movement | 38 (9) | 38 (9) |
| Night pain | 33 (8) | 31 (8) |
| Weakness | 31 (8) | 34 (8) |
| Stiffness | 26 (6) | 26 (6) |
| Mechanism of injury | ||
| Insidious | 5 (1) | 3 (<1) |
| Repetitive activities | 13 (32) | 16 (39) |
| Fall | 8 (20) | 7 (20) |
| Traumatic | 13 (32) | 7 (20) |
| Direct blow | 2 (<1) | 8 (20) |
| Rotator cuff pathology | ||
| Full-thickness tear | 37 (90) | 36 (88) |
| Partial-thickness tear | 4 (10) | 5 (12) |
| Biceps pathology | ||
| None | 21 (49) | 21 (51) |
| Partial tear | 18 (44) | 19 (46) |
| Full rupture | 2 (5) | 1 (2) |
| Associated surgeries | ||
| Acromioplasty | 41 (100) | 41 (100) |
| Lateral clavicle resection | 30 (73) | 25 (61) |
| Biceps tenodesis | 2 (5) | 2 (5) |
| Type of fixation | ||
| Single-row | 39 (95) | 36 (87) |
| Double-row | 2 (5) | 5 (13) |
| Number of anchors | ||
| 1 | 32 (78) | 27 (66) |
| 2 | 9 (22) | 14 (34) |
aData are presented as n (%) unless otherwise indicated. No statistically significant differences were detected between groups. NSAIDs, nonsteroidal anti-inflammatory drugs; PRP, platelet-rich plasma.
bBased on the Cumulative Illness Rating Scale7 (0 = no impairment, 52 = highest level of possible impairment).
Adverse Events
No adverse events related to PRP application were noted during the study. However, 2 patients had intraoperative complications: 1 study patient (72-year-old woman) had decreased oxygen saturation possibly related to pneumonia. This patient developed shoulder stiffness after surgery. The second patient was in the control group (57-year-old man) and developed pulmonary edema. Both patients required overnight observation and were discharged the next day. There was no surgical revision at the 1-year time frame.
Patient-Oriented Outcome Measures
Pain Scores
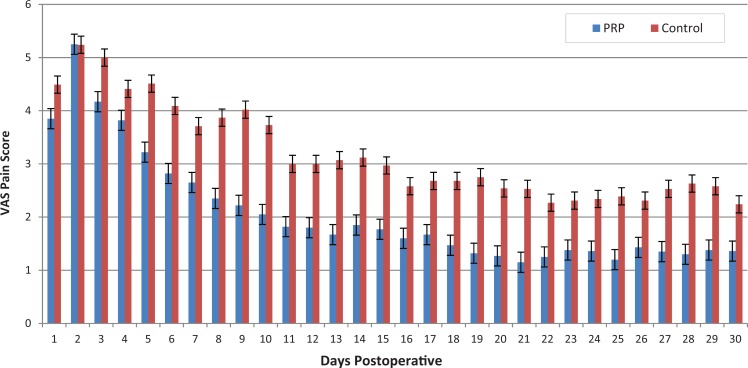
Figure 2 shows the change in VAS score over time. The repeated-measures ANOVA showed a significant improvement in pain level within the first 30 days (F = 34.60, P < .0001). There was a between-group difference in favor of the PRP group reporting statistically significantly less pain within this period (F = 6.61, P = .012). There was no time × group interaction (F = 1.35, P = .10). The difference in pain scores was clinically meaningful only on days 8 through 11, when it exceeded 1.4 points, considered as the MCID.34
Figure 2.
Daily comparison of group visual analog scale (VAS) pain scores (range, 0-10) for the first 30 days. Group differences (PRP vs control) were statistically and clinically significant from days 8 through 11. PRP, platelet-rich plasma.
Medication Use
Both groups had a statistically significant reduction of narcotic and NSAID use over the 30-day period postoperatively. The PRP group took less acetaminophen over this period, but groups were similar in taking narcotics and NSAIDs, with no statistically significant differences between groups (Table 2).
TABLE 2.
Postoperative Medication Use (Days 1-30)a
| Variable | Between-Group Difference | Within-Group Change | Time × Group Interaction |
|---|---|---|---|
| Acetaminophen | F = 5.13, P = .026 | F = 1.11, P = .308 | F = 0.58, P = .97 |
| NSAIDs | F = 0.00, P = .98 | F = 1.57, P = .026 | F = 1.45, P = .06 |
| Narcotics | F = 3.28, P = .07 | F = 47.26, P < .0001 | F = 1.20, P = .21 |
aNSAIDs, nonsteroidal anti-inflammatory drugs.
Shoulder-Related Outcome Measures
Both groups showed a significant improvement in their disability scores over time. There were no statistically significant differences between groups in ShortWORC or ASES or CMS over time (Table 3).
TABLE 3.
Shoulder-Related Disability Scores and Clinical Examination Findingsa
| PRP Group | Control Group | Repeated-Measures ANOVA Statisticsb | |
|---|---|---|---|
| Disability scores | |||
| ASES | F 1 = 1.39, P = .24 | ||
| Preop | 57 ± 19 | 52 ± 19 | F 2 = 78.63, P < .0001 |
| Final postop | 86 ± 18 | 84 ± 18 | F 3 = 0.32, P = .81 |
| ShortWORC | F 1 = 1.83, P = .18 | ||
| Preop | 42 ± 19 | 35 ± 22 | F 2 = 103.01, P < .0001 |
| Final postop | 85 ± 21 | 79 ± 27 | F 3 = 0.12, P = .95 |
| CMS | F 1 = 0.30, P = .58 | ||
| Preop | 65 ± 19 | 61 ± 23 | F 2 = 112.66, P < .0001 |
| Final postop | 108 ± 19 | 103 ± 24 | F 3 = 0.22, P = .81 |
| Range of motion, deg | |||
| Flexion: active | F 1 = 1.39, P = .24 | ||
| Preop | 143 ± 31 | 139 ± 33 | F 2 = 14.17, P < .0001 |
| Final postop | 170 ± 12 | 155 ± 24 | F 3 = 0.29, P = .75 |
| Flexion: passive | F 1 = 3.5, P = .0.06 | ||
| Preop | 158 ± 16 | 159 ± 19 | F 2 = 103.59, P < .0001 |
| Final postop | 164 ± 20 | 162 ± 18 | F 3 = 3.33, P = .02 |
| Abduction: active | F 1 = 1.45, P = .23 | ||
| Preop | 134 ± 37 | 129 ± 9 | F 2 = 15.50, P < .0001 |
| Final postop | 160 ± 24 | 150 ± 28 | F 3 = 0.15, P = .86 |
| Abduction: passive | F 1 = 0.50, P = .48 | ||
| Preop | 150 ± 28 | 150 ± 25 | F 2 = 12.11, P < .0001 |
| Final postop | 166 ± 18 | 157 ± 23 | F 3 = 1.34, P = .26 |
| External rotation: active | F 1 = 0.00, P = .95 | ||
| Preop | 54 ± 17 | 57 ± 13 | F 2 = 6.86, P = .001 |
| Final postop | 63 ± 16 | 67 ± 12 | F 3 = 0.76, P = .47 |
| External rotation: passive | F 1 = 0.32, P = .57 | ||
| Preop | 64 ± 16 | 62 ± 23 | F 2 = 62.25, P < .0001 |
| Final postop | 71 ± 65 | 69 ± 62 | F 3 = 1.46, P = .23 |
| Internal rotation: active | F 1 = 1.12, P = .29 | ||
| Preop | 39 ± 19 | 37 ± 23 | F 2 = 14.23, P < .0001 |
| Final postop | 56 ± 15 | 47 ± 18 | F 3 = 1.28, P = .28 |
| Internal rotation: passive | F 1 = 1.89, P = .17 | ||
| Preop | 52 ± 19 | 51 ± 22 | F 2 = 77.58, P < .0001 |
| Final postop | 66 ± 17 | 61 ± 16 | F 3 = 0.63, P = .53 |
aData are presented as mean ± SD. ANOVA, analysis of variance; ASES, American Shoulder and Elbow Surgeons; CMS, Constant-Murley score; postop, postoperative; preop, preoperative; PRP, platelet-rich plasma; ShortWORC, Short Western Ontario Rotator Cuff Index.
bF values provided for (1) between-group differences, (2) within-group differences, and (3) time × group interaction.
Clinical Examination (Range of Motion)
Both groups showed significant improvement in all directions of range of motion over time. However, no statistically significant differences were observed between groups. There was an interaction between passive flexion and group allocation, indicating that although there were no overall group differences, the pattern of change was different between groups, with the PRP group having better passive flexion at 6 weeks but not at other time points (Table 3).
Laboratory Tests
No group differences were observed in general blood tests or coagulation marker test results (P > .05), indicating that use of PRP did not introduce any detectable coagulation or inflammatory response at 6 weeks postoperatively (Table 4).
Table 4.
General Blood Test Resultsa
| Variable | PRP Group | Control Group | Statistics |
|---|---|---|---|
| WBC count, ×109/L | |||
| Preop | 6.5 ± 1.50 | 6.28 ± 1.84 | t = 0.62, P = .54 |
| 6 weeks postop | 6.8 ± 1.86 | 6.41 ± 1.72 | t = 0.97, P = .34 |
| Hemoglobin (Hb), g/L | |||
| Preop | 142.8 ± 10 | 143.3 ± 14 | t = 0.18, P = .86 |
| 6 weeks postop | 144.4 ± 9 | 143.2 ± 12 | t = 0.48, P = .63 |
| Hematocrit | |||
| Preop | 0.42 ± 0.03 | 0.43 ± 0.04 | t = 0.18, P = .86 |
| 6 weeks postop | 0.42 ± 0.03 | 0.43 ± 0.05 | t = 0.74, P = .46 |
| Platelet count, ×109/L | |||
| Preop | 231.5 ± 65 | 245 ± 61 | t = 0.96, P = .34 |
| 6 weeks postop | 235.1 ± 55 | 243.9 ± 65 | t = 0.63, P = .53 |
| A1c (HbA1c), % | |||
| Preop | 5.5 ± 0.03 | 5.5 ± 0.03 | t = 1.64, P = .10 |
| 6 weeks postop | 5.8 ± 0.07 | 5.7 ± 0.06 | t = 1.64, P = .10 |
| C-reactive protein | |||
| Preop | 2.35 ± 2.5 | 2.70 ± 2.96 | t = 1.21, P = .23 |
| 6 weeks postop | 2.51 ± 2.7 | 3.37 ± 3.4 | t = 0.53, P = .60 |
| Partial thromboplastin time, s | |||
| Preop | 30.65 ± 3 | 30.46 ± 2 | t = 0.31, P = .75 |
| 6 weeks postop | 31.21 ± 3 | 31.17 ± 2 | t = 0.06, P = .95 |
aData are presented as mean ± SD. Hb, hemoglobin; HbA1c, glycated hemoglobin; postop, postoperative; preop, preoperative; PRP, platelet-rich plasma; WBC, white blood cell.
Postsurgical Retear
MRIs of 74 patients were available for review (36 PRP, 38 control). Table 5 shows the retear rate in each group. Although the PRP group had more complete healing (23/36 vs 18/38), with a smaller number of partial- (8/36 vs 13/38) and full-thickness retears (5/36 vs 7/38), this was not statistically significant.
TABLE 5.
Pre- and Postoperative MRI Findingsa
| Variable | PRP Group | Control Group | Statistics |
|---|---|---|---|
| Retearb | FTE = 0.007, P = .44 | ||
| None | 23 (64) | 18 (47) | |
| Partial | 8 (22) | 13 (34) | |
| Full | 5 (14) | 7 (18) | |
| Stage I | 4 (11) | 4 (11) | |
| Stage II | 1 (3) | 3 (8) | |
| Stage III | 0 | 0 | |
| Fatty infiltrationc | FTE = 0.02, P = .22 | ||
| Preoperative | |||
| Stage 0 | 26 (72) | 29 (77) | |
| Stage 1 | 5 (14) | 7 (18) | |
| Stage 2 | 4 (11) | 1 (3) | |
| Stage 3 | 1 (3) | 0 | |
| Postoperative | FTE = 0.005, P = .08 | ||
| Stage 0 | 26 (72) | 33 (87) | |
| Stage 1 | 5 (15) | 5 (13) | |
| Stage 2 | 4 (12) | 0 | |
| Stage 3 | 1 (3) | 0 |
aData are presented as n (%). FTE, Fisher exact test; MRI, magnetic resonance imaging; PRP, platelet-rich plasma.
bClassified according to Thomazeau et al35: stage I tear = usually <1 cm in greatest diameter, stage II tear = 1-3 cm in greatest diameter, stage III tear = >5 cm in greatest diameter.
cAccording to Goutallier fatty index: stage 0 = no fat, stage 1 = some fatty streaks, stage 2 = more muscle than fat, stage 3 = as much fat as muscle, and stage 4 = more fat than muscle.
Fatty infiltration of muscle remained unchanged in all those who had a preoperative stage 2 (more muscle than fat) or stage 3 (as much muscle as fat) score regardless of the treatment they received (PRP vs no PRP). Therefore, use of PRP did not improve the fatty infiltration status. However, 3 patients in the control group improved from stage 1 or 2 to stage 0. Fatty infiltration in 4 patients in the study group with stage 1 did not improve (Table 5).
Discussion
The present study confirms the findings of previous studies on use of PRP injection in patients with small- to medium-sized RC tears2,19,39 with respect to lack of differences in disability, retear rate, and fatty infiltration. The only clinically significant difference was the PRP group having less pain from day 8 through day 11.
In a similar study to ours, Castricini et al2 examined 88 patients with small- to medium-sized RC tears randomized to arthroscopic double-row repair with or without autologous platelet-rich fibrin matrix (PRFM) augmentation. At 16 months’ follow-up, no significant difference was found in the CMS or MRI findings, which was based on tendon thickness, coverage of greater tuberosity, and intensity of signal. Their study did not support the use of PRFM to improve disability or healing of the small and medium RC tears.
Of 88 participants, MRI results were available for 78 (38/40). In 5 patients, there was MRI evidence of a retear: 4 patients in the control group (10.5%) and 1 (2.5%) in the PRP group (P = .07). None of the patients required further treatment.
In another prospective, randomized, double-blinded study of 54 patients by Malavolta et al,19 full-thickness supraspinatus tears of less than 3 cm were subjected to arthroscopic single-row repair. The outcomes were assessed by the University of California at Los Angeles (UCLA) scores, CMS, and VAS for pain. MRI was performed before surgery and repeated at 3, 6, 12, and 24 months after surgery. Both groups of patients exhibited significant clinical improvement (P < .001). Similar to our study, the investigators did not find any group differences in the UCLA, CMS, and VAS scores at 2 years postoperatively. The only difference was in UCLA scores at 12 months (30 in the control group and 32 in the PRP group; P = .046), which, if adjusted for multiple comparisons, would not be considered significant. In their study, the control group exhibited 1 case of a complete retear and 4 partial retears, and the PRP group exhibited 2 cases of partial retears. Although, the incidence of partial retears was 2-fold greater in the control group at the 6- and 12-month assessments compared with the PRP group, this was not statistically significant (P = .42). In our study, we had a greater incidence of partial and full retears (22% partial and 14% full retear in PRP vs 34% partial and 18% full retear in control), which may potentially be related to the older patients included in our study (59 years vs 54/55 years). We did find a trend toward a lower retear rate in the PRP group, which could be related to the impact of active growth factors being released at the repair site. However, this difference was not statistically significant.
Similar to our study, Weber et al,39 who randomized 60 patients with RC tears (mean size, 1.77 and 1.72 cm in the PRP and control groups, respectively) to PRFM reported that PRFM did not significantly improve clinical outcomes based on UCLA score at 12 months postoperative. Their retear rate was not statistically significant between groups (12/29 [43%] in the control vs 7/30 [30%] in the PRFM group).
The information on perioperative pain is more controversial. We found that pain was statistically significantly less during the first 30 days in the study group, being clinically different on days 8 through 11. Malavolta et al,19 who assessed postoperative pain on days 1 and 7 and at 3, 6, and 12 months, revealed no statistically significant between-group differences. Weber et al39 reported no group differences in perioperative pain on arrival to the recovery room or at 1 hour after surgery, which is similar to our findings of no group differences in VAS for at least 4 days after surgery. In the study by Randelli et al,25 who included small to massive tears, the PRP group showed a statistically significant reduction of VAS score as soon as day 3 after surgery (P = .04), which is more in line with our findings. Using a standardized pain diary is expected to have provided a more consistent picture of pain fluctuation during the first month after surgery, and overall, it appears that the PRP does not improve pain immediately on postoperative day 1 but has the potential to provide a short-term superior pain reduction.
The main growth factors in PRP are transforming growth factor β1 (TGFβ1), platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), hepatocyte growth factor (HGF), and insulin-like growth factor 1 (IGF1). The platelets are believed to synthesize and secrete additional factors for 7 to 10 days after the initial release of growth factors, which coincides with the inflammatory and repair phases of tendon healing.13,21 The role of autologous growth factors in pain reduction through increased tendon cell proliferation, collage synthesis, or vascularization within the first few days after surgery should be considered.13,21,42
Consistent with previous work, our results suggest that PRP augmentation for small- to medium-sized RC tears does not show significant improvement in patient-oriented outcome measures or repair integrity when compared with a conventional repair. At short-term follow-up, patients managed with PRP-augmented repair showed better control of postoperative pain. The subjective improvement in pain and disability after repair of the RC tendons is impressively high despite failure to achieve a full repair and the variable rate of structural healing.5,17,20,24,31 In light of no between-group differences in flexion, the interaction between passive flexion and group allocation also does not appear to be clinically significant as it does not correlate with active flexion or other range of motion directions. In a systematic review of studies published between 2010 and 2014 to assess effectiveness, safety, and cost-effectiveness of PRP augmented repair, Vavken et al37 reported lower retear rate in favor of PRP use in the subsample of patients with small- to medium-sized tears (P = .038). These findings were predominantly based on 3 studies that included small to massive tears,1,15,25 of which only 1 study was a randomized controlled study.25 Chahal et al3 added another randomized controlled trial and calculated the lower risk of retear in favor of PRP (P = .006). Of interest, Vavken et al37 conducted a unique cost analysis and reported that the use of PRP was not cost-effective for small- and medium-sized tears in spite of fewer retears. The cost-effectiveness analysis was based on an incremental ratio of US$127,893 per quality-adjusted life year gained, assuming a 5% revision rate. It is of value to note that small defects detected on MRI postoperatively do not predictably correlate with clinical outcomes5,17,20,24,31 and are not always subject to a revision surgery.
Intraoperative PRP injections do not change outcomes or retear rate, and the short-lived improved perioperative pain (days 8-11) does not justify the cost, increased chance of infection, or added operative time and should not be encouraged in small- to medium-sized tears. Identifying treatments and techniques that improve the structural integrity of RC repairs remains a clinical challenge and deserves ongoing investigation.
A limitation of the present study is that the number of platelets and levels of growth factors injected at the time of surgery were not measured. Future studies that incorporate this information will help to better determine the amount of the growth factors released within the RC repair. There was also no intertester reliability on rating of the retears due to involvement of only 1 musculoskeletal-trained radiologist. Our follow-up period was relatively short. The final follow-up with subjective outcomes and MRI examination was at 6 months after surgery, and rate of revision was examined at 1 year after completion of study. Considering the differences in subjective measures equalized at 6 months and the similarity of our results with previous studies, longer follow-up would not have had a significant impact on our conclusions. This study was conducted at academic centers with subspecialty trained musculoskeletal surgeons and radiologists, which limits the generalizability of the results.
Conclusion
The PRP biological augmentation for repair of small- to medium-sized RC tears has a short-term effect on perioperative pain, without any significant impact on patient-oriented outcome measures or structural integrity of the repair compared with a control group.
Footnotes
One or more of the authors has declared the following potential conflict of interest or source of funding: This study was funded by a grant from the Physicians Services Incorporated foundation.
References
- 1. Barber FA, Hrnack SA, Snyder SJ, Hapa O. Rotator cuff repair healing influenced by platelet-rich plasma construct augmentation. Arthroscopy. 2011;27:1029–1035. [DOI] [PubMed] [Google Scholar]
- 2. Castricini R, Longo UG, De Benedetto M, et al. Platelet-rich plasma augmentation for arthroscopic rotator cuff repair: a randomized controlled trial. Am J Sports Med. 2011;39:258–265. [DOI] [PubMed] [Google Scholar]
- 3. Chahal J, Van Thiel GS, Mall N, et al. The role of platelet-rich plasma in arthroscopic rotator cuff repair: a systematic review with quantitative synthesis. Arthroscopy. 2012;28:1718–1727. [DOI] [PubMed] [Google Scholar]
- 4. Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed Hillsdale, NJ: Lawrence Earlbaum Associates; 1988. [Google Scholar]
- 5. Colliver J, Wang A, Joss B, et al. Early postoperative repair status after rotator cuff repair cannot be accurately classified using questionnaires of patient function and isokinetic strength evaluation. J Shoulder Elbow Surg. 2016;25:536–542. [DOI] [PubMed] [Google Scholar]
- 6. Constant CR. An evaluation of the Constant-Murley shoulder assessment. J Bone Joint Surg Br. 1997;79:695–696. [PubMed] [Google Scholar]
- 7. de Groot V, Beckerman H, Lankhorst GJ, Bouter LM. How to measure comorbidity. A critical review of available methods. J Clin Epidemiol. 2003;56:221–229. [DOI] [PubMed] [Google Scholar]
- 8. DeOrio JK, Cofield RH. Results of a second attempt at surgical repair of a failed initial rotator-cuff repair. J Bone Joint Surg Am. 1984;66:563–567. [PubMed] [Google Scholar]
- 9. Diggle P, Heagert P, Liang KY, Zegar SL. Analysis of Longitudinal Data. 2nd ed New York, NY: Oxford University Press; 2002. [Google Scholar]
- 10. Eppley BL, Woodell JE, Higgins J. Platelet quantification and growth factor analysis from platelet-rich plasma: implications for wound healing. Plast Reconstr Surg. 2004;114:1502–1508. [DOI] [PubMed] [Google Scholar]
- 11. Gallagher EJ, Liebman M, Bijur PE. Prospective validation of clinically important changes in pain severity measured on a visual analog scale. Ann Emerg Med. 2001;38:633–638. [DOI] [PubMed] [Google Scholar]
- 12. Goutallier D, Postel JM, Bernageau J, Lavau L, Voisin MC. Fatty muscle degeneration in cuff ruptures. Pre- and postoperative evaluation by CT scan. Clin Orthop Relat Res. 1994;304:78–83. [PubMed] [Google Scholar]
- 13. Gulotta LV, Rodeo SA. Growth factors for rotator cuff repair. Clin Sports Med. 2009;28:13–23. [DOI] [PubMed] [Google Scholar]
- 14. Huijsmans PE, Pritchard MP, Berghs BM, van Rooyen KS, Wallace AL, de Beer JF. Arthroscopic rotator cuff repair with double-row fixation. J Bone Joint Surg Am. 2007;89:1248–1257. [DOI] [PubMed] [Google Scholar]
- 15. Jo CH, Kim JE, Yoon KS, et al. Does platelet-rich plasma accelerate recovery after rotator cuff repair? A prospective cohort study. Am J Sports Med. 2011;39:2082–2090. [DOI] [PubMed] [Google Scholar]
- 16. Lafosse L, Brozska R, Toussaint B, Gobezie R. The outcome and structural integrity of arthroscopic rotator cuff repair with use of the double-row suture anchor technique. J Bone Joint Surg Am. 2007;89:1533–1541. [DOI] [PubMed] [Google Scholar]
- 17. Lenart BA, Martens KA, Kearns KA, Gillespie RJ, Zoga AC, Williams GR. Treatment of massive and recurrent rotator cuff tears augmented with a poly-l-lactide graft, a preliminary study. J Shoulder Elbow Surg. 2015;24:915–921. [DOI] [PubMed] [Google Scholar]
- 18. Ma HL, Chiang ER, Wu HT, et al. Clinical outcome and imaging of arthroscopic single-row and double-row rotator cuff repair: a prospective randomized trial. Arthroscopy. 2012;28:16–24. [DOI] [PubMed] [Google Scholar]
- 19. Malavolta EA, Gracitelli ME, Ferreira Neto AA, Assuncao JH, Bordalo-Rodrigues M, de Camargo OP. Platelet-rich plasma in rotator cuff repair: a prospective randomized study. Am J Sports Med. 2014;42:2446–2454. [DOI] [PubMed] [Google Scholar]
- 20. McElvany MD, McGoldrick E, Gee AO, Neradilek MB, Matsen FA., 3rd Rotator cuff repair: published evidence on factors associated with repair integrity and clinical outcome. Am J Sports Med. 2015;43:491–500. [DOI] [PubMed] [Google Scholar]
- 21. Mei-Dan O, Carmont MR. The role of platelet-rich plasma in rotator cuff repair. Sports Med Arthrosc. 2011;19:244–250. [DOI] [PubMed] [Google Scholar]
- 22. Michener LA, McClure PW, Sennett BJ. American Shoulder and Elbow Surgeons Standardized Shoulder Assessment Form, patient self-report section: reliability, validity, and responsiveness. J Shoulder Elbow Surg. 2002;11:587–594. [DOI] [PubMed] [Google Scholar]
- 23. Molloy T, Wang Y, Murrell G. The roles of growth factors in tendon and ligament healing. Sports Med. 2003;33:381–394. [DOI] [PubMed] [Google Scholar]
- 24. Proctor CS. Long-term successful arthroscopic repair of large and massive rotator cuff tears with a functional and degradable reinforcement device. J Shoulder Elbow Surg. 2014;23:1508–1513. [DOI] [PubMed] [Google Scholar]
- 25. Randelli P, Arrigoni P, Ragone V, Aliprandi A, Cabitza P. Platelet rich plasma in arthroscopic rotator cuff repair: a prospective RCT study, 2-year follow-up. J Shoulder Elbow Surg. 2011;20:518–528. [DOI] [PubMed] [Google Scholar]
- 26. Razmjou H, Bean A, MacDermid JC, van Osnabrugge V, Travers N, Holtby R. Convergent validity of the Constant-Murley outcome measure in patients with rotator cuff disease. Physiother Can. 2008;60:72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Razmjou H, Bean A, van Osnabrugge V, MacDermid JC, Holtby R. Cross-sectional and longitudinal construct validity of two rotator cuff disease-specific outcome measures. BMC Musculoskelet Disord. 2006;7:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Razmjou H, Stratford PW, Holtby R. A shortened version of the Western Ontario Rotator Cuff Disability Index: development and measurement properties. Physiother Can. 2012;64:135–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Richards RR, An K, Bigliani LU, et al. A standardized method for the assessment of shoulder function. J Shoulder Elbow Surg. 1994;3:347–352. [DOI] [PubMed] [Google Scholar]
- 30. Roy JS, MacDermid JC, Woodhouse LJ. A systematic review of the psychometric properties of the Constant-Murley score. J Shoulder Elbow Surg. 2010;19:157–164. [DOI] [PubMed] [Google Scholar]
- 31. Russell RD, Knight JR, Mulligan E, Khazzam MS. Structural integrity after rotator cuff repair does not correlate with patient function and pain: a meta-analysis. J Bone Joint Surg Am. 2014;96:265–271. [DOI] [PubMed] [Google Scholar]
- 32. Saltzman BM, Jain A, Campbell KA, et al. Does the use of platelet-rich plasma at the time of surgery improve clinical outcomes in arthroscopic rotator cuff repair when compared with control cohorts? A systematic review of meta-analyses. Arthroscopy. 2016;32:906–918. [DOI] [PubMed] [Google Scholar]
- 33. Sugaya H, Maeda K, Matsuki K, Moriishi J. Repair integrity and functional outcome after arthroscopic double-row rotator cuff repair. A prospective outcome study. J Bone Joint Surg Am. 2007;89:953–960. [DOI] [PubMed] [Google Scholar]
- 34. Tashjian RZ, Deloach J, Porucznik CA, Powell AP. Minimal clinically important differences (MCID) and patient acceptable symptomatic state (PASS) for visual analog scales (VAS) measuring pain in patients treated for rotator cuff disease. J Shoulder Elbow Surg. 2009;18:927–932. [DOI] [PubMed] [Google Scholar]
- 35. Thomazeau H, Boukobza E, Morcet N, Chaperon J, Langlais F. Prediction of rotator cuff repair results by magnetic resonance imaging. Clin Orthop Relat Res. 1997;344:275–283. [PubMed] [Google Scholar]
- 36. Todd KH, Funk KG, Funk JP, Bonacci R. Clinical significance of reported changes in pain severity. Ann Emerg Med. 1996;27:485–489. [DOI] [PubMed] [Google Scholar]
- 37. Vavken P, Sadoghi P, Palmer M, et al. Platelet-rich plasma reduces retear rates after arthroscopic repair of small- and medium-sized rotator cuff tears but is not cost-effective. Am J Sports Med. 2015;43:3071–3076. [DOI] [PubMed] [Google Scholar]
- 38. Virchenko O, Aspenberg P. How can one platelet injection after tendon injury lead to a stronger tendon after 4 weeks? Interplay between early regeneration and mechanical stimulation. Acta Orthop. 2006;77:806–812. [DOI] [PubMed] [Google Scholar]
- 39. Weber SC, Kauffman JI, Parise C, Weber SJ, Katz SD. Platelet-rich fibrin matrix in the management of arthroscopic repair of the rotator cuff: a prospective, randomized, double-blinded study. Am J Sports Med. 2013;41:263–270. [DOI] [PubMed] [Google Scholar]
- 40. Yang J, Sun Y, Xu P, Cheng B. Can patients get better clinical outcomes by using PRP in rotator cuff repair: a meta-analysis of randomized controlled trials [published online October 16, 2015]. J Sports Med Phys Fitness. [PubMed] [Google Scholar]
- 41. Zhao JG, Zhao L, Jiang YX, Wang ZL, Wang J, Zhang P. Platelet-rich plasma in arthroscopic rotator cuff repair: a meta-analysis of randomized controlled trials. Arthroscopy. 2015;31:125–135. [DOI] [PubMed] [Google Scholar]
- 42. Zumstein MA, Rumian A, Lesbats V, Schaer M, Boileau P. Increased vascularization during early healing after biologic augmentation in repair of chronic rotator cuff tears using autologous leukocyte- and platelet-rich fibrin (L-PRF): a prospective randomized controlled pilot trial. J Shoulder Elbow Surg. 2014;23:3–12. [DOI] [PubMed] [Google Scholar]