Abstract
Background:
Heart transplantation remains the definitive therapy for patients with advanced heart failure; however, owing to limited donor organ availability and long wait times, continuous-flow left ventricular assist devices (LVADs) have become standard therapy.
Methods:
This review summarizes the history, progression, function, and basic management of LVADs. Additionally, we provide some clinical pearls and important caveats for managing this unique patient population.
Results:
Currently, the most common LVADs being implanted in the United States are second- and third-generation devices, the HeartMate II (Thoratec Corp., St. Jude Medical) and the HeartWare HVAD (HeartWare International, Inc.). A newer third-generation pump, the HeartMate III (Thoratec Corp., St. Jude Medical), is designed to create an artificial pulse and is currently under investigation in the United States.
Conclusion:
LVAD use is promising, will continue to grow, and has become standard therapy for advanced heart failure as a bridge to recovery, as destination therapy, and as a bridge to transplantation.
Keywords: Heart-assist devices, heart failure
INTRODUCTION
The incidence and prevalence of heart failure have steadily increased in the United States for the past several years. Currently, an estimated 5.7 million Americans >20 years of age have heart failure. Projections show that heart failure prevalence will continue to increase during the next several years, with estimates of more than 8 million people being affected by 2030.1
Heart failure is a broad spectrum of disease and ranges from patients who do well for many years with oral therapy to patients who require cardiac transplantation. For patients with advanced heart failure, multiple options are now available, including inotrope support (both inpatient and outpatient), cardiac transplantation, and long-term mechanical circulatory support (MCS).
Heart transplantation remains the definitive therapy for patients with advanced heart failure; however, owing to limited donor organ availability and long wait times, continuous-flow left ventricular assist devices (CF-LVADs) have become standard therapy for the management of advanced heart failure both for patients who will eventually receive a transplant (bridge to transplantation) and as an option for those who may not qualify for transplant but qualify for long-term MCS (destination therapy).2
In the past, providers have referred patients for CF-LVAD implantation, and the implanting center often managed most care postimplantation. However, because of improved survival, the increased number of implantations, and the increased number of patients with destination therapy, the concept of shared care has emerged.3 Shared care involves community physicians assisting in the long-term care of patients with CF-LVADs. As the numbers in this special population have increased, it has become crucial for the general cardiologist and internist to become familiar with this evolving technology. This review provides a fundamental understanding of the history, progression, function, and basic management of LVADs. Additionally, we provide some clinical pearls and important caveats for managing this unique patient population.
HISTORY OF CF-LVADs
The concept of using MCS began approximately 85 years ago when Dr Michael DeBakey, then a student at Tulane University, developed the roller pump.4 This important breakthrough eventually allowed for the development of the first heart and lung bypass machine.4 The first pulsatile LVAD HeartMate XVE (Thoratec Corp.) was approved in 1994 as a bridge to heart transplantation and in 2003 was approved for destination therapy, as shown in Figure 1.5-7 The initial devices tried to replicate the normal pulsatile flow of the heart, but they were large and noisy, required a large percutaneous lead, and were not durable.5-7
Figure 1.
Landmark events in the development of left ventricular assist devices and cardiac transplantation. FDA, US Food and Drug Administration; LVAD, left ventricular assist device; REMATCH, Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure.
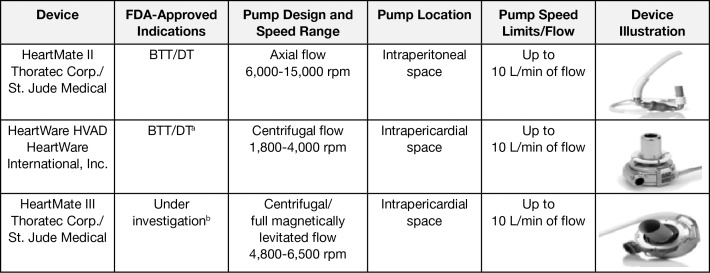
During the past several years, these devices have moved from pulsatile flow to a continuous-flow technology. The benefits of continuous-flow devices include being one-seventh the size of the original devices, one-quarter the weight, and quieter with a smaller percutaneous lead and improved durability. Currently, the most common LVADs being implanted in the United States are second- and third-generation devices, the HeartMate II (Thoratec Corp., St. Jude Medical) and the HeartWare HVAD (HeartWare International, Inc.). A newer third-generation pump (HeartMate III; Thoratec Corp., St. Jude Medical) designed to create an artificial pulse is currently under investigation in the United States (Figure 2).
Figure 2.
Left ventricular assist devices currently available in the United States. aThe HVAD trial for the DT indication was completed in 2015 and is awaiting FDA approval. bThe trial ongoing in 2016 for BTT and DT indications is the Momentum 3. BTT, bridge to transplantation; DT, destination therapy; FDA, US Food and Drug Administration. Images reproduced with permission from Thoratec Corp. and HeartWare.
BASICS OF CF-LVADs
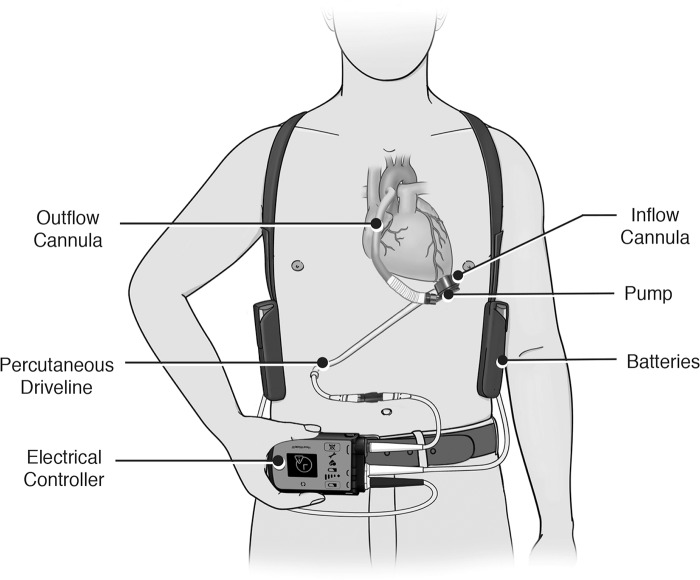
The current LVADs being implanted are all similar in function. Figure 3 highlights the 5 main components of an LVAD: an inflow cannula, a pump, an outflow cannula, a percutaneous driveline, and an electrical controller.7,8 The inflow cannula is most commonly inserted into the apex of the left ventricle, and the outflow cannula is usually anastomosed to the ascending aorta. Blood returns from the lungs to the left side of the heart and exits through the left ventricular apex and across an inflow valve into the prosthetic pumping chamber.7,8
Figure 3.
Basic left ventricular assist device components. Image reproduced with permission from Thoratec Corp.
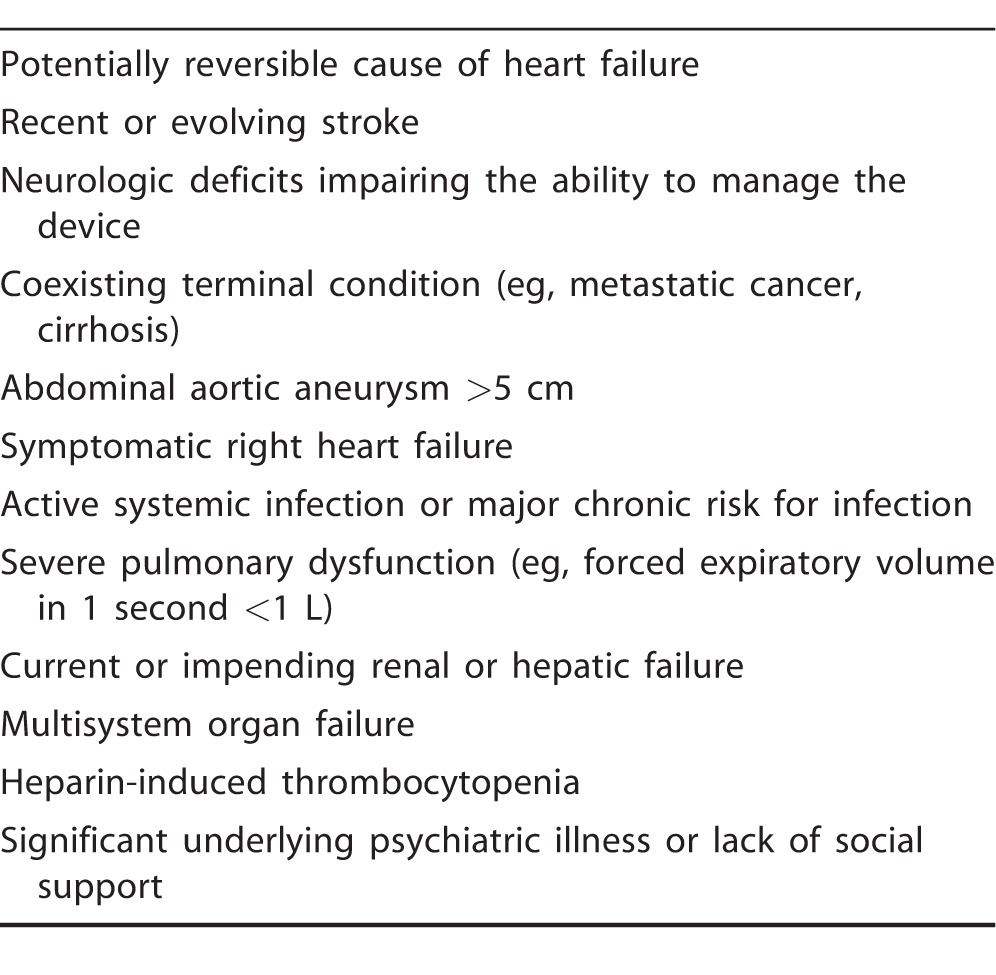
Blood is then actively pumped through an outflow valve into the ascending aorta. The pumping chamber is placed within the abdominal wall or peritoneal cavity. A percutaneous driveline carries the electrical cable and air vent to the battery packs and electronic controls that are worn on a shoulder holster and belt, respectively.7,8 Many considerations affect the evaluation of a patient for advanced mechanical therapy. Common contraindications for LVADs are summarized in Table 1.
Table 1.
Common Conditions Precluding Left Ventricular Assist Device Implant
BRIDGE TO TRANSPLANTATION VS DESTINATION THERAPY
Initially, mechanical assist devices were developed to provide patients with advanced heart failure an additional support option prior to transplantation. In the past several years, the indications have been expanded to include use as a bridge to transplantation, as a bridge to decision regarding transplantation, or as destination therapy for those who are not transplantation candidates. The Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATCH) trial,9 published in 2001, was a landmark study that showed improved outcomes in patients who received first-generation LVADs vs traditional medical therapy. At 12 months, survival was 52% in the LVAD group vs 25% in the medical therapy group. At 24 months, the trend continued, with 23% survival in the LVAD group and 8% in the medical therapy group.9 Currently, second- and third-generation LVADS provide similar 1-year survival (approximately 90%) compared to cardiac transplantation, further supporting their use as either a bridge to transplantation or destination therapy.10,11
PATIENT SELECTION AND TIMING OF IMPLANT
Patient selection and the timing of implant are key determinants of success for LVAD therapy. With few exceptions, patients with New York Heart Association (NYHA) functional Class IIIb/IV and American College of Cardiology/American Heart Association (ACC/AHA) stage D heart failure should be referred for evaluation for long-term MCS and cardiac transplantation. In addition, the current classification of patients with NYHA Class IV symptoms does not accurately define patient clinical risk profile. In this regard, the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS), the largest LVAD registry in the United States, has defined patient profiles that can help identify risks associated with the timing of implant (Table 2).12-14 These profiles are particularly important because current data indicate that patients in profile 1 (severe cardiogenic shock) have the lowest survival, and patients in profile 3 (stable on inotropes) have the best survival.12 This information implies that patients with cardiogenic shock may be too sick for permanent LVAD support and instead should be managed using temporary percutaneous MCS to optimize their condition prior to consideration for permanent LVAD placement. For these reasons, many centers have adopted the practice of LVAD implantation earlier in the progression of heart failure (eg, INTERMACS profiles 2-4). Data on post-LVAD outcomes of patients with INTERMACS profiles 4 and higher are lacking; therefore, no recommendations exist for these subgroups.
Table 2.
INTERMACS Patient Profiles

CLINICAL PEARLS
As the use of MCS continues to increase in patients with advanced heart failure, primary care providers and general cardiologists must become familiar with several of the unique characteristics of this patient population.
Pulse and Blood Pressure
CF-LVADs use centrifugal-flow or axial-flow pumps to provide continuous unloading of the left ventricle during all stages of the cardiac cycle. These pump designs can completely eliminate the arterial pulse or result in a dampened pulse. Despite continuous flow, contractility of the native left ventricle can still be in part preserved, resulting in the presence of an arterial pulse. Additionally, factors that affect left ventricular preload, such as right ventricular function and volume depletion and left ventricular afterload, will affect the pulsatility.8,15 The set speed of the pump will also affect pulsatility because of its effect on the opening and closing of the aortic valve at different speeds. As pump speed increases, diastolic pressure will increase. At some point, diastolic pressure will exceed the pressure gradient across the aortic valve, prohibiting the opening of the aortic valve and leading to an absent pulse. With numerous variables affecting pulsatility, patients with CF-LVADs may have complete, absent, or partial/intermittent aortic valve opening, producing a palpable pulse, no palpable pulse, or a weak and intermittently palpable pulse, respectively.3,8
Blood pressure monitoring and control are essential in the long-term management of patients with MCS. In patients with an adequate pulse pressure and palpable pulse, measured systolic and diastolic pressures by auscultation or automated cuffs are comparable to arterial line measurement. Because pulsatility can be diminished or absent in some patients, the use of a traditional blood pressure cuff may not be adequate. In patients with CF-LVADs whose blood pressure cannot be determined by auscultation or an automated cuff, Doppler ultrasound is recommended to detect flow. The Doppler probe is placed over the brachial artery prior to inflating the cuff. Once flow is heard, the cuff is inflated until flow is no longer audible by Doppler. Once inaudible, the cuff is slowly deflated until flow is restored. The pressure in mmHg at which flow becomes audible is thought to reflect the systolic blood pressure and approximate the mean arterial pressure.3
Importance of Blood Pressure Control
Effective blood pressure control allows for optimal pump performance and unloading of the left ventricle. The flow of CF-LVADs is reliant upon the pressure gradient, also known as the head pressure, across the pump as well as the speed of the LVAD. With the inflow cannula of the LVAD in the left ventricle and the outflow cannula in the ascending aorta, the head pressure is equivalent to the difference between the left ventricular pressure and the systemic blood pressure. The LVAD speed is a parameter measured in revolutions per minute. The normal range of speeds for each LVAD varies, and the speeds are manually adjusted to an optimal setting for each patient. The LVAD speed must be adequate in relation to the volume status, right ventricular function, amount of valvular regurgitation, and systemic blood pressure.3,8 Determination of optimal speed adjustment is discussed later in this article. At any constant speed, the variables that affect the head pressure, and thus flow, will affect end-organ perfusion. Left ventricular preload can be altered by volume status and right heart function. Uncontrolled hypertension results in increased afterload.3,8
CF-LVADs, especially centrifugal devices, are sensitive to excess afterload, making blood pressure control essential in optimizing pump performance and cardiac support through unloading of the left ventricle. The degree of unloading affects right ventricular function and the degree of mitral and aortic regurgitation, which in turn affect systemic blood pressure and end-organ perfusion. Adequate blood pressure control also minimizes adverse events, such as ischemic and hemorrhagic stroke.3,8
According to the most recent International Society for Heart and Lung Transplantation guidelines, no evidence base exists for blood pressure targets with CF-LVADS, but a mean arterial blood pressure goal of ≤80 mmHg is reasonable.2 Patients with pulsatile LVADs, in contrast, should have a blood pressure goal of systolic blood pressure <130 mmHg and diastolic blood pressure <85 mmHg.2 For patients with CF-LVADs and hypertension, defined as a mean arterial pressure >80 mmHg, treatment is similar to that for patients with heart failure. Medications such as angiotensin-converting enzyme inhibitors or angiotensin receptor blockers along with close monitoring of renal function, and beta blockers, with monitoring of right ventricular function, should be the primary medications used.3
CHRONIC MANAGEMENT OF LVADs
Patients with MCS are a unique population and require a multidisciplinary team approach for long-term outpatient management. Patients should have regularly scheduled appointments at an MCS center with the frequency of visits dictated by clinical stability. The focus of outpatient care will transition from rehabilitation in the early postimplantation period to screening for and addressing the complications of LVAD support and managing comorbidities.2
A cardiologist will oversee standard heart failure therapy and provide continued surveillance for device and non-device–related issues. Laboratory studies commonly obtained at regular intervals include blood urea nitrogen, creatinine, and liver enzymes to assess end-organ function; international normalized ratio (INR) and possibly platelet aggregation studies or thromboelastography to monitor the effectiveness of anticoagulation and antiplatelet therapy; hemoglobin and hematocrit, lactate dehydrogenase, and plasma free hemoglobin to screen for evidence of hemolysis; and lipid panel, hemoglobin A1c, and thyroid function panel to monitor comorbid illnesses. At each clinic visit, the driveline should be checked for proper positioning and the use of a binder or driveline immobilization. The exit site should be assessed for signs of infection or skin breakdown that would increase the risk of infection. The LVAD alarm history should be evaluated with proper adjustment of pump parameters as needed. All patients should be referred to cardiac rehabilitation after placement of an LVAD.2
Routine surveillance echocardiography should be performed to evaluate for signs of myocardial recovery and to determine the optimal pump parameters. Right heart catheterization may be useful to assess persistent or recurrent heart failure symptoms that can be secondary to right ventricular failure or device malfunction. In patients being bridged to transplantation with LVADs, right heart catheterization should be performed at regular intervals to document pulmonary artery pressure, as irreversible pulmonary hypertension is associated with early allograft failure.2
Additionally, patients with MCS are advised to follow general health maintenance guidelines, including vaccination recommendations according to the Centers for Disease Control that are appropriate for age and sex. Patients should receive regular dental care and have close follow-up of diabetes and renal dysfunction. Similar to the general population, smoking cessation and weight loss should be encouraged, if needed.2
Driveline Care
Great care must be taken to minimize the infection risk associated with the driveline. Percutaneous driveline infections are the most prevalent infections in patients with an LVAD and may reflect the presence of a deeper infection in the device hardware or pocket space. The majority of LVAD infections are bacterial, with the remainder primarily being fungal.15 Infection prophylaxis begins at the time of LVAD implantation when the device is anchored to stabilize the driveline. A stabilization belt or restraint device should be worn at all times. Movement of the percutaneous lead causes disruption of the subcutaneous tissue ingrowth in the velour lining of the lead, resulting in infection. All patients with LVADs and their caregivers should take precautions to avoid unnecessary exit-site movement to maintain the integrity of the fragile tissue.8
To minimize the risk of infection, a multidisciplinary team involves the patient, family members, and companions in care in the proper maintenance of the driveline. Patients begin to learn how to care for the percutaneous lead and exit site even before the LVAD is implanted, and the training continues throughout hospitalization following implantation. In some centers, the patient must be able to demonstrate his or her understanding and competency in performing routine care of the driveline prior to discharge.8 The daily sterile dressing care regimen for maintaining the driveline requires the use of chlorhexidine, masks, and sterile gloves.3
Anticoagulation/Antiplatelet Therapy
Thrombosis can occur in any of the components of the LVAD, including the inflow cannula, outflow cannula, and the rotor/propeller. Ventricular assist device thrombosis is a serious complication that can result in symptoms of hemolysis as well as a clinical range from mild heart failure to cardiogenic shock. Anticoagulation is required for patients supported with CF-LVADs to prevent these thrombotic complications. Each device has its own recommended goal INR to balance the potential risk of thromboembolism or pump thrombosis and bleeding risks. Many of the devices, including the HeartMate II and HeartWare HVAD, have an INR goal of 2.0-3.0. Novel oral anticoagulants are not approved at this time for anticoagulation in patients with MCS.2
In situations in which the INR becomes supratherapeutic and clinically significant bleeding is absent, acute reversal of anticoagulation is not recommended because of the lack of benefit and potential for harm. Patients with a subtherapeutic INR must be bridged with low molecular weight heparin or a heparin infusion until the INR reaches the therapeutic range. Patients requiring invasive procedures that require discontinuation of therapeutic anticoagulation with warfarin must be hospitalized and bridged with heparin.2
Antiplatelet therapy with aspirin 81 mg or 325 mg daily is recommended with the use of many MCS devices; however, the necessity, dosage, and frequency have not been well established. The newer antiplatelet medications, ticagrelor and prasugrel, are not approved for use in this patient population. Other antiplatelet medications, such as dipyridamole and clopidogrel, have been used in patients with LVADs, but the recommendations for use are specific to each device. As with other medications, the decision to use antiplatelet therapy must be tailored to each individual patient based on comorbid conditions that may require antiplatelet therapy, such as coronary artery disease with drug-eluting stents, prior stroke, or peripheral vascular disease.2
Bleeding is the most frequent adverse event associated with implantation of an LVAD, accounting for 9% of the total mortality associated with LVADs.15 The pathophysiology responsible for the increased risk of bleeding is complex but is thought to involve the development of acquired von Willebrand syndrome, secondary to polymer deformation by the rotating impeller of the LVAD that leads to deficiency of the von Willebrand factor.15 A mechanism of gastrointestinal bleed is the development of arteriovenous malformations that are believed to be associated with the reduction in pulse pressure and hypoperfusion of the gastrointestinal mucosa with resulting neovascularization with friable vessels that are prone to bleeding.15 Management of bleeding involves discontinuation of antiplatelet and anticoagulation therapy, as well as endoscopic evaluation and treatment. Reversal of anticoagulation can be considered but is not routinely performed because of the increased risk of LVAD thrombosis.15
Optimal LVAD Parameters and the Role of Echocardiography
Each patient who requires implantation of an LVAD will have a unique postoperative course. To adapt to these changes, the LVAD must be adjusted to each patient's needs by altering the one parameter that can be manually changed—the pump speed. At the optimal pump speed, the cardiac index and left ventricular size are within the normal ranges, and there is no rightward or leftward shift of the septum. Maintaining some degree of pulsatility with intermittent opening of the aortic valve is also desirable.8
The speed range is determined while the patient is hemodynamically stable, euvolemic, and not requiring support with inotropes or vasopressors. The various CF-LVADs have differing normal ranges for pump speed; however, transthoracic echocardiography is the primary imaging modality used to monitor patients supported by LVADs. During echocardiographic evaluation, the low end of the speed range is determined by gradually decreasing the speed until the aortic valve opens with each heartbeat and there are no signs of heart failure. In patients with severe underlying left ventricular dysfunction and no aortic valve opening, the low speed can be determined either invasively with a pulmonary artery catheter to assess for a decrease in cardiac index and an increase in pulmonary capillary wedge pressure or noninvasively by monitoring for left ventricular size enlargement and rightward shift of the septum. The high end of the speed range is determined by gradually increasing the speed of the pump until the short-axis or apical 4-chamber views show flattening of the septum and the left ventricular diastolic dimension decreases near the low end of the normal range. The appropriate fixed speed setting will fall approximately midway between the low-end and the high-end speed range. To accommodate for normal shifts in volume and hemodynamic status, the fixed speed should generally be set at least 400 rpm below the maximum speed.8
Surveillance echocardiography is performed at the pump baseline speed setting and includes views specific to LVADs, Doppler flow assessments, and all of the traditional elements of a standard transthoracic echocardiogram. Centers may add an LVAD optimization protocol in which limited-view echocardiography is performed at pump speeds higher and/or lower than the baseline speed to optimize LVAD and native heart function. Periodic surveillance echocardiography is performed approximately 2 weeks after implantation or prior to hospital discharge after implantation, followed by repeat examinations at 1, 3, 6, and 12 months postimplantation and every 6 to 12 months thereafter.16 The surveillance examinations may allow for early diagnosis of occult native heart abnormalities, such as left ventricular failure because of partial left ventricle unloading, right ventricular failure, inadequate left ventricular filling or excessive unloading, valvular abnormalities, intracardiac thrombus, pericardial effusion with or without tamponade physiology, or device-related complications, such as inflow cannula obstruction or malposition, outflow cannula thrombosis, or pump malfunction.3
Role of Computed Tomography in Diagnosing Thrombus
Echocardiography is the primary tool used for the assessment of LVADs; however, computed tomography (CT) may be helpful in certain clinical scenarios. CT allows for visualization of the native heart and MCS device without the limitation of acoustic windows and the presence of acoustic shadowing. If other imaging modalities have not been revealing, CT may be useful in patients with LVADs.2 In patients with LVADs and recurrent heart failure symptoms, CT allows assessment of the LVAD inflow and outflow cannulas to evaluate for obstruction, kinking, angulation, and placement. The inflow cannula should be directed toward the center of the left ventricular cavity with a midline septum and without thrombus formation. The outflow cannula should be without kinking and have a patent aortic anastomosis.17 The primary limitations of a cardiac CT include radiation exposure and the risk of nephrotoxicity when iodinated contrast is used.2
CONCLUSION
With the increasing age of the population, the prevalence of heart failure continues to grow. Heart transplantation remains the gold standard of care in treatment; however, the demand continues to exceed the consistently inadequate supply. LVAD use is promising, will continue to grow, and has become standard therapy for advanced heart failure as a bridge to recovery, as destination therapy, and as a bridge to transplantation. Questions regarding MCS are shifting from which patients are candidates to receive an LVAD to how to choose the proper LVAD for an individual patient and how to prevent the complications of thrombosis, stroke, and pump failure. Because of the increasing use of MCS, primary care physicians and general cardiologists must learn how to assess and care for the basic needs of this unique but growing patient population.
ACKNOWLEDGMENTS
The authors have no financial or proprietary interest in the subject matter of this article.
This article meets the Accreditation Council for Graduate Medical Education and the American Board of Medical Specialties Maintenance of Certification competencies for Patient Care, Medical Knowledge, and Practice-Based Learning and Improvement.
REFERENCES
- 1. Writing Group Members, Mozaffarian D, EJ Benjamin, Go AS, et al. American Heart Association Statistics Committee; Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics–2016 Update: A Report from the American Heart Association. Circulation. 2016. January 26; 133 4: e38- e60. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 2. Feldman D, Pamboukian SV, Teuteberg JJ, et al. The 2013 International Society for Heart and Lung Transplantation Guidelines for mechanical circulatory support: executive summary. J Heart Lung Transplant. 2013. February; 32 2: 157- 187. doi: 10.1016/j.healun.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 3. Estep JD, Trachtenberg BH, Loza LP, Bruckner BA. Continuous flow left ventricular assist devices: shared care goals of monitoring and treating patients. Methodist Debakey Cardiovasc J. 2015. Jan-Mar; 11 1: 33- 44. doi: 10.14797/mdcj-11-1-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. DeBakey Contributions to Medicine . Baylor College of Medicine. https://www.bcm.edu/about-us/debakey-museum/legacy-of-excellence. Accessed June 16, 2016. [Google Scholar]
- 5. Goldsmith MF. First implant of portable heart-assist device. JAMA. 1991. June 12; 265 22: 2930- 2933. [DOI] [PubMed] [Google Scholar]
- 6. Birks EJ, Tansley PD, Hardy J, et al. Left ventricular assist device and drug therapy for the reversal of heart failure. N Engl J Med. 2006. November 2; 355 18: 1873- 1884. [DOI] [PubMed] [Google Scholar]
- 7. Miller LW, Pagani FD, Russell SD, et al. HeartMate II Clinical Investigators. Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med. 2007. August 30; 357 9: 885- 896. [DOI] [PubMed] [Google Scholar]
- 8. Slaughter MS, Pagani FD, Rogers JG, et al. Clinical management of continuous-flow left ventricular assist devices in advanced heart failure. J Heart Lung Transplant. 2010. April; 29 4 Suppl: S1- S38. doi: 10.1016/j.healun.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 9. Rose EA, Gelijns AC, Moskowitz AJ, et al. Long-term use of a left ventricular assist device for end stage heart failure. N Engl J Med. 2001. November 15; 345 20: 1435- 1443. [DOI] [PubMed] [Google Scholar]
- 10. Lietz K, Long JW, Kfoury AG, et al. Outcomes of left ventricular assist device implantation as destination therapy in the post-REMATCH era: implications for patient selection. Circulation. 2007. July 31; 116 5: 497- 505. [DOI] [PubMed] [Google Scholar]
- 11. Starling RC, Naka Y, Boyle AJ, et al. Results of the post-U.S. Food and Drug Administration-approval study with a continuous flow left ventricular assist device as a bridge to heart transplantation: a prospective study using the INTERMACS (Interagency Registry for Mechanically Assisted Circulatory Support). J Am Coll Cardiol. 2011. May 10; 57 19: 1890- 1898. doi: 10.1016/j.jacc.2010.10.062. [DOI] [PubMed] [Google Scholar]
- 12. Kirklin JK, Naftel DC, Kormos RL, et al. Second INTERMACS annual report: more than 1,000 primary left ventricular assist device implants. J Heart Lung Transplant. 2010. January; 29 1: 1- 10. doi: 10.1016/j.healun.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Holman WL, Pae WE, Teutenberg JJ, et al. INTERMACS: interval analysis of registry data. J Am Coll Surg. 2009. May; 208 5: 755- 761 ; discussion 761-762. doi: 10.1016/j.jamcollsurg.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 14. Stevenson LW, Pagani FD, Young JB, et al. INTERMACS profiles of advanced heart failure: the current picture. J Heart Lung Transplant. 2009. June; 28 6: 535- 541. doi: 10.1016/j.healun.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 15. Birati EY, Rame JE. Left ventricular assist device management and complications. Crit Care Clin. 2014. July; 30 3: 607- 627. doi: 10.1016/j.ccc.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 16. Stainback RF, Estep JD, Agler DA, et al. Echocardiography in the management of patients with left ventricular assist devices: recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr. 2015. August; 28 8: 853- 909. doi: 10.1016/j.echo.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 17. Estep JD, Stainback RF, Little SH, Torre G, Zoghbi WA. The role of echocardiography and other imaging modalities in patients with left ventricular assist devices. JACC Cardiovasc Imaging. 2010. October; 3 10: 1049- 1064. doi: 10.1016/j.jcmg.2010.07.012. [DOI] [PubMed] [Google Scholar]