Abstract
The development of intensity-modulated radiation therapy has played a major role in improving outcomes and decreasing morbidity in head and neck cancer patients. This review addresses this vital modality with a focus on the important role of the head and neck surgeon. The technique as well as its benefits and points of caution are outlined, the definitions of tumor and treatment volumes are discussed, and the dose and fractionation are detailed. Following this are several sections dedicated to the role of the head and neck surgeon in the planning of both definitive and post-operative radiation therapy to the primary site and neck. There is a focus throughout on anatomic and surgical considerations; commonly encountered situations are illustrated. With a deeper understanding of this technique and their own pivotal contribution to target delineation, head and neck surgeons will be poised to expand their role and improve cancer care for their patients.
Indexing: Cancer, radiotherapy, IMRT, head and neck, target delineation
Introduction
Intensity-modulated radiation therapy (IMRT) is a focused type of three-dimensional conformal radiotherapy. IMRT has the ability to precisely deliver a very high dose to the tumor and at the same time minimize the amount of radiation received by the tumor’s surrounding normal tissues, thereby increasing the therapeutic ratio(1). While this factor allows for the intensification of the radiation dose to the tumor, it introduces the risk of under-treating any unappreciated tumor extension outside of the targeted volume, a phenomenon known as marginal miss(2–4). Marginal misses may result in unforgivable tumor recurrences. This can be devastating, as surgical salvage may not be possible. Therefore, with IMRT target definition is a critical step to ensure successful results. IMRT in the head and neck can be a complex endeavor—even for the most experienced radiation oncologist—and is an area in which head and neck surgeons can make valuable contributions to achieve cancer cure. Indeed, while accurate treatment planning software and a state of the art delivery system are essential in implementing IMRT for head and neck cancer, input from a multidisciplinary team made up of the radiation oncologist, medical physicist, medical dosimetrist, radiation therapist, radiologist, pathologist, and head and neck surgeon is also crucial(5). Head and neck surgeons involved in cancer treatment must therefore have some basic understanding of this new technology. Head and neck surgeons should be involved with the radiation oncologist in defining the tumor target volume in both definitive radiotherapy cases as well as in the post-operative setting, so that tumor recurrence secondary to inaccurate target delineation does not occur. Moreover, as we will discuss below, head and neck surgeons’ decisions during the surgery itself may fundamentally change the post-operative radiation planning.
Throughout this article we will review the current method of IMRT planning in the head and neck, paying special attention to the role of the head and neck surgeon during this procedure and highlighting the importance of a multidisciplinary case-by-case approach.
Intensity-Modulated Radiation Therapy: definition, benefits, risks and opportunities
IMRT is a high precision conformal radiotherapy technique in which multiple beams, each with a non-uniform intensity profile, create dose distributions that can exquisitely conform to convex and concave structures alike (see figures 1–3)(6, 7). The radiation oncologist delineates the target and normal structures on treatment planning computed tomography (CT) scans using specialized software. Computerized algorithms then determine the optimal radiation beam parameters (shape and intensity profiles, number of beams, incident directions, etc.) that are needed to meet the prescribed normal tissue tolerances while delivering the full dose to the target volumes. Multileaf collimators—devices made up of individual, independently moving metallic “leaves”—allow for the delivery of intensity-modulated fields by blocking the radiation beams, thereby permitting delivery of the calculated, non-homogenous dose. This sophisticated approach, at the core of which is the idea of inverse treatment planning (i.e. delineating structures first, calculating dose profiles using these structures second), is essential in head and neck radiation therapy and differs markedly from the historical method of forward planning, in which beam angles are chosen a priori and are then adjusted to meet target and tissue dose constraints.
Figure 1.
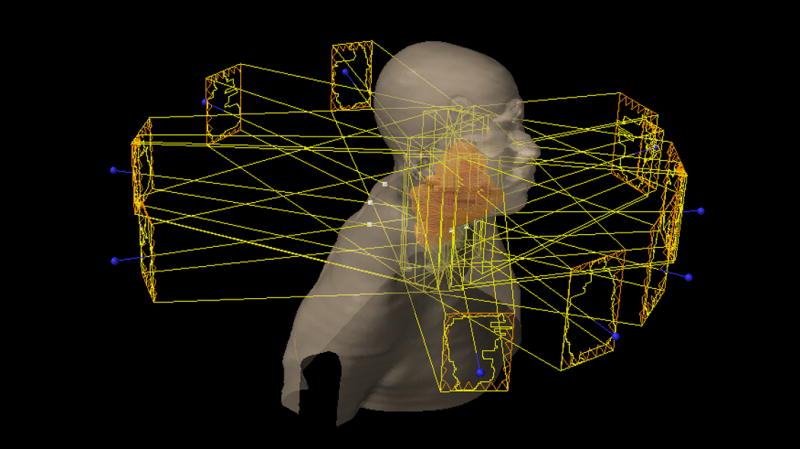
Figure 3.
All of the above factors allow IMRT to achieve sharp dose gradients near the targeted volumes, decreasing the volume of healthy tissue irradiated to high and moderate radiation dose levels. The major salivary glands, the mucosa of the pharynx or of the oral cavity, the larynx, the constrictor muscles, the mandible, and the inner and middle ears are examples of structures that can be partially spared when clinically feasible, due to the dose conformality afforded by IMRT(8–11). Including these anatomical structures in the low dose regions results in reductions in acute and, more significantly, late toxicity such as xerostomia and dysphagia. With the increasing use of more toxic chemoradiotherapy regimens(12, 13), the reduction in toxicity that can be achieved with IMRT by sparing functionally relevant tissues from high and moderate radiation doses becomes even more important. IMRT does not only reduce treatment-related morbidity: another of its promising capabilities is the potential improvement of loco-regional control through radiation dose intensification to the primary tumor (“dose escalation”)(14, 15).
There is, however, a dangerous consequence of the highly conformal dose distributions of IMRT: a higher sensitivity to improper target delineation, which can lead to under-dosing of regions that could be covered by a conventional field or over-dosing of normal structures not considered during the planning procedure. Put another way, on one hand IMRT may increase the risk of under-treating any unappreciated macroscopic or microscopic tumor extension, leading to a higher risk of recurrence, while on the other hand unnecessarily high radiation doses to normal tissues can be associated with considerable and potentially avoidable toxicity (i.e. acute mucosal and cutaneous reactions, trismus, neck fibrosis and spasm, osteoradionecrosis, etc.)(16, 17). Another less commonly discussed drawback of IMRT is a slightly elevated scattered dose to the rest of the body when compared with that occurring in earlier treatment modalities, raising concerns for a possible increased risk of radiation-induced malignancy (around 1.75%) in patients surviving more than 10 years after treatment(18, 19). However, in head and neck tumors this increased risk is controversial and remains under investigation(20). Moreover, it has not yet been specifically evaluated in non-smokers treated for head and neck cancers. Future studies will likely investigate this effect on the increasing and ever younger human papillomavirus positive patient population. IMRT must be used carefully, and proper delineation of the target is crucial to the technique’s success.
Recently, imaged guided IMRT or simply image-guided radiation therapy (IGRT) has become a complement to IMRT to ensure the safe delivery of a highly conformal treatment through the facilitation of convenient and frequent imaging of patient anatomy throughout the treatment course(21). These sophisticated imaging strategies help to avoid treatment setup errors secondary to internal soft tissue motion and deformation occurring due to breathing, weight loss, tissue involution, etc. Furthermore, the increasing precision and conformality afforded by IMRT coupled with the imaging capabilities of IGRT creates the entirely new possibility of re-planning(22). More specifically, during the 6–7 week treatment course the tumor volume and patient anatomy may visibly change and this can in turn modify doses to both the targets and to the organs at risk. Such patients may benefit from an adaptation of IMRT treatment plans. This re-planning procedure is called adaptive radiotherapy(23, 24).
Despite the latest advances in patient positioning and dose delivery, IMRT and IGRT remain therapeutic techniques whose results are highly dependent on the managing team, underscoring the importance of a multidisciplinary approach to radiation treatment planning.
Definition of Gross Tumor Volume, Clinical Target Volume, and Planning Target Volume
GTV: Gross Tumor Volume
The GTV comprises the macroscopic primary tumor and the grossly involved neck nodes, and it contains the highest tumor cell density. Delineation of the GTV must take into account radiological and clinical findings. CT is the main source of information used in defining the GTV for most head and neck sites. In some anatomical locations such as the tongue, the skull base, the nasopharynx or the paranasal sinuses, magnetic resonance imaging (MRI) may aid in defining the boundaries of the macroscopic tumor volume(25). Positron emission tomography (PET) provides functional information that may complement the anatomic delineation of CT and MRI, especially in difficult cases such as tumor recurrences(26).
CTV: Clinical Target Volume
The CTV encompasses the tissues suspicious for microscopic tumor invasion. In the head and neck, an area with a high density of lymphatics and complex anatomy, there is a high risk of subclinical local and nodal disease. Therefore, adequate delineation of the CTVs is crucial for loco-regional control and survival. The patterns of tumor spread are based classically on histological examination of post-mortem and surgical specimens(27–30) and, more recently, on models of cancer progression based on the cancerization field concept and the cancer stem cell network hypothesis(31, 32). Therefore, rather than using a simple and uniform expansion of the GTV, the precise definition of the CTV in IMRT requires a careful consideration of the above factors.
PTV: Planning Target Volume
To ensure a homogeneous dose to the GTV and the CTV during a fractionated course of radiation, margins must be added to account for both systematic and random set-up errors (patient movement, internal organ motion, deformation, and change, mechanical uncertainties, etc.). Usually a 3–5 mm margin is routinely added to generate the PTV from the GTV and the CTV to account for this daily setup variability(21). Due to its mobility, the larynx may require a 5–10 mm margin(33). As we have mentioned, utilizing advanced IGRT increases the precision and decreases the uncertainty of treatment. This allows the PTVs to be reduced, lowering the doses administered to adjacent normal tissues and hence the treatment sequelae.
Dose and fractionation
Radiotherapy regimens vary internationally. Fractions of 2.0Gy or less delivered 5 days a week are the usual standard of care. However, there are alternative fractionation schedules: hypofractionation, accelerated fractionation, and hyperfractionation(34, 35). Regardless of the fractionation schedule, the GTV, high-risk CTV (the CTV around the GTV and the first echelon nodes), and low-risk CTV (and their respective PTVs) receive different doses. The target volumes can be denoted by the dose level prescribed to those volumes: for example, CTV54 will indicate a particular CTV receiving 54Gy. Although there are differences across institutions, the most commonly prescribed doses are biologically equivalent to 70Gy, 60Gy and 54Gy at 2.0Gy per fraction to the PTV encompassing all gross disease and suspicious nodes, the high-risk PTV, and the low-risk PTV, respectively. At our institutions, we prefer a simultaneous integrated boost with a dose-painting IMRT technique in which different dose levels are assigned to the various target volumes in a once-a-day regimen. The median prescribed dose is 70Gy in 2.12Gy daily fractions over 33 days to the PTV70, 59.4Gy in 1.8Gy daily fractions to the PTV59.4, and 54Gy in 1.64Gy daily fractions to the PTV54(36).
The dose and fractionation may also be influenced by the inclusion or exclusion of concurrent chemotherapy. With higher doses per fraction the toxicity of concomitant treatment can increase. Conversely, low dose per fraction (such as 1.6Gy/fraction) to the low-risk CTV without chemotherapy may be insufficient to control microscopic disease(37–39). Another important point concerns the post-operative setting due to changes in tissue vascularization following surgery. Without concomitant chemotherapy, doses per fraction lower than 2Gy should be used cautiously during post-surgical adjuvant radiation treatment. This means that a previous surgical approach may compromise the ability to simultaneously deliver different doses of radiation to different areas.
The Head and Neck Surgeon’s role in Intensity-Modulated Radiation Therapy planning
During treatment planning, the radiation oncologist uses dedicated planning software to outline the GTV, the CTV, and the adjacent normal structures section by section on the simulation scan(5). Joint IMRT planning by the radiation oncologist, radiologist, and trained surgeons can improve target delineation and, by extension, tumor control.
The Head and Neck Surgeon’s role in GTV delineation
Despite numerous technical advances, the available imaging modalities have several potential pitfalls that may alter the interpretation of the gross disease’s maximal dimension. These include general factors such as the use of intravenous contrast agents and difficulties experienced when fusing images obtained using different imaging techniques, as well as modality specific factors (window and level setting in CT, the chosen method for segmenting PET, etc.)(26, 40). Such complexities illustrate the necessity of a close collaboration between radiologists, medical physicists, nuclear medicine physicians and radiation oncologists in order to achieve adequate GTV delineation.
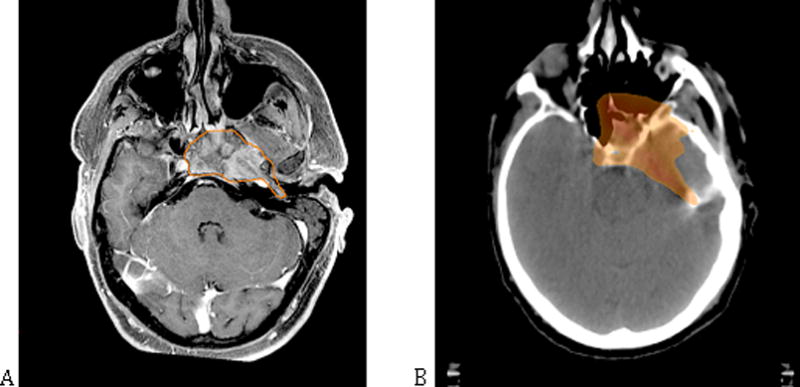
Of even greater importance to our current discussion is the fact that there are many cases in which imaging modalities struggle to delineate disease and specialized physical exploration remains a fundamental step in complex GTV definition. Anterior commissure involvement, subglottic invasion, base of tongue extension, and submucosal spread are examples of macroscopic tumor involvement that is difficult to define with conventional imaging techniques(41). In these situations, tumor palpation, mirror and fiber-optic exams, or even microscopic or endoscopic exploration under general anesthesia may provide information fundamental to target delineation(36).
Other treatment circumstances also require head and neck surgeon input. For example, in the case of induction chemotherapy, the GTV should encompass the pre-induction tumor volume and, therefore, the importance of the initial exploration is paramount, since the radiation oncologist must use this information to delineate the post-induction GTV(5). Another complex setting is that of re-irradiation: here, mucosal exploration and multidisciplinary evaluation may be even more crucial due to difficulties in distinguishing between treatment-related changes and tumor recurrence(42). These common scenarios underscore the importance of communication between the head and neck surgeon and the radiation oncologist; indeed, this dialogue is essential to GTV delineation.
The Head and Neck Surgeon’s role in Clinical Target Volume delineation
Variation in the CTV delineation is the greatest geometrical uncertainty in the entire treatment process and an inadequate delineation can lead to marginal misses. In conventional three-field radiation therapy, subclinical disease near the primary tumor was very often inevitably treated when targeting the GTV. In today’s IMRT era with regular achievement of sharp dose gradients, structures not specified as targets during the planning procedure will not be adequately irradiated. For example, retropharyngeal lymph nodes in oropharyngeal cancer or the clivus in nasopharyngeal cancer will not receive a clinically meaningful dose with IMRT if they are not intended as targets(43, 44). Therefore, adequate CTV contouring relies on the physician’s knowledge of the patterns of subclinical tumor spread. In this context, the experience gained by surgeons through the complex treatment of head and neck cancers of varying subsites and histology must be shared with the radiation oncologist in order to achieve improved tumor control via better CTV definition.
Clinical Target Volume delineation in the radical treatment of the primary tumor
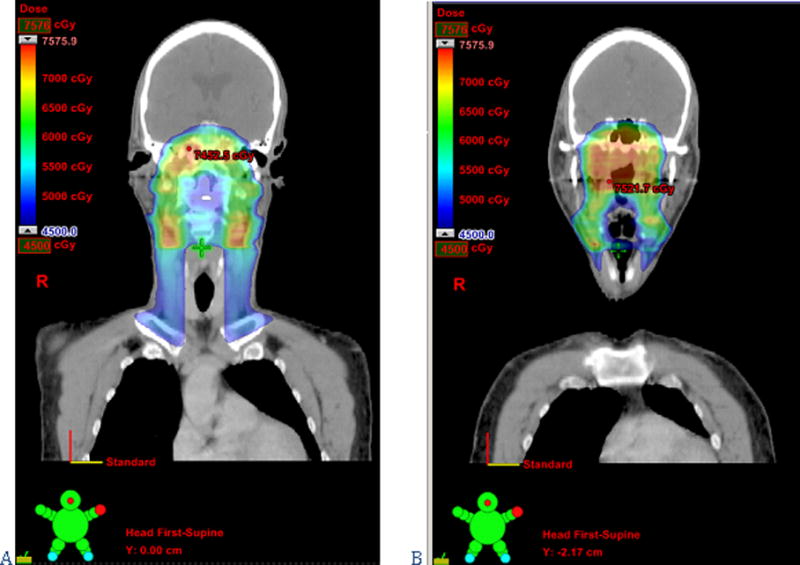
The natural history of head and neck cancer has driven classical surgical approaches to both the primary tumor and the neck for decades. Tumor site, size, stage, depth of invasion, grade of differentiation, perineural spread or lymphovascular invasion—all must be taken into account when planning surgery, since they effect the approach to the primary site as well as the need for and extent of a neck dissection. The predictability of the patterns of tumor progression justifies the use and explains the success of organ preserving and/or compartmental surgical approaches (for example, the commando operation or the supraglottic laryngectomy)(45, 46). Similarly, a meticulous consideration of the potential pathways of tumor spread (connective, muscular, lymphatic and vascular) at the time of CTV delineation results in better clinical outcomes. For example, the primary CTV for tonsil cancer must encompass the entire tonsillar fossa (from the pterygoid plate to the pharyngo-epiglottic fold), the primary CTV for base of tongue tumors should include the pre-epiglottic space, and the high-risk CTV for nasopharyngeal carcinoma should include the clivus(36). Furthermore, with IMRT microscopic tumor spread can be pursued not only adjacent to the primary site and through the lymphatic channels to the neck nodes but also to other routes of dissemination (figure 4). For instance, in tumors with a high risk of perineural spread such as adenoid cystic carcinomas, the nerves at risk can be safely and effectively treated to the skull base(47, 48).
Figure 4.
Clinical Target Volume delineation in the radical treatment of the neck
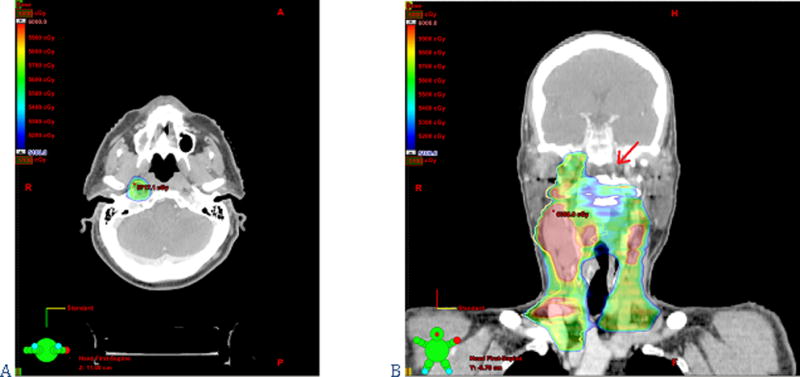
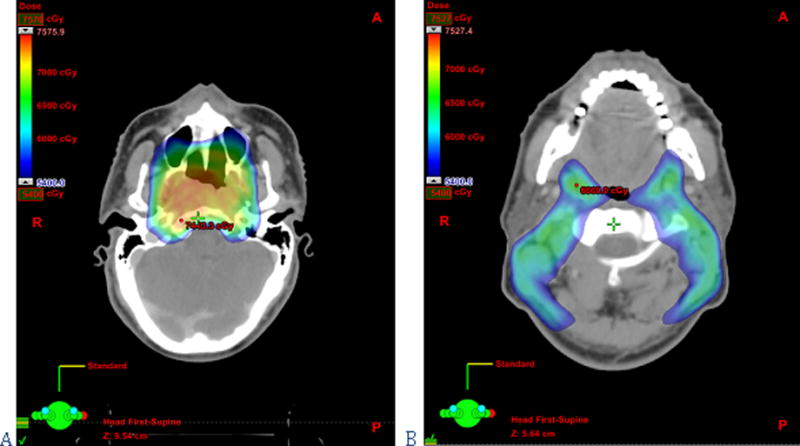
At the time of CTV definition in the neck, the radiation oncologist using IMRT can perform elective, selective and super-selective neck radiation—even using different doses depending on the risk of microscopic involvement—thereby mirroring the choices a surgeon makes with regards to neck dissections. If a comprehensive bilateral neck dissection were clinically indicated (for example in the case of a T1N2a tumor of the palatine tonsil), then a bilateral neck irradiation plan with adequate dosing must be outlined. Treatment of levels Ia, IIb, V and VI can be safely avoided in clinically N0 necks when indicated in order to decrease the sequelae of treatment (i.e. the oral cavity or the parotid gland will receive lower doses of radiation if levels Ia or IIb are not included in the CTV, respectively)(49–51). However, the aforementioned levels must be included in the CTV when clinically indicated, as is also the case in neck dissections (i.e. level Ia has to be treated in tumors of the floor of the mouth, level IIb in carcinomas of the oropharynx, or level V in nasopharyngeal carcinomas)(27, 52, 53). Neglecting to do so and proceeding with selective neck irradiation in such cases could result in regional recurrences. Lateral retropharyngeal lymph nodes are another important point of consideration with IMRT. In pharyngeal carcinomas managed with conventional 3-dimensional radiation therapy, these nodes are adequately covered while treating the primary tumor. With IMRT, they must be specifically included in the CTV to avoid under-dosing and recurrence (see figure 5). In situations where there is risk of retropharyngeal node metastases, bilateral retropharyngeal nodes should be treated stopping cranially at the level of C1 or at the base of the skull, depending on the risk(44, 50, 51).
Figure 5.
Clinical Target Volume delineation in the post-operative setting
In the postoperative setting, CTV delineation at the tumor bed can pose an even greater challenge. If the possibility of delivering post-surgical IMRT is considered, it must be taken into account at the time of pre-operative surgical planning. Meticulous description of the surgical procedure will complement the pre- and post-operative images and help the radiation oncologist define the remaining tissues at risk of involvement with microscopic tumor. In the case of nodal extra-capsular spread or close or involved surgical margins at the primary site, the radiation oncologist can use higher doses in the treatment of the affected tumor bed(5, 7, 54, 55). In large surgical fields that may employ loco-regional or free flaps for reconstruction, provision of details regarding the involved border to the radiation oncologist may allow for the sparing of a significant region either at the primary site or in the neck from unnecessarily high radiation doses. Therefore, good communication is essential between the head and neck surgeon and the radiation oncologist, especially given the subtleties that accompany surgical procedures. If post-operative radiation treatment is expected, a discussion in a multidisciplinary meeting must precede the surgical decision in order to reach a consensus regarding optimal treatment sequencing as well as the extent of the operation. Radiation oncologists should share their concerns with the treating surgeons and participate in the overall treatment plan from the outset. For example, in the case of a tumor with a high capacity of spreading via nerves, a pre-operative MRI evaluating the existence of radiologic perineural spread as well as an intra-operative nerve biopsy may allow for a significant reduction of dose delivery to that nerve (i.e. from 66Gy to 54Gy)(36, 48). The benefit of this reduction cannot be overstated when tracking a cranial nerve such as one of the trigeminal branches in its course near vital neurovascular structures, such as the optic chiasm or the cavernous sinus. It is also important that the surgeon bear in mind the need for eventual adjuvant radiotherapy at the time of surgical planning. For example, radiotherapy following neck dissection should encompass larger volumes than in the definitive setting due to lymphatic disruption, thereby resulting in lack of sparing of the sternocleidomastoid and the potential for increased neck fibrosis(56, 57). On the other hand, an incomplete surgery may require variable dose prescriptions to surgically treated and untreated tissues, resulting in difficulties for the radiation oncologist and medical physicist. As a hypothetical scenario, consider a T2N2b tonsillar cancer undergoing TORS for the primary and an excisional biopsy of a neck node: the radiation treatment will require an adjuvant dose to the pharynx but two different radical doses to the neck (for un-resected and resected disease). This will necessitate 2 or 3 plans, creating areas at risk of under and over dosing(50).
Finally, when planning the treating volumes and the prescribed doses in both the radical and postoperative settings, knowledge regarding the likelihood of success of salvage surgery may add helpful information to the target delineation process. For example, while a salvage neck dissection has a reasonable chance to rescue a neck recurrence, a second surgical procedure may not be feasible in the skull base(58). When indicated, this information can and must be factored in during radiation planning, in this situation potentially allowing a lower dose prescription to the uninvolved neck but not to the skull base.
Conclusions
IMRT is able to achieve a high rate of loco-regional control with low morbidity under optimal target delineation, appropriate physical quality control, and accurate patient setup. IMRT is more sensitive to inaccuracies in target definition than conventional 3-dimensional radiation therapy. Therefore, inadequate delineation of the treatment volumes can easily lead to loco-regional recurrences or unnecessary sequelae. In this setting, the head and neck surgeon can make valuable contributions to proper delineation of the target volumes. It is becoming increasingly important for the radiation oncologist and the head and neck surgeon to understand each other’s fields.
Figure 2.
Contributor Information
Stanley I. Gutiontov, Northwestern University Feinberg School of Medicine Chicago IL.
Edward J. Shin, New York Eye and Ear Infirmary of Mount Sinai New York NY.
Benjamin Lok, Memorial Sloan-Kettering Cancer Center New York NY.
Nancy Y. Lee, Memorial Sloan-Kettering Cancer Center New York NY.
Ruben Cabanillas, Institute of Molecular and Oncological Medicine of Asturias Oviedo Asturias Spain.
References
- 1.Mendenhall WM, Amdur RJ, Palta JR. Intensity-modulated radiotherapy in the standard management of head and neck cancer: promises and pitfalls. J Clin Oncol. 2006;24(17):2618–23. doi: 10.1200/JCO.2005.04.7225. [DOI] [PubMed] [Google Scholar]
- 2.Cannon DM, Lee NY. Recurrence in region of spared parotid gland after definitive intensity-modulated radiotherapy for head and neck cancer. Int J Radiat Oncol Biol Phys. 2008;70(3):660–5. doi: 10.1016/j.ijrobp.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 3.Chen AM, Farwell DG, Luu Q, Chen LM, Vijayakumar S, Purdy JA. Marginal misses after postoperative intensity-modulated radiotherapy for head and neck cancer. Int J Radiat Oncol Biol Phys. 2011;80(5):1423–9. doi: 10.1016/j.ijrobp.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 4.Damast S, Wolden S, Lee N. Marginal recurrences after selective targeting with intensity-modulated radiotherapy for oral tongue cancer. Head Neck. 2012;34(6):900–6. doi: 10.1002/hed.21677. [DOI] [PubMed] [Google Scholar]
- 5.Hartford AC, Galvin JM, Beyer DC, et al. American College of Radiology (ACR) and American Society for Radiation Oncology (ASTRO) Practice Guideline for Intensity-modulated Radiation Therapy (IMRT) Am J Clin Oncol. 2012;35(6):612–7. doi: 10.1097/COC.0b013e31826e0515. [DOI] [PubMed] [Google Scholar]
- 6.Galvin JM, De Neve W. Intensity modulating and other radiation therapy devices for dose painting. J Clin Oncol. 2007;25(8):924–30. doi: 10.1200/JCO.2007.10.6716. [DOI] [PubMed] [Google Scholar]
- 7.Hodapp N. The ICRU Report 83: prescribing, recording and reporting photon-beam intensity-modulated radiation therapy (IMRT) Strahlenther Onkol. 2012;188(1):97–9. doi: 10.1007/s00066-011-0015-x. [DOI] [PubMed] [Google Scholar]
- 8.Nutting CM, Morden JP, Harrington KJ, et al. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicentre randomised controlled trial. Lancet Oncol. 2011;12(2):127–36. doi: 10.1016/S1470-2045(10)70290-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eisbruch A, Schwartz M, Rasch C, et al. Dysphagia and aspiration after chemoradiotherapy for head-and-neck cancer: which anatomic structures are affected and can they be spared by IMRT? Int J Radiat Oncol Biol Phys. 2004;60(5):1425–39. doi: 10.1016/j.ijrobp.2004.05.050. [DOI] [PubMed] [Google Scholar]
- 10.Nguyen NP, Vock J, Chi A, et al. Effectiveness of intensity-modulated and image-guided radiotherapy to spare the mandible from excessive radiation. Oral Oncol. 2012;48(7):653–7. doi: 10.1016/j.oraloncology.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 11.Theunissen EA, Zuur CL, Yurda ML, et al. Cochlea sparing effects of intensity modulated radiation therapy in head and neck cancers patients: a long-term follow-up study. J Otolaryngol Head Neck Surg. 2014;43:30. doi: 10.1186/s40463-014-0030-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wanebo HJ, Lee J, Burtness BA, et al. Induction cetuximab, paclitaxel, and carboplatin followed by chemoradiation with cetuximab, paclitaxel, and carboplatin for stage III/IV head and neck squamous cancer: a phase II ECOG-ACRIN trial (E2303) Ann Oncol. 2014;25(10):2036–41. doi: 10.1093/annonc/mdu248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blanchard P, Bourhis J, Lacas B, et al. Taxane-cisplatin-fluorouracil as induction chemotherapy in locally advanced head and neck cancers: an individual patient data meta-analysis of the meta-analysis of chemotherapy in head and neck cancer group. J Clin Oncol. 2013;31(23):2854–60. doi: 10.1200/JCO.2012.47.7802. [DOI] [PubMed] [Google Scholar]
- 14.Gujral DM, Miah AB, Bodla S, et al. Final long-term results of a phase I/II study of dose-escalated intensity-modulated radiotherapy for locally advanced laryngo-hypopharyngeal cancers. Oral Oncol. 2014;50(11):1089–97. doi: 10.1016/j.oraloncology.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 15.Miah AB, Bhide SA, Guerrero-Urbano MT, et al. Dose-escalated intensity-modulated radiotherapy is feasible and may improve locoregional control and laryngeal preservation in laryngo-hypopharyngeal cancers. Int J Radiat Oncol Biol Phys. 2012;82(2):539–47. doi: 10.1016/j.ijrobp.2010.09.055. [DOI] [PubMed] [Google Scholar]
- 16.Hunter KU, Worden F, Bradford C, et al. Neck spasm after chemoradiotherapy for head and neck cancer: natural history and dosimetric correlates. Head Neck. 2014;36(2):176–80. doi: 10.1002/hed.23284. [DOI] [PubMed] [Google Scholar]
- 17.van der Molen L, Heemsbergen WD, de Jong R, et al. Dysphagia and trismus after concomitant chemo-Intensity-Modulated Radiation Therapy (chemo-IMRT) in advanced head and neck cancer; dose-effect relationships for swallowing and mastication structures. Radiother Oncol. 2013;106(3):364–9. doi: 10.1016/j.radonc.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 18.Hall EJ, Wuu CS. Radiation-induced second cancers: the impact of 3D-CRT and IMRT. Int J Radiat Oncol Biol Phys. 2003;56(1):83–8. doi: 10.1016/s0360-3016(03)00073-7. [DOI] [PubMed] [Google Scholar]
- 19.Purdy JA. Dose to normal tissues outside the radiation therapy patient’s treated volume: a review of different radiation therapy techniques. Health Phys. 2008;95(5):666–76. doi: 10.1097/01.HP.0000326342.47348.06. [DOI] [PubMed] [Google Scholar]
- 20.Ardenfors O, Josefsson D, Dasu A. Are IMRT treatments in the head and neck region increasing the risk of secondary cancers? Acta Oncol. 2014;53(8):1041–7. doi: 10.3109/0284186X.2014.925581. [DOI] [PubMed] [Google Scholar]
- 21.Chen AM, Yu Y, Daly ME, Farwell DG, Benedict SH, Purdy JA. Long-term experience with reduced planning target volume margins and intensity-modulated radiotherapy with daily image-guidance for head and neck cancer. Head Neck. 2014;36(12):1766–72. doi: 10.1002/hed.23532. [DOI] [PubMed] [Google Scholar]
- 22.Stoll M, Giske K, Debus J, Bendl R, Stoiber EM. The frequency of re-planning and its variability dependent on the modification of the re-planning criteria and IGRT correction strategy in head and neck IMRT. Radiat Oncol. 2014;9:175. doi: 10.1186/1748-717X-9-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olteanu LA, Berwouts D, Madani I, et al. Comparative dosimetry of three-phase adaptive and non-adaptive dose-painting IMRT for head-and-neck cancer. Radiother Oncol. 2014;111(3):348–53. doi: 10.1016/j.radonc.2014.02.017. [DOI] [PubMed] [Google Scholar]
- 24.Chen AM, Daly ME, Cui J, Mathai M, Benedict S, Purdy JA. Clinical outcomes among patients with head and neck cancer treated by intensity-modulated radiotherapy with and without adaptive replanning. Head Neck. 2014;36(11):1541–6. doi: 10.1002/hed.23477. [DOI] [PubMed] [Google Scholar]
- 25.Metcalfe P, Liney GP, Holloway L, et al. The potential for an enhanced role for MRI in radiation-therapy treatment planning. Technol Cancer Res Treat. 2013;12(5):429–46. doi: 10.7785/tcrt.2012.500342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greco C, Nehmeh SA, Schoder H, et al. Evaluation of different methods of 18F-FDG-PET target volume delineation in the radiotherapy of head and neck cancer. Am J Clin Oncol. 2008;31(5):439–45. doi: 10.1097/COC.0b013e318168ef82. [DOI] [PubMed] [Google Scholar]
- 27.Shah JP. Patterns of cervical lymph node metastasis from squamous carcinomas of the upper aerodigestive tract. Am J Surg. 1990;160(4):405–9. doi: 10.1016/s0002-9610(05)80554-9. [DOI] [PubMed] [Google Scholar]
- 28.Lee N, Xia P, Fischbein NJ, Akazawa P, Akazawa C, Quivey JM. Intensity-modulated radiation therapy for head-and-neck cancer: the UCSF experience focusing on target volume delineation. Int J Radiat Oncol Biol Phys. 2003;57(1):49–60. doi: 10.1016/s0360-3016(03)00405-x. [DOI] [PubMed] [Google Scholar]
- 29.Hirano M, Kurita S, Tanaka H. Histopathologic study of carcinoma of the hypopharynx: implications for conservation surgery. Ann Otol Rhinol Laryngol. 1987;96(6):625–9. doi: 10.1177/000348948709600602. [DOI] [PubMed] [Google Scholar]
- 30.Dubrulle F, Souillard R, Hermans R. Extension patterns of nasopharyngeal carcinoma. Eur Radiol. 2007;17(10):2622–30. doi: 10.1007/s00330-007-0616-z. [DOI] [PubMed] [Google Scholar]
- 31.Cabanillas R, Llorente JL. The Stem Cell Network model: clinical implications in cancer. Eur Arch Otorhinolaryngol. 2009;266(2):161–70. doi: 10.1007/s00405-008-0809-3. [DOI] [PubMed] [Google Scholar]
- 32.Simple M, Suresh A, Das D, Kuriakose MA. Cancer stem cells and field cancerization of Oral squamous cell carcinoma. Oral Oncol. 2015 doi: 10.1016/j.oraloncology.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 33.Nguyen NP, Chi A, Betz M, et al. Feasibility of intensity-modulated and image-guided radiotherapy for functional organ preservation in locally advanced laryngeal cancer. PLoS One. 2012;7(8):e42729. doi: 10.1371/journal.pone.0042729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bourhis J, Overgaard J, Audry H, et al. Hyperfractionated or accelerated radiotherapy in head and neck cancer: a meta-analysis. Lancet. 2006;368(9538):843–54. doi: 10.1016/S0140-6736(06)69121-6. [DOI] [PubMed] [Google Scholar]
- 35.Vargo JA, Ferris RL, Ohr J, et al. A prospective phase 2 trial of reirradiation with stereotactic body radiation therapy plus cetuximab in patients with previously irradiated recurrent squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 2015;91(3):480–8. doi: 10.1016/j.ijrobp.2014.11.023. [DOI] [PubMed] [Google Scholar]
- 36.Target Volume Delineation for Conformal and Intensity-Modulated Radiation Therapy. Springer International Publishing; Switzerland: 2015. p. 530. [Google Scholar]
- 37.Withers HR, Taylor JM, Maciejewski B. The hazard of accelerated tumor clonogen repopulation during radiotherapy. Acta Oncol. 1988;27(2):131–46. doi: 10.3109/02841868809090333. [DOI] [PubMed] [Google Scholar]
- 38.Schwaibold F, Scariato A, Nunno M, et al. The effect of fraction size on control of early glottic cancer. Int J Radiat Oncol Biol Phys. 1988;14(3):451–4. doi: 10.1016/0360-3016(88)90259-3. [DOI] [PubMed] [Google Scholar]
- 39.Yamazaki H, Nishiyama K, Tanaka E, Koizumi M, Chatani M. Radiotherapy for early glottic carcinoma (T1N0M0): results of prospective randomized study of radiation fraction size and overall treatment time. Int J Radiat Oncol Biol Phys. 2006;64(1):77–82. doi: 10.1016/j.ijrobp.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 40.Seitz O, Chambron-Pinho N, Middendorp M, et al. 18F-Fluorodeoxyglucose-PET/CT to evaluate tumor, nodal disease, and gross tumor volume of oropharyngeal and oral cavity cancer: comparison with MR imaging and validation with surgical specimen. Neuroradiology. 2009;51(10):677–86. doi: 10.1007/s00234-009-0586-8. [DOI] [PubMed] [Google Scholar]
- 41.Abraham J. Imaging for Head and Neck Cancer. Surg Oncol Clin N Am. 2015;24(3):455–471. doi: 10.1016/j.soc.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 42.Riaz N, Hong JC, Sherman EJ, et al. A nomogram to predict loco-regional control after re-irradiation for head and neck cancer. Radiother Oncol. 2014;111(3):382–7. doi: 10.1016/j.radonc.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang T, R N, Cheng S, Lu J, Lee N. Intensity-Modulated radiation therapy for nasopharyngeal carcinoma: a review. J Radiat Oncol. 2012;(1):129–146. [Google Scholar]
- 44.Eisbruch A, Marsh LH, Dawson LA, et al. Recurrences near base of skull after IMRT for head-and-neck cancer: implications for target delineation in high neck and for parotid gland sparing. Int J Radiat Oncol Biol Phys. 2004;59(1):28–42. doi: 10.1016/j.ijrobp.2003.10.032. [DOI] [PubMed] [Google Scholar]
- 45.Kowalski LP, Hashimoto I, Magrin J. End results of 114 extended “commando” operations for retromolar trigone carcinoma. Am J Surg. 1993;166(4):374–9. doi: 10.1016/s0002-9610(05)80336-8. [DOI] [PubMed] [Google Scholar]
- 46.Cabanillas R, Rodrigo JP, Llorente JL, Suarez C. Oncologic outcomes of transoral laser surgery of supraglottic carcinoma compared with a transcervical approach. Head Neck. 2008;30(6):750–5. doi: 10.1002/hed.20778. [DOI] [PubMed] [Google Scholar]
- 47.Singh FM, Mak SY, Bonington SC. Patterns of spread of head and neck adenoid cystic carcinoma. Clin Radiol. 2015;70(6):644–653. doi: 10.1016/j.crad.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 48.Gomez DR, Hoppe BS, Wolden SL, et al. Outcomes and prognostic variables in adenoid cystic carcinoma of the head and neck: a recent experience. Int J Radiat Oncol Biol Phys. 2008;70(5):1365–72. doi: 10.1016/j.ijrobp.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 49.Spencer CR, Gay HA, Haughey BH, et al. Eliminating radiotherapy to the contralateral retropharyngeal and high level II lymph nodes in head and neck squamous cell carcinoma is safe and improves quality of life. Cancer. 2014;120(24):3994–4002. doi: 10.1002/cncr.28938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhung J, S E, Lee NY, Cabanillas R. Target Delineation of the Neck in Head and Neck Carcinomas. In: Lee Nancy Y, Riaz Nadeem, Lu Jiade J., editors. Target Volume Delineation for Conformal and Intensity-Modulated Radiation Therapy. Spring International Publishing; Switzerland: 2015. pp. 147–166. [Google Scholar]
- 51.Gregoire V, Ang K, Budach W, et al. Delineation of the neck node levels for head and neck tumors: a 2013 update. DAHANCA, EORTC, HKNPCSG, NCIC CTG, NCRI, RTOG, TROG consensus guidelines. Radiother Oncol. 2014;110(1):172–81. doi: 10.1016/j.radonc.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 52.Shimizu K, Inoue H, Saitoh M, et al. Distribution and impact of lymph node metastases in oropharyngeal cancer. Acta Otolaryngol. 2006;126(8):872–7. doi: 10.1080/00016480500504259. [DOI] [PubMed] [Google Scholar]
- 53.Wang X, Hu C, Ying H, et al. Patterns of lymph node metastasis from nasopharyngeal carcinoma based on the 2013 updated consensus guidelines for neck node levels. Radiother Oncol. 2015 doi: 10.1016/j.radonc.2015.02.017. [DOI] [PubMed] [Google Scholar]
- 54.Bernier J, Cooper JS, Pajak TF, et al. Defining risk levels in locally advanced head and neck cancers: a comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (# 9501) Head Neck. 2005;27(10):843–50. doi: 10.1002/hed.20279. [DOI] [PubMed] [Google Scholar]
- 55.Cooper JS, Zhang Q, Pajak TF, et al. Long-term follow-up of the RTOG 9501/intergroup phase III trial: postoperative concurrent radiation therapy and chemotherapy in high-risk squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 2012;84(5):1198–205. doi: 10.1016/j.ijrobp.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gregoire V, Eisbruch A, Hamoir M, Levendag P. Proposal for the delineation of the nodal CTV in the node-positive and the post-operative neck. Radiother Oncol. 2006;79(1):15–20. doi: 10.1016/j.radonc.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 57.Straub JM, New J, Hamilton CD, Lominska C, Shnayder Y, Thomas SM. Radiation-induced fibrosis: mechanisms and implications for therapy. J Cancer Res Clin Oncol. 2015 doi: 10.1007/s00432-015-1974-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ho AS, Kraus DH, Ganly I, Lee NY, Shah JP, Morris LG. Decision making in the management of recurrent head and neck cancer. Head Neck. 2014;36(1):144–51. doi: 10.1002/hed.23227. [DOI] [PubMed] [Google Scholar]


