Abstract
Background:
Despite recent advances in the management of heart failure, cardiogenic shock remains a challenging and devastating condition with significant morbidity and mortality.
Methods:
We review currently available percutaneous mechanical circulatory support (MCS) devices and address each device's characteristics, mechanism of action, specific clinical indications, and contraindications.
Results:
Four types of percutaneous MCS devices are currently available: the intraaortic balloon pump (IABP), Impella devices, the TandemHeart, and extracorporal membrane oxygenation (ECMO). IABPs provide less hemodynamic support compared to the Impella, TandemHeart, and ECMO devices. However, because of its ease of placement and relatively small access catheter size, the IABP remains the most commonly used MCS device for the treatment of cardiogenic shock. When full cardiopulmonary support is needed, ECMO is the best option.
Conclusion:
Temporary MCS has emerged as a therapeutic option in the management of patients with acute cardiogenic shock. However, clinician familiarity with the indications, limitations, and benefits of individual MCS devices and enhanced patient comfort with the placement are paramount to improve patient outcomes.
Keywords: Heart-assist devices, intra-aortic balloon pumping, shock–cardiogenic
INTRODUCTION
An estimated 5.7 million people in the United States have heart failure. With our aging population, this number is expected to increase to >8 million by 2030.1 Additionally, acute coronary syndromes affect almost 700,000 patients annually,2 with cardiogenic shock complicating 5%-8% of patients presenting with acute ST-elevation myocardial infarction (STEMI).3 Although acute ischemic disease and its complications remain the most common etiologies of cardiogenic shock, nonischemic etiologies of left ventricular (LV) or right ventricular (RV) dysfunction, such as chronic systolic dysfunction with acute decompensation, myopericarditis, progressive and acute valvular disease, and takotsubo cardiomyopathy, remain common. Although the mortality of chronic heart failure has improved with medical therapy, the mortality rates associated with cardiogenic shock remain high.4
Clinically, cardiogenic shock manifests as hypotension with evidence of organ hypoperfusion, such as cool extremities and oliguria.5 Hemodynamically, it is defined as persistent hypotension with systolic blood pressure <90 mmHg or mean arterial pressure (MAP) <30 mmHg below baseline, a reduced cardiac index <1.8 L/min/m2 without support or <2.0 L/min/m2 with support, and adequate or elevated filling pressures (LV end-diastolic pressure >18 mmHg or RV end-diastolic pressure >10 mmHg).6
Treatment of cardiogenic shock includes reperfusion therapy when applicable, vasopressor and inotropic support, and mechanical circulatory support (MCS). These methods aim to improve hemodynamics, cardiac outflow, and tissue perfusion. Some of the currently available percutaneous temporary MCS devices can now be implanted in the cardiac catheterization laboratory without the need for surgical cutdown. In addition, recent data suggest that mortality among patients with cardiogenic shock who receive permanent MCS via surgically implanted LV assist devices (LVADs) is much higher than mortality among patients who receive an LVAD as an elective procedure.7 In this regard, percutaneous temporary LVADs are more suitable than surgically implanted LVADs and may be used as a bridge to permanent MCS and in some situations as a bridge to recovery.
We review currently available percutaneous MCS devices, including the intraaortic balloon pump (IABP), Impella devices (Abiomed), TandemHeart (Cardiac Assist, Inc.), and extracorporal membrane oxygenation (ECMO; veno-venous [VV] and veno-arterial [VA]). We address each device's characteristics, mechanism of action, contraindications, and specific scenarios in which one device may be more suitable than the others.
INTRAAORTIC BALLOON PUMP
The IABP was designed by Dr Adrian Kantrowitz in the 1960s to help with cardiac output and coronary perfusion, and its first reported clinical use was in 1968 at Maimonides Hospital in Brooklyn for a 45-year-old woman in cardiogenic shock.8 The IABP remains the most commonly used temporary MCS device, with >50,000 IABPs implanted annually.9 The balloon pump was designed on the concept of counterpulsation, with the augmentation of coronary blood flow during diastole improving coronary arterial flow.
The IABP is a balloon catheter, usually 30-50 cm3, that is inserted peripherally most often through the femoral artery via a 7.5-8 French (Fr) catheter. The pump console inflates the balloon with helium, a readily absorbable gas. The pump can be placed with fluoroscopic guidance or at the bedside with confirmatory x-ray, with the proximal tip of the balloon placed distal to the subclavian artery and the distal tip of the balloon proximal to the renal arteries so that flow is not obstructed. Using electrocardiographic or arterial pressure waveform timing, the console inflates the balloon during diastole, using counterpulsation to augment diastolic filling of coronary arteries. Deflation during systole provides decreased afterload and further enhances cardiac output. Advancements in IABP technology have improved the timing of inflation with aortic valve closure, using programmed algorithms to respond to tachycardia or arrhythmias.3
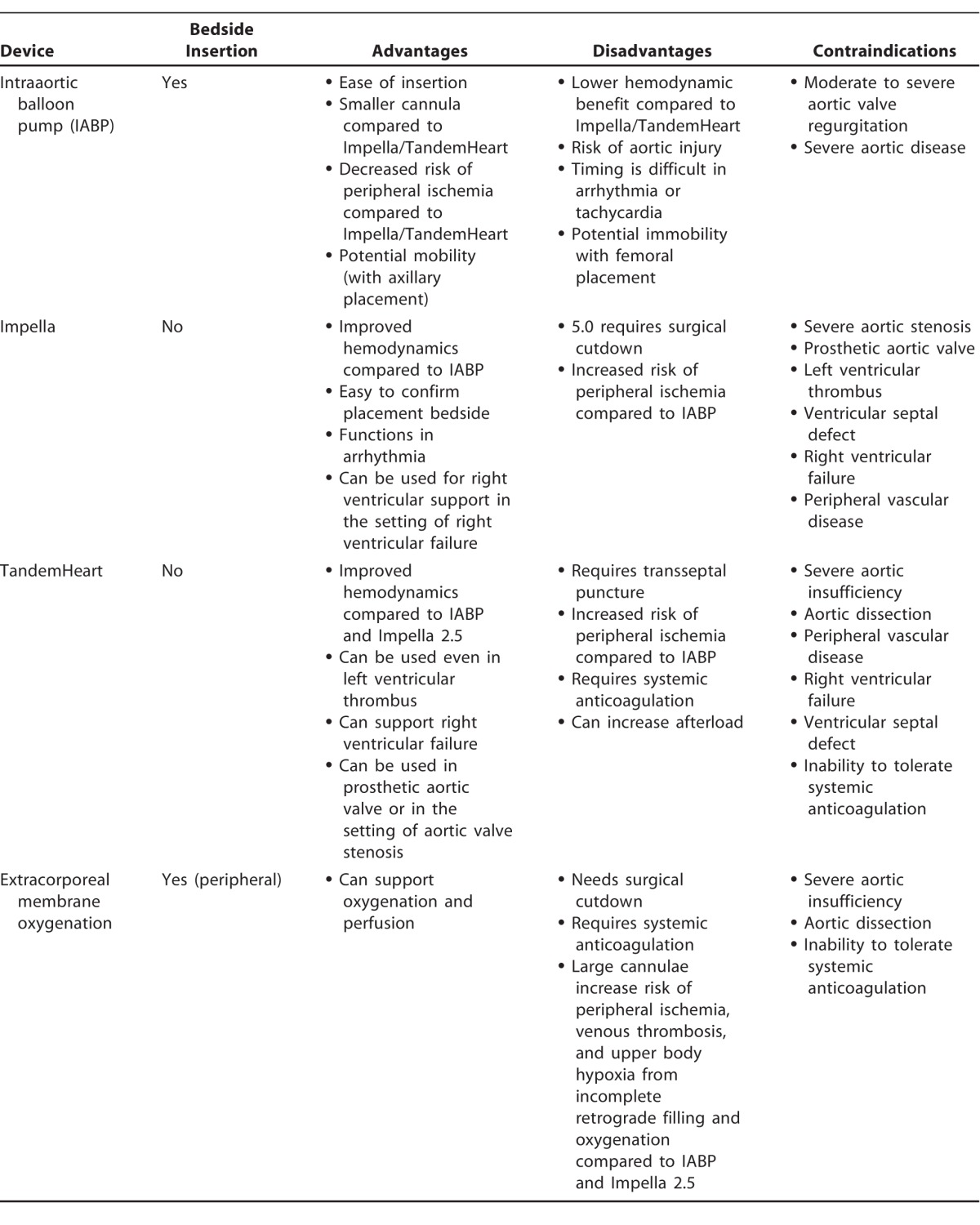
Hemodynamically, the IABP modestly improves cardiac output by 0.5 L/min. It increases coronary, cerebral, and peripheral perfusion pressure and decreases myocardial oxygen consumption and workload (Table 1).3,10-12 Other advantages are ease of placement (at the bedside or in the catheterization laboratory) and relatively small access catheter size. Contraindications include moderate or severe aortic valve regurgitation and severe peripheral arterial or aortic disease.10 Arrhythmia or tachycardia can make it difficult to time inflation and deflation of the balloon, an additional drawback of balloon counterpulsation (Table 2).3,10-12 Major complications, including acute limb ischemia, severe bleeding, IABP failure or leakage, and death, occur at a frequency of 2.6%.9
Table 1.
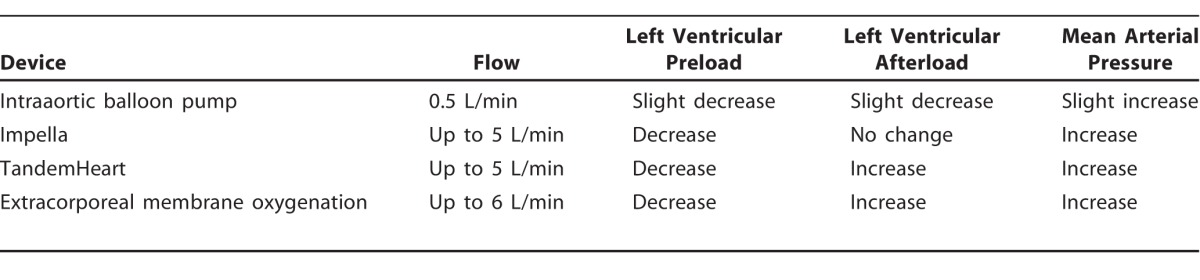
Table 2.
One disadvantage of femoral IABP is the resulting immobility because patients must lie still with the pump in the femoral artery, thus increasing their discomfort and deconditioning. In 2013, Estep and colleagues13 showed that an alternative percutaneous transaxillary approach is safe and effective and enables upright mobility. In a review of 50 patients who received a left axillary IABP as a bridge to transplantation for a median duration of 18 days, only 4 patients had clinically significant thromboembolic or bleeding events, and none suffered long-term sequelae, although 2 patients required left brachial artery embolectomy for left-hand ischemia. The most common adverse event was IABP malposition, occurring in 44% of patients and requiring bedside repositioning. Additionally, 10 patients required IABP exchange in the catheterization laboratory because of IABP malposition (7 patients) or balloon rupture (3 patients).13 Axillary IABP has been used in patients with expected prolonged mechanical support to help them stay mobile, to improve their comfort, and to help them participate in active physical therapy.
Despite the widespread use of IABP, the data suggesting benefit have been mixed, at best. The Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock (SHOCK) trial registry14 retrospectively observed that patients treated with IABPs and fibrinolytics had improved in-hospital and 1-year mortality compared to patients treated with fibrinolytics alone.14 The Intraaortic Balloon Pump in Cardiogenic Shock II (IABP-SHOCK II) trial,15 however, randomized 600 patients with cardiogenic shock secondary to acute myocardial infarction to either IABP or no IABP, with all patients expected to undergo early revascularization with percutaneous coronary intervention. At 30 days, no difference was seen in mortality or in secondary subgroups, such as length of stay in the intensive care unit, serum lactate levels, renal function, and time to hemodynamic stabilization.15 Similarly, no difference in mortality was observed at 12 months.15 Adverse effects, such as major bleeding, peripheral vascular complications, and stroke, were also not different between the 2 groups. Some criticisms of this trial are that some of the IABP candidates were hemodynamically stable and may not have needed mechanical support. Additionally, 10% of patients in the control group were crossed over to IABP therapy.15 The Counterpulsation to Reduce Infarct Size Pre-PCI Acute Myocardial Infarction (CRISP-AMI) trial16 randomized 337 patients in 9 countries who had anterior STEMI to IABP placed immediately before reperfusion or to no IABP. Results of this trial show no reduction in infarct size based on cardiac magnetic resonance imaging in patients with IABP.16
Although the mortality benefit of IABP therapy has not been demonstrated in randomized clinical trials, in the American College of Cardiology and the American Heart Association (ACC/AHA) 2013 guidelines for management of STEMI, the use of IABP counterpulsation is a Class IIa recommendation (level of evidence [LOE] B) for cardiogenic shock after STEMI in patients who do not quickly stabilize with pharmacologic therapy.2 It is important to note, however, that this is a downgrade from the Class I recommendation in the 1999 guidelines. Similarly, the European Society of Cardiology guidelines give IABP use a Class IIb recommendation in patients with cardiogenic shock after myocardial infarction, downgraded from a Class I indication.17 Nevertheless, this device remains widely used in the management of patients with cardiogenic shock.
IMPELLA DEVICES
The Impella devices are axial flow pumps inserted into the left ventricle from the aorta. Impella devices include the Impella 2.5, Impella CP, and Impella 5.0. The Impella 2.5 provides up to 2.5 L/min cardiac support, and the Impella 5.0 provides up to 5.0 L/min increased cardiac output. The Impella CP provides intermediate support, 3.0-4.0 L/min of increased cardiac output. With the exception of the Impella 5.0 that can be inserted via the axillary artery (allowing the patient to ambulate), these devices are inserted into the femoral artery. The Impella 2.5 and CP are placed percutaneously and use 12-Fr and 14-Fr sheaths, respectively. The Impella 5.0 is 21-Fr and requires a surgical cutdown for access.18
The tip of the Impella device is a pigtail that provides stabilization for the device in the left ventricle. The pigtail connects to a pump cannula with a motor housing and pump intake and output areas with a pump pressure monitor (Figure 1). The pump intake sits inside the left ventricle, with the outlet area sitting across the aortic valve into the aortic root.
Figure 1.
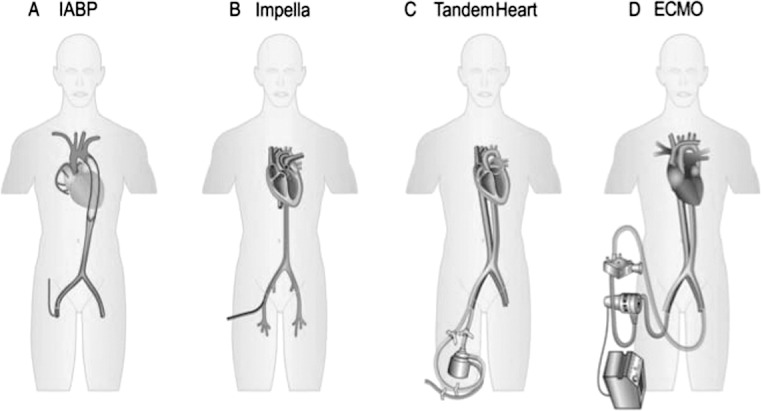
Schematic of placement comparison of (A) intraaortic balloon pump (IABP), (B) Impella, (C) TandemHeart, and (D) extracorporal membrane oxygenation (ECMO). Reproduced with permission from Werdan et al.21
Unlike the IABP, the Impella device uses continuous axial flow and consequently does not require pressure timing or electrocardiogram timing, allowing for stable output despite arrhythmias. The Impella device pumps blood directly from the left ventricle into the proximal aorta, thereby unloading the left ventricle, increasing cardiac output, and improving MAP, while reducing LV end-diastolic pressure, myocardial workload, and oxygen consumption.10 Because the Impella pulls blood directly from the left ventricle, its action is dependent on LV preload and is thus dependent in part on satisfactory RV function. To prevent suckdown events, wherein the left ventricle collapses on itself from Impella flow, central venous pressure should be kept at 8-12 mmHg. Positioning can readily be confirmed and readjusted by using bedside transthoracic echocardiography.
Contraindications to Impella usage include mechanical aortic valve or LV thrombus, ventricular septal defect, and severe peripheral arterial disease; relative contraindications include aortic stenosis and regurgitation. Adverse events include mechanical hemolysis, acquired von Willebrand syndrome with reduced platelet aggregation, peripheral vascular ischemia, and other peripheral complications associated with femoral procedures. As with an IABP, femoral access precludes mobility, although success has been reported with axillary and subclavian access.18,19
In the Efficacy Study of LV Assist Device to Treat Patients With Cardiogenic Shock (ISAR-Shock) trial,20,21 patients with myocardial infarction and cardiogenic shock were randomized to IABP or Impella. Patients with an Impella demonstrated improved cardiac output and index; however, no significant differences in in-hospital and 2-year mortality were seen.20 Large randomized trials comparing the Impella device to the IABP are lacking.
TANDEMHEART
The TandemHeart is an extracorporeal centrifugal pump with a 6-blade rotor that generates up to 5.0 L/min flow. The 21-Fr inflow cannula is placed percutaneously through the femoral vein, utilizing a transseptal puncture to pass the cannula across the intraatrial septum into the left atrium where the pump aspirates oxygenated blood. The outflow cannula (15-Fr to 19-Fr) returns the blood into the femoral artery utilizing the centrifugal pump. This method unloads the left ventricle and reduces LV end-diastolic pressure, but return of blood into the arterial system can increase afterload, limiting the degree to which LV workload is reduced.9
Advantages of the TandemHeart are the improved cardiac output and hemodynamic changes compared to the Impella 2.5 and IABP. The TandemHeart can also be used in patients with LV thrombus because it bypasses the left ventricle altogether. Likewise, aortic stenosis is not a contraindication to its usage. A metaanalysis showed improved hemodynamic profiles in patients with cardiogenic shock with TandemHeart vs IABP; however, the analysis showed no difference in short-term mortality.9
Disadvantages of the TandemHeart prevent its widespread use. The most limiting factor may be the technical expertise required to place the inflow cannula itself, which requires a transseptal puncture placed surgically or by experienced interventional cardiologists. Systemic anticoagulation must be maintained with heparin to prevent pump thrombosis, precluding use of this device in patients with contraindications to anticoagulation. Complications with placement of the transseptal puncture include perforation of adjacent structures, such as the coronary sinus, posterior right atrial wall, or aortic root; there does not seem to be significant shunting after the puncture itself. Additionally, repositioning of the cannula must be performed in a catheterization laboratory, and mobility is limited by risk of cannula migration. Because of the larger insertion cannulae, the TandemHeart has more bleeding and ischemic complications compared to the IABP. Contraindications to its use include ventricular septal defects (risk of right-to-left shunting from LV offloading), RV failure, aortic insufficiency, aortic dissection, and severe peripheral arterial disease that may prevent cannula insertion.18,19
EXTRACORPORAL MEMBRANE OXYGENATION
ECMO provides full cardiopulmonary support for patients who have concomitant respiratory and cardiac failure. ECMO can be either VV for oxygenation only or VA for complete cardiopulmonary support. ECMO involves an inflow cannula receiving deoxygenated blood pumped via an extracorporeal centrifugal pump over a membrane oxygenator for gas exchange and pumped back through the outflow cannula into VV or VA systems. VV ECMO utilizes an inflow cannula placed into the femoral vein or internal jugular vein, and oxygenated blood is returned via the internal jugular vein or right atrium. VA ECMO uses an inflow cannula in the same position; however, the outflow cannula is positioned into the femoral artery or aorta (Figure 1).11
While VV ECMO provides gas exchange, VA ECMO has the additional benefit of providing hemodynamic support, up to 6 L/min in increased output.10 Because VA ECMO outflows into the arterial system, LV afterload may be increased, as with the TandemHeart; thus, the net reduction in LV wall strain and LV workload from decreased preload may be reduced. Occasionally, additional support, such as an IABP or Impella, may be required for the left ventricle. The ability to oxygenate and provide cardiac output for the right and left ventricles makes ECMO a unique device in hypoxemic cardiogenic shock states. Peripheral ECMO using the femoral vein and artery can be placed bedside but still requires a surgical cutdown of the femoral artery for placement.12
For ECMO, systemic anticoagulation is required, most commonly with intravenous heparin. Thus, patients with contraindications to anticoagulation would be poor candidates. Aortic insufficiency is a relative contraindication, and increased outflow may worsen regurgitation and LV end-diastolic pressure, creating additional stress on the LV myocardium. Complications are similar to those of devices with large cannulae and include venous thrombosis, peripheral ischemia, and upper body hypoxia from incomplete retrograde filling and oxygenation.11
While 13,000 patients have been treated with ECMO,11 limited data and few randomized trials exist to assess when to initiate ECMO in cardiogenic shock. As such, the ACC/AHA guideline opinions give no recommendations on its use.
RIGHT VENTRICULAR DEVICES
While many ventricular assist devices have been developed to unload the left ventricle, right-sided heart failure remains problematic and has a high mortality rate. Two devices, the TandemHeart and Impella RP, have been used successfully for RV failure. The right-sided TandemHeart uses a centrifugal pump to provide blood flow from the right atrium or internal jugular vein into the main pulmonary artery. As with the left-sided TandemHeart, patient mobility is limited, and the provider must watch closely for cannula migration. Patients with antegrade migration of the outflow cannula may present with hypoxic respiratory failure, hemoptysis, hemothorax, and decreased cardiac output. The Impella RP is a catheter-based axial flow pump placed across the right ventricle using venous access. While use of these devices for RV support in the setting of acute myocardial infarction, post-LVAD RV failure, pulmonary hypertension, and acute cardiac allograft failure has been reported, the data are still limited on the benefits of their use.10
FUTURE DIRECTIONS
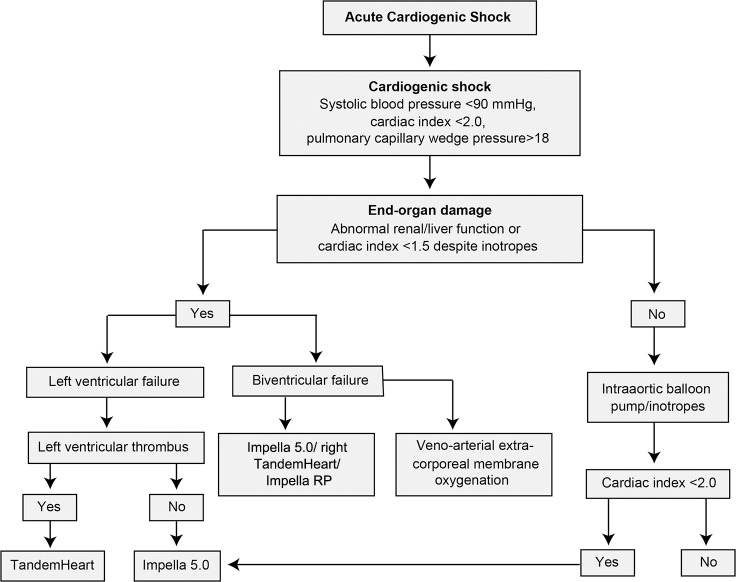
As highlighted in this review, tremendous progress has been made in the development of temporary MCS devices for the treatment of cardiogenic shock. While the armamentarium for mechanical support grows, further data need to be collected to help providers select the appropriate patient who may benefit from these devices, as current data show benefit in hemodynamics but not in clinical outcomes. More important, protocols and algorithms (Figure 2) are needed to help physicians choose the right device for the right patient. Additionally, as large randomized clinical trials are difficult to conduct in this patient population, multicenter collaboration and registries may provide some important insight regarding the use and efficacy of these novel devices.
Figure 2.
Proposed algorithm for temporary mechanical circulatory support use in acute cardiogenic shock.
In a study published in 2015, the Clinical Expert Consensus Statement on the Use of Percutaneous MCS Devices acknowledged that percutaneous MCS devices provide hemodynamic support that is superior to pharmacologic therapy and that these devices should be available and used appropriately in select patients with persistent cardiogenic shock despite appropriate revascularization and pharmacologic therapies.10 The European Society of Cardiology, in its 2012 STEMI guidelines, gives use of an IABP (LOE B) and LV assist devices (LOE C) a Class IIb recommendation for circulatory support in refractory shock. The guidelines mention that these devices should not be used as first-line therapies but can be considered on an individual basis, although evidence for their support is lacking.16
CONCLUSION
MCS aims to use mechanical means to support the physical hemodynamics associated with heart failure and cardiogenic shock. New devices that provide hemodynamic support are available to clinicians. In patients who do not respond to medical and inotropic therapy, mechanical devices may be a viable alternative. Clinician familiarity with the indications, limitations, and benefits of individual MCS devices, as well as enhanced patient comfort in the placement of these devices, will aid in selecting patients who may derive benefit from temporary mechanical support. In addition to cardiogenic shock, another use of MCS is as a bridge to LVAD, decision, recovery, or transplantation in select patients with acute heart failure.
ACKNOWLEDGMENTS
The authors have no financial or proprietary interest in the subject matter of this article.
This article meets the Accreditation Council for Graduate Medical Education and the American Board of Medical Specialties Maintenance of Certification competencies for Patient Care, Medical Knowledge, and Practice-Based Learning and Improvement.
REFERENCES
- 1. Writing Group Members. Mozaffarian D, Benjamin EJ, Go AS, et al. American Heart Association Statistics Committee; Stroke Statistics Subcommittee. Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation. 2016. January 26; 133 4: e38- e60. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 2. American College of Emergency Physicians. Society for Cardiovascular Angiography and Interventions. O'Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013. January 29; 61 4: e78- e140. doi: 10.1016/j.jacc.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 3. Khan MH, Corbett BJ, Hollenberg SM. Mechanical circulatory support in acute cardiogenic shock. F1000Prime Rep. 2014. October 1; 6: 91 doi: 10.12703/P6-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stretch R, Sauer CM, Yuh DD, Bonde P. National trends in the utilization of short-term mechanical circulatory support: incidence, outcomes, and cost analysis. J Am Coll Cardiol. 2014. October 7; 64 14: 1407- 1415. doi: 10.1016/j.jacc.2014.07.958. [DOI] [PubMed] [Google Scholar]
- 5. Hollenberg SM, Kavinsky CJ, Parrillo JE. Cardiogenic shock. Ann Intern Med. 1999. July 6; 131 1: 47- 59. [DOI] [PubMed] [Google Scholar]
- 6. Reynolds HR, Hochman JS. Cardiogenic shock: current concepts and improving outcomes. Circulation. 2008. February 5; 117 5: 686- 697. doi: 10.1161/CIRCULATIONAHA.106.613596. [DOI] [PubMed] [Google Scholar]
- 7. Kirklin JK, Naftel DC, Kormos RL, et al. Fifth INTERMACS annual report: risk factor analysis from more than 6,000 mechanical circulatory support patients. J Heart Lung Transplant. 2013. February; 32: 144- 156. doi: 10.1016/j.healun.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 8. Nanas JN, Moulopoulos SD. Counterpulsation: historical background, technical improvements, hemodynamic and metabolic effects. Cardiology. 1994; 84 3: 156- 167. [DOI] [PubMed] [Google Scholar]
- 9. Kapur NK, Esposito M, Shock C. Hemodynamic support with percutaneous devices in patients with heart failure. Heart Fail Clin. 2015. April; 11 2: 215- 230. doi: 10.1016/j.hfc.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 10. Rihal CS Naidu SS Givertz MM et al. Society for Cardiovascular Angiography and Interventions (SCAI); Heart Failure Society of America (HFSA); Society of Thoracic Surgeons (STS); American Heart Association (AHA), and American College of Cardiology (ACC) . 2015. SCAI/ACC/HFSA/STS clinical expert consensus statement on the use of percutaneous mechanical circulatory support devices in cardiovascular care: endorsed by the American Heart Association, the Cardiological Society of India, and Sociedad Latinoamericana de Cardiología Intervencionista; affirmation of value by the Canadian Association of Interventional Cardiology-Association Canadienne de Cardiologie d'Intervention J Am Coll Cardiol. 2015 May 19; 65 19: e7- e26. doi: 10.1016/j.jacc.2015.03.036. [DOI] [PubMed] [Google Scholar]
- 11. Gilotra NA, Stevens GR. Temporary mechanical circulatory support: a review of the options, indications, and outcomes. Clin Med Insights Cardiol. 2015. February 3; 8 Suppl 1: 75- 85. doi: 10.4137/CMC.S15718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lawson WE, Koo M. Percutaneous Ventricular Assist Devices and ECMO in the Management of Acute Decompensated Heart Failure. Clin Med Insights Cardiol. 2015. April 1; 9 Suppl 1: 41- 48. doi: 10.4137/CMC.S19701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Estep JD, Cordero-Reyes AM, Bhimaraj A, et al. Percutaneous placement of an intra-aortic balloon pump in the left axillary/subclavian position provides safe, ambulatory long-term support as bridge to heart transplantation. JACC Heart Fail. 2013. October; 1 5: 382- 388. doi: 10.1016/j.jchf.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 14. Hochman JS, Sleeper LA, Webb JG, et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock: SHOCK Investigators: should we emergently revascularize occluded coronaries for cardiogenic shock. N Engl J Med. 1999. August 26; 341 9: 625- 634. [DOI] [PubMed] [Google Scholar]
- 15. Thiele H, Zeymer U, Neumann FJ, et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med. 2012. October 4; 367 14: 1287- 1296. doi: 10.1056/NEJMoa1208410. [DOI] [PubMed] [Google Scholar]
- 16. Patel MR, Smalling RW, Thiele H, et al. Intra-aortic balloon counterpulsation and infarct size in patients with acute anterior myocardial infarction without shock: the CRISP AMI randomized trial. JAMA. 2011. September 28; 306 12: 1329- 1337. doi: 10.1001/jama.2011.1280. [DOI] [PubMed] [Google Scholar]
- 17. Task Force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology (ESC), Steg PG, SK James, Atar D. et al. ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2012. October; 33 20: 2569- 2619. doi: 10.1093/eurheartj/ehs215. [DOI] [PubMed] [Google Scholar]
- 18. Teuteberg JJ, Chou JC. Mechanical circulatory devices in acute heart failure. Crit Care Clin. 2014. July; 30 3: 585- 606. doi: 10.1016/j.ccc.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 19. Naidu SS. Novel percutaneous cardiac assist devices: the science of and indications for hemodynamic support. Circulation. 2011. February 8; 123 5: 533- 543. doi: 10.1161/CIRCULATIONAHA.110.945055. [DOI] [PubMed] [Google Scholar]
- 20. Seyfarth M, Sibbing D, Bauer I, et al. A randomized clinical trial to evaluate the safety and efficacy of a percutaneous left ventricular assist device versus intra-aortic balloon pumping for treatment of cardiogenic shock caused by myocardial infarction. J Am Coll Cardiol. 2008. November 4; 52: 1584- 1588. doi: 10.1016/j.jacc.2008.05.065. [DOI] [PubMed] [Google Scholar]
- 21. Werdan K, Gielen S, Ebelt H, Hochman JS. Mechanical circulatory support in cardiogenic shock. Eur Heart J. 2014. January; 35 3: 156- 167. doi: 10.1093/eurheartj/eht248. [DOI] [PubMed] [Google Scholar]