Abstract
Background
Thrombolytic therapy with intravenous alteplase within 4.5 hours of ischaemic stroke onset increases the overall likelihood of an excellent outcome (no, or non-disabling, symptoms). Any improvement in functional outcome distribution has value, and herein we provide an assessment of the effect of alteplase on the distribution of the functional level by treatment delay, age and stroke severity.
Methods
Pre-specified pooled analysis of 6756 patients from nine randomised trials comparing alteplase versus placebo/open control. Ordinal logistic regression models assessed treatment differences after adjustment for treatment delay, age, stroke severity, and relevant interaction term(s).
Results
Treatment with alteplase was beneficial for a delay in treatment extending to 4.5 hours after stroke onset, with a greater benefit with earlier treatment. Neither age nor stroke severity significantly influenced the slope of the relation between benefit and time to treatment initiation. For the observed case mix of patients treated within 4.5 h of stroke onset, (mean 3 hours and 20 minutes), the net absolute benefit of alteplase (i.e., the difference between those who would do better if given alteplase and those who would do worse) was 55 patients per 1,000 treated (95% CI 13-91; p=0.004).
Conclusions
Treatment with intravenous alteplase initiated within 4.5 hours of stroke onset increases the chance of achieving an improved level of function for all patients across the age spectrum including the over 80s and across all severities of stroke studied (top vs bottom fifth means: 22 vs 4); the earlier that treatment is initiated, the greater the benefit.
Background
There are essentially two widely accepted approaches to the statistical analysis of acute stroke outcome data. One may focus solely on good versus bad outcome, disregarding further details of the patient’s functional status. Thus, one may consider patients with an “excellent” outcome to be of equal value to those with a “good” outcome but otherwise patients left with only “fair” outcome, “poor” outcome or even death are all considered just as having a bad outcome. Alternatively, one could consider if treatment shifted the entire distribution of outcomes favourably, i.e. one seeks an increase in the average likelihood of achieving a better outcome.1 The traditional measure for a good stroke outcome, mRS 0-1, considers the former whereas ordinal approaches generally consider the latter.
These two approaches are not mutually incompatible, however, and both should usually be considered. Indeed, a good clinician could incorporate elements of each approach when counselling patients or relatives prior to offering intravenous thrombolysis for stroke.
The recent individual patient data (IPD) pooled analysis of 6756 patients from nine thrombolysis trials reported treatment differences that dichotomised outcomes into the “excellent” (mRS 0-1) and “bad” (death: mRS 6 versus mRS 0-5) ends of the scale.2 In those analyses, intravenous alteplase was shown to improve significantly the overall odds of an excellent stroke outcome (mRS 0-1) when delivered within 4·5 h of stroke onset. That analysis showed that earlier treatment resulted in larger benefit of treatment, with – at any given time delay – proportional treatment effects that were similar irrespective of age or stroke severity within the range studied. However, alteplase was also shown to increase the absolute risk of fatal intracranial haemorrhage within the first week by about 2%, with no significant effect on other early causes of death but perhaps partly offset by later causes of death.
An earlier IPD analysis carried out in 2010, which included data from 8 of the same 9 trials, suggested that 3 month mortality was increased when treatment was initiated beyond 4.5h after stroke onset,3 but in the updated analysis this trend with treatment delay lost statistical significance. However, death is just one potential adverse outcome: severe or even moderate disability will be considered by some patients to be as undesirable as death, or perhaps worse;4 and others still may consider any loss of dependence as a poor outcome.5 In addition, differences in the length of hospital stay, and the cost of care, increase substantially and monotonically with measures of disability.6 To help clinicians to discuss with their patients the pros and cons of a treatment that carries both potential risks and benefits, we now report various analyses that consider the full range of functional states 90-180 days after stroke.
For most circumstances in which the outcome measure involves a series of 3 or more levels of ordinal outcomes, ordinal analysis is generally considered a statistically powerful approach by expert groups for circumstances where treatment effects are not expected to concentrate at one end of the disability scale.1,7 For treatments that may have a dual effect (the extreme example would be ‘kill or cure’), risk and benefit may each be more reliably detected by a dichotomised analysis (“symptom-free survival”), but if survival despite continued symptoms is a desirable health state to some patients, then an analysis that considers all categories (symptom-free survival, symptomatic survival, severe disability, death) may be more discriminating for net benefit.1,8 Since it is well established that later treatment (eg 4.5 to 6 hours after stroke onset) is associated with reduced benefit and with a trend at least towards lower 90-day survival, we can surmise that the greatest treatment delay at which net benefit persists may be shorter than the ~5 hour window when indexed by defining a good outcome as mRS 0-1.2 However, the more sensitive ordinal analysis offers the opportunity to refine the characterisation of the time period when patients benefit from alteplase therapy. The aim of this report is to present the results from the pre-defined ordinal analyses,9 and to put them in context with the already published main dichotomous ones.2
Methods
The data sources and analysis methods have been described in detail previously, particularly the approach used to determine the influence of time from stroke onset on the outcome mRS 0-1 versus 2-6.2,9 For each other dichotomy of mRS, ie 0 versus 1-6, 0-2 versus 3-6 etc, we repeated that approach. We also examined the full range of the mRS to assess the common odds of better outcome with intravenous alteplase versus control, using ordinal logistic regression.10 The use of the common odds ratio invokes assumptions about the consistency of the treatment effect across the spectrum of outcome scores, but carries advantages over its alternatives in terms of greater statistical power and ease of adjustment for baseline prognostic factors.1,8 However, we also applied the ordinal approach of Howard et al (2012)11 that weights all steps of the modified Rankin scale equally, to estimate the net benefit of treatment (ie, the expected number who would do better if given alteplase minus the expected number who would do worse), among a cohort with the case mix included in the pooled trials.
As described previously, regression models, which for dichotomised outcomes were stratified by trial, were adjusted for allocation to alteplase, treatment delay, age, stroke severity (as measured by the pretreatment National Institutes of Health Stroke Scale; NIHSS), and relevant interaction term(s).9 Adjusted models are needed because of the strong inter-relationships between age, treatment delay and stroke severity in the 9 included trials.2;9 For example, patients treated earlier tended to be older and to have had more severe strokes than patients treated later. Eight trials assessed functional outcome after 3 months using the modified Rankin Scale whereas the third international stroke trial, IST-3, applied the Oxford Handicap Scale to patients at 6 months’ follow-up.12–18 Whereas our publication on mRS 0-1 described functional outcome at 3-6 months but mortality only at three months, for the present analyses we have used mortality and functional outcome at 6 months for IST-3 patients but at 3 months for all other trials’ patients (noting that a 3-month assessment was not performed in IST-3).2 Analyses were performed using SAS version 9.3 (SAS Institute, Cary) and R version 2.11.1 (www.R-project.org).
Where relevant, the significance of 3-way interaction terms was assessed using the likelihood ratio test of the change in deviance between two nested models that differed only by the relevant interaction term. We made no adjustment for multiplicity: ordinal analysis was planned9 and the primary dichotomised analysis of mRS 0-1 achieved statistical significance.
Results
The baseline characteristics of the patient population are provided in Table 1. There were 6756 patients with mean (SD) age 71 ± 13 years, NIHSS 12 ± 7 and onset to treatment delay 4.0 ± 1.2 hours.
Table 1. Baseline characteristics of included trials.
| Variable | NINDS A | NINDS B | ECASS I | ECASS II | ATLANTIS A | ATLANTIS B | ECASS III | EPITHET | IST-3 | TOTAL |
|---|---|---|---|---|---|---|---|---|---|---|
| Number randomized | 291 | 333 | 620 | 800 | 142 | 613 | 821 | 101 | 3035 | 6756 |
| Alteplase | 144 (49%) | 168 (50%) | 313 (50%) | 409 (51%) | 71 (50%) | 301 (49%) | 418 (51%) | 52 (51%) | 1515 (50%) | 3391 (50%) |
| Control | 147 (51%) | 165 (50%) | 307 (50%) | 391 (49%) | 71 (50%) | 312 (51%) | 403 (49%) | 49 (49%) | 1520 (50%) | 3365 (50%) |
| Treatment delay (hours) | 2.0 (0.6) | 2.0 (0.6) | 4.4 (1.1) | 4.3 (1.1) | 4.3 (1.1) | 4.4 (0.8) | 4.0 (0.4) | 4.9 (0.8) | 4.2 (1.2) | 4.0 (1.2) |
| >0, ≤3 | 290 (>99%) | 333 (100%) | 87 (14%) | 158 (20%) | 22 (15%) | 39 (6%) | - | - | 620 (20%) | 1549 (23%) |
| >3, ≤4.5 | 1 (<1%) | - | 233 (38%) | 265 (33%) | 53 (37%) | 249 (41%) | 788 (96%) | 31 (31%) | 1148 (38%) | 2768 (41%) |
| >4.5 | - | - | 295 (48%) | 370 (46%) | 67 (47%) | 321 (52%) | 6 (1%) | 69 (68%) | 1266 (42%) | 2394 (35%) |
| Missing | - | - | 5 (1%) | 7 (1%) | - | 4 (1%) | 27 (3%) | 1 (1%) | 1 (<1%) | 45 (1%) |
| Age (years) | 66 (11) | 68 (12) | 65 (12) | 66 (11) | 66 (13) | 66 (11) | 65 (12) | 72 (13) | 77 (12) | 71 (13) |
| ≤80 | 279 (96%) | 289 (87%) | 615 (>99%) | 792 (99%) | 142 (100%) | 608 (>99%) | 805 (98%) | 76 (75%) | 1418 (47%) | 5024 (74%) |
| >80 | 12 (4%) | 44 (13%) | 5 (1%) | 8 (1%) | - | 3 (<1%) | 15 (2%) | 25 (25%) | 1617 (53%) | 1729 (26%) |
| Missing | - | - | - | - | - | 2 (<1%) | 1 (<1%) | - | - | 3 (<1%) |
| Stroke severity (NIHSS) | 14 (7) | 15 (7) | 12 (6) | 12 (6) | 13 (7) | 11 (6) | 10 (5) | 13 (6) | 12 (7) | 12 (7) |
| >0, ≤4 | 16 (5%) | 13 (4%) | 34 (5%) | 47 (6%) | 10 (7%) | 47 (8%) | 98 (12%) | 1 (1%) | 400 (13%) | 666 (10%) |
| >4, ≤10 | 78 (27%) | 98 (29%) | 189 (30%) | 339 (42%) | 57 (40%) | 279 (46%) | 389 (47%) | 40 (40%) | 1064 (35%) | 2533 (37%) |
| >10, ≤15 | 68 (23%) | 63 (19%) | 183 (30%) | 232 (29%) | 28 (20%) | 128 (21%) | 163 (20%) | 22 (22%) | 601 (20%) | 1488 (22%) |
| >15, ≤21 | 76 (26%) | 78 (23%) | 146 (24%) | 113 (14%) | 25 (18%) | 106 (17%) | 142 (17%) | 29 (29%) | 618 (20%) | 1333 (20%) |
| >21 | 45 (15%) | 74 (22%) | 28 (5%) | 43 (5%) | 20 (14%) | 33 (5%) | 18 (2%) | 9 (9%) | 352 (12%) | 622 (9%) |
| Missing | 8 (3%) | 7 (2%) | 40 (6%) | 26 (3%) | 2 (1%) | 20 (3%) | 11 (1%) | - | * | 114 (2%) |
| Female | 120 (41%) | 142 (43%) | 231 (37%) | 331 (41%) | 45 (32%) | 250 (41%) | 325 (40%) | 43 (43%) | 1570 (52%) | 3057 (45%) |
| History of hypertension | 188 (65%) | 220 (66%) | 258 (42%) | 412 (52%) | 87 (61%) | 364 (59%) | 514 (63%) | 71 (70%) | 1954 (64%) | 4068 (60%) |
| History of stroke | 49 (17%) | 34 (10%) | 83 (13%) | 158 (20%) | 31 (22%) | 89 (15%) | 89 (11%) | 11 (11%) | 699 (23%) | 1243 (18%) |
| History of diabetes mellitus | 64 (22%) | 67 (20%) | 81 (13%) | 169 (21%) | 27 (19%) | 130 (21%) | 129 (16%) | 23 (23%) | 388 (13%) | 1078 (16%) |
| History of atrial fibrillation | 55 (19%) | 60 (18%) | 113 (18%) | 188 (24%) | 37 (26%) | 97 (16%) | 108 (13%) | 42 (42%) | 914 (30%) | 1614 (24%) |
| Antiplatelet use | 78 (27%) | 93 (28%) | 87 (14%) | 196 (25%) | 59 (42%) | 211 (34%) | 201 (24%) | 30 (30%) | 1306 (43%) | 2261 (33%) |
| Weight (kg) | 78 (17) | 78 (19) | 74 (12) | 75 (14) | 80 (20) | 79 (18) | 78 (15) | 75 (19) | 72 (15) | 75 (16) |
| Systolic blood pressure (mmHg) | 154 (21) | 152 (21) | 154 (23) | 152 (21) | 152 (24) | 152 (21) | 153 (21) | 148 (19) | 155 (24) | 154 (22) |
| Diastolic blood pressure (mmHg) | 85 (13) | 85 (14) | 87 (13) | 84 (13) | 81 (14) | 82 (14) | 84 (14) | 78 (13) | 82 (15) | 83 (14) |
Categorical data presented as n (%), continuous data presented as mean (SD). NINDS=National Institute of Neurological Disorders and Stroke; ECASS=European Cooperative Acute Stroke Study; ATLANTIS=Alteplase Thrombolysis for Acute Noninterventional Therapy in Ischemic Stroke; EPITHET=Echoplanar Imaging Thrombolytic Evaluation Trial; IST=International Stroke Trial.
In IST-3, 244 patients had their baseline NIHSS score predicted from other measurements recorded at their baseline assessment. Ignoring these patients, the numbers of IST-3 patients in each category of baseline NIHSS score above would be 385, 972, 531, 559 and 344 respectively.
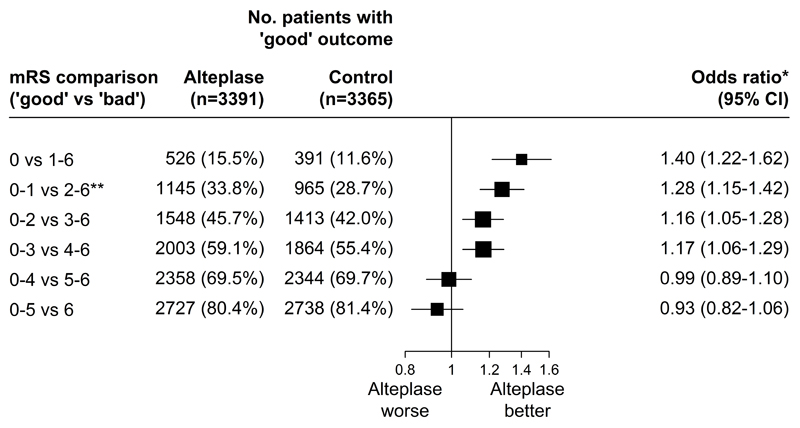
The average odds of achieving a better stroke outcome in alteplase-allocated compared with control-allocated patients across the whole 6-hour window (mean delay 4.0 h) diminished as the selected cutpoint used to define “better” outcome shifted from asymptomatic (mRS 0, OR 1.40, 95% CI: 1.22–1.62) to greatest residual disability (mRS 5, OR 0.93, 95% CI: 0.82–1.06; Figure 1). The p-value for the assumption of a common odds ratio is <0.0001, suggesting there is heterogeneity of the odds ratios across the various dichotomies. This heterogeneity between mRS thresholds would appear to be attributable to a general downward shift in the odds of better outcome at higher mRS thresholds, while the pattern of poorer outcomes with longer delays in the time to treatment was relatively consistent across the mRS spectrum.
Figure 1. Relative odds of a good stroke outcome with alteplase, for each alternative definition of 'good' outcome, among all randomized patients.
* Estimated from a logistic regression model stratified by trial and adjusted only for treatment allocation. ** Primary pre−specified mRS comparison. IST−3 applied the Oxford Handicap Scale to patients at 6 months’ follow−up. We have used mortality and functional outcome at 6 months for IST−3 patients but at 3 months for all other trials’ patients.
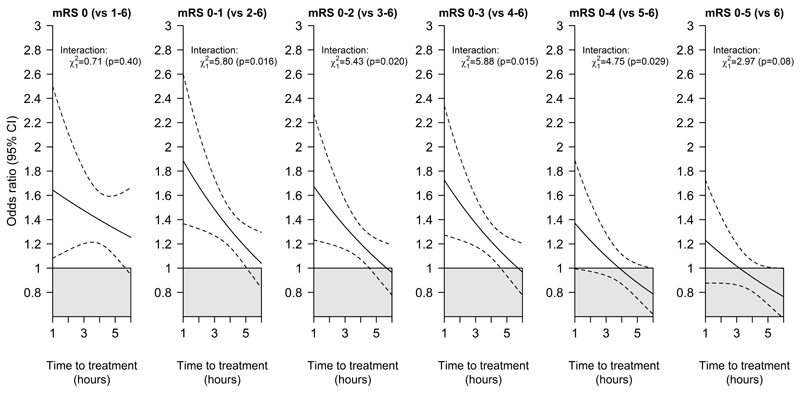
For each of the possible dichotomisations of mRS, earlier treatment was associated with bigger proportional benefits; this interaction with treatment delay was conventionally statistically significant (p<0.05) for each of the comparisons 0-1 v 2-6 through to 0-4 v 5-6, and, despite being qualitatively similar, with relatively few patients with mRS of either 0 or 6 was not statistically significant for mRS 0 vs 1-6 or mRS 0-5 vs 6 (Figure 2). The maximum delay between stroke onset and treatment that was associated with a significantly positive treatment effect was at least 4.5 hours for each dichotomisation of the mRS scale classifying 0, 0-1, 0-2 or 0-3 as good outcome. For mRS 0-4 versus 5-6 or 0-5 versus 6 (death), it was not established that alteplase significantly increased the overall odds of a better outcome (Figure 1) nor that earlier treatment (even at 1 hour) was associated with a clear benefit (Figure 2).
Figure 2. Relative odds of a good stroke outcome with alteplase by time to treatment, for each alternative definition of 'good' outcome.
In each panel the solid line represents the best linear fit between the log odds ratio for each mRS outcome among patients given alteplase compared with patients given control (vertical axis) and treatment delay (horizontal axis). Estimates are derived from a regression model in which alteplase, time to treatment, age and stroke severity (handled in a quadratic manner) are included as main effects but the only treatment interaction included is with time to treatment.
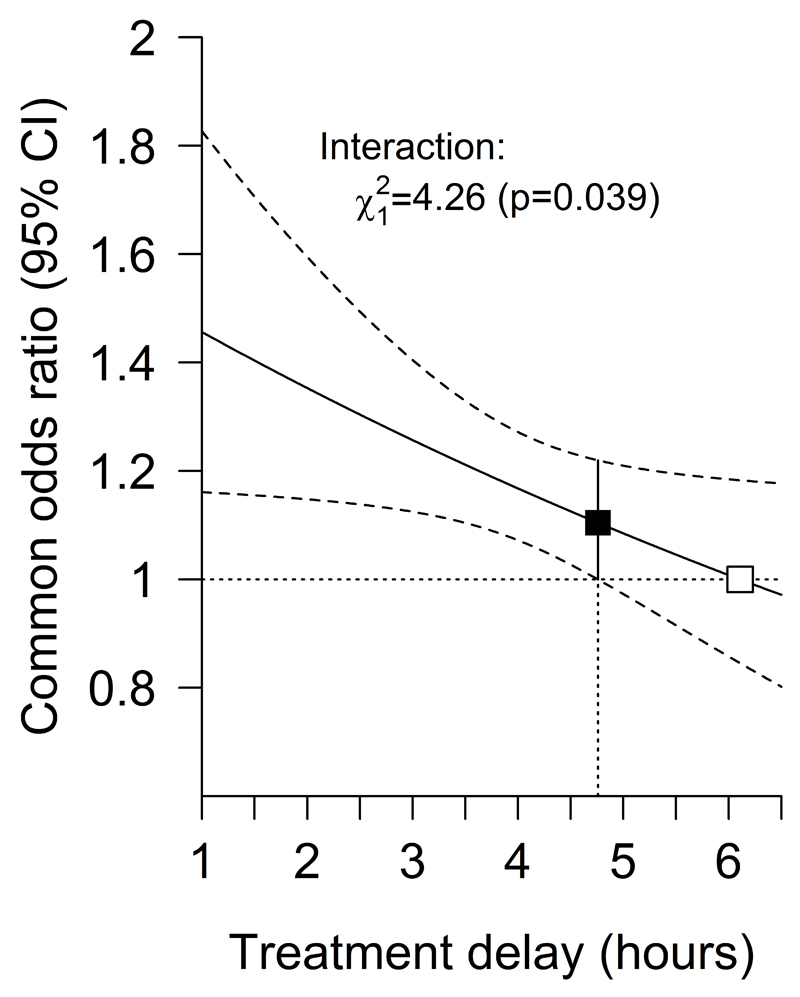
After combining the results of the alternative dichotomous analyses to model the average odds ratio for any upwards shift in mRS, treatment initiation within 4.5 hours was associated with statistically significant net benefit, with earlier treatment resulting in bigger proportional benefits (Figure 3). Given treatment delay, neither patient age or baseline stroke severity significantly altered the proportional effect of alteplase on the odds of an improved outcome (Supplemental Figure I); nor did stroke severity significantly alter the relationship between treatment delay and the odds of an improved outcome with alteplase (p = 0.72). The test for a three-way interaction among treatment, treatment delay and age as a continuous variable had a p value of 0.0019, in the direction of older age lengthening the window during which intravenous alteplase may be effective (Supplemental Figure II). The window remains open until at least 4.5 hours for both younger and more elderly (i.e. over 80 year old) patients.
Figure 3. Effect of alteplase on any upwards shift in mRS, by treatment delay.
The solid line represents the best linear fit between the log odds ratio for an improved stroke outcome among patients given alteplase compared with patients given control (vertical axis) and treatment delay (horizontal axis). Estimates are derived from a regression model in which alteplase, time to treatment, age and stroke severity (handled in a quadratic manner) are included as main effects but the only treatment interaction included is with time to treatment. Only 198 patients (159 of whom were from IST−3) had a time from stroke onset to treatment of more than 6 hours. The point at which the estimated treatment effect crosses 1 is therefore an extrapolation from the data.
The observed frequencies of patients in various outcome strata according to treatment delay and baseline stroke severity, without adjustment for other characteristics, are shown in Supplemental Figures III and IV.
An ordinal analysis, avoiding the proportionality assumption,11 of the patients treated within 4.5 hours of stroke onset (mean delay 3 hours 20 minutes), where benefit is expressed as any improvement in one or more divisions of the mRS, found that a net 55 patients (95% CI 13, 91) per 1,000 treated were better with alteplase, p=0.004. Earlier treatment was better: within 3 hours (average 2 h 20 m) net benefit was 122 patients (95% CI 61, 171) per 1,000 treated whereas initiating treatment beyond 4.5 hours (mean 5 hours 20 minutes) revealed a net benefit of only 20 patients (95% CI -31, 75) per 1,000 treated, p=0.45.
Discussion
Patients with acute ischaemic stroke show wide variation in the severity and range of their symptoms at presentation. Their functional outcomes are also diverse. The categories of the modified Rankin Scale capture this diversity well. Dichotomisation of the scale at mRS 0-1 (excellent outcome) versus 2-6 (poorer outcome), or at 0-2 (independent) versus 3-6 (dependent or dead) simplifies the presentation of the impact of treatment but implying cure or near cure oversimplifies clinical reality for most patients. Patients want to know if treatment will make a noticeable difference to their functional outcome: many would regard an improvement by any step in mRS level as worthwhile, though they would not necessarily weight all transitions as equal.5
In addition, such simplification tends to lead to situations where clinicians are eager to identify the “good responders”, whereas the reality may well be that the majority of patients improve somewhat, with a few showing a major improvement (or major deterioration and early death). An overemphasis on only treating those likely to have a major improvement may lead to the situation where the net benefit of thrombolysis would be lost.
While all trials except IST3 excluded patients with the most minor symptoms, our analysis reveals that if good outcome is represented by any contiguous group of mRS levels from 0 (asymptomatic) down to 3 (requiring help for activities of daily living), then the onset to treatment time window in which benefit is statistically more likely with alteplase is at least 4.5 hours, though earlier treatment is better and by 4.5 hours the benefit is becoming small. While generalizing these findings to those with the most minor symptoms (who were excluded from most trials) should be done with caution, our data cover a range of NIHSS from 4 (mean in bottom fifth of distribution) to 22 (top fifth mean). Within this, the uniformity of the proportional treatment effect across the spectrum of severity suggests a consistent effect. The absolute risk of bleeding is lower among patients with low NIHSS scores. Treatment with intravenous alteplase initiated within 4.5 hours reduces the proportion of patients with mRS 4 or higher: there will be fewer patients who cannot walk independently or require help with basic toileting. We did not find that intravenous alteplase alters mRS 0-4 versus 5-6, nor, as we have previously shown and examined in greater detail,2 did we have evidence that it alters survival excepting the small excess of early fatal bleeding (Figures 1 and 2). Thus, intravenous alteplase appears to enhance chances of achieving a good outcome by more than it improves the chances of achieving a merely acceptable outcome, which may be attractive to patients. Analysis that relies on a common odds ratio to combine all possible dichotomies of mRS is compromised by violation of the assumption that these ratios are similar. However, though the odds ratios diminish as the criterion for good outcome drops towards greater disability, we do not have evidence that they invert.
We found that if all categories of mRS are given equal weight and any net movement towards better outcome is regarded as desirable, then the onset to treatment window for benefit from intravenous alteplase is at least 4.5 hours, although earlier treatment is better than later treatment within this window. The ordinal approach11 that avoids the proportionality assumption gives a comparable result. Neither age nor baseline stroke severity shortens the treatment time window for any relative benefit. Note that the nine trials that we analysed include many patients who would fall outwith the labels for marketing authorisations that apply in USA, Europe and many other regions.
Since the magnitude of the treatment effect appears to decrease at higher thresholds (i.e., two higher dichotomies show no significant benefit from iv alteplase irrespective of treatment initiation delay), it was anticipated that the ordinal analysis may not confirm benefit until 4.5h. However, there was a clear benefit for lower mRS thresholds, and only a lack of effect for higher mRS thresholds. In contradistinction, although alteplase has a neutral effect on mRS 0-4 versus 5-6 and on survival, there is still a strong trend towards a time-related effect for these mRS divisions such that treatment initiated within 3 hours appears more useful than treatment initiation after 3 hours. This has biological plausibility.
The observed outcomes of the isolated cohorts of patients within each treatment delay window or severity category (Supplemental Figures IV and V) give a less reliable picture of treatment effects than the models that incorporate all data together, because case mix varies between cohorts and this has a strong influence on outcome and because smaller samples deliver lower statistical power.
We also note that the design of the IST differed from other trials in three major ways: 1) it was open label treatment, while other trials were placebo-controlled, 2) it assessed outcome after 6 months rather than three months, and 3) it had a broader eligibility criteria and included patients outside of usual treatment guidelines. However, as previously reported, the results from IST-3 were consistent with those from the other trials, both for measures of benefit and for measures of harm (eg, for mRS 0-1 vs 2-6, the p-value for inconsistency between IST-3 and the other trials was 0.92).19
Our current results extend our previous reports2,192 and are consistent with the conclusions of the individual trials that showed benefit within 4.5 hours of stroke onset, but especially within 3 hours.12,15 They also support the conclusions of the European Stroke Organisation Guidelines on stroke management.20 We have not discussed here the early risk of fatal bleeding that is a well recognised complication of intravenous alteplase treatment,2,19 because it neither has an impact on assessment of the outcome distribution nor, in the absence of a reliable means of identifying patients who will suffer an unacceptably high risk of bleeding, on the decision to treat. This does not detract from the importance of weighing risks and benefits when counselling patients.
Summary and Conclusions
The implications of our analysis that considers all levels of functional outcome including survival after stroke are that:
-
1)
on average, there is an increased chance of achieving an improved level of function if treatment with intravenous alteplase is initiated within 4.5 hours of stroke onset;
-
2)
treatment benefit is greater the earlier that it is initiated;
-
3)
neither age nor stroke severity has a detectable impact on the relation between delay and treatment benefit, and particularly not on the duration of the clinically justifiable treatment window; and
-
4)
any patient who fulfils the criteria for treatment with alteplase and who wishes to optimise his chance of survival with optimal function should be offered treatment with intravenous alteplase as a matter of extreme urgency, ideally within a maximum initiation delay of 4.5 hours.
Supplementary Material
Acknowledgments
Sources of Funding
This collaboration is coordinated by the Clinical Trial Service Unit & Epidemiological Studies Unit at the University of Oxford, UK. The Unit receives core funding from the UK Medical Research Council and the British Heart Foundation. This work also received support from the University of Glasgow and University of Edinburgh.
Appendix
Included Trials
ATLANTIS A and B (Gregory Albers, James Grotta, Maarten Lansberg, Jean Marc Olivot); ECASS-1, ECASS-2, ECASS-3 (Erich Bluhmki, Werner Hacke, Markku Kaste, Kennedy R Lees, Ruediger von Kummer, Danilo Toni, Nils Wahlgren); EPITHET (Stephen Davis, Geoffrey Donnan, Mark Parsons); IST-3 (Peter Sandercock, Joanna Wardlaw, Richard Lindley, Gordon Murray, Geoff Cohen, William Whiteley); NINDS A and B (Thomas Brott, James Grotta, Patrick Lyden, John Marler, Barbara Tilley).
STT Statistical Analysis Centre and Secretariat
Colin Baigent, Lisa Blackwell, Erich Bluhmki, Kelly Davies, Jonathan Emberson, Heather Halls, Lisa Holland, George Howard, Clare Mathews, Samantha Smith, Kate Wilson.
STT collaborative group
Gregory Albers, Colin Baigent, Lisa Blackwell, Erich Bluhmki, Thomas Brott, Geoffrey Cohen, Stephen Davis, Geoffrey Donnan, Jonathan Emberson, James Grotta, Werner Hacke, George Howard, Markku Kaste, Masatoshi Koga, Ruediger von Kummer, Maarten Lansberg, Kennedy R Lees, Richard I Lindley, Patrick Lyden, Gordon Murray, Jean Marc Olivot, Mark Parsons, Peter Sandercock, Barbara Tilley, Danilo Toni, Kazunori Toyoda, Nils Wahlgren, Joanna Wardlaw, William Whiteley, Gregory J del Zoppo
Footnotes
Conflicts of interest
KL reports fees or expenses from American Stroke Association, Applied Clinical Intelligence, Atrium, Boehringer Ingelheim, EVER NeuroPharma, Hilicon, Nestle, Novartis, Stroke Academic Industry Roundtable, University of Lancaster; and research funding to the University of Glasgow and to the Virtual International Stroke Trials Archive from Genentech. CB, LB, and JE have not accepted fees, honoraria, or paid consultancies but are involved in clinical trials of lipid-modifying treatment funded by Merck to the University of Oxford, with the University the trial sponsor in all cases. EB is employed by Boehringer Ingelheim. SD has received honoraria from AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Medtronic and Pfizer. GD is co-principal investigator for the EXTEND trial using alteplase and has received honoraria from Boehringer Ingelheim, Bayer, Pfizer, Sanofi and Merk Sharp & Dohme. JG has acted as a consultant for Frazer Ltd and Stryker, has received grant support from the American Heart Association, Genentech, and Behring, and has received grant support within the past 3 years from Haemonetics and Medtronics. MK reports fees and expenses from Lundbeck A/S, Mitsubishi Pharma Europe, Siemens AG. RvK reports fees from H. Lundbeck A/S, Boehringer Ingelheim, Covidien, Brainsgate, Synarc, and Penumbra, Inc. RIL has received honoraria from Boehringer Ingelheim, Covidien and Pfizer for lectures given in the past 3 years. PS has received honoraria for lectures which were paid to the department from Boehringer Ingelheim. DT reports honoraria from Boehringer Ingelheim, Bayer and Pfizer. KT has received research grant support from the Japan Agency for Medical Research and Development, and fees from Mitsubishi Tanabe Pharma. JW declares trial funding from the Medical Research Council, Efficacy and Mechanism Evaluation Programme, Stroke Association and Health Foundation. WNW is funded by a Medical Research Council Clinician Scientist Fellowship (G0902303). WH reports honoraria from Boehringer Ingelheim, Daiichi Sankyo and Bayer, receipt of an unrestricted research grant from Boehringer Ingelheim to perform the ECASS 4 EXTEND trial, and past chairmanship of the ECASS 1-3 thrombolysis trials. GH, MKa, ML and GM declare no conflicts of interest.
References
- 1.Bath PMW, Lees KR, Schellinger PD, Altman H, Bland M, Hogg C, et al. for the European Stroke Organisation Outcomes Working Group Statistical Analysis of the Primary Outcome in Acute Stroke Trials. Stroke. 2012;43:1171–1178. doi: 10.1161/STROKEAHA.111.641456. [DOI] [PubMed] [Google Scholar]
- 2.Emberson J, Lees KR, Lyden P, Albers G, Bluhmki E, Brott T, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet. 2014;379:2352–2363. doi: 10.1016/S0140-6736(14)60584-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lees KR, Bluhmki E, von Kummer R, Brott TG, Toni D, Grotta JC, et al. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet. 2010;375:1695–1703. doi: 10.1016/S0140-6736(10)60491-6. [DOI] [PubMed] [Google Scholar]
- 4.Gage BF, Cardinalli AB, Owens DK. The effect of stroke and stroke prophylaxis with aspirin or warfarin on quality of life. Arch Int Med. 1996;156:1829–1836. [PubMed] [Google Scholar]
- 5.Hong KS, Saver JL. Quantifying the value of stroke disability outcomes: WHO global burden of disease project disability weights for each level of the modified Rankin Scale. Stroke. 2009;40:3828–3833. doi: 10.1161/STROKEAHA.109.561365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dawson J, Lees JS, Chang TP, Walters MR, Ali M, Davis SM, et al. Association between disability measures and healthcare costs after initial treatment for acute stroke. Stroke; a journal of cerebral circulation. 2007;38:1893–1898. doi: 10.1161/STROKEAHA.106.472381. [DOI] [PubMed] [Google Scholar]
- 7.Lees KR, Bath PMW, Schellinger PD, Kerr DM, Fulton R, Hacke W, et al. and for the European Stroke Organisation Outcomes Working Group Contemporary Outcome Measures in Acute Stroke Research: Choice of Primary Outcome Measure. Stroke. 2012;43:227–230. doi: 10.1161/STROKEAHA.111.641423. [DOI] [PubMed] [Google Scholar]
- 8.Rahlfs VW, Zimmermann H, Lees KR. Effect size measures and their relationships in stroke studies. Stroke. 2014;45:627–633. doi: 10.1161/STROKEAHA.113.003151. [DOI] [PubMed] [Google Scholar]
- 9.Stroke Thombolysis Trialists’ Collaboration. Details of a prospective protocol for a collaborative meta-analysis of individual participant data from all randomized trials of intravenous rt-PA vs. control: statistical analysis plan for the Stroke Thrombolysis Trialists' Collaborative meta-analysis. International Journal of Stroke. 2013;8:278–283. doi: 10.1111/ijs.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCullagh P. Regression models for ordinal data (with discussion) J R Statist Soc B. 1980;42:109–142. [Google Scholar]
- 11.Howard G, Waller JL, Voeks JH, Howard VJ, Jauch EC, Lees KR, et al. A Simple, Assumption-Free, and Clinically Interpretable Approach for Analysis of Modified Rankin Outcomes. Stroke. 2012;43:664–559. doi: 10.1161/STROKEAHA.111.632935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 13.Hacke W, Kaste M, Fieschi C, Toni D, Lesaffre E, von Kummer R, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Cooperative Acute Stroke Study (ECASS) JAMA. 1995;274:1017–1025. [PubMed] [Google Scholar]
- 14.Hacke W, Kaste M, Fieschi C, Toni D, Lesaffre E, von Kummer R, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian Acute Stroke Study Investigators. Lancet. 1998;352:1245–1251. doi: 10.1016/s0140-6736(98)08020-9. [DOI] [PubMed] [Google Scholar]
- 15.Hacke W, Kaste M, Bluhmki E, Brozman M, Dávalos A, Guidetti D, et al. Thrombolxsis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359(13):1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 16.Clark WM, Wissman S, Albers GW, Jhamandas JH, Madden KP, Hamilton S, et al. Recombinant tissue-type plasminogen activator (Alteplase) for ischemic stroke 3 to 5 hours after symptom onset. The ATLANTIS Study: a randomized controlled trial. Alteplase Thrombolysis for Acute Noninterventional Therapy in Ischemic Stroke. JAMA. 1999;282:2019–2026. doi: 10.1001/jama.282.21.2019. [DOI] [PubMed] [Google Scholar]
- 17.Davis SM, Donnan GA, Parsons MW, Levi C, Butcher KS, Peeters A, et al. Effects of alteplase beyond 3 h after stroke in the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET): a placebo-controlled randomised trial. Lancet Neurol. 2008;7:299–309. doi: 10.1016/S1474-4422(08)70044-9. [DOI] [PubMed] [Google Scholar]
- 18.Sandercock P, Wardlaw JM, Lindley RI, Dennis M, Cohen G, Murray G, et al. and the IST-3 collaborative group The benefits and harms of intravenous thrombolysis with recombinant tissue plasminogen activator within 6 h of acute ischaemic stroke (the Third International Stroke Trial [IST-3]): a randomised controlled trial. Lancet. 2012;379:2352–2363. doi: 10.1016/S0140-6736(12)60768-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whiteley WN, Emberson J, Lees KR, Blackwell L, Albers G, Bluhmki E, Brott T, Cohen G, Davis S, Donnan G, Grotta J, et al. What are the risks of intracerebral haemorrhage due to alteplase after acute ischaemic stroke? Results from an individual patient data meta-analysis of randomised trials. Lancet Neurology. 2016 doi: 10.1016/S1474-4422(16)30076-X. in press. [DOI] [PubMed] [Google Scholar]
- 20.The European Stroke Organisation (ESO) Executive Committee and the ESO Writing Committee. Guidelines for Management of Ischaemic Stroke and Transient Ischaemic Attack 2008. Cerebrovasc Dis. 2008;25:457–507. doi: 10.1159/000131083. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.