Abstract
Background
Pediatric tuberculous meningitis leads to high rates of mortality and morbidity. Prompt diagnosis and initiation of treatment are challenging; imaging findings play a key role in establishing the presumptive diagnosis. General brain imaging findings are well reported; however, specific data on cerebral vascular and spinal involvement in children are sparse.
Methods
This prospective cohort study examined admission and follow up computed tomography brain scans and magnetic resonance imaging scans of the brain, cerebral vessels (magnetic resonance angiogram) and spine at 3 weeks in children treated for tuberculous meningitis with hydrocephalus (inclusion criteria). Exclusion criteria were no hydrocephalus on admission, treatment of hydrocephalus or commencement of anti-TB treatment before study enrolment. Imaging findings were examined in association with outcome at 6 months.
Results
Forty-four patients (median age 3.3 [0.3-13.1] years) with definite (54%) or probable tuberculous meningitis were enrolled. Good clinical outcome was reported in 72%; the mortality rate was 16%. Infarcts were reported in 66% of patients and were predictive of poor outcome. Magnetic resonance angiogram abnormalities were reported in 55% of patients. Delayed tuberculomas developed in 11% of patients (after starting treatment). Spinal pathology was more common than expected, occurring in 76% of patients. Exudate in the spinal canal increased the difficulty of lumbar puncture and correlated with high cerebrospinal fluid protein content.
Conclusion
Tuberculous meningitis involves extensive pathology in the central nervous system. Severe infarction was predictive of poor outcome although this was not the case for angiographic abnormalities. Spinal disease occurs commonly and has important implications for diagnosis and treatment. Comprehensive imaging of the brain, spine and cerebral vessels adds insight into disease pathophysiology.
Keywords: pediatric tuberculous meningitis, radiology, brain, spine, cerebral vessels
Introduction
Tuberculous meningitis (TBM) is the most serious form of extrapulmonary tuberculosis (TB) affecting children and leading to high rates of mortality, and physical and cognitive morbidity 1–3. Prompt diagnosis and commencement of treatment are key to minimizing the risk of poor outcome 4; however, early diagnosis remains challenging due to the non-specific presentation, delayed cerebrospinal fluid (CSF) culture results, and poor culture yield. Radiological features suggestive of TBM are important in establishing the presumptive diagnosis, combined with the history, clinical examination, and CSF findings 5, 6. Follow up imaging is vital in guiding the treatment of intracranial pressure (ICP), providing information on disease evolution and predicting outcome 4, 7.
Basal enhancement, due to meningeal inflammation and the presence of exudate, is considered one of the most sensitive imaging findings in TBM 5. This exudate blocks the normal flow of CSF, contributing to the hydrocephalus (HCP) that accompanies TBM in 80-90% of patients 1, 4, 8. Hydrocephalus is of two forms; communicating hydrocephalus if the obstruction to CSF flow by the exudate occurs in the subarachnoid space, mostly at the level of basal cisterns, and non-communicating hydrocephalus when the obstruction occurs at the outlet foramina of the 4th ventricle at the cerebral aqueduct, caused by the circumferential compression of meningeal exudates, mesencephalic edema, intraventricular exudate or the presence of a tuberculoma 9, 10, although this occurs less frequently (12-25%) 1, 4, 11. The exudate may also lead to vasculitis and occlusion of the basal vessels 12. Consequently, the brain is at risk of ischemia and infarcts are reported in 30- 60% of pediatric patients with TBM 1, 4, 7, 8, 13. Vascular involvement has been documented on angiography 14–16, however, the literature on the subject is sparse, especially in children. Spinal pathology associated with TBM has not been widely investigated 17, 18 and has largely focused on adult patients presenting with neurological deficits 19–21, although spinal disease may be clinically silent.
To date, there has been no study in children with TBM examining brain parenchymal findings in conjunction with cerebrovascular and spinal involvement. This prospective study describes the imaging features on computed tomography (CT) brain on admission and magnetic resonance imaging (MRI) of the brain, cerebral vessels and spine after 3 weeks, and their association with outcome in a cohort of children with TBM and HCP.
Materials and Methods
Patient cohort
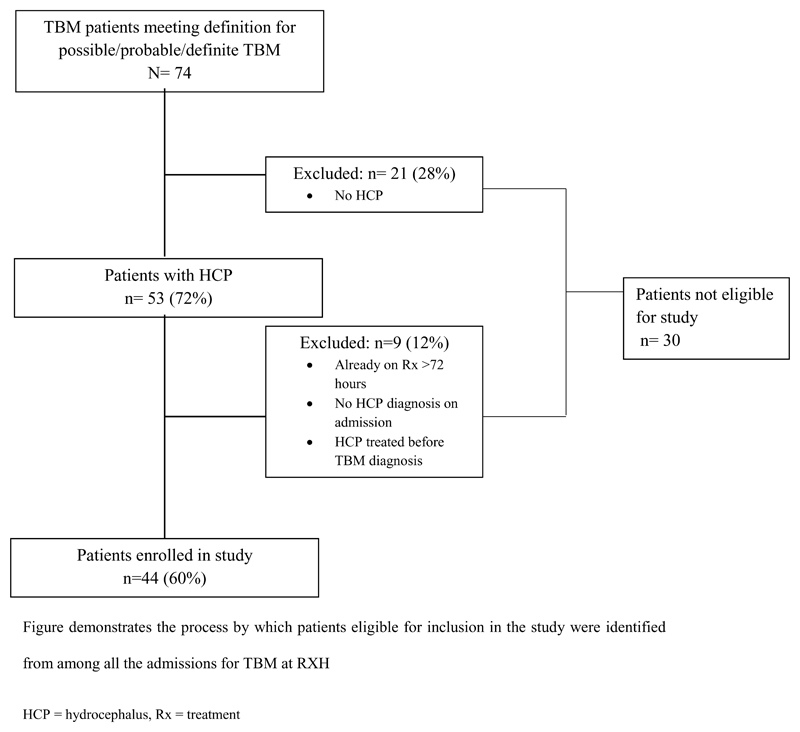
This study was approved by the Institutional Review Boards of the University of Cape Town, informed consent was obtained for all participants. Patients were selected as part of a prospective study on biomarkers conducted at Red Cross War Memorial Children’s Hospital over a 3-year period. All children treated for definite or probable TBM with associated HCP were enrolled (Figure 1). Definite TBM was defined by CSF positive for mycobacterium tuberculosis culture or visualization of acid-fast bacilli. Gene-Xpert (Cepheid) testing was not routine at the time of this study. Probable TBM was diagnosed according to a combination of clinical, bacteriological and radiological criteria 6. General demographic and clinical data were collected. Severity of disease was scored according to the refined British Medical Research Council criteria 22. Clinical outcome was assessed using the Pediatric Cerebral Performance Category Scale (PCPCS) 23 at 6 months after admission; scores were dichotomized as 1-3 (good outcome: normal function to moderate disability) and 4-6 (poor outcome: severe disability or death).
Figure 1. Flow diagram of patient selection.
Figure demonstrates the process by which patients eligible for inclusion in the study were identified from among all the admissions for TBM at Red Cross War Memorial Children's Hospital
HCP = hydrocephalus, Rx = treatment
All patients were treated with the standard anti-TB drugs rifampicin, isoniazid, pyrazinamide and ethambutol with adjunctive prednisone 24, 25. HCP was managed according to a previously described protocol 10. Briefly, patients underwent air encephalography 26–28 to establish the communicating nature of the HCP; this involved injecting 5-10 ml of air into the lumbar subarachnoid space (SAS) after which the patient was sat in an upright position for 30 minutes. Air in the ventricular system suggests communicating hydrocephalus and air in the basal cisterns or over the convexity of the brain but not in the ventricular system suggests non-communication. Non-communicating HCP was treated with an endoscopic third ventriculostomy or ventriculoperitoneal shunt (VPS); communicating HCP was treated medically with repeated lumbar punctures (LPs) and acetazolamide and Furosemide for 3 weeks or until the ICP had normalized 29.
Imaging protocol
As per standard imaging protocol all patients had a pre and post contrast-enhanced CT brain scan on admission. These patients commonly present with a decreased level of consciousness, which is not specific to TBM and may be due to multiple causes including trauma and bleeds. The pre-contrast scans are performed to rule out these other possible causes of the decreased level of consciousness and only patients with a suspected infection will proceed to a contrast-enhanced scan. Further non-contrasted CT scans were indicated for surveillance and in the case of deteriorating clinical status. An MRI of the brain and spine was scheduled for 3 weeks post-admission. MRI was not performed on admission due to limited resources. The 3 week time period was selected to allow for better evaluation of infarcts or tuberculomas that developed after admission. CT scans after discharge were performed to follow-up HCP and the evolution of intracranial pathology.
Admission CT brains pre- and post- administration of intravenous non-ionic contrast (Ultravist 1ml/kg) were performed on a 64 slice Phillips Brilliance scanner. MRI scans (Philips Achieva 1.5 Tesla) included axial T2, axial FLAIR, 3D T1 (acquired sagitally and reformatted in 3 planes), time of flight (TOF) magnetic resonance angiogram (MRA), diffusion weighted imaging (DWI) and axial T1 post-contrast (Magnevist –gadopentetic acid) of the brain and sagittal T2 and T1 pre and post contrast of the whole spine. Axial T2 and T1 post-contrast sequences were obtained through the area of pathology if a focal lesion was detected. Patients were either sedated or had a general anesthetic as per standard hospital protocol. Imaging was performed immediately after gadolinium contrast was administered. Chest X-Rays (CXRs) were performed routinely.
Imaging data
Scans were reviewed by three senior pediatric radiologists and a senior pediatric neurosurgeon according to criteria set by the reviewers due to the paucity of standardized criteria. Reviewers were blinded to patient clinical characteristics and outcome. Specific features recorded included severity of hydrocephalus (Figure 2), focal or diffuse enhancement (Figure 3), presence of precontrast hyperdensity, development and pattern of infarction (Figure 4), presence of tuberculomas, angiographic abnormalities, spinal arachnoiditis and pathology, and CXR findings. Details of how these variables were recorded are included in appendix. Each radiologic feature was assigned to one primary reviewer who examined that feature across all scans included for analysis. Thereafter the scans were all reviewed for a second time by second reviewer from amongst the team to ensure uniformity. Results were compared to the original radiology and neurosurgery reports. Where discrepancies in reporting were noted, scans were re-evaluated by the team until consensus was reached.
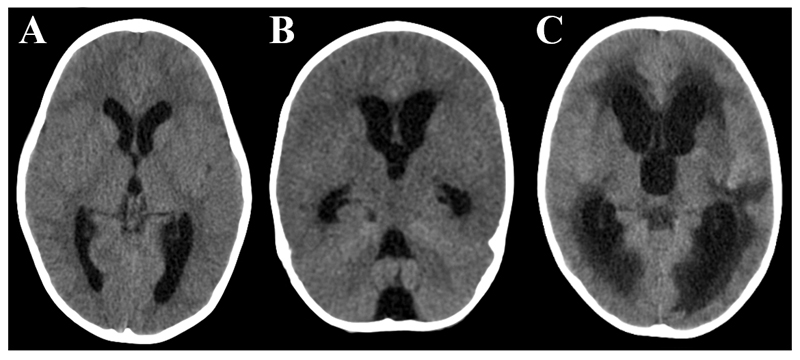
Figure 2. Hydrocephalus (HCP).
Axial uncontrasted CT showing A: mild hydrocephalus with a rounded appearance of the 3rd ventricle, B: moderate HCP with diffuse ventricular dilatation but no transependymal fluid shift (periventricular hypodensity), C: severe HCP with diffuse ventricular dilatation and marked transependymal fluid shift.
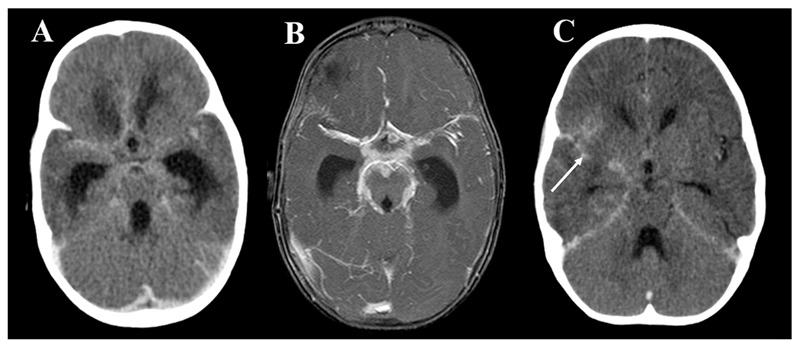
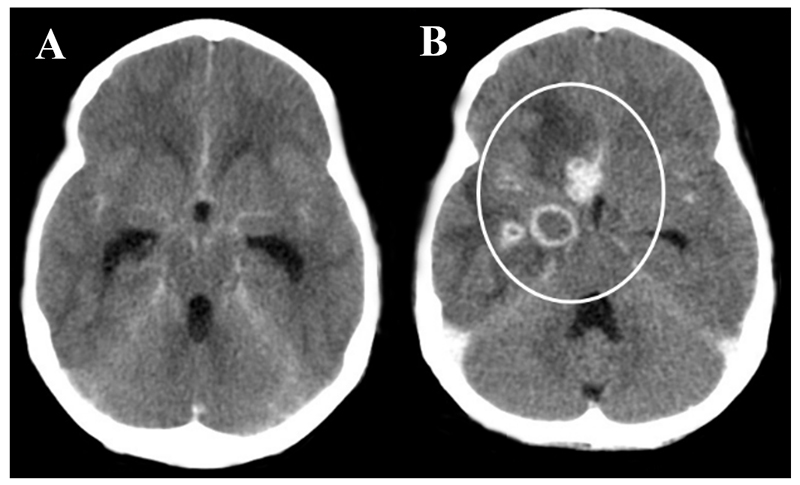
Figure 3. Enhancement.
A: Post-contrast axial CT and B: T1 weighted post-contrast MRI both showing hydrocephalus and exudate within the basal cisterns and surrounding the major vessels. C: post-contrast axial CT showing focal enhancement involving the right Sylvian fissure. Arrow indicates right sylvian fissure.
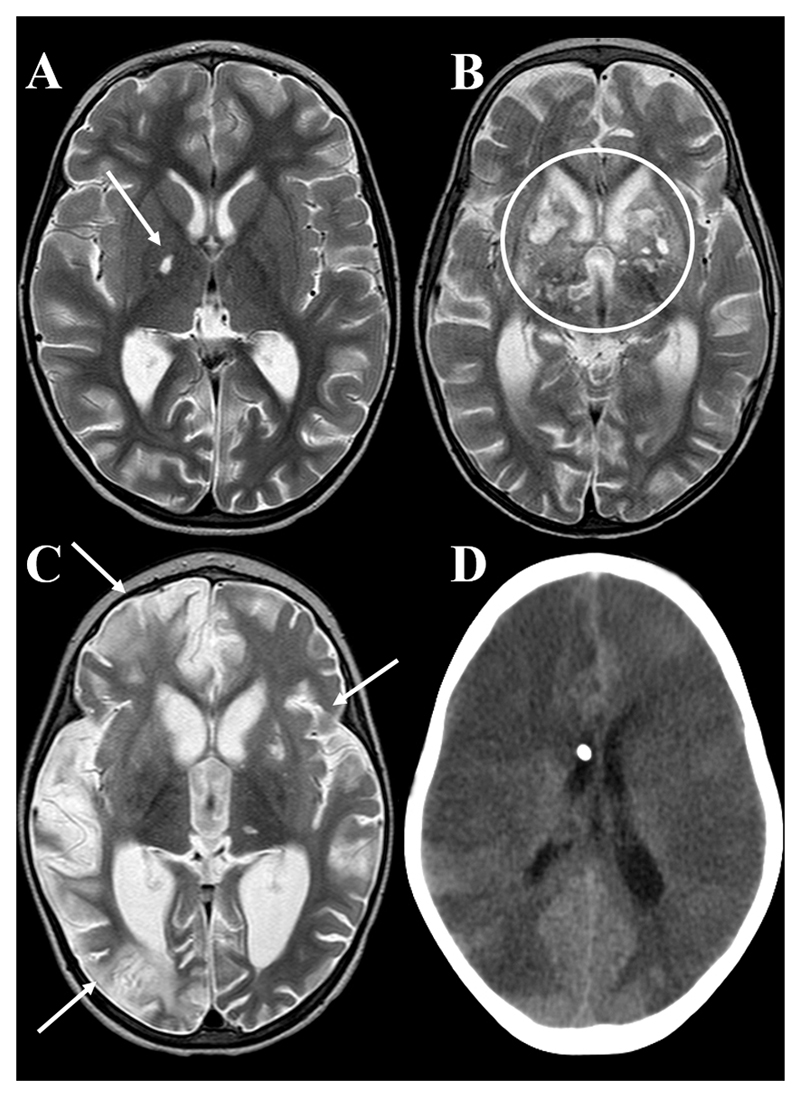
Figure 4. Infarcts.
Axial T2 weighted MRI showing A: small lacunar infarct involving the right globus pallidus (arrow), B: multiple bilateral MCA/ACA territory basal ganglia infarcts (circled), C: multiple hemispheric (arrows) and basal ganglia infarcts, D: axial uncontrasted CT showing global infarction in a patient who died before an MRI could be performed.
Analysis
Analyses examined the relationships between radiological features, patient characteristics (age, MRC staging on admission, culture result, seizures, symptom duration) and 6 month outcome. Data are presented as medians with a (min-max) range, or as number (percentage). Categorical variables were analyzed with chi-square tests, or Fisher’s exact tests for smaller sample sizes. Comparison of continuous variables across two groups was done with Student’s t test (normal distribution) or the Mann–Whitney U test (non-normal distribution). Comparisons across three groups were done with one-way analysis of variance for continuous variables (normal distribution) or Kruskall– Wallis test (non-normal distribution).
Results
Forty-four patients with a median age of 3.3 (0.3 – 13.1) years with definite (54%) or probable TBM (46%) and HCP were enrolled. All patients had drug sensitive TBM. Most patients presented in Stage II or III with an altered level of consciousness (90%, n=40) and focal neurology (n=21, 48%). The human immunodeficiency virus (HIV) positivity rate was 5% (n=2) – Table 1. Good clinical outcomes were recorded in 32 patients (73%), of whom 21 made a full clinical recovery (48%). Poor outcomes occurred in 12 patients (27%); 7 patients died (16%), 4 within the first 10 days post-admission. Poor outcome was associated with severe TBM according to MRC staging (p=0.01) and a shorter duration of symptoms (p=0.05), headaches and irritability were more frequently reported in patients who survived (p=0.02 and p=0.02 respectively).
Table 1. Admission clinical characteristics and outcome.
| Characteristic | Value |
|---|---|
| Demographic characteristics | |
| Age | 3.3 (0.3 – 13.1) years |
| Gender | Male 28 (64) |
| Admission characteristics | |
| MRC Staging admission | |
| 1 | 4 (9) |
| 2 | 31 (71) |
| 3 | 9 (21) |
| Symptom duration | 7.5 (1 – 90) days |
| Vomiting | 24 (55) |
| Headache a | 15 (40) |
| Fever | 30 (68) |
| Seizures | 14 (31) |
| Focal neurology b | 21 (48) |
| Altered level of consciousness | 40 (90) |
| Meningism | 34 (77) |
| Bulging fontanelle c | 5 (56) |
| HIV infection d | 2 (5) |
| Diagnostics | |
| CSF TB culture of AFB positive e | 22 (56) |
| Outcome | |
| Good outcome | 32 (72) |
| PCPS 1 | 21 (48) |
| PCPS 2 | 8 (18) |
| PCPS 3 | 3 (7) |
| Poor outcome | 12 (27) |
| PCPS 4 | 2 (5) |
| PCPS 5 | 3 (7) |
| PCPS 6 | 7 (16) |
Values reported as median and range or number (percentage)
Pre-verbal children under the age of 1.5 years excluded (n=7)
Focal neurology includes pupillary response, paresis, cranial nerve palsies and aphasia
For children with open fontanelles (n=9)
For children had HIV testing (n=43)
Only 39 patients had CSF sent for TB diagnostics, in 5 patients TB investigations were not requested in error or the volume of CSF was insufficient, all culture confirmed cases were drug sensitive, 4 of these cases were positive on Gene Xpert as well, this was not a routine test performed in all suspected TBM patients. PCPS: Pediatric Cerebral Performance Category Scale
Admission CT scans
HCP severity was moderate in half the cohort (n=24, 55%) -the inter-observer agreement in grading HCP severity was 88% (Kappa, p<0.001). Enhancement was present on 96% of scans (n=41), basal in almost all (n=40, 98%), and most often diffuse (n=39, 95%). Pre-contrast hyperdensity was visible in two thirds of the scans that demonstrated enhancement post-contrast (n=27, 66%). Seven patients (16%) presented with infarcts visible on admission CT, largely involving the MCA territory. Tuberculomas were reported in 3 patients (7%) - Table 2.
Table 2. Summary of radiology features overall.
| Radiology features | Admission CT (n=44) | Early deaths (n=4) | MRI (n= 39) | Overall (n=44) | Association with poor outcome |
|---|---|---|---|---|---|
| HCP present | n = 44 (100) | Treated a | n = 24 (62) | ||
| Severity | p=0.7 | ||||
| Mild | 11 (25) | 18 (75) | |||
| Moderate | 24 (55) | 6 (25) | |||
| Severe | 9 (21) | 2 (8) | |||
| Periventricular low density | 32 (73) | 9 (38) | |||
|
| |||||
| Type of HCP | |||||
|
| |||||
| Communicating | 34 (77) | ||||
|
| |||||
| Non-communicating | 3 (7) | ||||
|
| |||||
| Uncertain b | 7 (16) | ||||
|
| |||||
| Enhancement present | n = 41 (96) | No contrast given | n =38 (100) c | p=0.6 | |
| Focal | 2 (5) | 4 (11) | |||
| Diffuse | 39 (95) | 34 (90) | |||
| Basal | 40 (98) | 35 (92) | |||
| Pre-contrast hyperdensity | 27 (66) | p=0.18 | |||
|
| |||||
| Infarcts present | 9 (20) | 4 (100) | 25 (64) | 29 (66) | p=0.01* |
| No. of vascular territories | p<0.001* | ||||
| 1 | 6 (67) | 0 | 12 (48) | 12 (41) | |
| 2 | 3 (33) | 0 | 6 (24) | 6 (21) | |
| 3 | 0 | 0 | 4 (16) | 4 (14) | |
| 4 | 0 | 0 | 1 (4) | 1 (3) | |
| 5 | 0 | 0 | 1 (4) | 1 (3) | |
| 6 | 0 | 0 | 0 | 1 (3) | |
| 7 | 0 | 4 (100) | 1 (4) | 4 (14) | |
| MCA | 7 (78) | 4 (100) | 23(92) | ||
| ACA | 1 (11) | 4 (100) | 5 (20) | ||
| PCA | 0 | 4 (100) | 3 (12) | ||
| Vertebrobasilar | 1 (1) | 4 (100) | 6 (24) | ||
| Infarct laterality | p<0.001* | ||||
| Unilateral | 6 (67) | 14 (56) | 14 (48) | ||
| Bilateral | 3 (33) | 4 (100) | 11 (44) | 15 (51) | |
| Infarct number | p=0.003* | ||||
| Single | 5 (56) | 9 (36) | 11 (38) | ||
| Multiple | 4 (44) | 4 (100) | 14 (56) | 18 (62) | |
| Infarct size | p<0.001* | ||||
| Small | 3 (33) | 0 | 10 (40) | 10 (35) | |
| Moderate | 6 (67) | 0 | 15 (60) | 16 (55) | |
| Large | 0 | 4 (100) | 0 | 3 (10) | |
| DWI infarct evolution | n= 23 | p=0.08 | |||
| Acute d | 1 (4) | 1 (4) | |||
| Old e | 20 (87) | 20 (87) | |||
| Mixed f | 2 (9) | 2 (9) | |||
|
| |||||
| Tuberculoma present | 3 (7) | No contrast | 23 (59) | p=0.04* | |
|
| |||||
| Tuberculoma number | p=0.98 | ||||
| Single | 1 (33) | 10 (44) | |||
| Multiple | 2 (67) | 13 (57) | |||
This table summarises the frequency of radiology features reported on admission CT scans, final CT scans of the 4 patients who died before 3 week MRI (Early deaths), on 3 week MRI, and early deaths combined with MRI findings (Overall). The associations between radiology features and poor 6 month clinical outcome are indicated. Data are presented as number (percent) of patients.
HCP was treated with EVDs or VPS,
4 of these patients had a dry tap and the air encephalogram could not be definitively performed, and 1 patient was thought to have bacterial meningitis and underwent an immediate shunt procedure
Only 38 patients received contrast
infarcts < 10 days old
infarcts > 14 days old,
combination of infarcts < 10 days and >10 days old
MRI Brain Scans
MRI brain scans were conducted in 39 patients, an average of 26 ±11 days post-admission. Four patients died before an MRI could be performed and 1 patient was not scanned for logistical reasons. Ventriculomegaly was still present in 24 patients (61.5%), in 12 patients (30.8%) HCP was treated with an external ventricular drain (EVD) or VPS at the time of the scan, and in 3 patients (7.7%) HCP had resolved. Enhancement, predominantly basal (92%) and diffuse (90%), was present on all scans. Infarcts were reported in 25 patients (64%), largely limited to 1 or 2 vascular territories (n=18, 72%), most commonly involving the MCA territory (n=23, 92%) and basal ganglia (n=20, 80%). Only 8 of these 25 infarcted patients (24%) demonstrated infarcts on their admission CT scans; and by the time of DWI almost 90% of infarcts were more than 2 weeks old. Tuberculomas were observed in 23 patients (59%), largely multiple (57%) and bilateral (48%) and occurring in the cerebellum (27%).
Early deaths
Four patients died within 10 days of admission (median 4 days). Their admission scans demonstrated moderate to severe HCP (all received an EVD or VPS), mild enhancement and no tuberculomas. Infarcts were visible in only 2 patients’ admission scans, however, global infarction involving all 7 vascular territories was observed in all patients by their last scan, a median of 4 days (3-11 days) after admission (Figure 4D) - Table 2. One of these 4 patients was HIV infected.
Radiologic deterioration
Delayed or ‘paradoxical’ tuberculoma development was defined as the progression of established tuberculomas or the occurrence of new tuberculomas while on treatment as demonstrated on follow-up CT relative to admission CT scans. These were observed in 4 of the 37 patients (11%) who survived till 6 months, a median of 78 (47-106) days after admission and treatment; a positive culture of drug sensitive TB was confirmed in 3 of these patients. These delayed tuberculomas frequently occurred in the cisterns (50%), persistent basal enhancement was also present. In 2 asymptomatic patients the lesions were discovered incidentally on routine follow-up imaging, and 2 patients presented with signs of progressive HCP. In 7 patients (5 with culture-confirmed drug sensitive TBM and 2 with probable TBM) a ‘paradoxical’ worsening was also noted with respect to precontrast hypderdensity in the basal cisterns on follow up CT scans while on treatment (n=7, 54%) - Figure 5.
Figure 5. Late onset tuberculomas.
Axial post-contrast CT in a 10 year old, HIV uninfected patient with TBM A: on admission showing basal meningeal enhancement with no tuberculomas, B: 4 months after diagnosis and treatment showing multiple peripherally enhancing tuberculomas with surrounding edema (circled).
MR Angiograms
The MRA was a late addition to the study protocol and was performed in 29 patients. MRA abnormalities were detected in 16 patients (55%), predominantly involving the MCA (94%) singly or in combination with other vessels; the median number of vessels per patient was 2 (range 1-7). Abnormalities included focal stenosis, multifocal stenosis, irregular vessel caliber and occlusion (Figure 6).
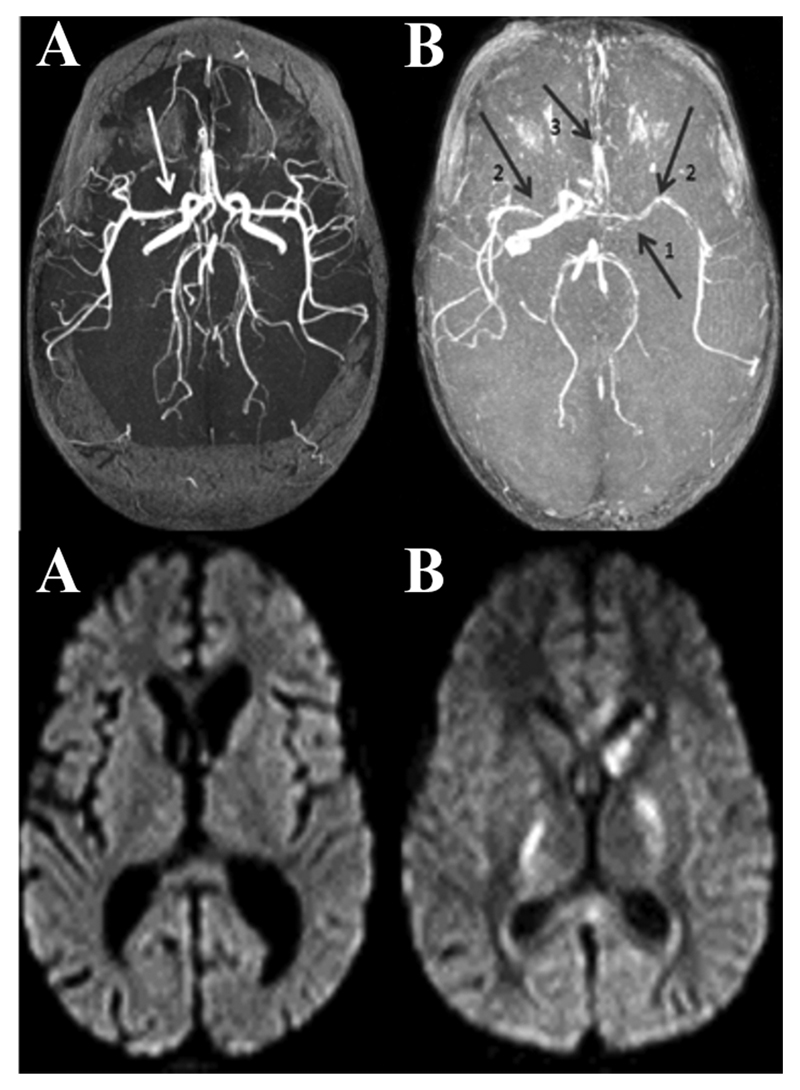
Figure 6. Maximum Intensity projection (MIP) MRA images.
Axial TOF MRA showing A: focal area of narrowing of the right MCA in a TBM patient who did not develop infarcts as demonstrated on the FLAIR image below, B: arrow 1: occluded left ICA, arrow 2: narrow irregular right and left MCA’s, and arrow 3: bilateral ACA narrowing3 in a TBM patient who developed multiple bilateral infarcts as demonstrated on the FLAIR image below.
Spinal imaging
MRI of the spine was obtained in 33 patients (4 died, 7 excluded due to technical reasons). Spinal disease was present in 25 children (76%) – Table 3. Features included spinal arachnoiditis in 24 patients (72% - Figure 7B-D), spinal tuberculomas in 6 patients (18% - Figure 7C) and intradural extramedullary plaque-like collections of exudate in 3 patients (9% - Figure 7D). There was no bony involvement. Spinal arachnoiditis involved enhancement of the cord (n=19/24, 79%) and the nerve roots (n= 23/24, 96%). Exudate filled the thecal sac below the conus and/or caused clumping of the spinal nerve roots in 12 patients (50%). The severity of exudate in the spinal canal was mild in 12 patients (50 %), moderate in 6 patients (25%) and severe in 6 patients (25 %), occasionally completely obliterating the caudal thecal sac (Figure 8B-D). Patients with spinal arachnoiditis were 4 times more likely to have a dry tap on LP (p=0.04- unable to release CSF or check opening pressure [OP]), the severity of spinal arachnoiditis was significantly associated with a dry tap (p=0.002) and all patients with spinal arachnoiditis had significantly higher CSF protein (median 2.9 (0.78-40.88)g/l). These patients also more commonly experienced a deterioration in ICP after it appeared to have normalized (p=0.03); this was not true for cerebral enhancement (p=0.9). Among the 6 patients with spinal tuberculomas, 5 had tuberculomas on the surface of the cord (83%) and 1 had an intramedullary tuberculoma (17%) – Figure 7C. Most patients with intradural spinal disease were asymptomatic (n=23/25, 92%), 2 patients complained of sensory deficit or pain in the legs.
Table 3. MR characteristics of the spine accompanying TBM (n=33).
| Feature | Value |
|---|---|
| Spinal disease overall | 25 (76) |
| Spinal arachnoiditis | 24 (73) |
| Severity | |
| Mild | 12 (50) |
| Moderate | 6 (25) |
| Severe | 6 (25) |
| Cord arachnoiditis | 19 (79) |
| Nerve root arachnoiditis | 23 (96) |
| Arachnoid nodules | 4 (17) |
| Exudate in the lumbar thecal sac | 7 (29) |
| Clumping of nerve roots | 10 (42) |
| Spinal Tuberculoma | 6 (18) |
| Single | 3 (50) |
| Multiple | 3 (50) |
| Number of patients with symptomatic spinal cord disease | 2 (8) |
Data are presented as number (percent) of patients reported to have the various forms of spinal pathology
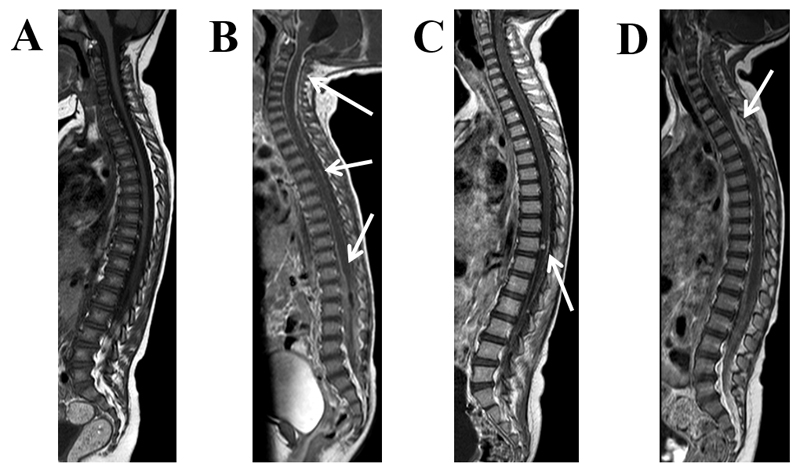
Figure 7. Pathology of the spine.
Sagittal T1 weighted MRI post-contrast in TBM: A: no enhancement, B: diffuse enhancement surrounding the cord and filling the thecal sac (arrows), C: cord and nerve root enhancement with an enhancing intramedullary tuberculoma (arrow), D: diffuse enhancement within the thecal sac and a focal intradural extramedullary plaque-like collection (arrow).
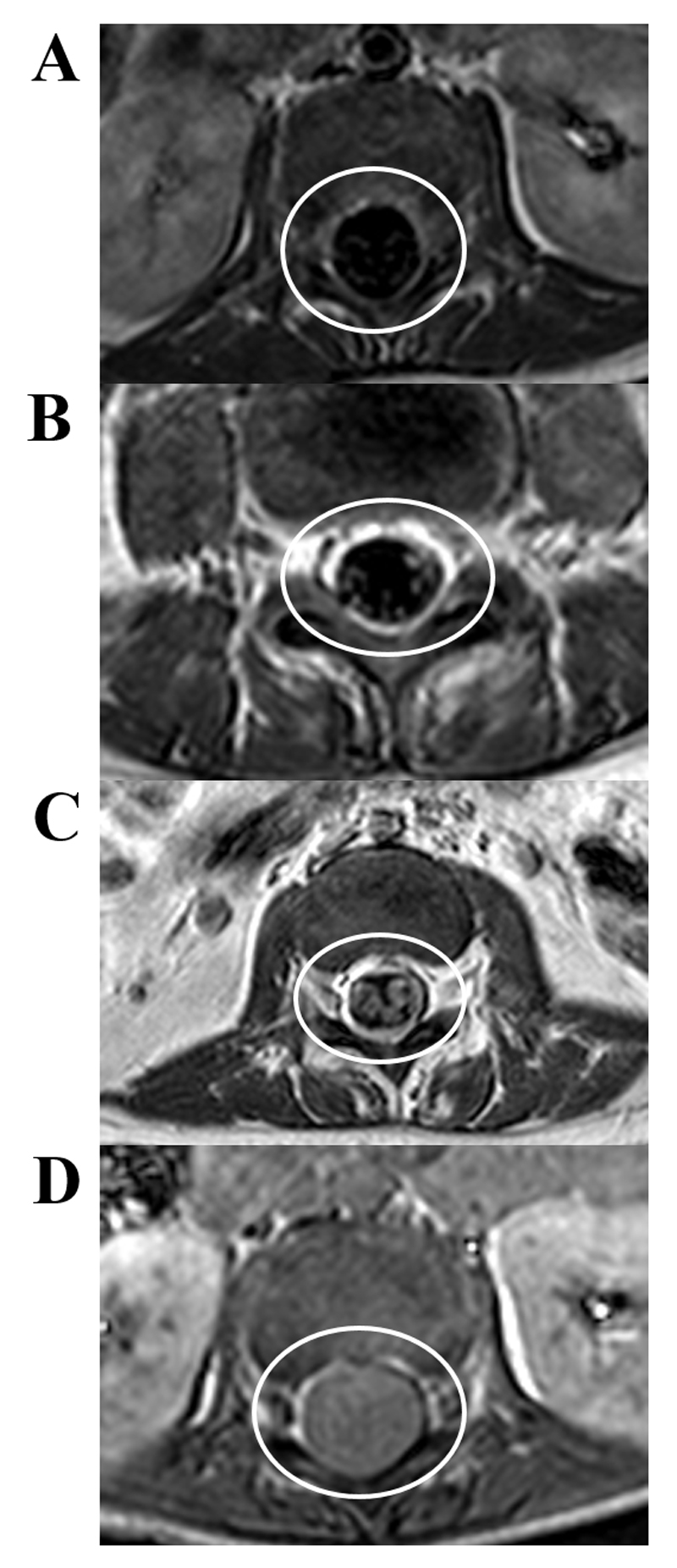
Figure 8. Spinal arachnoiditis.
Axial T1 weighted post-contrast MRI at the level of the cauda equina in TBM: A: normal thecal sac, no enhancement, B: mild arachnoiditis with nerve root enhancement, C: moderate arachnoiditis with enhancing thickened clumped nerve roots, D: severe arachnoiditis, hyperintense exudate filling the thecal sac obscuring all roots.
Chest imaging
CXRs were performed in 42 patients. Features suggestive of TB were documented in 22 patients (52%) and miliary TB in 5 of those patients (12%).
Association with patient characteristics and outcome
HCP severity was not associated with LP OP (p=0.48). Neither HCP severity nor infarcts (admission and follow-up) were related to MRC staging on admission (p=0.23 and p=0.43 respectively) or a history of seizures (p=0.24 and p=0.31 respectively). The severity of spinal arachnoiditis was not associated with severity of MRC Staging (p>0.05). A suggestive CXR was associated with older age (p=0.03). The sample size (n=2) was too small to examine imaging features in patients co-infected with HIV, however, previous studies in adults and children have shown that basal enhancement, non-communicating HCP, and parenchymal tuberculomas occur more frequently in HIV-uninfected (HIV-) patients. Mortality - Death was significantly associated with multiple (p=0.03), bilateral (p=0.006), and large infarcts (p<0.0001). The number of vascular territories involved in infarction was positively associated with death (p<0.001); however, vascular pathology on MRA was not (p=0.41).
Six month outcome
Tuberculomas (p=0.04) were associated with good outcomes. Infarcts were associated with poor outcome (p=0.01), particularly if they were bilateral (p<0.001) or large infarcts (p<0.001) involving multiple vascular territories (p<0.001), but this relationship was not found for vascular pathology on MRA (p=0.44). Multivariate analysis could not be performed for mortality or 6 month outcome because of the small sample size and too few events (death or poor outcome).
Discussion
This cohort of TBM patients demonstrated typical brain imaging features suggestive of TBM. Descriptions of MRA and spinal pathology provide insight into the pathology of TBM in the cerebral vessels, the extension of disease in the spinal canal in pediatric TBM and clinical implications thereof.
Radiology findings
As previously documented contrast enhancement was largely diffuse and involved the basal cisterns 1, 4, 11, 30, 31. Our findings confirmed the reliability of pre-contrast hyperdensity as an indicator of moderate-severe enhancement 5; however, cases with mild post-contrast enhancement may be missed, and contrast-enhanced scans are optimal in the diagnostic workup.
Infarcts were rarely visible on the admission CT scan; however, DWI at 3 weeks demonstrated that most infarcts were more than 2 weeks old. This suggests that progression to infarction likely occurs within the first week or 2 and/or that early CT scans may not reveal developing infarcts. In some cases a pathological process was clearly ongoing even after treatment initiation as evidenced by the progression to infarction and the late development of tuberculomas, even for antibiotic-sensitive organisms. For patients with a fatal aggressive disease process, infarcts progressed rapidly to involve all vascular territories within the first 10 days, even when no infarcts were visible on admission. Infarcts were associated with poor outcome; however, the nature of infarcts most likely affects the strength of this association: small infarcts occurred frequently in patients who made good recoveries; however, poor outcome was more likely if infarcts were large, multiple, and/or bilateral. Infarcts developed at locations throughout the brain, including the brain stem and cerebellum; however, as previously reported, the MCA territory and basal ganglia were most affected4, 8, 14, 30, 32 likely reflecting the predilection of the inflammatory exudate to collect in the Sylvian fissure.33. Similarly vascular pathology on MRA predominantly affected the MCA. The absence of a statistical association between vessel abnormality on MRA and infarction may be due to several reasons. Firstly, dynamic vascular pathology, like vasospasm, may have resolved by the time of MRA. Secondly, infarction may also result from other injurious processes including raised ICP, cytotoxic and vasogenic edema, seizures, and uncoupling of cerebral blood flow and metabolism. Thirdly, there are likely inter-individual differences in tolerance for vascular occlusion due to anatomical variation. Fourthly, MRA may be insensitive to pathology in small perforator arteries.
Tuberculomas were uncommon on admission CT scans. CT may not be sensitive to detect small lesions or lesions may develop late, despite a drug-sensitive microbe 8, 30, 34, 35. It has been suggested that the delayed or ‘paradoxical’ development of tuberculomas, observed in 11% of this cohort, may result from a hypersensitivity reaction to bacillary debris released in response to the bactericidal effects of TB medication 34, 36 or could represent an immune reconstitution response in immunosuppressed patients. Tuberculomas and infarcts were negatively correlated, and tuberculomas were predictive of survival and infarcts predictive of death. This may suggest that the underlying immunological processes in these children are different and that processes leading to tuberculoma development may be protective against infarction.
To our knowledge, this is the first study to prospectively describe spinal cord disease associated with TBM in a large pediatric cohort. Importantly, there were clinical consequences. Spinal arachnoiditis was present in over 70% of patients and ranged from a mild coating of the cord to near-complete obliteration of the subarachnoid space at the cauda equina. The resulting difficulty in releasing CSF and measuring the OP on LPs has significant clinical implications for establishing a presumptive or confirmed diagnosis, assessing raised ICP and establishing HCP management. Findings of extensive disease (such as in Figure 8D) were associated with failure to obtain CSF in the lumbar cistern, failure of air encephalography, and very high CSF protein content in lumbar CSF when obtained. Although often considered to be a marker of cranial subarachnoid disease, the high protein content may also reflect spinal disease and importantly stagnation of CSF due to a block in flow, typically described as Froin’s syndrome in association with mass lesions causing a block to CSF flow. This may also partly explain the failure of CSF protein values to correlate with clinical outcome and shunt survival in previous studies. These observations have implications for the reliability of CSF pressure measurement, the ability of air encephalography to distinguish between communicating and non-communicating hydrocephalus, the indication and safety of medical treatment of hydrocephalus, and decision-making between ventriculoperitoneal shuting or endoscopic third ventriculostomy if surgery is required. Most of these patients were asymptomatic and the spinal pathology would not have been detected without a MRI. The true incidence of neurological sequelae may have been underestimated as subtle neurological signs may have been missed, especially in patients who were young, had a depressed level of consciousness, and whose lack of mobility was attributed to general illness. Follow up over 1-2 years revealed subtle neurological signs in only 2 patients (8%) with spinal disease, and did not reveal worsening disease in this patient cohort. However, in our experience, occasionally patients present with late neurological deterioration due to progressive spinal disease after a previous presentation with TBM.
With respect to indications for spinal imaging, most centers have resource constraints and so routine spinal imaging may be unnecessary. At our institution, based on our clinical experience and the findings of this study, we request spinal imaging for patients in whom we suspect spinal disease due to failure to obtain CSF via LP (or minimal CSF), failed air encephalography, very high protein levels in the lumbar CSF, or obvious neurological symptoms or signs related to suspected spinal cord disease. Marked differences between lumbar and ventricular CSF, where available, may add to this suspicion.
Associations with patient variables
Our findings confirm that HCP severity on CT and CSF OPs are not significantly associated 4, 37. Factors that may contribute to this discrepancy include non-communicating HCP and spinal arachnoiditis blocking CSF flow in the spinal canal. This emphasizes the limitations of imaging in predicting ICP and the value of actual ICP measurement. No association was shown between infarcts overall and MRC staging. This may be due to the fact that initial neurological findings can be the result of pathophysiological phenomena other than permanent cerebral tissue death; including raised ICP, cerebral edema, hyponatremia, seizures and cranial arachnoiditis, which may be reversible and, therefore, account for the early clinical improvement in some patients. Additionally only multiple large infarcts were associated with poor outcome, lacunar infarcts were reported in patients who presented with less severe TBM and who made good recoveries. HCP severity and severity of spinal arachnoiditis were also not significantly associated with MRC staging, again emphasizing the role of other pathophysiological processes in determining clinical condition. Similarly, the absence of an association between seizure history and HCP, infarcts and tuberculomas probably reflects the same principle. The sample size of HIV infected patients was too small to meaningful evaluate differences between HIV infected and uninfected patients, however, previous studies in adults and children have shown that basal enhancement, non-communicating HCP, and parenchymal tuberculomas occur more frequently in HIV-uninfected (HIV-) patients 38–40.
Limitations
Due to differences in the sensitivity of CT and MRI in detecting TBM pathology 41 it is not possible to directly compare CT and MRI findings and comment comprehensively on progression of features over time. Additionally, the relative poorer sensitivity of CT may have underestimated infarcts on admission. However, in resource limited settings where TBM is most prevalent, admission MRI scanning is not likely to be standard of care. We chose to perform MRIs at 3 weeks to maximise detection of infarcts, however, the timing may have influenced our findings. MRIs could not be performed in patients who died early; therefore, MR data is skewed towards patients who survived, and more extensive vascular and spinal pathology may have been missed. However, these data still demonstrate the high frequency of abnormalities on spinal and MRA imaging; future studies involving earlier MRI, MRA or CT angiography may add further insights into the pathogenesis and evolution of TBM and clarify their role for clinical care. Timing of the MRI scan was subject to the requirements of clinical management and the availability of the scanner in a busy resource constrained center. The consequent variability in the timing of the scan may have led to some variability in the reported presence and severity of hydrocephalus and enhancement on MRI. Acute infarcts were reported in the 3 patients who had imaging within 14 days of admission, however, no additional infarcts developed between the MRI and follow up imaging and it is unlikely that the frequency of infarction overall was underestimated. Similarly, the frequency of tuberculomas overall was calculated using all imaging including follow up scans.
Conclusion
Radiologic features suggestive of TBM are readily identifiable on admission CT scans and are important in establishing a presumptive diagnosis. However, extended imaging with MRI, MRA, and spinal images provide a more complete picture of the extent of central nervous system involvement, important both for individual management of patients and a deeper understanding of the disease process. Repeat imaging provides valuable information on disease progression and resolution. However, the degree of follow up imaging may be limited by access to MR imaging and the risks of CT radiation. Similarly more advanced imaging techniques like perfusion imaging may add further to our understanding of this disease, but are not frequently available in locations that see the burden of TBM disease. Infarcts predict poor outcome, especially if large, multiple, and/or bilateral, emphasizing the need to better understand and detect the underlying ischemic process. Spinal disease associated with TBM occurs commonly in children. Although it is often clinically silent, it may have important clinical consequences. The reliability of LP opening pressure, the ability to obtain CSF from the lumbar cistern, the interpretation of the protein content in CSF, and the success of air encephalography may be affected by spinal disease and so affects our understanding about intracranial dynamics and decisions about medical treatment, ventriculoperitoneal shunting or endoscopic third ventriculostomy in the management of hydrocephalus. Although perhaps not indicated in all patients, spinal imaging may be of value in selected patients.
Supplementary Material
Source of Funding
This work was supported by the Clinical Infectious Diseases and Research Initiative-Wellcome Trust [084323] to [UKR]. AF is supported by the National Research Foundation SARChI Chair of Clinical Neurosciences.
Footnotes
Conflicts of interest
The authors have no conflicts of interest to declare.
References
- 1.van Well GT, Paes BF, Terwee CB, Springer P, Roord JJ, Donald PR, van Furth AM, Schoeman JF. Twenty years of pediatric tuberculous meningitis: A retrospective cohort study in the Western Cape of South Africa. Pediatrics. 2009;123:e1–8. doi: 10.1542/peds.2008-1353. [DOI] [PubMed] [Google Scholar]
- 2.Springer P, Swanevelder S, van Toorn R, van Rensburg AJ, Schoeman J. Cerebral infarction and neurodevelopmental outcome in childhood tuberculous meningitis. Eur J Paediatr Neurol. 2009 Jul;13:343–9. doi: 10.1016/j.ejpn.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Ramzan A, Nayil K, Asimi R, Wani A, Makhdoomi R, Jain A. Childhood tubercular meningitis: An institutional experience and analysis of predictors of outcome. Pediatr Neurol. 2013 Jan;48:30–5. doi: 10.1016/j.pediatrneurol.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 4.Schoeman JF, Van Zyl LE, Laubscher JA, Donald PR. Serial CT scanning in childhood tuberculous meningitis: Prognostic features in 198 cases. J Child Neurol. 1995 Jul;10:320–9. doi: 10.1177/088307389501000417. [DOI] [PubMed] [Google Scholar]
- 5.Andronikou S, Smith B, Hatherhill M, Douis H, Wilmshurst J. Definitive neuroradiological diagnostic features of tuberculous meningitis in children. Pediatr Radiol. 2004 Nov;34:876–85. doi: 10.1007/s00247-004-1237-1. [DOI] [PubMed] [Google Scholar]
- 6.Marais S, Thwaites G, Schoeman JF, Torok ME, Misra UK, Prasad K, Donald PR, Wilkinson RJ, Marais BJ. Tuberculous meningitis: A uniform case definition for use in clinical research. Lancet Infect Dis. 2010 Sep 3;10:803–12. doi: 10.1016/S1473-3099(10)70138-9. [DOI] [PubMed] [Google Scholar]
- 7.Andronikou S, Wieselthaler N, Smith B, Douis H, Fieggen AG, van Toorn R, Wilmshurst J. Value of early follow-up CT in paediatric tuberculous meningitis. Pediatr Radiol. 2005 Nov;35:1092–9. doi: 10.1007/s00247-005-1549-9. [DOI] [PubMed] [Google Scholar]
- 8.Farinha NJ, Razali KA, Holzel H, Morgan G, Novelli VM. Tuberculosis of the central nervous system in children: A 20-year survey. J Infect. 2000 Jul;41:61–8. doi: 10.1053/jinf.2000.0692. [DOI] [PubMed] [Google Scholar]
- 9.Lamprecht D, Schoeman J, Donald P, Hartzenberg H. Ventriculoperitoneal shunting in childhood tuberculous meningitis. Br J Neurosurg. 2001 Apr;15:119–25. doi: 10.1080/02688690020036801. [DOI] [PubMed] [Google Scholar]
- 10.Figaji AA, Fieggen AG. The neurosurgical and acute care management of tuberculous meningitis: Evidence and current practice. Tuberculosis (Edinb) 2010 Oct 20;90:393–40. doi: 10.1016/j.tube.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 11.Ranjan P, Kalita J, Misra UK. Serial study of clinical and CT changes in tuberculous meningitis. Neuroradiology. 2003 May;45:277–82. doi: 10.1007/s00234-003-0958-4. [DOI] [PubMed] [Google Scholar]
- 12.Misra UK, Kalita J, Maurya PK. Stroke in tuberculous meningitis. J Neurol Sci. 2011 Apr 15;303:22–30. doi: 10.1016/j.jns.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 13.Kalita J, Misra UK, Nair PP. Predictors of stroke and its significance in the outcome of tuberculous meningitis. J Stroke Cerebrovasc Dis. 2009 Jul-Aug;18:251–8. doi: 10.1016/j.jstrokecerebrovasdis.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 14.Kalita J, Prasad S, Maurya PK, Kumar S, Misra UK. MR angiography in tuberculous meningitis. Acta Radiol. 2012 Feb 27;3:324–9. doi: 10.1258/ar.2012.110712. [DOI] [PubMed] [Google Scholar]
- 15.Gupta RK, Gupta S, Singh D, Sharma B, Kohli A, Gujral RB. MR imaging and angiography in tuberculous meningitis. Neuroradiology. 1994;36:87–92. doi: 10.1007/BF00588066. [DOI] [PubMed] [Google Scholar]
- 16.Singh B, Garg RK, Singh MK, Verma R, Malhotra HS, Jain A, Singh R, Kohli N, Phadke RV, Shukla R, Parihar A. Computed tomography angiography in patients with tuberculous meningitis. J Infect. 2012 Mar 22;64:565–72. doi: 10.1016/j.jinf.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 17.Lan SH, Chang WN, Lu CH, Lui CC, Chang HW. Cerebral infarction in chronic meningitis: A comparison of tuberculous meningitis and cryptococcal meningitis. QJM. 2001 May;94:247–53. doi: 10.1093/qjmed/94.5.247. [DOI] [PubMed] [Google Scholar]
- 18.Srivastava T, Kochar DK. Asymptomatic spinal arachnoiditis in patients with tuberculous meningitis. Neuroradiology. 2003 Oct;45:727–9. doi: 10.1007/s00234-003-1077-y. [DOI] [PubMed] [Google Scholar]
- 19.Moghtaderi A, Alavi Naini R. Tuberculous radiculomyelitis: Review and presentation of five patients. Int J Tuberc Lung Dis. 2003 Dec;7:1186–90. [PubMed] [Google Scholar]
- 20.Chotmongkol V, Kitkuandee A, Limpawattana P. Tuberculous radiculomyelitis (arachnoiditis) associated with tuberculous meningitis. Southeast Asian J Trop Med Public Health. 2005 May;36:722–4. [PubMed] [Google Scholar]
- 21.Poon TL, Ho WS, Pang KY, Wong CK. Tuberculous meningitis with spinal tuberculous arachnoiditis. Hong Kong Med J. 2003 Feb;9:59–61. [PubMed] [Google Scholar]
- 22.van Toorn R, Springer P, Laubscher JA, Schoeman JF. Value of different staging systems for predicting neurological outcome in childhood tuberculous meningitis. Int J Tuberc Lung Dis. 2012 Mar 8; doi: 10.5588/ijtld.11.0648. [DOI] [PubMed] [Google Scholar]
- 23.Fiser DH. Assessing the outcome of pediatric intensive care. J Pediatr. 1992;121:68–74. doi: 10.1016/s0022-3476(05)82544-2. [DOI] [PubMed] [Google Scholar]
- 24.van Toorn R, Schaaf HS, Laubscher JA, Elsland SL, Donald PR, Schoeman JF. Short intensified treatment in children with drug-susceptible tuberculous meningitis. Pediatr Infect Dis J. 2013 Oct 28;33:248–52. doi: 10.1097/INF.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 25.Donald PR, Schoeman JF, Van Zyl LE, De Villiers JN, Pretorius M, Springer P. Intensive short course chemotherapy in the management of tuberculous meningitis. Int J Tuberc Lung Dis. 1998 Sep;2:704–11. [PubMed] [Google Scholar]
- 26.Figaji AA, Fieggen AG, Peter JC. Air encephalography for hydrocephalus in the era of neuroendoscopy. Childs Nerv Syst. 2005 Jul;21:559–65. doi: 10.1007/s00381-004-1119-8. [DOI] [PubMed] [Google Scholar]
- 27.Schoeman J, Donald P, van Zyl L, Keet M, Wait J. Tuberculous hydrocephalus: Comparison of different treatments with regard to ICP, ventricular size and clinical outcome. Dev Med Child Neurol. 1991 May;33:396–405. doi: 10.1111/j.1469-8749.1991.tb14899.x. [DOI] [PubMed] [Google Scholar]
- 28.Figaji AA, Fieggen AG. Endoscopic challenges and applications in tuberculous meningitis. World Neurosurg. 2013 Feb;79:S24.e9–14. doi: 10.1016/j.wneu.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 29.Avery RA, Shah SS, Licht DJ, Seiden JA, Huh JW, Boswinkel J, Ruppe MD, Chew A, Mistry RD, Liu GT. Reference range for cerebrospinal fluid opening pressure in children. N Engl J Med. 2010 Aug 26;363:891–3. doi: 10.1056/NEJMc1004957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thwaites GE, Macmullen-Price J, Tran TH, Pham PM, Nguyen TD, Simmons CP, White NJ, Tran TH, Summers D, Farrar JJ. Serial MRI to determine the effect of dexamethasone on the cerebral pathology of tuberculous meningitis: An observational study. Lancet Neurol. 2007 Mar;6:230–6. doi: 10.1016/S1474-4422(07)70034-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schoeman J, Hewlett R, Donald P. MR of childhood tuberculous meningitis. Neuroradiology. 1988;30:473–7. doi: 10.1007/BF00339685. [DOI] [PubMed] [Google Scholar]
- 32.Andronikou S, Wilmshurst J, Hatherill M, VanToorn R. Distribution of brain infarction in children with tuberculous meningitis and correlation with outcome score at 6 months. Pediatr Radiol. 2006 Dec;36:1289–94. doi: 10.1007/s00247-006-0319-7. [DOI] [PubMed] [Google Scholar]
- 33.Dastur DK, Manghani DK, Udani PM. Pathology and pathogenetic mechanisms in neurotuberculosis. Radiol Clin North Am. 1995 Jul;33:733–52. [PubMed] [Google Scholar]
- 34.Shah I, Borse S. Paradoxical tuberculomas after completion of antituberculous treatment. Trop Med Health. 2012 Apr;40:15–7. doi: 10.2149/tmh.2012-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ravenscroft A, Schoeman JF, Donald PR. Tuberculous granulomas in childhood tuberculous meningitis: Radiological features and course. J Trop Pediatr. 2001 Feb;47:5–12. doi: 10.1093/tropej/47.1.5. [DOI] [PubMed] [Google Scholar]
- 36.Bernaerts A, Vanhoenacker FM, Parizel PM, Van Goethem JW, Van Altena R, Laridon A, De Roeck J, Coeman V, De Schepper AM. Tuberculosis of the central nervous system: Overview of neuroradiological findings. Eur Radiol. 2003 Aug;13:1876–90. doi: 10.1007/s00330-002-1608-7. [DOI] [PubMed] [Google Scholar]
- 37.Schoeman JF, Laubscher JA, Donald PR. Serial lumbar CSF pressure measurements and cranial computed tomographic findings in childhood tuberculous meningitis. Childs Nerv Syst. 2000 Apr;16:203–8. doi: 10.1007/s003810050497. discussion 209. [DOI] [PubMed] [Google Scholar]
- 38.van der Weert EM, Hartgers NM, Schaaf HS, Eley BS, Pitcher RD, Wieselthaler NA, Laubscher R, Donald PR, Schoeman JF. Comparison of diagnostic criteria of tuberculous meningitis in human immunodeficiency virus-infected and uninfected children. Pediatr Infect Dis J. 2006 Jan;25:65–9. doi: 10.1097/01.inf.0000183751.75880.f8. [DOI] [PubMed] [Google Scholar]
- 39.Katrak SM, Shembalkar PK, Bijwe SR, Bhandarkar LD. The clinical, radiological and pathological profile of tuberculous meningitis in patients with and without human immunodeficiency virus infection. J Neurol Sci. 2000 Dec 1;181:118–26. doi: 10.1016/s0022-510x(00)00440-8. [DOI] [PubMed] [Google Scholar]
- 40.Dekker G, Andronikou S, van Toorn R, Scheepers S, Brandt A, Ackermann C. MRI findings in children with tuberculous meningitis: A comparison of HIV-infected and non-infected patients. Childs Nerv Syst. 2011 Apr 15; doi: 10.1007/s00381-011-1451-8. [DOI] [PubMed] [Google Scholar]
- 41.Pienaar M, Andronikou S, van Toorn R. MRI to demonstrate diagnostic features and complications of TBM not seen with CT. Childs Nerv Syst. 2009 Aug;25:941–7. doi: 10.1007/s00381-008-0785-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.