Abstract
Background:
Worldwide, more than 700,000 pacemakers are implanted annually with more than 250,000 implanted in the United States. Since the first fully transvenous pacemaker implantations in the early 1960s, great technologic advances have been made in pacing systems. However, the combination of subcutaneous pulse generators and transvenous pacing leads has remained constant for more than 50 years. Leadless pacing systems offer an alternative to traditional pacing systems by eliminating the need for permanent transvenous leads while providing therapy for patients with bradyarrhythmias.
Methods:
We discuss the 2 leadless cardiac pacemakers (LCPs), the Nanostim Leadless Pacemaker and Micra Transcatheter Pacing System, and the 1 ultrasound-powered device, the WiCS-LV, that have been studied in humans. Currently LCPs are restricted to single-chamber pacing, specifically, ventricular pacing. Dual-chamber pacing and multichamber pacing with leadless systems have yet to be studied.
Results:
LCPs represent the greatest advancement in bradycardia therapy since the first transvenous pacemaker implantation more than 50 years ago.
Conclusion:
Initial studies of both the Nanostim and Micra LCPs show favorable efficacy and safety results compared to transvenous pacemakers. Pending US Food and Drug Administration approval, these devices will transform our ability to provide pacing for patients with bradyarrhythmias. Future developments may allow for completely leadless single-chamber and multichamber pacing, ushering in an era of pacing without wires.
Keywords: Bradycardia, cardiac pacing–artificial, pacemaker–artificial
INTRODUCTION
Worldwide, more than 700,000 pacemakers are implanted annually with more than 250,000 implanted in the United States.1 Since the first fully transvenous pacemaker implantations in the early 1960s,2 great technologic advances have been made in pacing systems. However, the combination of subcutaneous pulse generators and transvenous pacing leads has remained constant for more than 50 years. Traditionally, pulse generators are implanted above the pectoral fascia with pacing leads entering the venous system in the upper extremity veins. The presence of transvenous leads carries short-term risk to the patient at the time of implantation, as well as long-term risks.3,4 Leadless pacing systems offer an alternative to traditional pacing systems by eliminating the need for permanent transvenous leads while providing therapy for bradyarrhythmias. To date, 2 leadless pacing devices, the Nanostim Leadless Pacemaker5,6 (St. Jude Medical) and the Micra Transcatheter Pacing System (Medtronic),7,8 and 1 ultrasound-powered device, the WiCS-LV (EBR Systems),9 have been studied in humans. Currently, leadless cardiac pacemakers (LCPs) are restricted to single-chamber pacing, specifically, ventricular pacing. Dual-chamber pacing and multichamber pacing with leadless systems have yet to be studied.
RISKS OF TRANSVENOUS LEADS AND SUBCUTANEOUS POCKETS
Transvenous leads have been used for more than 50 years for permanent pacing indications. While the traditional route for transvenous leads is via the upper extremity veins, these leads can be implanted via the lower extremity (femoral/iliac) system. Every heart chamber can be paced with transvenous leads. The left ventricle is often paced epicardially with a transvenous lead guided through the coronary sinus system. While these leads have great flexibility, reliable performance, and ease of use, they are not without risk. At implantation, the incidence of traumatic injury, including pneumothorax and cardiac perforation, has been reported to be 1%-2.7%.3,4 Lead dislodgement rates at the time of implant and within 30 days are 2.4%-3.3%.3,4 Long-term transvenous lead–associated risks include fracture (1%-4%),10,11 moderate to severe tricuspid regurgitation (5%),12,13 venous obstruction (8%-21%),14 and infection (1%-2%).15,16 Furthermore, pocket infection rates for transvenous systems are 1%-2% at initial implant and 3%-4% after generator changes.15 Because of the communication between subcutaneous pockets and transvenous leads, a pacemaker pocket infection carries the risk of bacteremia and endocarditis. Bacteremia in the presence of a transvenous pacing system most often requires device extraction for definitive therapy. Overall, traditional pacing systems have a 10% complication rate including lead- and pocket-related events as well as implant and long-term events.3 Despite more than 50 years of use, transvenous leads remain the weakest link in traditional pacing systems and are the most common source of device complications. Development of LCPs represents a technologic leap forward for treatment of bradyarrhythmias.
LEADLESS CARDIAC PACEMAKERS
Nanostim Leadless Pacemaker
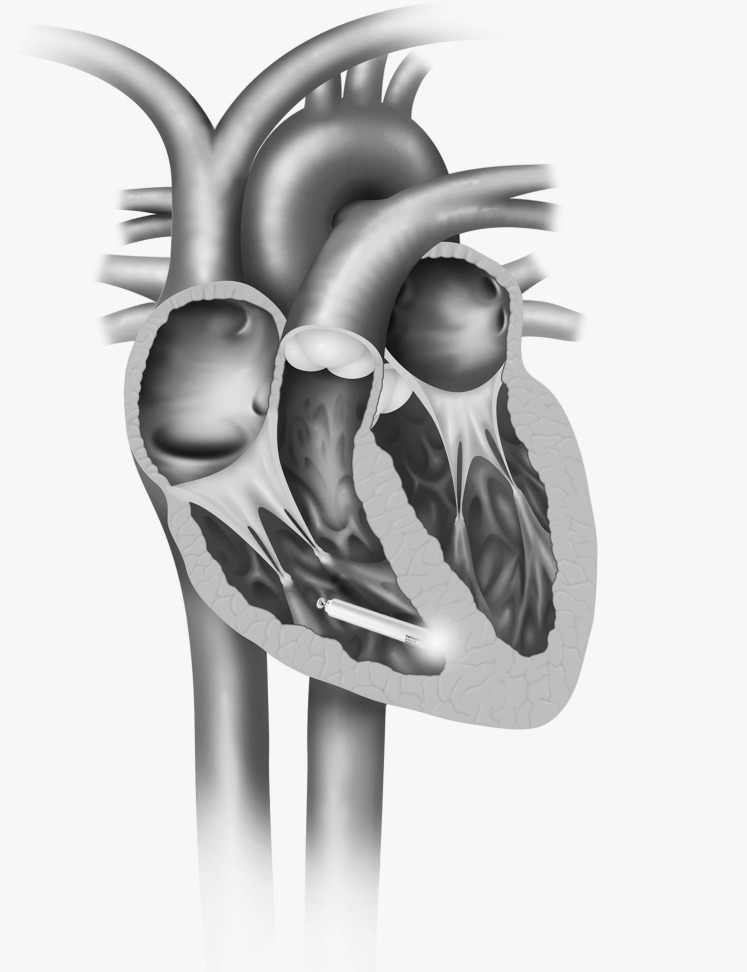
The first self-contained leadless pacing device implanted in humans was the Nanostim Leadless Pacemaker (Figure 1).5 The device is cylindrical with dimensions of 42 mm in length and 6 mm in diameter. The proximal portion of the device has a docking interface with most of the surface of the device serving as the ring electrode. The distal end has a fixation helix surrounding a tip electrode that is 10 mm from the ring electrode. The device is delivered through an 18 French femoral vein sheath on a steerable delivery catheter with an integrated guide catheter. The leadless pacemaker is attached to the docking cap at the distal end of the delivery catheter. The delivery catheter is deflectable and guides the device to the lower septum of the right ventricle where it is affixed into the myocardium by turning the device with the leadless pacemaker control knob. The device is placed into tether mode, and a gentle deflection test is performed for implant integrity. Capture threshold, impedance, and sensing tests are performed to confirm the adequacy of the implant site. If electrical measurements are deemed inadequate, the docking interface can be used to redock the device, unscrew it, and reposition it in a more favorable site. Separately, Nanostim offers 2 retrieval catheters (single and tri-loop) that allow the clinician to snare the back of the pacemaker (docking button), dock it to the retrieval catheter, and unscrew it from the myocardium (Figure 2). In the LEADLESS trial, 33 patients requiring ventricular pacing were enrolled in a single-arm study and were followed for 90 days.5 Eligibility included permanent atrial fibrillation with atrioventricular (AV) block; sinus rhythm with high-grade AV block and limited expected activity; or sinus bradycardia with infrequent pauses, syncope, or His-Purkinje disease. Exclusions included current pacing or defibrillator leads, pacemaker dependence, prosthetic tricuspid valves, pulmonary hypertension, and inferior vena cava (IVC) filter. Devices were programmed to VVIR pacing with the lower rate left to the discretion of the implanter. Primary safety endpoints were serious adverse device complications, and the secondary safety endpoint was success of implant. The device was successfully implanted in 32/33 (97%) patients. The overall complication-free rate was 31/33 (94%). No lead dislodgements occurred. Pacing sensing, thresholds, and impedance values were stable at 3 months. In the LEADLESS II trial, which used the same design as the LEADLESS trial, 667 patients were enrolled, and analysis was performed for the first 526 patients.6 The success rate for implantation was 95.8% (504/526). Of the 300 patients with 6 months of follow-up, the complication-free rate was 93.3%. The most common complications making up the overall 6.7% complication rate were dislodgement and retrieval in 1.7%, cardiac perforation in 1.3%, poor thresholds requiring retrieval and replacement in 1.3%, and vascular injury in 0.7% of patients. Average time for implantation was 50 minutes, with more than 87% of the implants requiring no or 1 repositioning of the device. Catheter-in to catheter-out time was 30.4 ± 18.23 minutes.
Figure 1.
Nanostim Leadless Pacemaker. Nanostim and St. Jude Medical are trademarks of St. Jude Medical, Inc. or its related companies. Reproduced with permission of St. Jude Medical, © 2016. All rights reserved.
Figure 2.
Nanostim Leadless Pacemaker delivery. Nanostim and St. Jude Medical are trademarks of St. Jude Medical, Inc. or its related companies. Reproduced with permission of St. Jude Medical, ©2016. All rights reserved.
At 6 months, the average battery life was 15 years. Results of the LEADLESS II trial support the conclusion that the Nanostim Leadless Pacemaker has favorable implantation, performance, safety, and battery life compared to traditional transvenous pacing systems.
Micra Transcatheter Pacing System
The Micra Transcatheter Pacing System is 26 mm long and 6.7 mm in diameter (Figure 3). It is delivered through a 23 French femoral vein sheath. The device is fixed to a delivery catheter that guides the device to the right ventricular apex. The anode is a circumferential ring located in the proximal portion of the device, and the cathode is at the tip of the device. Four nitinol fixation tines at the distal end of the device are used to secure the device to the endocardium. Once the tip is at the desired location, the sheath is retracted and the nitinol tines engage the myocardium, fixing the device in place. The sheath is then disconnected from the device and removed from the body. In the largest published study of the Micra device, the outcomes of 725 patients were analyzed in a single-arm investigation for safety and performance.8 Inclusion criteria were patients at least 18 years of age with Class I and II indications for pacing who were suitable for ventricular (VVI) pacing and had the ability to complete the study follow-up. Exclusions included pacemaker dependence, existing or prior pacemaker or implantable cardioverter defibrillator (ICD), IVC filter, prosthetic mechanical tricuspid valve, and the presence of a left ventricular assist device. The device was successfully implanted in 719/725 (99%) patients. The safety endpoint was achieved in 700/725 (96%) patients with 28 major complications occurring in 25 patients. Major adverse events included cardiac perforation (1.6%), vascular injury (0.7%), and high thresholds requiring revision (0.3%). The primary efficacy endpoint of the device was met in more than 98% of implants. Pacing parameters were stable at 12 months. Average battery life at 6 months was 12.5 years. When compared to a cohort of transvenous implantations, the Micra device performed favorably with a lower overall complication rate but a slightly higher perforation rate. Overall, Micra had comparable success, performance, and safety rates to the Nanostim device.
Figure 3.

Micra Transcatheter Pacing System. Reproduced with permission of Medtronic.
Leadless Cardiac Pacemaker Limitations
The most significant limitation of the Nanostim and Micra devices is the restriction to single-chamber, ventricular pacing. Single-chamber pacemakers, both atrial and ventricular devices, make up <10% of pacemaker implants.17 The most common reasons for implantation in the LEADLESS II study were chronic atrial fibrillation with slow ventricular response (56%), sinus rhythm with infrequent pauses or syncope (34%), and sinus rhythm with high-grade AV block (9%).6 In the Micra study, advanced AV block was the most common indication (49%), followed by sinus node dysfunction (43%).8 Dual-chamber pacing allows for atrioventricular synchrony, which has been shown to minimize pacemaker syndrome.17 Furthermore, chronic ventricular pacing can lead to ventricular dyssynchrony and systolic heart failure. For patients with an expected high burden of ventricular pacing, implantation on the right ventricle septum was left to the discretion of the implanting physician in both the LEADLESS II and Micra studies. Right ventricle septal implants theoretically would minimize the interaction of the device with the free wall of the right ventricle and thereby limit cardiac perforation. Also, pacing from the right ventricle septum would result in a narrower QRS, which could lead to less dyssynchrony. In the LEADLESS II study, the device was implanted in the apex 39% of the time, with more than 60% of the implants occurring in the right ventricle septum. In the Micra study, the device was implanted in the right ventricle apex 66% of the time, with more than 30% of implants occurring at the right ventricle septum. Other limitations include inadequate venous anatomy, exclusion of patients with pulmonary hypertension (common in patients with chronic atrial fibrillation), and inability to upgrade to ICD and/or cardiac resynchronization therapy devices. For patients who do not need AV synchrony or for patients with low expected pacing burden, LCPs are a suitable alternative to traditional pacing systems.
Retrieval and Replacement
Both Nanostim and Micra leadless pacemakers incorporate a docking feature on the back of the device to allow for acute repositioning and potentially chronic retrieval. The Nanostim pacemaker's proximal docking button is grasped by the snare of the retrieval catheter, docked, and then unscrewed and detached from the endocardium and removed from the body. Seven attempted chronic retrievals were performed during the LEADLESS II study.6 The reasons for retrieval were elevated pacing thresholds (4), worsening heart failure (2), and elective explantation (1). All 7 attempts were successful without complications. The retrieved devices had an implant duration ranging from 1-413 days. Micra retrievals use a retrieval catheter with a snare designed by Cook Medical. Three attempted retrievals of the Micra were performed in the Reynolds et al study, and one was successful.8 The longest duration retrieval of a Micra device was 61 days after implantation. Retrieval is accomplished by using a snare to capture the distal portion of the device and guide the delivery sheath over the nitinol tines to undeploy them. At the time of battery depletion, it remains unclear if the existing devices will be removed or if a new device will be implanted at an alternative location in the ventricle. Cases of complete encapsulation of LCPs have been reported, possibly making retrieval challenging.18,19 No long-term data from human subjects have been obtained regarding the safety and success of device removal after the expected battery life has been depleted (10-15 years). Although only performed in animal studies, multiple LCPs have been implanted in the right ventricle.20
Dislodgements
Six dislodgements of the Nanostim occurred, recognized 1-14 days after implantation: 4 in the pulmonary artery and 2 in the femoral vein. No long-term dislodgements occurred. All 6 dislodged devices were successfully removed without complication by using off-the-shelf snares.6 No dislodgements were reported from the Micra study.8
MULTICHAMBER LCPs AND INTERACTION WITH OTHER CARDIAC RHYTHM DEVICES
While LCPs offer treatment for bradyarrhythmias, they are restricted to ventricular pacing as their primary modality. Although no published reports are available, the capacity for dual-chamber and multichamber leadless pacing is under development. Challenges for development of dual-chamber and cardiac resynchronization leadless pacing stem from the need for interdevice communication, minimization of interference, ability to interrogate simultaneous devices, and the likely need for a device large enough to accommodate the required circuitry without significantly impacting battery life. Furthermore, delivery and deployment in the atrium and left ventricle may require adaptation of the current device profile and fixation methods. One method to circumvent these issues was studied in the Wireless Stimulation Endocardially for Cardiac Resynchronization Therapy (WiSE-CRT) study.9 The trial was designed to enroll 100 patients who had failed transvenous left ventricular lead implant, who were nonresponders to traditional CRT therapy, or who were eligible for upgrade to CRT therapy from an existing device. The concept was to pace the left ventricle triggered by an ultrasound stimulus timed to right ventricular pacing. The benefit of such a device would allow for an external power source (ultrasound waves) that could be replaced with minimum risk to the patient while preserving the optimal left ventricular pacing site. The device was delivered through a retrograde aortic approach and fixed to the lateral left ventricular endocardium. A separate pulse generator powering an ultrasound transmitter was implanted subcutaneously near the left costal margin so that the ultrasound transmitter was approximated near the left lateral wall of the heart. The study was stopped prematurely because of safety concerns: 3 hemodynamically significant pericardial effusions occurred as a direct result of device delivery. Despite the premature termination of the WiSE-CRT study, the prospect for ultrasound-based pacing stimulation is a promising mechanism for multichamber leadless pacing.
Given the exclusion criteria for both the LEADLESS and Micra studies, no data are available regarding the interaction between transvenous defibrillators and LCPs. However, subcutaneous ICDs (S-ICDs) were not part of the exclusion criteria. There is a single report of a patient with both an S-ICD and an LCP.21 No interference of function occurred between the LCP and the S-ICD during normal rhythm, LCP pacing, and defibrillation threshold testing via the S-ICD. While LCPs do not have the capability for defibrillation, they could perform antitachycardia pacing given proper programming. In a proof-of-concept study, a prototype LCP manufactured by Boston Scientific was implanted in a patient with an existing S-ICD.22 Ventricular tachycardia was detected successfully by the S-ICD, and communication to the LCP triggered antitachycardia pacing that terminated the arrhythmia. While this technology is in its infancy, the potential for full bradycardia and tachycardia therapy without permanent transvenous leads signals a new era in device-based cardiac rhythm management.
CONCLUSION
Leadless cardiac pacing systems represent the greatest advancement in bradycardia therapy since the first transvenous pacemaker implantation more than 50 years ago. LCPs circumvent the weakest link in traditional pacing systems, the transvenous leads. While LCP use is restricted to ventricular pacing, dual-chamber and multichamber leadless pacing will likely be developed in the near future. Because only 10%-20% of current transvenous implants are single-chamber devices, the potential clinical use of LCPs is limited; however, they offer an option for patients with difficult venous anatomy and/or for patients who are not candidates for transvenous systems. Preliminary data suggest that retrieval is feasible and safe, but more longitudinal studies are required. LCPs offer longer battery life than transvenous systems. At this time, the method of replacement once the initial device has reached the end of battery life has yet to be determined. Animal models show that implantation of multiple LCPs in the right ventricle is possible; therefore, placement of an additional LCP once the original battery has been depleted may be the recommended course of action. More intriguing possibilities include the potential for coordinated function between subcutaneous ICDs and LCPs to provide both bradycardia and tachycardia therapy without transvenous leads. Initial studies of both the Nanostim and Micra LCPs show favorable efficacy and safety results compared with transvenous pacemakers. Pending US Food and Drug Administration approval, these devices will transform our ability to provide pacing for patients with bradyarrhythmias. Future developments may allow for completely leadless single-chamber and multichamber pacing, ushering in an era of pacing without wires.
ACKNOWLEDGMENTS
The author has no financial or proprietary interest in the subject matter of this article.
This article meets the Accreditation Council for Graduate Medical Education and the American Board of Medical Specialties Maintenance of Certification competencies for Patient Care, Medical Knowledge, and Practice-Based Learning and Improvement.
REFERENCES
- 1. Mond HG, Proclemer A. The 11th world survey of cardiac pacing and implantable cardioverter-defibrillators: calendar year 2009—a World Society of Arrhythmia's project. Pacing Clin Electrophysiol. 2011. August; 34 8: 1013- 1027. doi: 10.1111/j.1540-8159.2011.03150.x. [DOI] [PubMed] [Google Scholar]
- 2. Parsonnet V, Zucker IR, Gilbert L, Maxim ASA M. An intracardiac bipolar electrode for interim treatment of complete heart block. Am J Cardiol. 1962. August; 10: 261- 265. [DOI] [PubMed] [Google Scholar]
- 3. Udo EO, Zuithoff NP, van Hemel NM, et al. Incidence and predictors of short- and long-term complications in pacemaker therapy: the FOLLOWPACE study. Heart Rhythm. 2012. May; 9 5: 728- 735. doi: 10.1016/j.hrthm.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 4. Ellenbogen KA, Hellkamp AS, Wilkoff BL, et al. Complications arising after implantation of DDD pacemakers: the MOST experience. Am J Cardiol. 2003. September 15; 92 6: 740- 741. [DOI] [PubMed] [Google Scholar]
- 5. Reddy VY, Knops RE, Sperzel J, et al. Permanent leadless cardiac pacing: results of the LEADLESS trial. Circulation. 2014. April 8; 129 14: 1466- 1471. doi: 10.1161/CIRCULATIONAHA.113.006987. [DOI] [PubMed] [Google Scholar]
- 6. Reddy VY, Exner DV, Cantillon DJ, et al. LEADLESS II Study Investigators. Percutaneous implantation of an entirely intracardiac leadless pacemaker. N Engl J Med. 2015. September 17; 373 12: 1125- 1135. doi: 10.1056/NEJMoa1507192. [DOI] [PubMed] [Google Scholar]
- 7. Ritter P, Duray GZ, Steinwender C, et al. Micra Transcatheter Pacing Study Group. Early performance of a miniaturized leadless cardiac pacemaker: the Micra Transcatheter Pacing Study. Eur Heart J. 2015. October 1; 36 37: 2510- 2519. doi: 10.1093/eurheartj/ehv214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Reynolds D, Duray GZ, Omar R, et al. Micra Transcatheter Pacing Study Group. A leadless intracardiac transcatheter pacing system. N Engl J Med. 2016. February 11; 374 6: 533- 541. doi: 10.1056/NEJMoa1511643. [DOI] [PubMed] [Google Scholar]
- 9. Auricchio A, Delnoy PP, Butter C, et al. Collaborative Study Group. Feasibility, safety, and short-term outcome of leadless ultrasound-based endocardial left ventricular resynchronization in heart failure patients: results of the wireless stimulation endocardially for CRT (WiSE-CRT) study. Europace. 2014. May; 16 5: 681- 688. doi: 10.1093/europace/eut435. [DOI] [PubMed] [Google Scholar]
- 10. Magney JE, Flynn DM, Parsons JA, et al. Anatomical mechanisms explaining damage to pacemaker leads, defibrillator leads, and failure of central venous catheters adjacent to the sternoclavicular joint. Pacing Clin Electrophysiol. 1993. March; 16 3 Pt 1: 445- 457. [DOI] [PubMed] [Google Scholar]
- 11. Alt E, Völker R, Blömer H. Lead fracture in pacemaker patients. Thorac Cardiovasc Surg. 1987. April; 35 2: 101- 104. doi: 10.1055/s-2007-1020206. [DOI] [PubMed] [Google Scholar]
- 12. Al-Bawardy R, Krishnaswamy A, Bhargava M, et al. Tricuspid regurgitation in patients with pacemakers and implantable cardiac defibrillators: a comprehensive review. Clin Cardiol. 2013. May; 36 5: 249- 254. doi: 10.1002/clc.22104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. de Cock CC, Vinkers M, Van Campe LC, Verhorst PM, Visser CA. Long-term outcome of patients with multiple (> or = 3) noninfected transvenous leads: a clinical and echocardiographic study. Pacing Clin Electrophysiol. 2000. April; 23 4 Pt 1: 423- 426. [DOI] [PubMed] [Google Scholar]
- 14. Spittell PC, Hayes DL. Venous complications after insertion of a transvenous pacemaker. Mayo Clin Proc. 1992. March; 67 3: 258- 265. [DOI] [PubMed] [Google Scholar]
- 15. Johansen JB, Jørgensen OD, Møller M, Arnsbo P, Mortensen PT, Nielsen JC. Infection after pacemaker implantation: infection rates and risk factors associated with infection in a population-based cohort study of 46299 consecutive patients. Eur Heart J. 2011. April; 32 8: 991- 998. doi: 10.1093/eurheartj/ehq497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Baddour LM, Bettmann MA, Bolger AF, et al. AHA. Nonvalvular cardiovascular device-related infections. Circulation. 2003. October 21; 108 16: 2015- 2031. doi: 10.1161/01.CIR.0000093201.57771.47. [DOI] [PubMed] [Google Scholar]
- 17. Epstein AE, DiMarco JP, Ellenbogen KA, et al. American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices); American Association for Thoracic Surgery; Society of Thoracic Surgeons. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices): developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. Circulation. 2008. May 27; 117 21: e350- e408. doi: 10.1161/CIRCUALTIONAHA.108.189742. [DOI] [PubMed] [Google Scholar]
- 18. Kypta A, Blessberger H, Lichtenauer M, Steinwender C. Complete encapsulation of a leadless cardiac pacemaker. Clin Res Cardiol. 2016. January; 105 1: 94 doi: 10.1007/s00392-015-0929-x. [DOI] [PubMed] [Google Scholar]
- 19. Tjong FV, Stam OC, van der Wal AC, et al. Postmortem histopathological examination of a leadless pacemaker shows partial encapsulation after 19 months. Circ Arrhythm Electrophysiol. 2015. October; 8 5: 1293- 1295. doi: 10.1161/CIRCEP.115.003101. [DOI] [PubMed] [Google Scholar]
- 20. Chen K, Zheng X, Dai Y, et al. Multiple leadless pacemakers implanted in the right ventricle of swine. Europace. 2016. January 13. [Epub ahead of print]. doi: 10.1093/europace/euv418. [DOI] [PubMed] [Google Scholar]
- 21. Tjong FV, Brouwer TF, Smeding L, et al. Combined leadless pacemaker and subcutaneous implantable defibrillator therapy: feasibility, safety, and performance. Europace. 2016. March 3. [Epub ahead of print]. doi: 10.1093/europace/euv457. [DOI] [PubMed] [Google Scholar]
- 22. Tjong FV, Brouwer TF, Kooiman KM, et al. Communicating antitachycardia pacing-enabled leadless pacemaker and subcutaneous implantable defibrillator. J Am Coll Cardiol. 2016. April 19; 67 15: 1865- 1866. doi: 10.1016/j.jacc.2016.02.039. [DOI] [PubMed] [Google Scholar]