Abstract
Obesity induces chronic inflammation and is an established risk and progression factor for triple-negative breast cancers, including basal-like (BL) and claudin-low (CL) subtypes. We tested the effects of dietary supplementation with ethyl esters of the marine-derived anti-inflammatory omega-3 fatty acids eicosapentaenoic and docosahexaenoic acid (EPA+DHA; Lovaza®) on growth of murine BL and CL mammary tumors. Female ovariectomized C57BL/6 mice were fed a control diet or a diet-induced obesity (DIO) diet with or without EPA+DHA (0.025%, resulting in blood levels of EPA and DHA comparable to women taking Lovaza 4 g/day) for 6 weeks. All mice were then orthotopically injected with Wnt-1 cells (a BL tumor cell suspension derived from MMTV-Wnt-1 transgenic mouse mammary tumors) or M-Wnt cells (a CL tumor cell line cloned from the Wnt-1 tumor cell suspension). Mice were killed when tumors were 1 cm in diameter. EPA+DHA supplementation did not significantly impact Wnt-1 or M-Wnt mammary tumor growth in normoweight control mice. However, EPA+DHA supplementation in DIO mice reduced growth of Wnt-1 and M-Wnt tumors; reduced leptin:adiponectin ratio and pro-inflammatory eicosanoids in the serum; improved insulin sensitivity; and decreased tumoral expression of cyclooxygenase-2 and phospho-p65. Thus, EPA+DHA supplementation in mouse models of postmenopausal BL and CL breast cancer offsets many of the pro-tumorigenic effects of obesity. These preclinical findings, in combination with results from parallel biomarker studies in women, suggest EPA+DHA supplementation may reduce the burden of BL and CL breast cancer in obese women.
Keywords: basal-like breast cancer, claudin-low breast cancer, diet and cancer, inflammation, omega-3 fatty acids, animal/transgenic models in promotion and prevention
Introduction
Obesity induces chronic inflammation in adipose and other tissues, resulting in an increase in multiple factors known to promote breast cancer development and progression. These obesity-related factors include insulin, bioavailable insulin-like growth factor (IGF)-1, and pro-inflammatory adipokines and cytokines, as well as increased mammary gland expression of aromatase and markers of inflammation, proliferation and cell survival [1]. Obesity is associated with poorer prognoses of breast cancer, independent of menopausal status and across subtypes [2], including the triple-negative breast cancer (TNBC) subtype. TNBC, which primarily encompasses both the basal-like (BL) and claudin-low (CL) breast cancer subtypes, is highly aggressive and is associated with a higher risk of distant metastases and mortality [3]. Although significant (>10%) weight loss is associated with favorable modulation of intermediates known to promote breast cancer in animal models and in human interventional studies [4-5], achievement and maintenance of significant weight loss is an elusive goal for most obese individuals. Consequently, improved treatment regimens for the prevention of TNBC recurrence and mortality, particularly in the obese patient population, are urgently needed.
Omega-3 fatty acids and omega-6 fatty acids are long-chain polyunsaturated fatty acids incorporated into lipid membranes, whose derivatives play major roles in inflammation, cell membrane fluidity, and cell signaling [6]. Omega-3 and -6 fatty acids also have profound effects on insulin sensitivity and can modulate cancer-related cell proliferation and survival pathways [7]. Epidemiological studies suggest an inverse correlation between omega-3 levels (including the ratio of omega-3 to omega-6 intake and the ratio of phospholipid omega-3 to omega-6 fatty acid levels in erythrocytes or adipocytes and breast cancer risk and/or progression [8-9].
In the US, most omega-3 fatty acids obtained from the diet are from alpha-linolenic acid, commonly found in plant oils and nuts. Due to poor conversion efficiency, less than 5% of alpha-linolenic acid is converted into the biologically relevant long-chain fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) that impact cell signaling. Lovaza® is a highly concentrated combination of marine-derived omega-3 ethyl esters, principally EPA and DHA, approved in the US for reducing hypertriglyceridemia. Although its impact on cancer risk or progression has not been previously reported, Lovaza (henceforth referred to as EPA+DHA) has been suggested for inclusion in future breast cancer chemoprevention trials [10].
We have previously demonstrated the beneficial effects of calorie restriction on murine BL and CL mammary tumors [11]; both of these breast cancer subtypes are associated with an increase in pro-inflammatory markers [12-13]. Given that omega-3 fatty acids mimic many of the anti-inflammatory and metabolic effects of calorie restriction, we hypothesized that dietary supplementation with EPA+DHA would produce a favorable blood and mammary tissue profile of obesity-related inflammatory and metabolic markers and reduce progression of CL and/or BL mammary tumors. We tested this hypothesis using our Wnt-driven mouse models of BL and CL mammary cancer in preclinical tumor and biomarker studies performed in parallel with human biomarker trials [14]. Specifically, we assessed the effects of dietary supplementation with EPA+DHA, in the context of a control or diet-induced obesity (DIO) diet, on BL and CL mammary tumor growth, serum fatty acids, hormones, insulin sensitivity and adipokines as well as tissue proliferation, cyclooxygenase (COX)-2 expression, measures of apoptotic activity, and pro- and anti-inflammatory eicosanoid intermediates.
Materials and Methods
Mice, diets and study design
All animal procedures were approved by the University of Texas at Austin Institutional Animal Care and Use Committee.
A pilot study was completed to determine the EPA+DHA concentration in the diet needed to achieve similar serum and erythrocyte EPA and DHA levels in mice as observed in the parallel human studies in women at high risk for breast cancer and receiving Lovaza 4 g/day [14]. For this pilot study, 14 week old female ovariectomized (surgery performed at 9 weeks of age) C57BL/6 mice (n=18) were purchased from Charles River (Wilmington, MA) and allowed to acclimate on a 12-hour light/dark cycle for one week. Mice were then placed on a control diet (Research Diets, New Brunswick, NJ; D12450B) for 4 weeks, then randomly assigned (n=6 per group) to receive (ad libitum) the control diet, a low dose EPA+DHA diet (0.025%, Research Diets, D11051403) or a high dose EPA+DHA diet (0.05%, Research Diets, D11051404) for 8 weeks. Lovaza capsules were purchased from the Kansas University Medical Center pharmacy, and the contents removed then mixed with diet. Diets were stored at 4°C in sealed bags and replaced daily to prevent oxidation. At the conclusion of the study, mice were killed by cervical dislocation, exsanguinated by cardiac puncture, and tissues were excised and flash frozen in liquid nitrogen and then stored at −80°C for lipid analysis.
For the mammary tumor growth study, ovariectomized female C57BL/6 mice (10 weeks of age; n=92) were purchased from Charles River and allowed to acclimate on the control diet for one week. Mice were then randomly assigned (n=23 per diet) to the control diet (10% kcal from fat), the control diet supplemented with 0.025% EPA+DHA (control with EPA+DHA), a 60 kcal % fat diet (DIO; Research Diets, D12492) or a DIO diet supplemented with 0.025% EPA+DHA (DIO with EPA+DHA), all fed ad libitum. Diet was stored at 4°C in sealed bags and replaced daily to prevent oxidation. Body weight and caloric intake were measured weekly. After 6 weeks on the diet treatments, a subset of mice (n=3 per diet) was killed for pre-tumor biological analysis. The remaining mice were orthotopically injected with 5×104 Wnt-1 (BL; n=10 per diet) or 5×104 M-Wnt (CL; n=10 per diet) murine mammary tumor cells into the fourth (abdominal) mammary fat pad. Tumors were measured twice weekly and in vivo tumor area was approximated using the formula πr2. When tumors reached 1.0 cm in any direction, all mice were fasted for 6 hours and anesthetized with isoflurane followed by cardiac puncture. Tumors were excised and either fixed in 10% neutral-buffered formalin or flash frozen in liquid nitrogen and stored at −80 °C until further analysis.
Wnt-1 and M-Wnt tumor cell suspensions
The Wnt-1 mammary tumor cell suspension was prepared from spontaneous mammary tumors excised from MMTV-Wnt-1 transgenic mice as previously described [4]. M-Wnt cells were clonally derived from a MMTV-Wnt-1 tumor cell suspension, cultured, and prepared for transplantation as previously described [11]. The M-Wnt cells were tested for species verification, karyotyping, and analysis of genomic instability and were authenticated by the Molecular Cytogenetics Core facility at the University of Texas MD Anderson Cancer Center, Houston, TX in March 2011.
Body composition, glucose tolerance and serum hormones
Prior to tumor implantation, body fat and lean mass were assessed by quantitative magnetic resonance (qMRI; Echo Instruments, Houston, TX) (n=10 per group). A glucose tolerance test (GTT) was done as previously described [15] after 5 weeks of dietary intervention (n=10 per group). After 6 weeks of dietary intervention, serum was collected (following a 6-hour fast) from the retro-orbital venous plexus and assayed using LINCOplex® Mouse Adipokine Multiplex Assays (Millipore, Inc., Billerica, MA), according to the manufacturer’s instructions, on a BioRad Bioplex 200 analysis system (BioRad, Inc., Hercules, CA) to determine serum levels of insulin, leptin, and adiponectin. Serum tumor necrosis factor alpha (TNFα) and monocyte chemoattractant protein 1 (MCP-1) levels were measured using Mouse Singleplex assays (BioRad) on the BioPlex analyzer. Total circulating levels of insulin-like growth factor (IGF)-I and IGF binding protein 3 (IGFBP3) were measured using Mouse/Rat IGF-I and Mouse IGFBP3 Quantikine ELISA Kits (R&D System, Inc., Minneapolis, MN) per manufacturer’s instructions on a BioTek Synergy plate reader (Winooski, VT). Blood collected by tail nick following a 6-hour fast was analyzed for fasting glucose using an Ascencia Elite XL 3901G glucose analyzer (Bayer Corporation, Mishawaka, IN).
Histopathology and immunohistochemical staining
Paraffin embedded tumor tissue (6 per group, randomly selected) was cut into 4 μm thick sections and processed for either hematoxlin and eosin (H&E) staining or immunohistochemical (IHC) analysis as previously described [16]. Slides were then incubated with the following primary antibodies (source, dilution, and incubation conditions presented parenthetically): Ki-67 (Dako; 1:200, 4°C overnight); phospho-p65Ser276 (Santa Cruz; 1:100, overnight at 4°C), cleaved caspase-3 (R&D systems; 1:500, 30 minutes at RT) and COX-2 (Cayman, 1:500, 1 hour at RT). Finally, slides were incubated with Dako EnVision™ labeled polymer for 30 minutes at RT, followed by incubation with Dako diaminobenzidine and counterstained with hematoxylin. Tumor slides were scanned using the ScanScope XT (Aperio Technologies, Vista, CA). Quantitation was performed using the Aperio Digital Pathology Platform. Briefly, 3-4 representative areas/tumor were viewed at 20-40× magnification and scored based upon the percentage of cells with positive staining or by stain intensity using individually developed scoring algorithms.
Serum and erythrocyte lipid analysis
The serum and erythrocyte fatty acid composition in pilot study mice was measured using a modified Folch et al. method [17] as previously described [18]. Fatty acid methyl esters were isolated following transmethylation with boron trifluoride-methanol and analyzed by gas chromatography using a Varian 3900 (Agilent Technologies, Santa Clara, CA). To analyze the phospholipid content of the erythrocyte samples, equal volumes of sample from mice in the same group were combined. Fatty acid levels are reported as weight % of total phospholipid (for erythrocytes) and weight % of total cholesterol ester and phospholipid (for serum).
Eicosanoid analysis
Eicosanoids in the mammary tumor and visceral adipose tissues were extracted and analyzed by liquid chromatography/tandem mass spectroscopy (LC/MS/MS) using the modified method described previously [19]. LC/MS/MS analyses were performed using an Agilent 6460 triple quadrupole (QqQ) mass spectrometer (Agilent Technologies) equipped with an Agilent 1200 binary pump high-performance liquid chromatography system as described previously [20].
Statistics
Fatty acid compositions in serum and erythrocytes, plus fatty acid derivatives in adipose and tumor tissue are described by means ± SEM; but comparison between groups is assessed with the non-parametric Mann-Whitney test due to small sample sizes and non-normal distributions. All other data are presented as means ± SEM and analyzed by 1-way analysis of variance (ANOVA) followed by Tukey’s post-hoc test with the exception of feed intake, body weight, in vivo tumor area and glucose tolerance test which were analyzed by repeated measures ANOVA. P ≤ 0.05 was considered statistically significant.
Results
EPA+DHA supplementation increases serum and erythrocyte EPA and DHA levels and modulates visceral adipose tissue accumulation of inflammatory lipid derivatives
In the dose-establishing pilot study, serum and erythrocyte fractions were analyzed for EPA, DHA, arachidonic acid (AA) and total n-3 and n-6 fatty acids from mice that consumed diets supplemented with EPA+DHA (0%, 0.025% or 0.05% of total diet) for 8 weeks (Table 1). EPA levels significantly increased in both the sera (P<0.05) and erythrocyte (P<0.05) phospholipid fractions (Table 1) in response to both EPA+DHA diets although the higher dose (0.05%) did not increase EPA accumulation relative to lower supplementation levels (0.025%). DHA levels increased in the erythrocytes in response to both levels of EPA+DHA supplementation, but the increase was only statistically significant for the higher dose (0.05%) (P<0.05). As the proportion of arachidonic acid (AA) decreased in the sera and erythrocyte fractions, the ratio of EPA + DHA to AA significantly increased, as did the total n-3 to n-6 ratio (P<0.05). EPA+DHA supplementation (n=2 randomly selected per group) in general reduced accumulation of COX and LOX derived AA pro-inflammatory mediators, including 6-keto prostaglandin (PG)F1α, thromboxane (TX)B2, PGF2α, PGE2, PGD2, 12-hydroxyeicosatetraenoic acid (12-HETE), and 5-HETE, and substantially increased EPA derived COX-2 metabolites, such as PGE3, in visceral adipose tissue. Moderate reductions of 13-hydroxyoctadecadienoic acid (13-HODE) and 15-HETE (metabolites of omega-6 fatty acids), were also observed in the visceral adipose tissue from mice treated with EPA+DHA compared to the control group (Supplementary Table 1).
Table 1.
Pilot dosing study – 8 weeks ad libitum consumption.
| For Erythrocytes For Serum |
Control Diet n = 4 n = 5 |
0.025%EPA+DHA Diet n = 5 n = 6 |
0.05%EPA+DHA Diet n = 5 n = 7 |
|---|---|---|---|
| Erythrocyte EPA | 0.19 ± 0.01a | 2.53 ± 0.28b | 1.41 ± 0.11b |
|
| |||
| Serum EPA | 0.16 ± 0.02a | 1.66 ± 0.09b | 1.05 ± 0.08b |
|
| |||
| Erythrocyte DHA | 5.59 ± 0.52a | 7.86 ± 0.90 | 7.99 ± 0.81b |
|
| |||
| Serum DHA | 5.01 ± 0.38 | 5.13 ± 0.17 | 5.51 ± 0.32 |
|
| |||
| Erythrocyte AA | 14.5 ± 1.01a | 9.06 ± 0.92b | 10.5 ± 0.80b |
|
| |||
| Serum AA | 11.6 ± 0.91a | 5.47 ± 0.58b | 7.59 ± 0.51b |
|
| |||
| Erythrocyte (EPA + DHA):AA | 0.40 ± 0.01a | 1.16 ± 0.09b | 0.89 ± 0.03b |
|
| |||
| Serum (EPA + DHA):AA | 0.45 ± 0.01a | 1.31 ± 0.15b | 0.88 ± 0.05b |
|
| |||
| Erythrocyte n-3: n-6 | 0.25 ± 0.01a | 0.60 ± 0.04b | 0.52 ± 0.02b |
|
| |||
| Serum n-3: n-6 | 0.31 ± 0.01a | 0.52 ± 0.06b | 0.43 ± 0.02b |
Erythrocyte (% total phospholipid) and serum (% total cholesterol ester and phospholipid) levels of eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), arachidonic acid (AA), the ratio of EPA + DHA: AA, and the ratio of n-3: n-6 fatty acids. Statistically significant (P≤0.05) differences are indicated by different letters, e.g.: a group designated with is a different than a group designated with b; but two groups with b are not different.
EPA+DHA supplementation does not alter body composition, body weight or feed intake
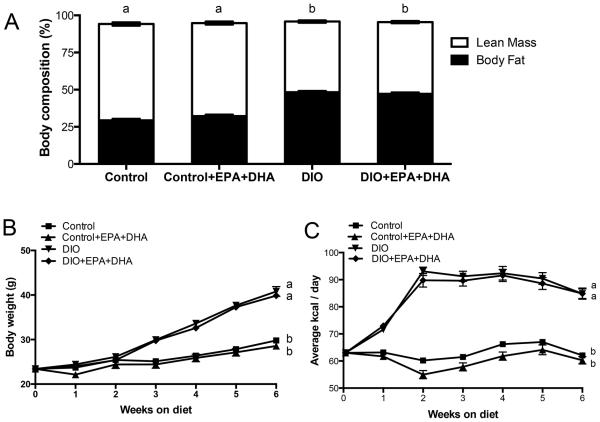
Body composition was analyzed by qMRI for lean mass and fat mass after 5 weeks on diet and prior to tumor implantation. Control (29.3 ± 1.0%) and control with EPA+DHA (32.2 ± 1.0%) mice had significantly less fat mass relative to DIO (48.2 ± 0.8%) and DIO with EPA+DHA (47.2 ± 1.0%) (P<0.001, Figure 1A). Average body weight is shown for the first 6 weeks on dietary intervention and prior to tumor implantation. Mice that consumed the DIO diets (+/− EPA+DHA) were significantly heavier relative to mice fed the control diet (+/− EPA+DHA) (P<0.001) at 6 weeks (Figure 1B) and throughout the remainder of the study (data not shown). The DIO and DIO with EPA+DHA dietary regimens resulted in a significantly greater average caloric consumption compared to the control and control with EPA+DHA groups for the first 6 weeks of dietary intervention (P<0.001, Figure 1C) and throughout the remainder of the study (data not shown).
Figure 1.
EPA+DHA supplementation does not alter body composition, body weight or feed intake. A, body composition is assessed as the percentage of fat and lean tissue at 5 weeks, B, average body weight (1-6 weeks on diet) and C, average kcals consumed per day (1-6 weeks on diet); n=10 per group. Means ± SEM, statistically significant (P<0.05) differences are indicated by different letters.
EPA+DHA supplementation modulates production of inflammatory fatty acid derivatives
The accumulation of pro-inflammatory and anti-inflammatory fatty acid metabolites was assessed in Wnt-1 and M-Wnt tumor tissue (Table 2). The DIO diet, relative to the control diet, led to increased accumulation of 5-HETE and 18-Hydroxyeicosapentaenoic acid (HEPE) within M-Wnt tumor tissue (P<0.0.5), but did not significantly modify 5-HETE within Wnt-1 tumors (18-HEPE was not assayed). EPA+DHA supplementation to the DIO diet significantly increased PGE3 in M-Wnt mammary tumors (P<0.05). . EPA+DHA did not significantly change other fatty acid metabolites in the mammary tumors of DIO mice, but there were numerous instances where supplementation reversed a trend resulting from the DIO diet. For example, TXB2, PGD2, 15-HETE, and 5-HETE were all decreased in M-Wnt mammary tumors, and 6ketoPGF1α, TXB2, PGF2α, PGE2, PGD2, 15-HETE, 12-HETE, and 5-HETE were all reduced in Wnt-1 tumors with supplementation. In visceral adipose tissue, mice receiving either the control or DIO diets with EPA+DHA had significantly elevated levels of 18-HEPE compared to control without EPA+DHA (P<0.05). PGE3 was increased with EPA+DHA supplementation to either diet, but the increase was only statistically significant for the control with EPA+DHA mice (P<0.0.5) (Table 3).
Table 2.
Fatty acid derivatives in tumor tissue.
| Eicosanoid (ng/mg protein) for M-Wnt for Wnt-1 |
Control Diet n = 9 n = 9 |
Control + EPA+DHA n = 5 n = 5 |
DIO n = 9 n = 8 |
DIO + EPA+DHA n = 9 n = 9 |
|---|---|---|---|---|
| M-Wnt 6ketoPGF1α | 12.2 ± 4.7 | 3.9 ± 1.7a | 12.7 ± 3.3b | 22.9 ± 8.9b |
|
| ||||
| Wnt-1 6ketoPGF1α | 3.5 ± 1.3 | 3.2 ± 1.8 | 9.4 ± 6.1 | 4.2 ± 2.3 |
|
| ||||
| M-Wnt TXB2 | 44.6 ± 12.7 | 13.5 ± 5.3 | 128.2 ± 45.2 | 80.4 ± 23.1 |
|
| ||||
| Wnt-1 TXB2 | 3.9 ± 1.4 | 1.2 ± 0.4 | 7.9 ± 4.8 | 3.2 ± 1.4 |
|
| ||||
| M-Wnt PGF2α | 3.3 ± 0.9a | 1.1 ± 0.7a | 8.8 ± 3.3 | 7.6 ± 1.8b |
|
| ||||
| Wnt-1 PGF2α | 1.9 ± 0.8 | 0.23 ± 0.06 | 3.1 ± 1.9 | 1.7 ± 0.8 |
|
| ||||
| M-Wnt PGE2 | 9.0 ± 2.8 | 4.4 ± 1.0a | 10.0 ± 2.8 | 13.6 ± 1.6b |
|
| ||||
| Wnt-1 PGE2 | 8.6 ± 3.7 | 2.3 ± 0.9 | 18.3 ± 9.4 | 5.4 ± 2.3 |
|
| ||||
| M-Wnt PGE3 | 0.11 ± 0.04 | 0.085 ± 0.037a | 0.071 ± 0.017a | 0.25 ± 0.08b |
|
| ||||
| Wnt-1 PGE3 | 0.08 ± 0.03 | 0.03 ± 0.01 | 0.14 ± 0.07 | 0.15 ± 0.09 |
|
| ||||
| M-Wnt PGD2 | 144.2 ± 51.1 | 99.8 ± 42.4a | 385.1 ± 62.4b | 220.6 ± 67.3 |
|
| ||||
| Wnt-1 PGD2 | 5.7 ± 2.3 | 1.4 ± 0.6 | 8.9 ± 4.4 | 4.3 ± 1.6 |
|
| ||||
| M-Wnt 13HODE | 40.3 ± 10.3 | 12.4 ± 6.7a | 47.9 ± 17.6 | 99.0 ± 30.2b |
|
| ||||
| Wnt-1 13HODE | 2.6 ± 1.1a | 2.1 ± 1.7a | 12.8 ± 5.3 | 11.0 ± 2.6b |
|
| ||||
| M-Wnt 15HETE | 9.5 ± 2.8 | 3.2 ± 0.8a | 28.4 ± 9.5b | 17.5 ± 4.9b |
|
| ||||
| Wnt-1 15HETE | 1.3± 0.5 | 0.61 ± 0.17a | 4.1 ± 1.9b | 2.1 ± 0.6 |
|
| ||||
| M-Wnt 12HETE | 28.6 ± 9.8 | 12.2 ± 3.8a | 31.5 ± 9.8 | 32.9 ± 8.1b |
|
| ||||
| Wnt-1 12HETE | 21.9 ± 6.8 | 5.6 ± 1.5a | 39.3 ± 17.6 | 20.1 ± 4.8b |
|
| ||||
| M-Wnt 5HETE | 3.2 ± 0.7a | 2.1 ± 0.5a | 28.9 ± 11.9b | 8.9 ± 2.6b |
|
| ||||
| Wnt-1 5HETE | 0.65 ± 0.15 | 0.60 ± 0.19 | 1.6 ± 0.5 | 0.87 ± 0.19 |
|
| ||||
| M-Wnt 18HEPE | 0.004 ± 0.004a | 0.038 ± 0.016b | 0.037 ± 0.012b | 0.053 ± 0.030 |
|
| ||||
| Wnt-1 18HEPE | NA | NA | NA | NA |
6-ketoPGF1α, 6-keto prostaglandin F1α; TXB2, thromboxane B2; PGE3, prostaglandin E3; PGF2α, prostaglandin F2α; PGE2, prostaglandin E2; PGD2, prostaglandin D2; 13HODE, 13-hydroxyoctadecadienoic acid; 15HETE, 15-hydroxyeicosatetraenoic acid; 12HETE, 12-hydroxyeicosatetraenoic acid; 5HETE, 5-hydroxyeicosatetraenoic acid; 18HEPE, 18-hydroxyeicosapentaenoic acid. Statistically significant (P≤0.05) differences are indicated by different letters, e.g., a group designated with is a different than a group designated with b; but two groups with a are not different.
Table 3.
Fatty acid derivatives in adipose tissue of non-tumor bearing mice after 6 weeks EPA+DHA supplementation.
| Adipose Tissue (ng/mg protein) |
Control (n = 3) |
Control + EPA+DHA (n = 3) |
DIO (n = 3) |
DIO + EPA+DHA (n = 3) |
|---|---|---|---|---|
| 6ketoPGF1α | 148 ± 58 | 144 ± 33 | 211 ± 103 | 47.6 ± 47 |
| TXB2 | 6.7 ± 1.5 | 5.4 ± 2.2 | 10.0 ± 5.3 | 4.2 ± 4.2 |
| PGF2α | 11.3 ± 3.5 | 8.7 ± 2.7 | 22.8 ± 11.0 | 4.4 ± 3.5 |
| PGE2 | 35.8 ± 9.9 | 31.3 ± 6.6 | 51.4 ± 34.5 | 9.3 ± 9.2 |
| PGD2 | 47 ± 22 | 37 ± 11 | 199 ± 92 | 113 ± 106 |
| 13HODE | 973 ± 456 | 648 ± 123 | 1175 ± 268 | 769 ± 542 |
| 15HETE | 171 ± 61 | 85 ± 5 | 159 ± 39 | 84 ± 62 |
| 12HETE | 753 ± 216 | 400 ± 72 | 776 ± 158 | 320 ± 230 |
| 5HETE | 11.0 ± 1.2 | 8.6 ± 3.1 | 15.6 ± 3.7 | 18.8 ± 12.6 |
| 18-HEPE | 0.16 ± 0.08a | 2.34 ± 0.44b | 0.79 ± 0.22b | 7.46 ± 6.32b |
| PGE3 | 0.29 ± 0.06a | 3.35 ± 0.94b | 0.73 ± 0.47 | 1.07 ± 0.60 |
6-ketoPGF1α, 6-keto prostaglandin F1α; TXB2, thromboxane B2; PGE3, prostaglandin E3; PGF2α, prostaglandin F2α; PGE2, prostaglandin E2; PGD2, prostaglandin D2; 13HODE, 13-hydroxyoctadecadienoic acid; 15HETE, 15-hydroxyeicosatetraenoic acid; 12HETE, 12-hydroxyeicosatetraenoic acid; 5HETE, 5-hydroxyeicosatetraenoic acid; 18HEPE, 18-hydroxyeicosapentaenoic acid. Statistically significant (P≤0.05) differences are indicated by different letters, e.g., a group designated with is a different than a group designated with b; but two groups with b are not different.
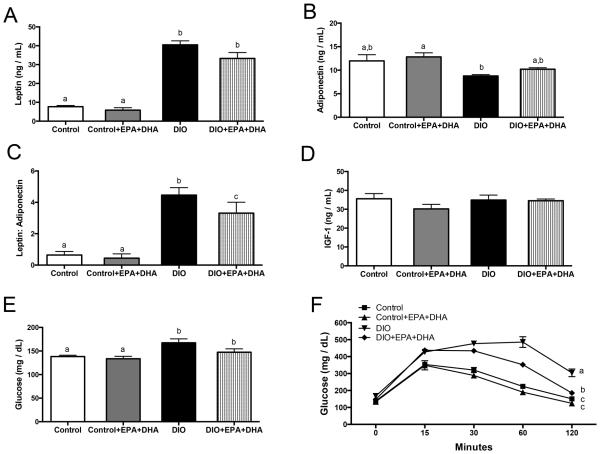
EPA+DHA supplementation improves glucose tolerance and adipokine levels in DIO mice
Fasting serum leptin levels were higher in DIO mice and DIO with EPA+DHA mice compared to control and control with EPA+DHA (both P<0.001, Figure 2A). Adiponectin concentrations were lower in DIO mice relative to all other groups (Figure 2B), and DIO mice also had a significantly higher leptin to adiponectin ratio compared to DIO with EPA+DHA (P<0.05), and compared to control and control with EPA+DHA mice (P<0.001, Figure 2C). There were no significant differences between groups in total IGF-1 levels (Figure 2D). However, fasting blood glucose was significantly greater in mice on the DIO and DIO with EPA+ DHA regimens compared to control mice (P<0.05) and mice on the control with EPA+DHA regimen (P<0.005) (Figure 2E). Mice receiving the DIO with EPA+DHA regimen had significantly better glucose tolerance than DIO mice, but worse tolerance in comparison to the control and control with EPA+DHA mice (P<0.05, Figure 2F). TNFα, MCP-1 and insulin (Supplemental Figure 1A-C) were not significantly altered by EPA+DHA supplementation regardless of diet or tumor type. IGFBP3 and bioavailable IGF-1, indicated by the ratio of total IGF-1 to IGFBP3, were also not significantly altered by EPA+DHA supplementation (Supplemental Figure 1D-E).
Figure 2.
The effect of diet regimens on serum hormones. A, Leptin, B, Adiponectin, C, Leptin:Adiponectin ratio, D, IGF-1, E, Glucose, F, GTT conducted at 6 weeks on diet, n=10 per group. Means ± SEM, statistically significant (P<0.05) differences are indicated by different letters.
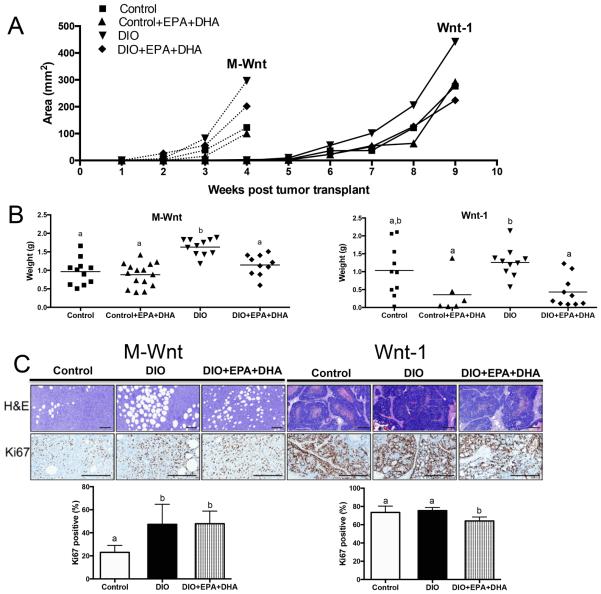
EPA+DHA supplementation reduces rate of Wnt-1 and M-Wnt murine mammary tumor growth in obese mice
M-Wnt mammary tumors grew significantly faster than Wnt-1 tumors (median time to 1 cm tumor is ~4 weeks after M-Wnt tumor transplant vs. ~9 weeks after Wnt-1 tumor transplant) (P<0.001, Figure 3A). For this reason, mice with M-Wnt tumor transplants were killed at 4 weeks and mice with Wnt-1 tumor transplants were killed at 9 weeks post implantation. EPA+DHA supplementation reduced tumor area of M-Wnt and Wnt-1 tumors in obese mice compared with the DIO regimen alone (Figure 3A). The average DIO with EPA+DHA mouse tumor weight (M-Wnt, 1.14 ± 0.09 g; Wnt-1, 0.43 ± 0.1 g) was significantly less than the average DIO mouse tumor weight (M-Wnt, 1.63 ± 0.07 g; Wnt-1, 1.26 ± 0.1 g) (both P<0.05, Figure 3B). However, tumors in the control with EPA+DHA mice (M-Wnt, 0.88 ± 0.08 g; Wnt-1, 0.36 ± 0.2 g) were non-significantly different in weight relative to their non-supplemented counterparts (M-Wnt, 0.97 ± 0.1 g; Wnt-1, 1.03 ± 0.2 g) (Figure 3B). Since the strongest and most consistent anticancer effects of EPA+DHA were observed in DIO mice, all subsequent comparisons will be made between the control, DIO, and DIO with EPA+DHA groups.
Figure 3.
The effect of a DIO regimen +/− EPA+DHA supplementation on tumor growth. A, in vivo M-Wnt (claudin—low) and Wnt-1 (basal-like) mammary tumor growth, n=10 per group. B, M-Wnt and Wnt-1 average ex vivo tumor weight, n=10 per group. C, representative photomicrographs of pathology and IHC staining of tumors for Ki-67 and bar graphs representing the Aperio image quantitation, scale bars indicate 100 μm, n=6 per group. Means ± SEM, statistically significant (P<0.05) differences are indicated by different letters.
H&E staining of M-Wnt tumors reveals accumulation of adipocytes, which were not present in Wnt-1 tumors (Figure 3C). Although it is not clear whether adipocytes differentiated from the tumor initiating cell-enriched M-Wnt cell population or whether there was increased recruitment of adipocytes from local mammary fat pad tissue, it is apparent that dietary energy balance modulated the accumulation of adipocytes within the tumor tissue. Specifically, tumors from DIO mice had much greater accumulation of adipocytes than tumors from control mice. Moreover, EPA+DHA supplementation reduced the DIO-associated increase in prevalence and size of adipocytes within the tumor tissue (Figure 3C).
The influence of dietary interventions on cell proliferation was assessed in tumor tissues by IHC staining against Ki-67 (Figure 3C). Tumor cell proliferation rates for the M-Wnt and Wnt-1 tumors from control-fed mice varied widely, with 23.0 ± 2.5% and 73.4 ± 2.8% positive cells, respectively. The DIO diet did not further increase the proliferation rate in the highly proliferative Wnt-1 tumors (75.4 ± 1.5% positive cells) relative to control mice. However, M-Wnt tumors in the DIO mice had double the proliferation rate (47.3 ± 7.1% positive cells, P<0.05) in comparison to control mice. Wnt-1 tumors from DIO with EPA+DHA-treated mice, relative to DIO without EPA+DHA, showed significantly reduced tumor Ki-67 staining (64.2 ± 1.9% positive cells, P<0.005). In contrast, M-Wnt tumors from DIO with EPA+DHA-treated mice (47.9 ± 4.5% positive cells) showed no significant reduction in proliferation relative to DIO without supplementation. EPA+DHA did not significantly alter the expression of several cell proliferation-related proteins, including: phospho-mTORSer2448, mTOR, phospho-S6 ribosomal proteinSer235/236, cyclin D1, and phospho-AktS473 (data not shown).
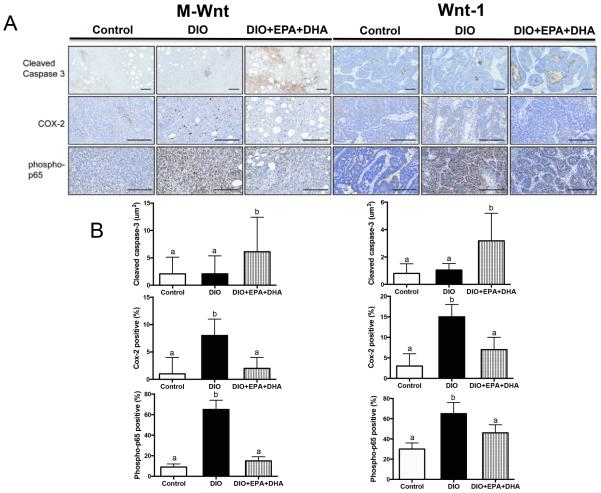
EPA+DHA supplementation increases cleaved caspase-3 and reduces COX-2 and phospho-p65 in DIO mouse tumors
The influence of dietary interventions on markers of cellular apoptosis and inflammation was assessed in tumor tissues by IHC staining against cleaved caspase-3, COX-2 and phospho-p65 (Figure 4A-B). Cleaved caspase-3 staining, a marker of apoptosis, clustered in small groups rather than throughout the entire tumor sections; therefore, average “positive staining island” area was measured. M-Wnt tumors from the DIO with EPA+DHA group had significantly more staining for cleaved caspase-3 (3.18 ± 1.2 μm2) than M-Wnt tumors from DIO without supplementation (1.04 ± 0.27 μm2, P<0.05) or control mice (0.8 ± 0.4 μm2, P<0.05). The DIO with EPA+DHA regimen also significantly increased staining for cleaved caspase-3 in Wnt-1 tumors (6.1 ± 2.6 μm2) relative to DIO (2.1 ± 1.1 μm2, P<0.05) or control (2.07 ± 1.0 μm2, P<0.05) diets. Expression of COX-2, the enzyme responsible for the production of prostanoids from 20 carbon polyunsaturated fatty acids [21], and phospho-p65, the active form of a subunit of the pro-inflammatory NFκB transcription factor [22], were analyzed for the percentage of total positive staining per tumor section. COX-2 and phospho-p65 expression levels were significantly increased with the DIO regimen in the Wnt-1 (P<0.001, P<0.0001 respectively) and M-Wnt (P<0.01, P<0.05 respectively) tumors, and this DIO-associated increase in staining was reduced with EPA+DHA supplementation (P<0.05). EPA+DHA did not significantly alter staining for the macrophage markers CD31 or F4/80 regardless of diet group or tumor type (data not shown).
Figure 4.
The effect of a DIO regimen +/− EPA+DHA supplementation on IHC tumoral staining. A, representative photomicrographs of pathology and IHC staining of tumors for cleaved caspase-3, COX-2 and phospho-p65. B, bar graphs representing the Aperio image quantitation, scale bars indicate 100 μm, n=6 per group. Means ± SEM, statistically significant (P<0.05) differences are indicated by different letters.
Discussion
We found that supplementation with EPA+DHA ethyl esters reduced tumor growth in obese immunocompetent mice orthotopically transplanted with aggressive types of TNBC (BL or CL). The anticancer effects of EPA+DHA administration were associated with: 1) reduced production of inflammatory signaling molecules and lowered expression of key inflammatory proteins within mammary tumor tissue; 2) normalization (to control levels) of assorted obesity-associated factors, including leptin:adiponectin ratio and insulin sensitivity, in DIO mice without normalization of body weight; and 3) induced expression of the apoptotic protein cleaved caspase-3. Our results suggest that EPA+DHA supplementation may be a promising strategy for the inhibition of hormone receptor-negative breast cancer progression in obese postmenopausal women.
To our knowledge this is the first study to model the effects of obesity, with and without EPA and DHA supplementation, on two very aggressive breast cancer subtypes (BL and CL) with poor prognosis and few therapeutic options. Wnt/beta-catenin pathway activation is known to be enriched in human TNBCs and is associated with poor outcome [23], so our murine models of Wnt-driven BL and CL are relevant to human TNBC. The M-Wnt cell line (which when orthotopically transplanted generate tumors with molecular profiles mimicking human CL) was subcloned from Wnt-1 cells (which when orthotopically transplanted generate tumors reflective of human BL breast tumors). Thus, our murine models of BL and CL breast cancer share the same genetic background but differ in molecular and clinical characteristics. Another important aspect of our Wnt-1 and M-Wnt tumor models is the use of C57BL/6 mice, which are syngeneic to our Wnt-1 and M-Wnt cells. Unlike most other commonly used genetic backgrounds for BL and CL mammary tumor models, C57BL/6 mice have a fully functioning immune system and are sensitive to DIO. This facilitates the characterization of the effects of obesity and omega-3 fatty acid supplementation on inflammation, a hallmark of cancer [24] that is inherent in obese individuals and that cannot be adequately modeled in immunodeficient or obesity-resistant mouse models. Moreover, the tissue levels of DHA and EPA attained in this study are in the physiological range [25-26] and were matched with women receiving daily supplementation with 4g of EPA+DHA per day (14). This dose of EPA+DHA is considered safe and well tolerated in postmenopausal women and is currently approved for treatment of hypertriglyceridemia by the FDA [27].
Insulin resistance, glucose intolerance, elevated leptin levels, and low adiponectin levels are associated with the metabolic syndrome and are established risk factors for breast cancer [28-31]. As normal cells become neoplastic there is a switch in glucose metabolism from oxidative phosphorylation to oxidative glycolysis in order to supply essential macromolecules for new cell production [32]. The increased proliferation rate associated with most cancer cells can only be sustained by a constant supply of glucose, sometimes referred to as glucose addiction. Here, we demonstrated a decreased leptin:adiponectin ratio and improved glucose tolerance in DIO mice with EPA+DHA supplementation, relative to DIO mice without supplementation, as previously seen with other studies testing dietary regimens containing high omega-3 fatty acids [33-34].
Regardless of tumor type, EPA+DHA in DIO mice reduced (relative to DIO alone) tumorla staining of two important regulators of inflammatory signals: COX-2 (an enzyme that catalyzes the conversion of arachidonic acid to PGE2) and phospho-p65 (the major subunit of the cytokine-regulating transcription factor NF-κB) [35]. EPA+DHA also enhanced staining for cleaved caspase-3, a pro-apoptotic protein that is responsible for chromatin condensation and DNA fragmentation [36]. Taken together, these findings suggest that EPA+DHA can dampen inflammation and promote apoptosis in Wnt-1 and M-Wnt tumors. Supplementation with EPA+DHA did not significantly reduce serum levels of obesity-associated inflammatory markers unrelated to polyunsaturated fatty acid metabolism in the DIO mice, including TNFα and MCP-1, but these cytokines were also not significantly elevated in the DIO mice compared to control. It may be that the six week obesity induction period in this study was too short to produce significant increases in the circulating levels of these cytokines. Given that all of the anti-inflammatory effects of EPA+DHA supplementation demonstrated in this study involved markers that had been significantly changed by obesity in the DIO mice, it is possible that EPA+DHA may only reduce levels of these cytokines if they are significantly elevated by obesity or another cause. In support of this hypothesis, there was a trend towards increased concentrations of both TNFα and MCP-1 in the DIO mice that was reduced by EPA+DHA supplementation, but these differences did not reach statistical significance.
While enhanced apoptosis may partially explain why EPA+DHA supplementation in obese mice significantly reduced M-Wnt (CL) and Wnt-1 (BL) tumor size and weight, EPA+DHA also significantly decreased Ki-67 within Wnt-1 mammary tumors, which had relatively high proliferation rates (in comparison to the M-Wnt tumors) that were not further enhanced by obesity. In contrast, Ki-67 in M-Wnt mammary tumors was not affected by EPA+DHA supplementation, but was elevated in the tumors of DIO mice relative to control. These differences in proliferation response to obesity and EPA+DHA between tumor types are likely related to the difference in baseline proliferation rates. However, despite the inhibitory effects of EPA+DHA on Wnt-1 tumor proliferation, supplementation had no significant impact on the levels of key proliferative proteins (phosphor-mTORSer2448, mTOR, phosphor-S6 ribosomal proteinSer235/236, cyclin D1, phosphor-AktS473) in either model of breast cancer. Thus, combining EPA+DHA with practical weight loss interventions or agents that better target proliferation and survival pathways, such as the mTOR pathway, may more effectively suppress BL or CL breast cancer progression [37-39].
A reduction in obesity-driven pro-inflammatory signaling may contribute to the reduced tumor progression in DIO mice, but not control mice. Chronic inflammation, commonly associated with obesity, is linked with increased risk for breast cancer [40]. The inflammatory microenvironment exerts tumor-promoting effects specifically via genetic instability and cellular proliferation, survival and angiogenesis [41-42]. In this study, EPA+DHA supplementation increased the anti-inflammatory fatty acid derivative PGE3 in M-Wnt mammary tumors. Additionally, other EPA and DHA derivatives in both tumor types trended towards a reduction in pro-inflammatory signaling in response to EPA+DHA, although differences were not statistically significant. Tumoral staining for COX-2, the enzyme responsible for the production of pro-inflammatory eicosanoids from omega-6 fatty acids, was also significantly reduced by EPA+DHA supplementation. Selective COX-2 inhibitors are commercially available and could potentially be used for the prevention or therapy of breast cancer. However, many of these drugs have been removed from the market or are limited in their use due to an elevated risk for cardiovascular events. In contrast, EPA+DHA is an FDA approved drug that has shown no enhanced risk for cardiovascular events and in fact may be cardioprotective, as it is currently prescribed for individuals with elevated levels of triglycerides [43].
Increased adipocyte infiltration into breast tumors has been correlated with a worse patient outcome [44-45], and numerous in vivo and in vitro studies have demonstrated that cross-talk between adipocytes and mammary cancer cells promotes greater tumor aggressiveness via multiple mechanisms [46]. Previously, we have shown that DIO promotes greater M-Wnt tumor adipocyte accumulation in comparison to control diet [11]. This study produced similar results, finding that the DIO diet induces greater intra-tumoral adipocyte number and size relative to control. It further demonstrated that EPA+DHA supplementation partially prevents these DIO-related effects, suggesting that the inhibition of intra-tumoral adipocyte accumulation may be one additional mechanism by which EPA+DHA supplementation can help prevent CL breast cancer progression. In contrast, there was very low adipocyte accumulation in any of the Wnt-1 tumors, consistent with previous studies utilizing Wnt-1 mammary tumor cells [4,47], and EPA+DHA thus had no impact on this variable in the Wnt-1 tumors. This difference between the Wnt-1 and M-Wnt tumors may be related to the latter’s enrichment in tumor initiating cells, which may have increased secretion of chemoattractants that recruit adipocytes to t he tumors and/or may have the potential to differentiate into adipocytes. Both possibilities are being explored in current studies.
In conclusion, EPA+DHA ethyl ester supplementation countered the DIO-induced acceleration of tumor growth in murine CL and BL models of TNBC. The anticancer effects of EPA+DHA were associated with improved glucose tolerance, decreased serum leptin:adiponectin ratio, decreases in tumoral pro-inflammatory markers, and increased tumoral cleaved caspase-3. These findings, in combination with parallel studies in women [14], suggest EPA+DHA supplementation should be further explored for reducing the burden of TNBC (including BL and CL) in obese women.
Supplementary Material
Acknowledgments
Financial Support: This work was supported by a Breast Cancer Research Foundation Grant (UTA13-001068; S.D. Hursting). N.A. Ford was supported by an American Institute for Cancer Research Postdoctoral Fellowship. E.L. Rossi was supported by a UNC University Cancer Fund Fellowship. L.W. Bowers was supported by a grant from the National Cancer Institute (R25CA057726).
Footnotes
Conflicts of interest: The authors disclose no potential conflicts of interest.
REFERENCES
- 1.Hursting SD, Digiovanni J, Dannenberg AJ, Azrad M, Leroith D, Demark-Wahnefried W, et al. Obesity, energy balance, and cancer: new opportunities for prevention. Cancer Prev Res. 2012;5:1260–72. doi: 10.1158/1940-6207.CAPR-12-0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Protani M, Coory M, Martin JH. Effect of obesity on survival of women with breast cancer: systematic review and meta-analysis. Breast Cancer Res Treat. 2010;123:627–635. doi: 10.1007/s10549-010-0990-0. [DOI] [PubMed] [Google Scholar]
- 3.Millikan RC, Newman B, Tse CK, Moorman PG, Conway K, Dressler LG, et al. Epidemiology of basal-like breast cancer. Breast Cancer Res Treat. 2008;109:123–39. doi: 10.1007/s10549-007-9632-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nogueira LM, Dunlap SM, Ford NA, Hursting SD. Calorie restriction and rapamycin inhibit MMTV-Wnt-1 mammary tumor growth in a mouse model of postmenopausal obesity. Endocr Relat Cancer. 2012;19:57–68. doi: 10.1530/ERC-11-0213. [DOI] [PubMed] [Google Scholar]
- 5.Fabian CJ, Kimler BF, Donnelly JE, Sullivan DK, Klemp JR, Petroff BK, et al. Favorable modulation of benign breast tissue and serum risk biomarkers is associated with > 10 % weight loss in postmenopausal women. Breast Cancer Res Treat. 2013;142:119–32. doi: 10.1007/s10549-013-2730-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riediger ND, Othman RA, Suh M, Moghadasian MH. A systemic review of the roles of n-3 fatty acids in health and disease. J Am Dietetic Assoc. 2009;109:668–79. doi: 10.1016/j.jada.2008.12.022. [DOI] [PubMed] [Google Scholar]
- 7.Flachs P, Rossmeisl M, Bryhn M, Kopecky J. Cellular and molecular effects of n-3 polyunsaturated fatty acids on adipose tissue biology and metabolism. Clin Sci (Lond) 2009;116:1–16. doi: 10.1042/CS20070456. [DOI] [PubMed] [Google Scholar]
- 8.Maillard V, Bougnoux P, Ferrari P, Jourdan ML, Pinault M, Lavillonniere F, et al. N-3 and N-6 fatty acids in breast adipose tissue and relative risk of breast cancer in a case-control study in Tours, France. Int J Cancer. 2002;98:78–83. doi: 10.1002/ijc.10130. [DOI] [PubMed] [Google Scholar]
- 9.Kuriki K, Hirose K, Wakai K, Matsuo K, Ito H, Suzuki T, et al. Breast cancer risk and erythrocyte compositions of n-3 highly unsaturated fatty acids in Japanese. Int J Cancer. 2007;121:377–85. doi: 10.1002/ijc.22682. [DOI] [PubMed] [Google Scholar]
- 10.Signori C, DuBrock C, Richie JP, Prokopczyk B, Demers LM, Hamilton C, et al. Administration of omega-3 fatty acids and Raloxifene to women at high risk of breast cancer: interim feasibility and biomarkers analysis from a clinical trial. Eur J Clin Nutr. 2012;66:878–84. doi: 10.1038/ejcn.2012.60. [DOI] [PubMed] [Google Scholar]
- 11.Dunlap SM, Chiao LJ, Nogueira L, Usary J, Perou CM, Varticovski L, et al. Dietary energy balance modulates epithelial-to-mesenchymal transition and tumor progression in murine claudin-low and basal-like mammary tumor models. Cancer Prev Res. 2012;5:930–42. doi: 10.1158/1940-6207.CAPR-12-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuo WH, Chang YY, Lai LC, Tsai MH, Hsiao CK, Chang KJ, et al. Molecular characteristics and metastasis predictor genes of triple-negative breast cancer: a clinical study of triple-negative breast carcinomas. PloS One. 2012;7:e45831. doi: 10.1371/journal.pone.0045831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hartman ZC, Poage GM, den Hollander P, Tsimelzon A, Hill J, Panupinthu N, et al. Growth of triple-negative breast cancer cells relies upon coordinate autocrine expression of the proinflammatory cytokines IL-6 and IL-8. Cancer Res. 2013;73:3470–80. doi: 10.1158/0008-5472.CAN-12-4524-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fabian CF, Kimler BF, Petroff BK, Zalles CM, Metheny T, Box JA, et al. High dose omega-3 fatty acid (FA) supplementation modulates breast tissue biomarkers in pre-menopausal women at high risk for development of breast cancer. J Clin Oncol. 2013;31 (suppl; abstr 1515) [Google Scholar]
- 15.De Angel RE, Berrigan D, Nunez NP, Hursting SD, Perkins SN. Dietary calcium source influences body composition, glucose metabolism and hormone levels in a mouse model of postmenopausal obesity. In Vivo. 2009;23:527–35. [PubMed] [Google Scholar]
- 16.Lashinger LM, Malone LM, Brown GW, Daniels EA, Goldberg JA, Otto G, et al. Rapamycin partially mimics the anticancer effects of calorie restriction in a murine model of pancreatic cancer. Cancer Prev Res. 2011;4:1041–51. doi: 10.1158/1940-6207.CAPR-11-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 18.Hidaka BH, Li S, Harvey KE, Carlson SE, Sullivan DK, Kimler BF, et al. Omega-3 and Omega-6 fatty acids in blood and breast tissue of high-risk women and association with atypical cytomorphology. Cancer Prev Res. 2015 doi: 10.1158/1940-6207.CAPR-14-0351. In Press. [DOI] [PubMed] [Google Scholar]
- 19.Yee LD, Lester JL, Cole RM, Richardson JR, Hsu JC, Li Y, et al. Omega-3 fatty acid supplements in women at high risk of breast cancer have dose-dependent effects on breast adipose tissue fatty acid composition. Am J Clin Nutr. 2010;91:1185–94. doi: 10.3945/ajcn.2009.29036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang P, Chan D, Felix E, Madden T, Klein RD, Shureiqi I, et al. Determination of endogenous tissue inflammation profiles by LC/MS/MS: COX- and LOX-derived bioactive lipids. Prostaglandins Leukot Essent Fatty Acids. 2006;75:385–95. doi: 10.1016/j.plefa.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 21.Dubois RN, Abramson SB, Crofford L, Gupta RA, Simon LS, Van De Putte LB, et al. Cyclooxygenase in biology and disease. FASEB J. 1998;12:1063–73. [PubMed] [Google Scholar]
- 22.Gasparini C, Feldmann M. NF-κB as a target for modulating inflammatory responses. Curr Pharm Des. 2012;18:5735–45. doi: 10.2174/138161212803530763. [DOI] [PubMed] [Google Scholar]
- 23.Dey N, Barwick BG, Moreno CS, Ordanic-Kodani M, Chen Z, Oprea-Ilies G, et al. Wnt signaling in triple negative breast cancer is associated with metastasis. BMC Cancer. 2013;13:537–43. doi: 10.1186/1471-2407-13-537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 25.Harris WS, Pottala JV, Sands SA, Jones PG. Comparison of the effects of fish and fish-oil capsules on the n 3 fatty acid content of blood cells and plasma phospholipids. Am J Clin Nutr. 2007;86:1621–5. doi: 10.1093/ajcn/86.5.1621. [DOI] [PubMed] [Google Scholar]
- 26.Schuchardt JP, Schneider I, Meyer H, Neubronner J, von Schacky C, Hahn A. Incorporation of EPA and DHA into plasma phospholipids in response to different omega-3 fatty acid formulations—a comparative bioavailability study of fish oil vs. krill oil. Lipids Health Dis. 2011;10:145. doi: 10.1186/1476-511X-10-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoy SM, Keating GM. Omega-3 ethylester concentrate: a review of its use in secondary prevention post-myocardial infarction and the treatment of hypertriglyceridaemia. Drugs. 2009;69:1077–105. doi: 10.2165/00003495-200969080-00008. [DOI] [PubMed] [Google Scholar]
- 28.Ford ES, Li C, Zhao G. Prevalence and correlates of metabolic syndrome based on a harmonious definition among adults in the US. J Diabetes. 2010;2:180–93. doi: 10.1111/j.1753-0407.2010.00078.x. [DOI] [PubMed] [Google Scholar]
- 29.Hursting SD, Berger NA. Energy balance, host-related factors, and cancer progression. J Clin Oncol. 2010;28:4058–65. doi: 10.1200/JCO.2010.27.9935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carter JC, Church FC. Obesity and breast cancer: the roles of peroxisome proliferator-activated receptor-gamma and plasminogen activator inhibitor-1. PPAR Res. 2009;2009:345320. doi: 10.1155/2009/345320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, et al. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss. Arterioscler Thromb Vasc Biol. 2006;26:968–76. doi: 10.1161/01.ATV.0000216787.85457.f3. [DOI] [PubMed] [Google Scholar]
- 32.Pollak M. The insulin and insulin-like growth factor receptor family in neoplasia: an update. Nat Rev Cancer. 2012;12:159–69. doi: 10.1038/nrc3215. [DOI] [PubMed] [Google Scholar]
- 33.Rossmeisl M, Jilkova ZM, Kuda O, Jelenik T, Medrikova D, Stankova B, et al. Metabolic effects of n-3 PUFA as phospholipids are superior to triglycerides in mice fed a high-fat diet: possible role of endocannabinoids. PloS One. 2012;7:e38834. doi: 10.1371/journal.pone.0038834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dangardt F, Chen Y, Gronowitz E, Dahlgren J, Friberg P, Strandvik B. High physiological omega-3 fatty acid supplementation affects muscle fatty acid composition and glucose and insulin homeostasis in obese adolescents. J Nutr Metab. 2012;2012:395757. doi: 10.1155/2012/395757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Del Prete A, Allavena P, Santoro G, Fumarulo R, Corsi MM, Mantovani A. Molecular pathways in cancer-related inflammation. Biochem Med (Zagreb) 2011;21:264–75. doi: 10.11613/bm.2011.036. [DOI] [PubMed] [Google Scholar]
- 36.Porter AG, Janicke RU. Emerging roles of caspase-3 in apoptosis. Cell Death Diff. 1999;6:99–104. doi: 10.1038/sj.cdd.4400476. [DOI] [PubMed] [Google Scholar]
- 37.Hursting SD, Dunlap SM, Ford NA, Hursting MJ, Lashinger LM. Calorie restriction and cancer prevention: a mechanistic perspective. Cancer Metab. 2013;1:10. doi: 10.1186/2049-3002-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mizuno NK, Rogozina OP, Seppanen CM, Liao DJ, Cleary MP, Grossman ME. Combination of intermittent calorie restriction and eicospentaenoic acid for inhibition of mammary tumors. Cancer Prev Res. 2013;6:540–7. doi: 10.1158/1940-6207.CAPR-13-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Basu S, Nachat-Kappes R, Caldefie-Chezet F, Vasson MP. Eicosanoids and adipokines in breast cancer: from molecular mechanisms to clinical considerations. Antioxid Redox Signal. 2013;18:323–60. doi: 10.1089/ars.2011.4408. [DOI] [PubMed] [Google Scholar]
- 40.Howe LR, Subbaramaiah K, Hudis CA, Dannenberg AJ. Molecular pathways: adipose inflammation as a mediator of obesity-associated cancer. Clin Cancer Res. 2013;19:6074–83. doi: 10.1158/1078-0432.CCR-12-2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ono M. Molecular links between tumor angiogenesis and inflammation: inflammatory stimuli of macrophages and cancer cells as targets for therapeutic strategy. Cancer Sci. 2008;99:1501–6. doi: 10.1111/j.1349-7006.2008.00853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kundu JK, Surh YJ. Inflammation: gearing the journey to cancer. Mutation research. 2008;659:15–30. doi: 10.1016/j.mrrev.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 43.Glueck CJ, Khan N, Riaz M, Padda J, Khan Z, Wang P. Titrating Lovaza from 4 to 8 to 12 grams/day in patients with primary hypertriglyceridemia who had triglyceride levels >500 mg/dl despite conventional triglyceride lowering therapy. Lipids Health Dis. 2012;11:143. doi: 10.1186/1476-511X-11-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kimijima I, Ohtake T, Sagara H, Watanabe T, Takenoshita S. Scattered fat invasion: an indicator for poor prognosis in premenopausal, and for positive estrogen receptor in postmenopausal breast cancer patients. Oncology. 2000;59(Suppl 1):25–30. doi: 10.1159/000055284. [DOI] [PubMed] [Google Scholar]
- 45.Yamaguchi J, Ohtani H, Nakamura K, Shimokawa I, Kanematsu T. Prognostic impact of marginal adipose tissue invasion in ductal carcinoma of the breast. Am J Clin Pathol. 2008;130:382–388. doi: 10.1309/MX6KKA1UNJ1YG8VN. [DOI] [PubMed] [Google Scholar]
- 46.Tan J, Buache E, Chenard MP, Dali-Youcef N, Rio MC. Adipocyte is a non-trivial, dynamic partner of breast cancer cells. Int J Dev Biol. 2011;55:851–9. doi: 10.1387/ijdb.113365jt. [DOI] [PubMed] [Google Scholar]
- 47.De Angel RE, Conti CJ, Wheatley KE, Brenner AJ, Otto G, Degraffenried LA, et al. The enhancing effects of obesity on mammary tumor growth and Akt/mTOR pathway activation persist after weight loss and are reversed by RAD001. Mol Carcinog. 2013;52:446–58. doi: 10.1002/mc.21878. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.