Abstract
The layer-by-layer (LbL) technique was introduced in the early 90s by Profs Moehwald, Lvov and Decher. Since then, it has undergone a series of technological developments, making it possible to engineer various theranostic platforms such as films and capsules, with precise control at the nanometer and micrometer scales. This Research News article highlights recent progress in the applications of LbL assemblies in the field of cancer therapy, diagnosis and fundamental biology study. The potentials of LbL-based systems as drug carriers are discussed, especially with regard to the engineering of innovative stimuli-responsive systems, and their advantageous multifunctionality in the development of new therapeutic tools. Then, the diagnostic functions of LbL assemblies are illustrated for detection and capture of rare cancer cells. Finally, LbL mimicking extracellular environments demonstrate the emerging potential for the study of cancer cell behaviors in vitro. We conclude by highlighting the advantages of LbL systems, important challenges that need to be overcome, and future perspectives in clinical practice.
Keywords: layer-by-layer, cancer, drug delivery, diagnosis, cellular behaviors
Introduction
Layer-by-layer (LbL) assembly is a simple method that makes it possible to design multilayer architectures with nanometer precision. The technique is based on the alternating adsorption of synthetic and natural polyelectrolytes and other components, such as RNA and DNA, or nanoparticles on surfaces.[1] It was introduced in the 90s by Decher et al.,[2] and has triggered a large number of fundamental studies aiming to understand and control the mechanisms of film growth in different physical and chemical conditions. The technologies used to assemble LbL systems form five distinct categories: immersive, spin, spray, electromagnetic, and fluidic assembly.[3] According to Caruso and coworkers,[3] two main themes are identified for current developments in assembly technologies: The first is the move away from random diffusion-driven kinetics for layer deposition, and the second is the advancement from manual assembly toward automated systems. The simplicity, versatility, and nanoscale control of LbL assembly has made it one of the most widely used technologies for biomedical applications in diagnosis, the coatings of medical implants, tissue engineering and drug delivery.[4–7]
In the field of cancer diagnosis and therapy, polyelectrolyte microcapsules and LbL self-assembled nanoshells have emerged as carriers that are an attractive means of obtaining advanced functionalities in drug delivery, given their versatility and controlled structures.[8, 9] Advantageously, LbL films can coat a wide variety of nanometer- to micrometer-sized objects, including nanoparticles, lipid vesicles and microcapsules.[4] This makes it possible to take advantage of the nano/micro-object, particularly the possibility for loading a drug “in the bulk”, as well as of its surface, which can be coated with a LbL film. In this context, LbL-based hollow lipid/polymeric vesicles and microcapsules are emerging as a new potential therapeutic tool, consisting of an aqueous cavity surrounded by a shell.
This edition of Research News highlights progress made in LbL assemblies in cancer research in the past 4 years. We will focus on the potentials of LbL drug delivery systems for efficient cancer therapy, the development of stimuli-responsive systems and multifunctional LbL platforms. We also highlight its applications for cancer diagnosis and capture of rare cancer cells, as well as on the engineering of microenvironments to study cancer cell behaviors in vitro.
1. The potentials of LbL technique on the fabrication of drug carriers
1.1. Delivering therapeutic agents
Numerous challenges hamper effective drug delivery in cancer treatment, such as the low drug concentrations at tumor sites due to their limited penetration through the tumor tissues,[10] and serious side-effects by uncontrolled distribution in the body.[11] The LbL technique makes it possible to precisely control various parameters, such as film architecture,[2, 12] thickness, chemistry, and mechanical properties,[13, 14] as well as to show the potential for controlling both the amount of cancer drug entrapped - by varying the number of layers deposited[15, 16] - and its subsequent release.[17, 18]
A range of hydrophilic drugs, including small peptides, nucleic acids and small interferent RNA (siRNA) can be loaded into the films, either by “pre-loading” or “post-loading” as these components are hydrophilic. For hydrophobic drugs, several strategies have been developed in order to trap them inside the nanoarchitectures. The first strategy consists in using a hydrophobic carrier, such as an oil phase containing the drug,[19] polymeric micelles[20] or a hydrophobically-modified polyelectrolyte.[21] For instance, the anti-cancerous drug paclitaxel (PTX) was trapped in the nanoshell using hyaluronan (HA) modified with long alkyl chains assembled with quaternized chitosan derivatives as polycations.[21] Using this method, PTX-loaded LbL microcapsules were shown to release the drug slowly under physiological conditions without initial burst. The presence of an enzyme, hyaluronidase, accelerated the release of PTX by deconstructing the film.[16] The second strategy consists in using a nano- or mesoscale carrier for the drug, such as a liposomes[22] or mesoporous silica templates.[23]
1.2. Targeting cancer cells
A major objective for cancer drug delivery is to both specifically target cancer cells – and ideally not the non-cancerous cells – as well as to deliver the drugs in a timely manner. Surface targeting relies on the over-expression of different receptors at the cell surface, such as integrins, folate receptors[24] and CD44 glycoproteins.[25] Antibodies and specific ligands have also been widely used, depending on the cells of interest. For instance, Caruso et al.[26] demonstrated the specific binding and uptake of A33 antigen-targeted LbL coated particles to colorectal cancer cells. Galactose-functioned LbL hollow microcapsules may specifically target hepatocytes expressing asialoglycoprotein receptors.[27]
1.3. Improving the stability of LbL carriers
The stability of LbL carriers under physiological conditions is also an important issue, especially their accumulation in the organs of the reticulo-endothelial system (e.g. the liver and spleen) after administration. Grafting or adding long hydrophilic chains at the film surface may provide stealth properties. Cheng et al.[28] proved that nanoparticles coated with LbL films can be further conjugated with branched poly(ethylene glycol) (PEG), thus improving stability in a physiological environment (including saline, cell medium, and serum) for several days. When injected intravenously, they exhibited stealth-like behavior with a long blood circulation half-life of ~20h. In addition, Poon et al.[29] showed that the in vivo stability of LbL assembled nanoparticles was improved by increasing the number of layers deposited. They showed that the circulation time was extended by adding HA as a hydrophilic outer layer, with the blood elimination half-life being ~9 h with low levels of accumulation in the liver (~10-15% recovered fluorescence/g).
1.4. Co-delivering two therapeutic agents
LbL hollow capsules, which are made of a core and a shell, have the potential to co-deliver drugs by loading at least two kinds of drug into their cavity and multilayer shells.
Delivering two drugs
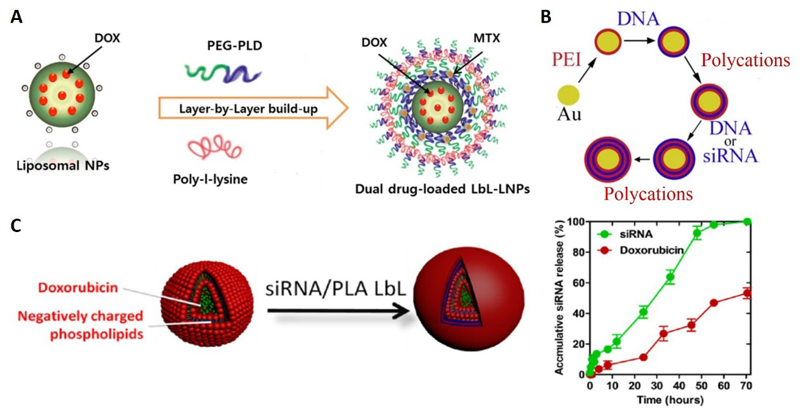
Ramasamy et al.[30] developed LbL liposomal nanoparticles combining the advantages of the LbL technique with those of liposomes. The particles were prepared with alternating layers of poly(ethylene glycol)-block-poly(L-aspartic acid) (PEG-b-PLD) and poly-L-lysine (PLL). Doxorubicin (DOX) was incorporated into the core and mitoxantrone (MTX) was loaded into the nanoshell (Figure 1A). The pharmacokinetic study in male Sprague Dawley rats showed that the LbL nanoarchitecture significantly reduced the elimination rates of both drugs, and markedly extended their systemic circulation times.
Figure 1. Three examples of co-delivery systems based on the LbL technique.
(A) LbL liposomal nanoparticles (LbL-LNPs) manufactured for a dual-drug delivery system with doxorubicin (DOX) loaded into the core and mitoxantrone (MTX) on to the shell layers;[30] (B) LbL degradable polymer coatings on AuNPs for the simultaneous co-delivery of DNA and siRNA;[31] (C) The co-delivery of siRNA and doxorubicin using the modular DOX-liposome/PLA/siRNA/PLA/HA LbL nanoparticle platform (Left); Release profile of the two therapeutic components (Right).[25]
Delivering two genes
LbL depositing on a surface or a core with multiple layers of polyelectrolytes provides a simple way of simultaneously delivering large, negatively-charged molecules (such as DNA) and small, negatively-charged molecules (such as siRNA). Bishop et al.[31] reported an LbL platform for co-delivery of DNA and siRNA, with two types of biodegradable synthetic polymers as the outer layers deposited on to gold nanoparticles (AuNPs) (Figure 1B). The hybrid nanoparticles led to both exogenous DNA expression and siRNA-mediated knockdown of the green fluorescent protein (GFP) in GFP-positive breast cancer cells, with the knockdown efficacy greater than that of the commercially available Lipofectamine 2000.
Co-delivering drugs and genes
Co-delivering drugs and genes has synergistic advantages in cancer treatments. Ji’s group[32] prepared hydrophobic micelles loaded with DOX inside a ferrocene core and with polyethyleneimine (PEI) as the outer cationic shell. DNA was incorporated into the shells, to form positively-charged PEI-Fc–DOX–DNA nanocomplexes, several hundred nanometers in size. The nanocomplexes were used to construct LbL multilayers with negatively-charged dextran sulfate. Cancer cells were found to be killed by the burst release of DOX from the multilayers. Furthermore, the multilayers directly transfected cancer cells by simple contact with the film containing the GFP plasmid.
Hammond et al.[25] recently developed a combined drug-siRNA delivery platform using DOX-loaded negatively-charged phospholipid liposomes as the carrier and an siRNA-containing film as the shell. The film was made of poly-L-arginine (PLA) and siRNA against the multidrug resistance protein. The two components showed staggered release over 72 h with a faster release rate of siRNA from the film, compared to the drug from the liposome core (Figure 1C). They used this system for triple-negative breast cancer treatment and found a 4-fold increase in DOX efficacy in vitro as well as an 8-fold decrease in tumor volume in vivo compared to the saline control, with no observed toxicity.
2. Stimuli-responsive LbL drug delivery systems
The controlled-release drug-delivery systems play an important role in the treatment of diseases, to reduce doses of drug while achieiving the same therapeutic effect. Different strategies have thus been proposed to engineer stimuli-responsive LbL nanoshells, which are reviewed in,[33] with the aim of adjusting the permeability of the LbL and controlling the release of cargo. The stimuli can be classified as chemical (pH, solvent, electrochemistry), or physical (temperature, light, ultrasounds, magnetic fields, mechanical deformation). In past years, stimuli-responsive LbL systems have shown their potential in drug delivery, and selected examples are given here in the context of cancer therapy.
pH responsive
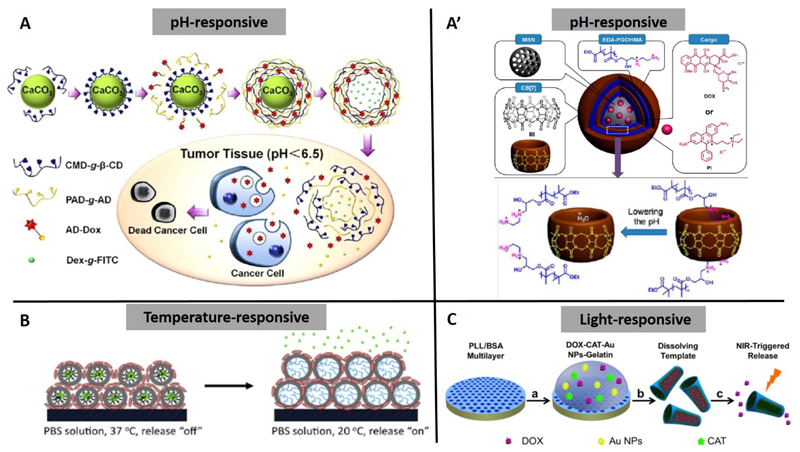
The change in pH from normal tissue (pH ~7.4) to cancerous tissue, which has an acidic pH of ~5, has motivated researchers to design pH-sensitive drug delivery vehicles for tumor-targeted delivery. Zhao’s study[27] showed that polyelectrolyte hollow microspheres made of modified chitosan (CS) and HA exhibited pH-sensitivity. The DOX release from the DOX-loaded (hollow microspheres at pH 7.4 was much slower than that at pH 5.0 at the same temperature. Luo et al.[34] prepared pH-responsive microcapsules via host-guest interaction between polyaldenhyde dextran-graft-adamantane (PAD-g-AD) and carboxymethyl dextran-graft-β-cyclodextran (CMD-g-β-CD). The pH-responsiveness was due to acid-sensitive hydrazine bonds in the PAD-g-AD. DOX was loaded into the wall of the microcapsules by conjugation with AD through hydrazones. At the tumor sites, the hydrazone bonds were disrupted and AD groups were removed, leading to the destruction of the microcapsules and the release of DOX (Figure 2A). Yang and Gao et al.[35, 36] reported an integrated nanosystem, with mesoporous silica nanoparticles coated with bis-aminated poly(glycerol methacrylate) and cucurbit[7]uril as the LbL components. The LbL nanoparticles possessed excellent biostability, negligible premature drug leakage at pH 7.4, and pH-responsive drug release performance in acidic endosomal compartments (Figure 2A’).
Figure 2. Diagrams of recent developments in stimuli-responsive LbL systems for drug delivery.
(A) pH-induced DOX release from adamantine-doxorubicin (AD-Dox) loaded LbL hollow microcapsules made by polyaldenhyde dextran-graft-adamantane (PAD-g-AD) and carboxymethyl dextran-graft-β-cyclodextran (CMD-g-β-CD);[34] (A’) The LbL film at the surface of mesoporous silica nanoparticles (MSNs) operated by lowering the pH value to regulate the release of cargo, i.e., DOX or propidium iodide (PI);[35] (B) Temperature-triggered “on-demand” DOX release from LbL films containing temperature-responsive micelles;[37] (C) Light-triggered drug release from (PLL/BSA)10-DOX-catalase (CAT)-AuNPs-gelatin rockets.[39]
Temperature responsive
Sukhishvili’s group[37] reported on pulsed, on-demand release of drugs from biocompatible hydrogen-bonded temperature-responsive LbL films containing block copolymer micelles. The temperature-responsive micelles made of poly(N-vinylpyrrolidone)-b-poly(N-isopropylacrylamide) provided the films with temperature-controlled swelling/deswelling transitions. At 37 °C, DOX was efficiently retained within the hydrophobic cores of micelles, whereas exposure to a lower temperature (20 °C) triggered fast DOX release (Figure 2B). The films were able to withstand at least 15 temperature-induced swelling/deswelling cycles and could be reused for repeated DOX loading and release.
Light responsive
Release can be triggered by light activation, such as near-infrared (NIR) light in the presence of AuNPs, which can adsorb electromagnetic energy and induce local heating.[38] Wu et al.[39] described a proof-of-concept application of a multilayer “rocket” as a vehicle for drug delivery and NIR light-controlled release induced by the film-loaded AuNPs. They first coated a porous template with poly(L-lysine)/bovine serum albumin (PLL/BSA) films and then added a temperature-sensitive gelatin hydrogel that was loaded with DOX and AuNPs. After the template dissolved, the rocket was formed and DOX could be released into the surrounding medium via light-induced melting of the gelatin hydrogel (Figure 2C).
3. Multifunctional therapeutic LbL systems
3.1. Combining phototherapy with gene therapy
Light-based treatments of cancer are complementary to drug-based treatments. Photothermal therapy (PTT) and photodynamic therapy (PDT) are currently in use. In PTT, a photothermal agent is used for selective local heating because tumor tissue is more sensitive to temperature increases than normal tissue.[40] In PDT, a series of photochemical reactions are triggered by photoactivated molecules or materials called photosensitizer drugs.[41] Jin et al.[42] successfully produced a type of LbL microcapsule for PTT therapy, by depositing graphene oxide on to the surface of PLA microcapsules containing AuNP using the LbL technique. In vivo therapeutic examinations showed that the NIR light completely ablated the tumor within 9 days in the presence of the microcapsules, and that tumor growth inhibition was 83.8%. LbL assembly has recently been further applied to different carriers, in order to develop multifunctional platforms combining phototherapy with gene therapy.
Shen et al.[43] used Au nanorods (NRs) as their platform. The surface of the AuNRs was modified with PEI, through LbL assembly, and complexed with siRNA against pyruvate kinase isoenzyme type M2 (PKM2), which is over-expressed in breast cancer cells. siRNAs were efficiently delivered to the breast cancer cells, resulting in subsequent PKM2 gene silencing. The cells were then irradiated with NIR light, causing heat-induced PTT anticancer activity. The combination of gene silencing and photothermal therapy resulted in effective inhibition of cell proliferation in vitro.
A strategy combining PDT and gene therapy was recently proposed for cancer by Wang et al.[44] Positively-charged nanoparticles made of NaGdF4:Yb,Er (named “upconversion” nanoparticles, UCNPs) were synthesized and loaded simultaneously with a photosensitizing molecule (Ce6) and siRNA against the polo-like kinase 1(Plk1) oncogene, which plays a role in DNA replication and is over-expressed in many types of cancer. Upon photon absorption, Ce6 underwent energy transition to the surrounding oxygen, and then generated singlet oxygen or other reactive oxygen species, which are cytotoxic for cancer cells. At the same time, silencing the Plk1 gene by siRNA induced significant cancer cell apoptosis. Interestingly, UCNP have also shown potential in the field of imaging, which would make it possible to use them in the future as a multifunctional theranostic nanoplatform.
3.2. Dual-targeting capacities
A successful delivery system is needed to complete the transfer and release of objects at the right moment and in the right place. The effective targeting function has thus attracted considerable attention in controlled drug release.
Du et al.[45] recently developed a dual targeting hollow microsphere combining magnetic and molecular targeting properties. The magnetic properties were provided by a composite LbL shell made of the polycation CS and the hybrid anion, citrate modified ferroferric oxide nanoparticles (Fe3O4−CA). The targeting property was made of folic acid, which was coupled to PEG. DOX was post-loaded into the hollow microsphere by simple diffusion. The combined magnetic and targeting properties for DOX delivery induced a pronounced cytotoxic effect in vitro on cancer cells. Dreaden et al.[46] designed a pH-responsive LbL polymer drug carrier that actively targeted tumors based on two independent mechanisms: pH-dependent cellular uptake at hypoxic tumor pH, and HA-directed targeting of cell-surface CD44 receptors, a well-characterized biomarker for breast and ovarian cancer. This dual-targeting system selectively bound CD44 and diminished cancer cell migration in vitro, while co-localizing with the CD44 receptor in vivo.
4. LbL technique for cancer diagnosis
Several techniques have been developed to detect and isolate cancer cells in human samples, including flow cytometry[47] and magnetic-activated cell sorting.[48, 49] However, most of these methods are high cost and time-consuming in either the experimental process or instrumentation.[50] LbL magnetic platforms have been engineered to develop rapid, simple and non-destructive methods for early detection of cancer using a magnetic field.[51, 52] Wang et al.[52] designed a type of hollow magnetic microcapsule, synthesized using alkyne-modified poly(acrylic acid) (PAA) as polyanions, while a mixture of magnetic Fe3O4-NH2 and poly(allylamine hydrochloride) (PAH) polycations was used as positively-charged layers on calcium carbonate cores. The magnetic microcapsules were further modified with arginine-glycine-aspartate (RGD) in order to specifically target and isolate cancer cells using the magnetic field. These authors found that the RGD-modified microcapsules sensitively detected and efficiently captured nearly 80% of the Hela cells within 20 min of co-incubation.
Circulating tumor cells (CTCs) are cells that travel via physiological fluids. They are shed from cancerous tumors, enter the circulatory system, and migrate to distant organs to form metastases.[53] As a new cancer “biomarker” for disease progression and a guide for implementing therapy, two strategies based on LbL assemblies have been developed to detect and enumerate CTCs. One is to combine a fast magnetic response with targeting properties of the LbL film. Wen et al.[54] constructed magnetic nanospheres (MNs) by depositing alternate layers of PEI and γ-Fe2O3 magnetic nanoparticles. The MNs were modified with an anti-epithelial cell adhesion molecule (EpCAM) antibody, as CTCs express EpCAM which is often used as a biotarget in CTC sensing methodologies.[55] They succeeded in detecting CTCs in whole blood samples from 19 patients with cancer using a commercially-available magnetic field. Xie et al.[56] constructed MNs using the LbL self-assembly of Ca2+ and alginate on the surface of fluorescent-magnetic nanospheres. They also immobilized a biotin-labeled anti-EpCAM in order to target cancer cells. EDTA was used to destabilize the alginate in view of its stronger interaction with Ca2+, resulting in a disconnection between the anti-EpCAM and the MNs. This made it possible to reverse capture and release the target cells. Their system specifically recognized CTCs with 86% capture efficiency in blood samples. 65% of the captured SK-BR-3 cells were released after EDTA treatment, and nearly 70% of the released cells retained their viability. The second strategy used temperature-responsive gels. The Stott group[57] developed a dual-mode gelatin-based nanostructured coating that had both temperature-responsive release and mechano-sensitive release of CTCs captured from peripheral blood. The bulk-population release of CTCs was achieved by raising the temperature of the device to 37 °C, which deconstructed the gelatin-based nanocoating from the surface within minutes. The single cell release of CTCs was achieved via mechanical stress from a frequency-controlled microtip used to dissolve selected regions of the nanocoating.
5. Engineering microenvironments in vitro to study the behavior of cancer cells
The LbL technique has started to show great potential in the study of understanding and controlling the behavior of cancer cells. Of the engineered biomaterials mimicking the in vivo extracellular microenvironment, LbL assemblies are promising candidates, allowing precise control over the properties of the architecture (e.g. thickness, stiffness), as well as presenting biomolecules such as peptides and proteins.[6] In Lee’s study,[58] AuNPs were used as nanotopological structures and protein nanocluster-forming substrates. Adhesion proteins (fibronection and ephrinB3) were conjugated to the AuNPs, and these particles were then modified with an LbL polymer surface by PAH and PAA. They used the biomolecule and nanoparticle-functionalized LbL polymer platforms to study and control the phenotypic changes in breast metastatic cancer cells. The existence of AuNPs induced more dramatic changes in metastatic cell adhesion, protrusion, polarity, and motility than the presence of a cell adhesion protein on the surface.
6. Conclusions and outlook
In summary, we first reviewed how LbL-based systems function as potential candidates for drug delivery, with stimuli-responsive and multifunctional properties. For cancer diagnosis application, LbL magnetic platforms have been successfully developed for rapid, simple and non-destructive detection of rare cancer cells. Furthermore, the LbL assemblies have started to be applied for the study and control of behavior of cancer cells, due to their capacities of precise control over the architecture and biomolecule presentation.
Each type of delivery carrier has its advantages and disadvantages. LbL systems have demonstrated their unique advantage in providing a longer release profile as compared to other delivery methods.[59] LbL nanoparticles and capsules can load individually at least two kinds of cargos into their cavity and multilayer shells, showing great potential for next generation drug delivery systems, with the controlled release of two or more drugs from a single carrier. Moreover, the drug can be utilized as one component of LbL assembly, which would lead to high drug loading densities. Although considerable achievements have been obtained, a number of challenges still remain and need to be overcome for further applications, including 1) the optimization of size of LbL capsules to fit the enhanced permeability and retention (EPR) effect, which allows macromolecules to escape the circulation and accumulate in tumors. 2) The mechanical properties need to be improved for long time in vivo circulation. 3) The downstream cellular responses and signaling pathways during the interactions of LbL-based systems with cells or organs require further investigation. Ultimately, the ability to generate one LbL delivery system capable of loading high concentrations of multiple drugs with tunable sustained release will provide new avenues for clinical therapeutic applications.
References
- [1].de Villiers MM, Lvov YM. Adv Drug Deliver Rev. 2011;63:699. doi: 10.1016/j.addr.2011.06.001. [DOI] [PubMed] [Google Scholar]
- [2].Decher G. Science. 1997;277:1232. [Google Scholar]
- [3].Richardson JJ, Bjornmalm M, Caruso F. Science. 2015;348:aaa2491. doi: 10.1126/science.aaa2491. [DOI] [PubMed] [Google Scholar]
- [4].Monge C, Almodovar J, Boudou T, Picart C. Adv Healthcare Mater. 2015;4:811. doi: 10.1002/adhm.201400715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Tang Z, Wang Y, Podsiadlo P, Kotov NA. Adv Mater. 2006;18:3203. [Google Scholar]
- [6].Boudou T, Crouzier T, Ren K, Blin G, Picart C. Adv Mater. 2010;22:441. doi: 10.1002/adma.200901327. [DOI] [PubMed] [Google Scholar]
- [7].Zelikin AN. ACS Nano. 2010;4:2494. doi: 10.1021/nn100634r. [DOI] [PubMed] [Google Scholar]
- [8].Vergaro V, Scarlino F, Bellomo C, Rinaldi R, Vergara D, Maffia M, Baldassarre F, Giannelli G, Zhang X, Lvov YM, Leporatti S. Adv Drug Deliver Rev. 2011;63:847. doi: 10.1016/j.addr.2011.05.007. [DOI] [PubMed] [Google Scholar]
- [9].Ariga K, Lvov YM, Kawakami K, Ji Q, Hill JP. Adv Drug Deliver Rev. 2011;63:762. doi: 10.1016/j.addr.2011.03.016. [DOI] [PubMed] [Google Scholar]
- [10].Minchinton AI, Tannock IF. Nat Rev Cancer. 2006;6:583. doi: 10.1038/nrc1893. [DOI] [PubMed] [Google Scholar]
- [11].Chen ZG. Trends Mol Med. 2010;16:594. doi: 10.1016/j.molmed.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ai H, Jones SA, Lvov YM. Cell Biochem Biophys. 2003;39:23. doi: 10.1385/CBB:39:1:23. [DOI] [PubMed] [Google Scholar]
- [13].Thompson MT, Berg MC, Tobias IS, Rubner MF, Van Vliet KJ. Biomaterials. 2005;26:6836. doi: 10.1016/j.biomaterials.2005.05.003. [DOI] [PubMed] [Google Scholar]
- [14].Schneider A, Francius G, Obeid R, Schwinte P, Hemmerle J, Frisch B, Schaaf P, Voegel JC, Senger B, Picart C. Langmuir. 2006;22:1193. doi: 10.1021/la0521802. [DOI] [PubMed] [Google Scholar]
- [15].Choi J, Konno T, Takai M, Ishihara K. Biomaterials. 2009;30:5201. doi: 10.1016/j.biomaterials.2009.06.003. [DOI] [PubMed] [Google Scholar]
- [16].Boudou T, Kharkar P, Jing J, Guillot R, Pignot-Paintrand I, Auzely-Velty R, Picart C. J Controlled Release. 2012;159:403. doi: 10.1016/j.jconrel.2012.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Wood KC, Boedicker JQ, Lynn DM, Hammond PT. Langmuir. 2005;21:1603. doi: 10.1021/la0476480. [DOI] [PubMed] [Google Scholar]
- [18].Hammond PT. Mater Today. 2012;15:196. [Google Scholar]
- [19].Sivakumar S, Bansal V, Cortez C, Chong SF, Zelikin AN, Caruso F. Adv Mater. 2009;21:1820. [Google Scholar]
- [20].Zhu Y, Tong W, Gao C, Mohwald H. Langmuir. 2008;24:7810. doi: 10.1021/la801119z. [DOI] [PubMed] [Google Scholar]
- [21].Cui D, Jing J, Boudou T, Pignot-Paintrand I, De Koker S, De Geest BG, Picart C, Auzely-Velty R. Adv Mater. 2011;23:H200. doi: 10.1002/adma.201100600. [DOI] [PubMed] [Google Scholar]
- [22].Hosta-Rigau LS, Yan Y, Nice E, Heath J, Albericio F, Caruso F. Adv Funct Mater. 2010;20:59. [Google Scholar]
- [23].Wang YY, Cui J, Hosta-Rigau LS, Heath J, Nice E, Caruso F. Adv Mater. 2010;22:4293. doi: 10.1002/adma.201001497. [DOI] [PubMed] [Google Scholar]
- [24].Zhou J, Romero G, Rojas E, Ma L, Moya S, Gao C. J Colloid Interface Sci. 2010;345:241. doi: 10.1016/j.jcis.2010.02.004. [DOI] [PubMed] [Google Scholar]
- [25].Deng ZJ, Morton SW, Ben-Akiva E, Dreaden EC, Shopsowitz KE, Hammond PT. ACS Nano. 2013;7:9571. doi: 10.1021/nn4047925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Cortez C, Tomaskovic-Crook E, Johnston AP, Scott AM, Nice EC, Heath JK, Caruso F. ACS Nano. 2007;1:93. doi: 10.1021/nn700060m. [DOI] [PubMed] [Google Scholar]
- [27].Zhao X, Liu P. Mol Pharm. 2014;11:1599. doi: 10.1021/mp400774v. [DOI] [PubMed] [Google Scholar]
- [28].Cheng L, Yang K, Chen Q, Liu Z. ACS nano. 2012;6:5605. doi: 10.1021/nn301539m. [DOI] [PubMed] [Google Scholar]
- [29].Poon Z, Lee JB, Morton SW, Hammond PT. Nano Lett. 2011;11:2096. doi: 10.1021/nl200636r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ramasamy T, Haidar ZS, Tran TH, Choi JY, Jeong JH, Shin BS, Choi HG, Yong CS, Kim JO. Acta Biomater. 2014;10:5116. doi: 10.1016/j.actbio.2014.08.021. [DOI] [PubMed] [Google Scholar]
- [31].Bishop CJ, Tzeng SY, Green JJ. Acta Biomater. 2015;11:393. doi: 10.1016/j.actbio.2014.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Sun JK, Ren KF, Zhu LZ, Ji J. Colloids Surf B. 2013;112:67. doi: 10.1016/j.colsurfb.2013.07.044. [DOI] [PubMed] [Google Scholar]
- [33].Delcea M, Mohwald H, Skirtach AG. Adv Drug Deliver Rev. 2011;63:730. doi: 10.1016/j.addr.2011.03.010. [DOI] [PubMed] [Google Scholar]
- [34].Luo GF, Xu XD, Zhang J, Yang J, Gong YH, Lei Q, Jia HZ, Li C, Zhuo RX, Zhang XZ. ACS Appl Mater Inter. 2012;4:5317. doi: 10.1021/am301258a. [DOI] [PubMed] [Google Scholar]
- [35].Li QL, Sun Y, Sun YL, Wen J, Zhou Y, Bing QM, Isaacs LD, Jin Y, Gao H, Yang YW. Chem Mater. 2014;26:6418. doi: 10.1021/cm503304p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Sun Y, Wang L, Ma J, Yang YW, Gao H. Micropor Mesopor Mat. 2014;185:245. [Google Scholar]
- [37].Zhu Z, Gao N, Wang H, Sukhishvili SA. J Controlled Release. 2013;171:73. doi: 10.1016/j.jconrel.2013.06.031. [DOI] [PubMed] [Google Scholar]
- [38].Hu M, Chen J, Li ZY, Au L, Hartland GV, Li X, Marquez M, Xia Y. Chem Soc Rev. 2006;35:1084. doi: 10.1039/b517615h. [DOI] [PubMed] [Google Scholar]
- [39].Wu Z, Lin X, Zou X, Sun J, He Q. ACS Appl Mater Inter. 2015;7:250. doi: 10.1021/am507680u. [DOI] [PubMed] [Google Scholar]
- [40].Levine EM, Robbins EB. J Cell Physiol. 1970;76:373. doi: 10.1002/jcp.1040760315. [DOI] [PubMed] [Google Scholar]
- [41].Edakkattuparambil Sidharth Shibu MH, Murase Norio, Biju Vasudevanpillai. J Photochem Photobiol C. 2013;15:53. [Google Scholar]
- [42].Jin Y, Wang J, Ke H, Wang S, Dai Z. Biomaterials. 2013;34:4794. doi: 10.1016/j.biomaterials.2013.03.027. [DOI] [PubMed] [Google Scholar]
- [43].Shen J, Kim HC, Mu C, Gentile E, Mai J, Wolfram J, Ji LN, Ferrari M, Mao ZW, Shen H. Adv Healthcare Mater. 2014;3:1629. doi: 10.1002/adhm.201400103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Wang X, Liu K, Yang G, Cheng L, He L, Liu Y, Li Y, Guo L, Liu Z. Nanoscale. 2014;6:9198. doi: 10.1039/c4nr02495h. [DOI] [PubMed] [Google Scholar]
- [45].Du P, Zeng J, Mu B, Liu P. Mol Pharm. 2013;10:1705. doi: 10.1021/mp300534a. [DOI] [PubMed] [Google Scholar]
- [46].Dreaden EC, Morton SW, Shopsowitz KE, Choi JH, Deng ZJ, Cho NJ, Hammond PT. ACS Nano. 2014;8:8374. doi: 10.1021/nn502861t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Cohen SJ, Alpaugh RK, Gross S, O'Hara SM, Smirnov DA, Terstappen LW, Allard WJ, Bilbee M, Cheng JD, Hoffman JP, Lewis NL, et al. Clin Colorectal Cancer. 2006;6:125. doi: 10.3816/CCC.2006.n.029. [DOI] [PubMed] [Google Scholar]
- [48].Fan Z, Shelton M, Singh AK, Senapati D, Khan SA, Ray PC. ACS nano. 2012;6:1065. doi: 10.1021/nn2045246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Song EQ, Hu J, Wen CY, Tian ZQ, Yu X, Zhang ZL, Shi YB, Pang DW. ACS nano. 2011;5:761. doi: 10.1021/nn1011336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].de la Rica R, Thompson S, Baldi A, Fernandez-Sanchez C, Drain CM, Matsui H. Anal Chem. 2009;81:10167. doi: 10.1021/ac9021049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Liu J, Qin Y, Li D, Wang T, Liu Y, Wang J, Wang E. Biosens Bioelectron. 2013;41:436. doi: 10.1016/j.bios.2012.09.002. [DOI] [PubMed] [Google Scholar]
- [52].Wang Y, Zhang J, Jia HZ, Yang J, Qin SY, Liu CW, Zhuo RX, Zhang XZ. Macromol Biosci. 2012;12:1321. doi: 10.1002/mabi.201200205. [DOI] [PubMed] [Google Scholar]
- [53].Balic M, Williams A, Lin H, Datar R, Cote RJ. Annu Rev Med. 2013;64:31. doi: 10.1146/annurev-med-050311-163404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Wen CY, Wu LL, Zhang ZL, Liu YL, Wei SZ, Hu J, Tang M, Sun EZ, Gong YP, Yu J, Pang DW. ACS Nano. 2014;8:941. doi: 10.1021/nn405744f. [DOI] [PubMed] [Google Scholar]
- [55].Joosse SA, Pantel K. Cancer Res. 2013;73:8. doi: 10.1158/0008-5472.CAN-12-3422. [DOI] [PubMed] [Google Scholar]
- [56].Xie M, Lu NN, Cheng SB, Wang XY, Wang M, Guo S, Wen CY, Hu J, Pang DW, Huang WH. Anal Chem. 2014;86:4618. doi: 10.1021/ac500820p. [DOI] [PubMed] [Google Scholar]
- [57].Reategui E, Aceto N, Lim EJ, Sullivan JP, Jensen AE, Zeinali M, Martel JM, Aranyosi AJ, Li W, Castleberry S, Bardia A, et al. Adv Mater. 2015;27:1593. doi: 10.1002/adma.201404677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Lee H, Jang Y, Seo J, Nam JM, Char K. ACS Nano. 2011;5:5444. doi: 10.1021/nn202103z. [DOI] [PubMed] [Google Scholar]
- [59].Wohl BM, Engbersen JF. J Controlled Release. 2012;158:2. doi: 10.1016/j.jconrel.2011.08.035. [DOI] [PubMed] [Google Scholar]