Abstract
Background
Cixutumumab, a human monoclonal antibody (HuMAb), targets the insulin-like growth factor receptor. Ramucirumab is a recombinant HuMAb that binds to vascular endothelial growth factor receptor-2. A non-comparative randomized phase II study evaluated cixutumumab or ramucirumab plus mitoxantrone and prednisone (MP) in metastatic castration-resistant prostate cancer (mCRPC).
Patients and Methods
Men with progressive mCRPC during or after docetaxel therapy received mitoxantrone 12 mg/m2 on day 1 and prednisone 5 mg twice daily and were randomized 1:1 to receive either cixutumumab or ramucirumab 6 mg/kg intravenously weekly in a 21-day cycle. Primary endpoint was composite progression-free survival (cPFS). Secondary endpoints included safety, response, radiographic PFS, and overall survival (OS). Sample size was based on a 50% increase in median cPFS from 2.6 (MP) to 3.9 months (either combination).
Results
132 men were treated (66 per arm). Median cPFS was 4.1 months (95% CI, 2.2–5.6) for cixutumumab and 6.7 months (95% CI, 4.5–8.3) for ramucirumab. Median time to radiographic progression was 7.5 months for cixutumumab and 10.2 months for ramucirumab, with a median OS of 10.8 and 13.0 months, respectively. Fatigue was the most frequent adverse event (AE). Incidence of most non-hematologic grade 3-4 AEs was <10% on both arms. Grade 3 cardiac dysfunction occurred in 7.6% of patients on ramucirumab.
Conclusion
Combinations of cixutumumab or ramucirumab plus MP were feasible and associated with moderate toxicities in docetaxel pretreated men with mCRPC. Of the two regimens, the ramucirumab regimen is worthy of further testing based on the observed cPFS relative to the historical control.
Keywords: Ramucirumab, cixutumumab, mitoxantrone, prednisone, prostate cancer
Introduction
Despite significant progress in therapy development for patients with metastatic castration resistant prostate cancer (mCRPC), survival is limited and better treatments are needed [1-3]. Insulin-like growth factor (IGF) and type-1 receptor (IGF-IR)-mediated signaling can potentiate androgen-receptor activation [4], and IGF-IR signaling contributes to proliferation, tumor-stromal interactions, invasion, and metastasis [5-9] in preclinical models of prostate cancer (PC). Anti–IGF-IR antibodies, IGF-IR kinase inhibitors, and antisense oligonucleotides to IGF-IR inhibit PC growth in vitro and in vivo [10-12].
Cixutumumab (IMC-A12) is a human immunoglobulin G, subclass 1 (IgG1) monoclonal antibody (MAb) with high affinity and specificity for IGF-IR and is an antagonist of IGF-I and IGF-II ligand binding and signaling [13,14]. Cixutumumab inhibits the proliferation and growth of a variety of human tumor cell lines, both in vitro and in vivo [13]. Cixutumumab inhibited growth of androgen-dependent and androgen-independent xenograft prostate tumors and growth inhibition was enhanced when cixutumumab was co-administered with docetaxel in CRPC models [14,15]. Preclinical data suggest that cixutumumab monotherapy inhibits but does not completely arrest tumor growth, with the most profound effects observed when IGF-IR inhibitors are combined with other agents [16]. In a phase II study of cixutumumab monotherapy in mCRPC patients, 9 of 31 (29%) had disease stabilization for at least 6 months and cixutumumab was found to be well tolerated [17].
Vascular endothelial growth factor (VEGF) is up-regulated in PC, and higher expression has been associated with higher grade [18], more advanced disease, rapid progression, and shorter survival [19-22]. Microvessel density and VEGF expression are increased in PC and higher levels of circulating and tumor VEGF are associated with aggressive clinical and preclinical PC phenotypes [18,20,21,22]. Inhibition of VEGF receptor-2 (VEGFR-2) with the antibody DC101 inhibits PC growth and bone metastasis in murine models [23]. Ramucirumab is a recombinant human IgG1 MAb that binds specifically and with high affinity to VEGFR-2, and inhibits receptor activation [24]. Preclinical cellular and animal models of solid and liquid tumors have demonstrated that ramucirumab attacks its intended target with inhibition of VEGF-induced VEGFR-2 activation and inhibition of VEGF-stimulated cellular migration and proliferation, and efficacy has been demonstrated in phase I trials, particularly in heavily pretreated refractory patients [25].
At the time of the study design, mCRPC patients progressing on docetaxel had no life-prolonging therapy choices and the only available treatment was the combination of mitoxantrone and prednisone, which was approved for pain palliation [26].
Based on the biological and preclinical data, we hypothesized that cixutumumab or ramucirumab would enhance the activity of mitoxantrone and prednisone in men with docetaxel-pretreated mCRPC. The study was designed and completed before the regulatory approvals of cabazitaxel, abiraterone, enzalutamide, and radium-223 in the post-docetaxel setting. Thus, we conducted a randomized, open-label, non-comparative phase II study of cixutumumab or ramucirumab plus mitoxantrone and prednisone in patients with mCRPC.
Methods
Eligibility Criteria
Eligible patients were men ≥18 years old with histologically confirmed prostate adenocarcinoma, castration-resistant disease, radiographic evidence of metastases, an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0 to 2, and prostate-specific antigen (PSA) ≥2 ng/mL. Patients had disease progression during or within 120 days of completion of or documented intolerance of docetaxel. Disease progression was defined as at least one of the following: 1) progressive measurable disease using Response Evaluation Criteria in Solid Tumors (RECIST 1.0) criteria, 2) bone scan progression, with at least two new lesions, and/or 3) increasing PSA, with at least two consecutive rising PSA values over a reference value taken at least one week apart. Patients were required to have surgical or medical castration with a serum testosterone level <50 ng/mL. Nonsurgically castrated patients continued using luteinizing hormone releasing hormone agonists during study treatment.
Patients were excluded for prior therapy with mitoxantrone, radionuclide therapy with ongoing evidence of bone marrow dysfunction or inadequate symptom control, or left ventricular ejection fraction (LVEF) that was ≥10% below the lower limit of normal (multigated acquisition scan [MUGA]).
The study was undertaken in accordance with principles of the Declaration of Helsinki and Good Clinical Practice guidelines, and with local ethics committee approval. Written informed consent was obtained from all participants.
Randomization, Treatment, and Disease Monitoring
Randomization was stratified by ECOG PS of 2 (versus 0 or 1), the presence (versus absence) of PC-related bone pain requiring frequent opiate analgesic therapy (defined as use ≥50% of days during the week before randomization), and stable disease (SD) or better (complete response [CR], partial response [PR]) as best response to prior docetaxel therapy versus progressive disease (Supplemental Table A.1).
Patients were randomized 1:1 to open-label cixutumumab 6 mg/kg or ramucirumab 6 mg/kg intravenously over 1 hour on days 1, 8, and 15 of 3-week (21-day) cycles. Patients received oral prednisone 5 mg twice daily and mitoxantrone 12 mg/m2 intravenously on day 1 every 21 days for a maximum of 12 cycles. Treatment continued until disease progression, death, intolerable toxicity, or other withdrawal criteria were met. Experimental drug was continued if mitoxantrone was stopped. Patients were followed until the cutoff date for analysis or until death.
Baseline evaluations included medical history, physical examination, biochemistry, hematology, PSA, and electrocardiogram. Patients were monitored throughout the study for PS, adverse event (AE) assessment, recording of concomitant medications, and echocardiogram or MUGA to assess LVEF. AEs were collected weekly and graded according to National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE), version 3.0. Computerized tomography scan or magnetic resonance imaging of abdomen, pelvis, and bone scans were repeated after the initial 9 weeks of therapy and thereafter every 6 weeks.
The primary endpoint of composite progression-free survival (cPFS) was defined as time from randomization to any of the following: 1) tumor progression by RECIST 1.0; 2) at least two new lesions detected on bone scan [27]; 3) new skeletal events (pathologic bone fracture in an area of metastatic disease, bone lesions requiring radiation therapy or surgery, or spinal cord or nerve root compression); 4) symptomatic progression, defined as a deterioration in ECOG PS of 2 or more points or weight loss of 20% or more from baseline; 5) other prostate cancer-related clinical events requiring major interventions, or; 6) death.
Biomarker Analyses
Biomarkers were measured as exploratory analyses (Intertek Alta Analytical Laboratory, San Diego, CA). A total of 23 analytes were measured using non-GLP quantitative sandwich electrochemiluminescence prototype kits. Analytes assayed, in all treated patients from whom samples were provided and who signed the appropriate consent, included: VEGF (VEGF-A), VEGF-C, VEGF-D, placental growth factor (PlGF), soluble VEGFR-1 (sFlt-1), soluble VEGFR-2 (KDR), angiopoietin-1, angiopoietin-2, HGF, SDF-1A, bFGF/FGF2, thrombomodulin, E-Selectin, P-Selectin, SAA, CRP, VCAM-1, ICAM-1, ICAM-3, IL-12, IL-4, IL-8, and C-KIT.
Statistical Analyses
This randomized non-comparative trial was designed to evaluate two promising regimens in a comparable population to select the best combination for potential future phase III testing. Considering available data regarding cPFS associated with minimally effective chemotherapy in a randomized phase III trial at the time of study design [28], and to increase the efficiency of conducting this trial, the study was designed without a mitoxantrone-prednisone control arm. A sample size of 66 patients per arm (132 patients total) was required to detect an increase of 50% in median cPFS to 3.9 months with either combination as compared with 2.6 months cPFS with mitoxantrone-prednisone in either arm. This yielded 90% power for a one-tail test at a 0.025 significance level, assuming a 52-week accrual period and total study duration of 104 weeks. The arms were analyzed separately using SAS, version 8.2 or higher (SAS Institute, Cary, NC). The PSA response rate was calculated based on the proportion of patients with a decrease in PSA ≥50% from baseline. Composite-PFS and OS were analyzed using the Kaplan-Meier method. Cox regression was used to assess correlations between the time-to-event outcomes and biomarkers at baseline (additional details in Data Supplement).
Results
Patient Characteristics
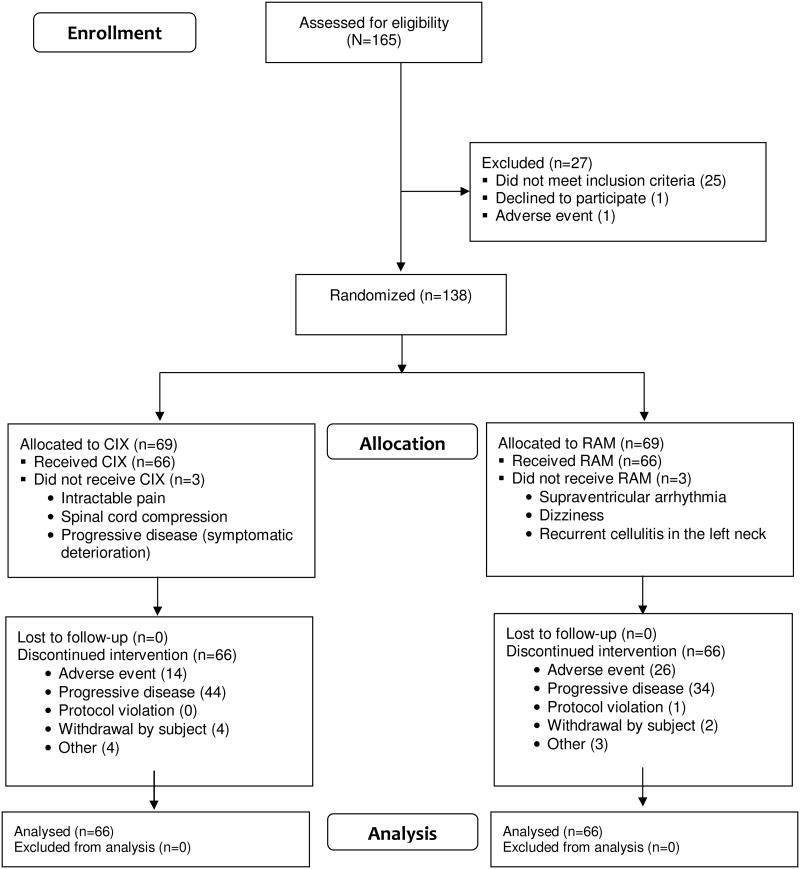
Between August 2008 and September 2011, 132 mCRPC patients (66 per arm) were randomized and treated at 35 centers across the United States (Figure 1). Baseline demographics and disease-related characteristics were similar for both arms (Table 1). Approximately one-third of the study population had metastases to liver, lung, peritoneum, pleura or adrenal gland, with or without involvement of other sites (43.9% cixutumumab; 33.3% ramucirumab).
Figure 1.
CONSORT diagram.
Table 1. Baseline Patient Demographics and Clinical Characteristics.
| Cixutumumab + M + P (n=66) | Ramucirumab + M + P (n=66) | |
|---|---|---|
| Age, years | ||
| Median (range) | 65 (48–88) | 68 (46–86) |
| 18 to <65 | 30 (45.5) | 21 (31.8) |
| ≥65 | 36 (54.5) | 45 (68.2) |
| Race, n (%) | ||
| Black or African American | 4 (6.1) | 6 (9.1) |
| White | 61 (92.4) | 58 (87.9) |
| Other | 1 (1.5) | 2 (3.0) |
| Ethnic origin, n (%) | ||
| Hispanic or Latino | 1 (1.5) | 3 (4.5) |
| Not Hispanic or Latino | 65 (98.5) | 63 (95.5) |
| ECOG PS, n (%) | ||
| 0 | 23 (34.8) | 19 (28.8) |
| 1 | 38 (57.6) | 41 (62.1) |
| 2 | 5 (7.6) | 6 (9.1) |
| Disease site, n (%) | ||
| Bone only | 13 (19.7) | 18 (27.3) |
| Lymph nodes with/without bones | 17 (25.8) | 19 (28.8) |
| Viscera | 29 (43.9) | 22 (33.3) |
| Skin/soft tissue with/without others | 7 (10.6) | 7 (10.6) |
| Prior docetaxel therapy, n (%) | ||
| 1 regimen | 56 (84.8) | 54 (81.8) |
| 2 regimens | 9 (13.6) | 11 (16.7) |
| 3 regimens | 1 (1.5) | 1 (1.5) |
| PD on prior docetaxel, n (%) | ||
| During therapy | 38 (57.6) | 41 (62.1) |
| Within 3 m of last dose | 13 (19.7) | 9 (13.6) |
| >3 m of last dose | 6 (9.1) | 9 (13.6) |
| Not complete/available | 9 (13.6) | 7 (10.6) |
| Pain during week prior to randomization, n (%) | ||
| Required opiate ≥50% of days | 28 (42.4) | 33 (50.0) |
| Did not require opiate ≥50% of days | 38 (57.6) | 33 (50.0) |
| Stratification, n (%) | ||
| PS=2 or opiate ≥50% of days | 31 (47.0) | 35 (53.0) |
| PS 0–1 and opiate <50% of days | 35 (53.0) | 31 (47.0) |
| Stratification (best response prior therapy), n (%) | ||
| CR, PR, SD on docetaxel | 29 (43.9) | 27 (40.9) |
| PD on or intolerant to docetaxel | 37 (56.1) | 39 (59.1) |
| PSA, µg/mL | ||
| Median, range | 133.45 (0.1–5530.0) | 107.30 (2.2–5826.4) |
| Duration of disease (from diagnosis to first dose), months | ||
| Mean, (SD) | 65.9 (50.6) | 71.2 (56.2) |
Abbreviations: CR, complete response; ECOG PS, Eastern Cooperative Oncology Group performance status; m, months; M, mitoxantrone; P, prednisone; PD, progressive disease; PR, partial response; SD, stable disease; PSA, prostate-specific antigen.
Treatment Summary
Most patients on both arms (62 [93.9%] and 65 [98.5%]) received two or more doses of cixutumumab or ramucirumab, respectively. Median duration of therapy was 15.0 weeks (range, 1.0–117.1) on cixutumumab and 19.0 weeks (range, 1.0–86.0) on ramucirumab. Most patients (89.4% cixutumumab; 87.9% ramucirumab) had no dose reductions. Discontinuations due to AEs occurred in 14/69 (20.3%) patients on cixutumumab and 26/69 (37.7%) patients on ramucirumab (Figure 1). Administration and exposure of cixutumumab, ramucirumab, and mitoxantrone are provided in Supplemental Table A.2.
Approximately half of the patients (cixutumumab 59.1%; ramucirumab 50.0%) received additional post-study therapy. The most frequent types of post-study therapy received were chemotherapy (42.4% and 36.4%) and radiotherapy (24.2% and 15.2%) (Supplemental Table A.3).
Efficacy
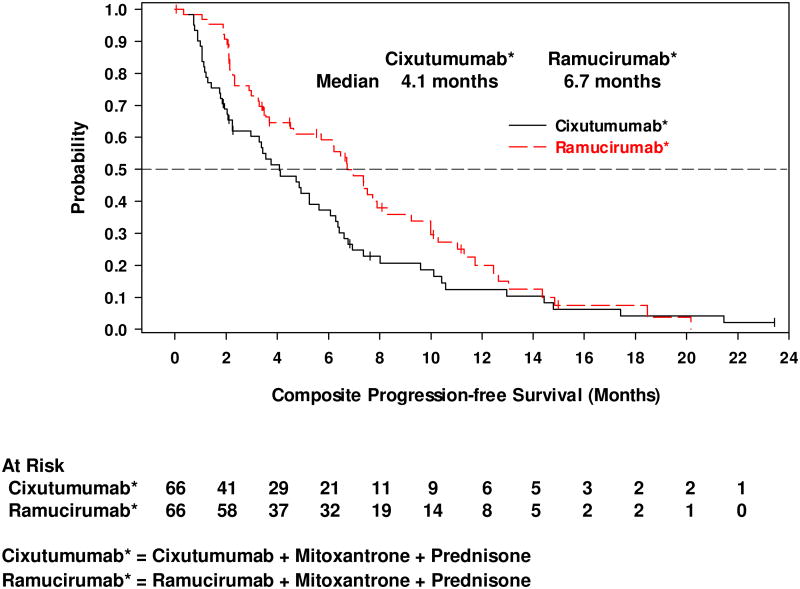
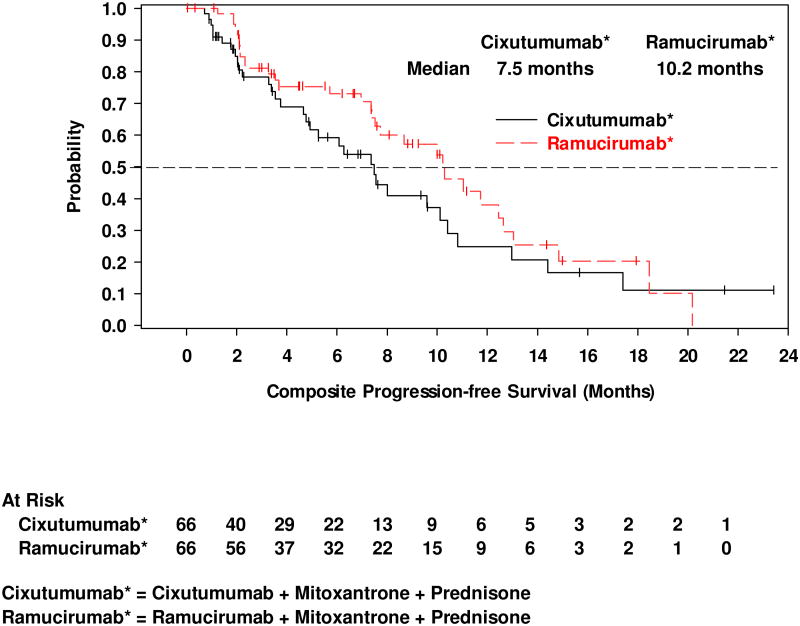
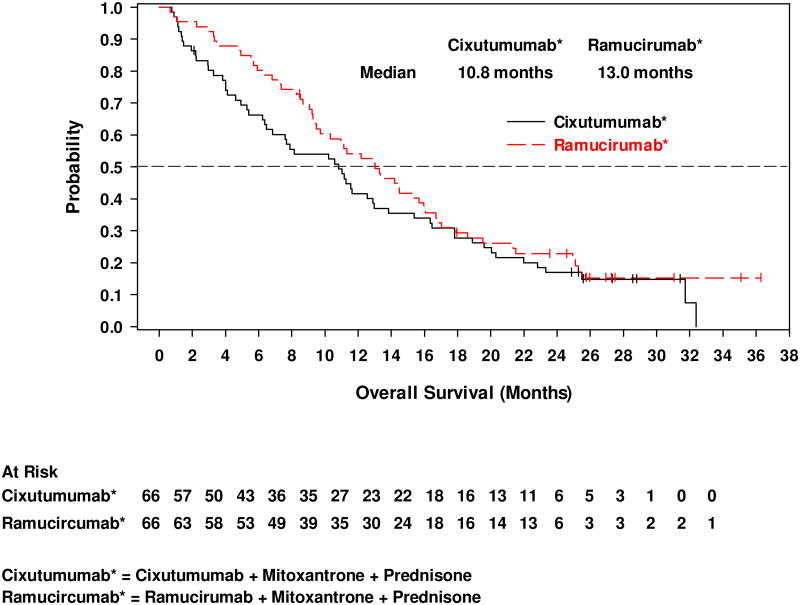
The median cPFS was 4.1 months (95% CI, 2.2–5.6) for cixutumumab and 6.7 months (95% CI, 4.5–8.3) for ramucirumab (Figure 2a). The 6-month cPFS rates were 37.2% for cixutumumab and 59.2% for ramucirumab (Table 2). Median time to radiographic disease progression (RECIST or bone scan criteria) was 7.5 months (95% CI, 4.8–10.1) on cixutumumab and 10.2 months (95% CI, 7.5–12.6) on ramucirumab (Figure 2b). Median OS was 10.8 months (95% CI, 6.5–13.0) on cixutumumab and 13.0 months (95% CI, 9.5–16.0) on ramucirumab (Figure 2c). A PSA decline of ≥50% from baseline occurred in 18.5% of patients on cixutumumab and 21.4% of patients on ramucirumab (Figure 3). In the subset of patients with measurable disease, the ORR was 15.2% (7/46) (cixutumumab) and 31.6% (12/38) (ramucirumab) (Table 2). The disease control rate (DCR, defined as CR+PR+SD) for all patients was 65.2% (cixutumumab: 95% CI, 52.4–76.5) and 77.3% (ramucirumab: 95% CI, 65.3–86.7).
Figure 2.
Kaplan-Meier plots of (a) composite progression-free survival; (b) time to radiographically evident disease progression, and; (c) overall survival.
Table 2. Efficacy Results.
| Cixutumumab + M + P | Ramucirumab + M + P | |
|---|---|---|
| Composite progression-free survival | (n=66) | (n=66) |
| Median, months | 4.1 | 6.7 |
| 95% CI | 2.2–5.6 | 4.5–8.3 |
| Rate at 6 months, % | 37.2 | 59.2 |
| 95% CI | 25.0–49.4 | 45.8–70.4 |
| Rate at 12 months, % | 12.4 | 20.0 |
| 95% CI | 5.2–22.9 | 10.1–32.3 |
| Overall survival | (n=66) | (n=66) |
| Median, months | 10.8 | 13.0 |
| 95% CI | 6.5–13.0 | 9.5–16.0 |
| Rate at 6 months, % | 66.3 | 80.3 |
| 95% CI | 53.5–76.4 | 68.5–88.1 |
| Rate at 12 months, % | 41.6 | 54.2 |
| 95% CI | 29.6–53.2 | 41.4–65.3 |
| PSA response | (n=54) | (n=56) |
| ≥50% decline from baseline, % | 18.5 | 21.4 |
| 95% CIa | 9.3–31.4 | 11.6–34.4 |
| Objective response (CR + PR) | ||
| Measurable disease | (n=46) | (n=38) |
| Response rate, % | 15.2 | 31.6 |
| 95% CIa | 6.3–28.9 | 17.5–48.7 |
| Disease control rate (CR + PR + SD) | (n=66) | (n=66) |
| Response rate, % | 65.2 | 77.3 |
| 95% CIa | 52.4–76.5 | 65.3–86.7 |
| Duration of follow-upb | ||
| Median, months | 28.6 | 26.9 |
Binomial exact confidence interval.
Duration of follow-up calculation was made using the Kaplan-Meier method of analysis.
Abbreviations: CI, confidence interval; CR, complete response; M, mitoxantrone; P, prednisone; PR, partial response; PSA, prostate-specific antigen; SD, stable disease.
Figure 3.
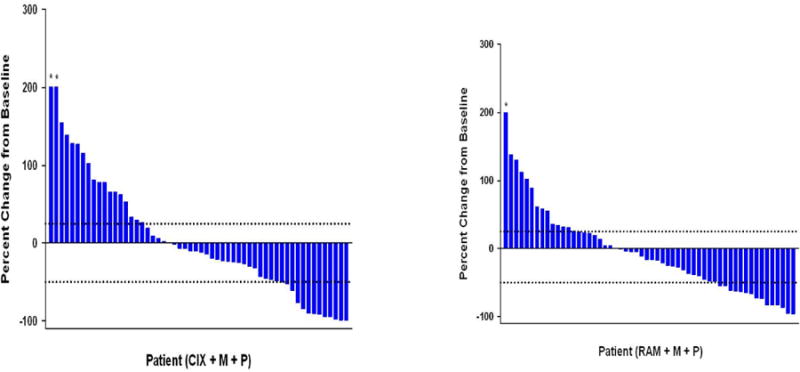
Waterfall plot for best percent change in PSA from baseline. Response defined as ≥50% PSA decrease from baseline and progression defined as 25% PSA increase.
CIX, cixutumumab; M, mitoxantrone; P, prednisone; RAM, ramucirumab. (*Truncated at 200%)
Safety
Regardless of causality, fatigue (any grade) was the most frequent AE (Table 3), and the incidence of most non-hematologic grade 3–4 AEs was <10% on both arms. The incidence of hyperglycemia and dehydration occurred in 47.0% and 28.8% of patients on cixutumumab, respectively. Hypertension (34.8%), thrombocytopenia (34.8%), and dyspnea (31.8%) were seen in >20% of patients on ramucirumab. Treatment-related serious AEs occurred in 22 patients (33.3%) on cixutumumab and 16 patients (24.2%) on ramucirumab.
Table 3. Most Frequent Treatment-emergent Non-hematologic and Hematologic Adverse Events (≥20% Any Grade on Either Arm)a.
| Cixutumumab + M + P (n=66) | Ramucirumab + M + P (n=66) | |||||
|---|---|---|---|---|---|---|
| Non-hematologic AE | Any G, % | G3, % | G4, % | Any G, % | G3, % | G4, % |
| Fatigue | 74.2 | 16.7 | 0 | 71.2 | 7.6 | 0 |
| Weight decreased | 65.2 | 4.5 | 0 | 60.6 | 1.5 | 0 |
| Anorexia | 53.0 | 0 | 0 | 47.0 | 3.0 | 0 |
| Nausea | 53.0 | 1.5 | 0 | 47.0 | 4.5 | 0 |
| Diarrhea | 43.9 | 7.6 | 0 | 45.5 | 1.5 | 0 |
| Constipation | 40.9 | 0 | 0 | 37.9 | 1.5 | 0 |
| Hyperglycemia | 47.0 | 7.6 | 1.5 | 12.1 | 1.5 | 1.5 |
| Vomiting | 28.8 | 1.5 | 0 | 28.8 | 3.0 | 0 |
| Dehydration | 28.8 | 6.1 | 0 | 7.6 | 1.5 | 0 |
| Arthralgia | 25.8 | 6.1 | 0 | 24.2 | 4.5 | 0 |
| Back pain | 24.2 | 4.5 | 0 | 24.2 | 1.5 | 0 |
| Dyspnea | 18.2 | 3.0 | 0 | 31.8 | 7.6 | 0 |
| Peripheral edema | 15.2 | 1.5 | 0 | 21.2 | 0 | 0 |
| Ecchymosis | 15.2 | 0 | 0 | 24.2 | 0 | 0 |
| Stomatitis | 10.6 | 0 | 0 | 22.7 | 0 | 0 |
| Hypertension | 7.6 | 1.5 | 0 | 34.8 | 9.1 | 0 |
| Hematologic AE | ||||||
| Neutropenia | 42.4 | 16.7 | 15.2 | 37.9 | 22.7 | 9.1b |
| Anemia | 34.8 | 3.0 | 0 | 36.4 | 10.6 | 0 |
| Leukopenia | 31.8 | 16.7 | 6.1 | 25.8 | 15.2 | 1.5 |
| Thrombocytopenia | 18.2 | 3.0 | 1.5 | 34.8 | 7.6 | 0 |
Deaths assessed to be related to study treatment: ramucirumab arm, septic shock and pneumonia aspiration; cixutumumab arm, cachexia.
Includes 1 incidence (1.5%) of grade 5 neutropenia on ramucirumab.
Abbreviations: AE, adverse event; G, grade; M, mitoxantrone; P, prednisone.
At the time of analysis, fifty-seven patients (86.4%) had died in the cixutumumab arm and 54 patients (81.8%) in the ramucirumab arm; 10 patients (15.2%) and 6 patients (9.1%) died while either on study or within 30 days of last dose of study drug in the cixutumumab and ramucirumab arms, respectively. Four deaths on each arm were attributed to AEs, three of which were considered related to study treatment (one on cixutumumab arm and two on ramucirumab) (Table 3).
Cardiac dysfunction occurred in 16 patients (24.2%; 5 [7.6%] with grade 3) and 9 patients (13.6%; no grade 3 events reported) on ramucirumab and cixutumumab, respectively (Supplemental Table A.4). No grade 4–5 cardiac dysfunction was observed on either study arm. The median time to >10% decrease in LVEF from baseline was 6.0 months (range, 2.1–16.1) for cixutumumab and 5.1 months (range, 1.9–9.0) for ramucirumab. In patients who experienced cardiac dysfunction as an AE, the median time to event was 5.6 months (range, 2.1–16.1) on cixutumumab and 5.0 months (range, 2.0–7.9) on ramucirumab.
Biomarkers
For the majority of biomarkers assessed, there were no significant associations between baseline levels and cPFS or OS (data not shown). However, a potential association between higher baseline levels of IL-8 and both shorter cPFS and OS was identified (Supplemental Table A.5) for patients on both arms. The treatment benefit, as estimated by the model, of ramucirumab over cixutumumab is greater for patients with higher baseline levels of IL-8. However, these relationships were not consistent across endpoints and models. These results have not been adjusted for statistical testing of multiple hypotheses, and hence should only be considered as hypothesis-generating. Increases in pharmacodynamic markers PlGF and VEGF-A were observed following ramucirumab but not cixutumumab administration (data not shown).
Discussion
In this randomized, non-comparative phase II study, the combination of cixutumumab or ramucirumab with mitoxantrone-prednisone resulted in moderate disease control. The median cPFS of 4.1 months for the cixutumumab arm marginally exceeded the projected median target of 3.9 months used to estimate sample size, whereas the median cPFS for the ramucirumab arm of 6.7 months exceeded the projected median.
The benchmark for cPFS (median of 2.6 months) was based on contemporary data available for mitoxantrone-predniosone following docetaxel at the time of the study design (SPARC phase III study in mCRPC). Although the cPFS of both combinations exceeded that observed in SPARC [29], disease control and survival appeared longer on the ramucirumab arm.
During the conduct of this study, results from a randomized phase III study evaluating cabazitaxel versus mitoxantrone-prednisone in a post-docetaxel setting (TROPIC) were published. A median OS for mitoxantrone-prednisone of 12.7 months was reported [30], which is comparable to that observed for ramucirumab plus mitoxantrone-prednisone in the current study (13.0 months), although cross-study comparisons are inherently difficult.
The AEs reported for cixutumumab and ramucirumab in combination with mitoxantrone-prednisone were generally consistent with known safety profiles of cixutumumab or ramucirumab and mitoxantrone. A higher incidence of cardiac dysfunction (including grade 3) was observed with ramucirumab plus mitoxantrone-prednisone. It is likely that ramucirumab enhances the cardiotoxicity associated with mitoxantrone. Future studies of this agent should consider combinations that have a higher benefit-to-risk potential.
In an exploratory biomarker analysis, evidence of a potential association was observed between IL-8 levels and efficacy outcomes. This association appears to be primarily prognostic, with higher IL-8 levels associated with worse clinical outcome for both arms.
Limitations of the current study include absence of a chemotherapy control arm and availability of improved treatment options in mCRPC since study inception, including several efficacious systemic agents rendering mitoxantrone’s utility in this disease less certain. Although progress in mCRPC therapy has recently occurred, improvements in OS remain modest and newer agents and rational combinations targeting biologically relevant pathways remain important.
Several anti-angiogenic therapies have failed to impact survival in patients with mCRPC. Whether this is a function of the agent/target, disease setting, or biological context remains to be determined. In early-phase clinical trials of cixutumumab, targeting IGF-IR in patients with mCRPC has demonstrated both biologic and clinical activity [17]. However, the addition of cixutumumab to androgen-deprivation therapy failed to meet the primary endpoint of undetectable PSA response (ie, ≤0.2 ng/mL) compared with androgen-deprivation therapy [31]. The totality of the biological data do indicate the importance of angiogenesis in mCRPC, thus targeting other elements of the angiogenic pathway may be relevant. In phase III trials, ramucirumab has demonstrated improved OS in patients with resistant gastric cancer [32,33], metastatic colorectal carcinoma [34], and metastatic lung cancer [35]. A recent randomized phase II study of docetaxel with or without ramucirumab demonstrated a significant improvement in PFS for docetaxel plus ramucirumab in second-line metastatic urothelial carcinoma [36]. These data coupled with the observation from this study provide the rationale for further evaluation of ramucirumab in mCRPC. This should be informed by preclinical evaluation in CRPC models resistant to enzalutamide and abiraterone to better elucidate the potential utility of ramucirumab in the current clinical context.
Supplementary Material
Highlights.
Phase II trial of cixutumumab or ramucirumab in prostate cancer.
Primary endpoint was composite progression-free survival.
Median cPFS for ramucirumab/mitoxantrone/prednisone of 6.7 months exceeded projected median.
Additional evaluation of ramucirumab in mCRPC is potentially warranted.
Acknowledgments
This work was supported by and ramucirumab and cixutumumab were provided by Eli Lilly and Company. The study was designed under the responsibility of Eli Lilly and Company, in conjunction with the steering committee. Eli Lilly and Company collected and analyzed the data and contributed to the interpretation of the study. All authors had full access to all of the data in the study and had final responsibility for the decision to submit for publication. Eli Lilly and Company contracted with inVentiv Health Clinical for writingand editorial support, provided by Mary Ann Weller, PhD, and Noelle Gasco, respectively.
Footnotes
Previoulsy Presented: Presented in part at the Innovative Minds in Prostate Cancer Today (IMPaCT) conference, March 9–12, 2011, Orlando, FL and the American Society of Clinical Oncology Annual Genitourinary Cancers Symposium, February 2–4, 2012, San Francisco, CA (abstr 97).
ClinicalTrials.gov identifier: NCT00683475
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tannock IF, deWit R, Berry WR, Horti J, Pluzanska A, Chi KN, et al. TAX 327 Investigators: Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–12. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 2.Petrylak DP, Tangen CM, Hussain MH, Lara PN, Jr, Jones JA, Taplin ME, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–20. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 3.Alva A, Hussain M. The changing natural history of metastatic prostate cancer. Cancer J. 2013;19:19–24. doi: 10.1097/PPO.0b013e318281197e. [DOI] [PubMed] [Google Scholar]
- 4.Khandwala HM, McCutcheon IE, Flyvbjerg A, Friend KE. The effects of insulin-like growth factors on tumorigenesis and neoplastic growth. Endocr Rev. 2000;21:215–44. doi: 10.1210/edrv.21.3.0399. [DOI] [PubMed] [Google Scholar]
- 5.Kawada M, Inoue H, Masuda T, Ikeda D. Insulin-like growth factor I secreted from prostate stromal cells mediates tumor-stromal cell interactions of prostate cancer. Cancer Res. 2006;66:4419–25. doi: 10.1158/0008-5472.CAN-05-4239. [DOI] [PubMed] [Google Scholar]
- 6.Bähr C, Groner B. The IGF-1 receptor and its contributions to metastatic tumor growth-novel approaches to the inhibition of IGF-1R function. Growth Factors. 2005;23:1–14. doi: 10.1080/08977190400020229. [DOI] [PubMed] [Google Scholar]
- 7.Mauro L, Salerno M, Morelli C, Boterberg T, Bracke ME, Surmacz E. Role of the IGF-I receptor in the regulation of cell-cell adhesion: implications in cancer development and progression. J Cell Physiol. 2003;194:108–16. doi: 10.1002/jcp.10207. [DOI] [PubMed] [Google Scholar]
- 8.Montagnani Marelli M, Moretti RM, Procacci P, Motta M, Limonta P. Insulin-like growth factor-I promotes migration in human androgen-independent prostate cancer cells via the alphavbeta3 integrin and PI3-K/Akt signaling. Int J Oncol. 2006;28:723–30. [PubMed] [Google Scholar]
- 9.Rubin J, Chung LW, Fan X, Zhu L, Murphy TC, Nanes MS, et al. Prostate carcinoma cells that have resided in bone have an upregulated IGF-I axis. Prostate. 2004;58:41–9. doi: 10.1002/pros.10299. [DOI] [PubMed] [Google Scholar]
- 10.Blum G, Gazit A, Levitzki A. Development of new insulin-like growth factor-1 receptor kinase inhibitors using catechol mimics. J Biol Chem. 2003;278:40442–54. doi: 10.1074/jbc.M305490200. [DOI] [PubMed] [Google Scholar]
- 11.Burfeind P, Chernicky CL, Rininsland F, Ilan J. Antisense RNA to the type I insulin-like growth factor receptor suppresses tumor growth and prevents invasion by rat prostate cancer cells in vivo. Proc Natl Acad Sci U S A. 1996;93:7263–8. doi: 10.1073/pnas.93.14.7263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pandini G, Mineo R, Frasca F, Roberts CT, Jr, Marcelli M, Vigneri R, et al. Androgens up-regulate the insulin-like growth factor-I receptor in prostate cancer cells. Cancer Res. 2005;65:1849–57. doi: 10.1158/0008-5472.CAN-04-1837. [DOI] [PubMed] [Google Scholar]
- 13.Burtrum D, Zhu Z, Lu D, Anderson DM, Prewett M, Pereira DS, et al. A fully human monoclonal antibody to the insulin-like growth factor I receptor blocks ligand-dependent signaling and inhibits human tumor growth in vivo. Cancer Res. 2003;63:8912–21. [PubMed] [Google Scholar]
- 14.Wu JD, Odman A, Higgins LM, Haugk K, Vessella R, Ludwig DL, et al. In vivo effects of the human type I insulin-like growth factor receptor antibody A12 on androgen-dependent and androgen-independent xenograft human prostate tumors. Clin Cancer Res. 2005;11:3065–74. doi: 10.1158/1078-0432.CCR-04-1586. [DOI] [PubMed] [Google Scholar]
- 15.Wu JD, Haugk K, Coleman I, Woodke L, Vessella R, Nelson P, et al. Combined in vivo effect of A12, a type 1 insulin-like growth factor receptor antibody, and docetaxel against prostate cancer tumors. Clin Cancer Res. 2006;12(20 Pt 1):6153–60. doi: 10.1158/1078-0432.CCR-06-0443. [DOI] [PubMed] [Google Scholar]
- 16.Rowinsky EK, Schwartz JD, Zojwalla N, Youssoufian H, Fox F, Pultar P, et al. Blockade of insulin-like growth factor type-1 receptor with cixutumumab (IMC-A12): a novel approach to treatment for multiple cancers. Curr Drug Targets. 2011;12:2016–33. doi: 10.2174/138945011798829401. [DOI] [PubMed] [Google Scholar]
- 17.Higano C, Alumkal J, Ryan CJ, Yu EY, Beer TM, Chandrawansa K, et al. Prostate Cancer Clinical Trials Consortium. A phase II study evaluating the efficacy and safety of single agent IMC A12, a monoclonal antibody (MAb), against the insulin-like growth factor-1 receptor (IGF-IR), as monotherapy in patients with metastastic, asymptomatic castration-resistant prostate cancer (CRPC) J Clin Oncol. 2009;27(suppl 15S):abstr 5142. [Google Scholar]
- 18.Doll JA, Reiher FK, Crawford SE, Pins MR, Campbell SC, Bouck NP. Thrombospondin-1, vascular endothelial growth factor and fibroblast growth factor-2 are key functional regulators of angiogenesis in the prostate. Prostate. 2001;49:293–305. doi: 10.1002/pros.10025. [DOI] [PubMed] [Google Scholar]
- 19.Hwang C, Heath EI. Angiogenesis inhibitors in the treatment of prostate cancer. J Hematol Oncol. 2010;3:26. doi: 10.1186/1756-8722-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strohmeyer D, Rössing C, Bauerfeind A, Kaufmann O, Schlechte H, Bartsch G, et al. Vascular endothelial growth factor and its correlation with angiogenesis and p53 expression in prostate cancer. Prostate. 2000;45:216–24. doi: 10.1002/1097-0045(20001101)45:3<216::aid-pros3>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 21.Borre M, Nerstrøm B, Overgaard J. Association between immunohistochemical expression of vascular endothelial growth factor (VEGF), VEGF-expressing neuroendocrine-differentiated tumor cells, and outcome in prostate cancer patients subjected to watchful waiting. Clin Cancer Res. 2000;6:1882–90. [PubMed] [Google Scholar]
- 22.Bok RA, Halabi S, Fei DT, Rodriquez CR, Hayes DF, Vogelzang NJ, et al. Vascular endothelial growth factor and basic fibroblast growth factor urine levels as predictors of outcome in hormone-refractory prostate cancer patients: a cancer and leukemia group B study. Cancer Res. 2001;61:2533–6. [PubMed] [Google Scholar]
- 23.Sweeney P, Karashima T, Kim SJ, Kedar D, Mian B, Huang S, et al. Anti-vascular endothelial growth factor receptor 2 antibody reduces tumorigenicity and metastasis in orthotopic prostate cancer xenografts via induction of endothelial cell apoptosis and reduction of endothelial cell matrix metalloproteinase type 9 production. Clin Cancer Res. 2002;8:2714–24. [PubMed] [Google Scholar]
- 24.Spratlin JL, Cohen RB, Eadens M, Gore L, Camidge DR, Diab S, et al. Phase I pharmacologic and biologic study of ramucirumab (IMC-1121B), a fully human immunoglobulin G1 monoclonal antibody targeting the vascular endothelial growth factor receptor-2. J Clin Oncol. 2010;28:780–7. doi: 10.1200/JCO.2009.23.7537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spratlin J. Ramucirumab (IMC-1121B): Monoclonal antibody inhibition of vascular endothelial growth factor receptor-2. Curr Oncol Rep. 2011;13:97–102. doi: 10.1007/s11912-010-0149-5. [DOI] [PubMed] [Google Scholar]
- 26.Tannock IF, Osoba D, Stockler MR, Ernst DS, Neville AJ, Moore MJ, et al. Chemotherapy with mitoxantrone plus prednisone or prednisone alone for symptomatic hormone-resistant prostate cancer: a Canadian randomized trial with palliative end points. J Clin Oncol. 1996;14:1756–64. doi: 10.1200/JCO.1996.14.6.1756. [DOI] [PubMed] [Google Scholar]
- 27.Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA, et al. Prostate Cancer Clinical Trials Working Group. Design and end points of phase 2 trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group (PCWG2) J Clin Oncol. 2008;26:1148–59. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sternberg CN, Petrylak D, Witjes F, Ferrero J, Eymard J, Falcon S, et al. Satraplatin (S) demonstrates significant clinical benefits for the treatment of patients with HRPC: results of a randomized phase III trial. J Clin Oncol. 2007;25(suppl 18S):abstr 5019. [Google Scholar]
- 29.Sternberg CN, Petrylak DP, Sartor O, Witjes JA, Demkow T, Ferrero JM, et al. Multinational, double-blind, phase III study of prednisone and either satraplatin or placebo in patients with castrate-refractory prostate cancer progressing after prior chemotherapy: the SPARC trial. J Clin Oncol. 2009;27:5431–8. doi: 10.1200/JCO.2008.20.1228. [DOI] [PubMed] [Google Scholar]
- 30.de Bono JS, Oudard S, Ozguroglu M, Hansen S, Machiels JP, Kocak I, et al. TROPIC Investigators. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376:1147–54. doi: 10.1016/S0140-6736(10)61389-X. [DOI] [PubMed] [Google Scholar]
- 31.Yu EY, Li H, Higano CS, Agarwal N, Pal SK, Alva A, et al. SWOG S0925: A randomized phase II study of androgen deprivation combined with cixutumumab versus androgen deprivation alone in patients with new metastatic hormone-sensitive prostate cancer. J Clin Oncol. 2015 Apr 6; doi: 10.1200/JCO.2014.59.4127. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fuchs CS, Tomasek J, Yong CJ, Dumitru F, Passalacqua R, Goswami C, et al. REGARD Trial Investigators. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014;383:31–9. doi: 10.1016/S0140-6736(13)61719-5. [DOI] [PubMed] [Google Scholar]
- 33.Wilke H, Muro K, Van Cutsem E, Oh SC, Bodoky G, Shimada Y, et al. RAINBOW Study Group. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15:1224–35. doi: 10.1016/S1470-2045(14)70420-6. [DOI] [PubMed] [Google Scholar]
- 34.Tabernero J, Cohn AL, Obermannova R, Bodoky G, Garcia-Carbonero R, Ciuleanu TE, et al. RAISE Study Investigators. RAISE: a randomized, double-blind, multicenter phase III study of irinotecan, folinic acid, and 5-fluorouracil (FOLFIRI) plus ramucirumab (RAM) or placebo (PBO) in patients (pts) with metastatic colorectal carcinoma (CRC) progressive during or following first-line combination therapy with bevacizumab (bev), oxaliplatin (ox), and a fluoropyrimidine (fp) J Clin Oncol. 2015;33(suppl 3):abstr 512. [Google Scholar]
- 35.Garon EB, Ciuleanu TE, Arrieta O, Prabhash K, Syrigos KN, Goksel T, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet. 2014;384:665–73. doi: 10.1016/S0140-6736(14)60845-X. [DOI] [PubMed] [Google Scholar]
- 36.Petrylak DP, Tagawa ST, Kohli M, Tang S, Zhang H, Hamid O, et al. Interim results of a randomized phase 2 study of docetaxel with ramucirumab versus docetaxel in second-line advanced or metastatic urothelial carcinoma. J Clin Oncol. 2015;33(suppl 7):abstr 295. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.