Abstract
Background:
Heart disease is a major cause of death in industrialized nations, with approximately 50% of these deaths attributable to sudden cardiac arrest. If patients at high risk for sudden cardiac arrest can be identified, their odds of surviving fatal arrhythmias can be significantly improved through prophylactic implantable cardioverter defibrillator placement. This review summarizes the current knowledge pertaining to surface electrocardiogram (ECG) predictors of sudden cardiac arrest.
Methods:
We conducted a literature review focused on methods of predicting sudden cardiac arrest through noninvasive electrocardiographic testing.
Results:
Several electrocardiographic-based methods of risk stratification of sudden cardiac arrest have been studied, including QT prolongation, QRS duration, fragmented QRS complexes, early repolarization, Holter monitoring, heart rate variability, heart rate turbulence, signal-averaged ECG, T wave alternans, and T-peak to T-end. These ECG findings have shown variable effectiveness as screening tools.
Conclusion:
At this time, no individual ECG finding has been found to be able to adequately stratify patients with regard to risk for sudden cardiac arrest. However, one or more of these candidate surface ECG parameters may become useful components of future multifactorial risk stratification calculators.
Keywords: Arrhythmias–cardiac, death–sudden–cardiac, defibrillators–implantable, electrocardiography, primary prevention, ventricular fibrillation
INTRODUCTION
Heart disease is a major cause of death in industrialized nations.1 Sudden cardiac arrest (SCA) accounts for approximately 50% of these deaths, amounting to 350,000-400,000 per year and making SCA second only to all cancers combined in terms of the number of American lives lost.2,3 SCA deaths are most often attributed to ventricular fibrillation (VF).2
Through large multicenter randomized controlled trials such as the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) and the Multicenter Automatic Defibrillator Implantation Trial II (MADIT-II), it is well known that placement of an implantable cardioverter defibrillator (ICD) for the purpose of primary prevention of SCA results in a significant reduction in mortality in patients with a reduced left ventricular ejection fraction (LVEF).4,5 However, LVEF alone is neither sensitive enough nor specific enough a risk stratifier for SCA6: only a minority of patients implanted with these devices receive appropriate ICD therapies, and the majority of victims of SCA have no prior known cardiac disease.7 It is important to consider closely the risk/benefit relationship of ICD implantation, as patients with ICDs sometimes receive inappropriate therapies that have been associated with increased mortality.8 In addition to this adverse effect, patients receiving these devices are at risk for additional short- and long-term complications, including bleeding, pneumothorax, and device-related infection.9,10
ICDs are effective at aborting sudden cardiac death (SCD) resulting from ventricular tachyarrhythmia (VT).11 Considering the significant benefit of ICD therapy, an improved risk stratification method is needed to enable clinicians to better identify the patients who are most likely to benefit from these devices. The optimal risk stratification tool would be noninvasive, cost effective, expeditious, widely accessible, and effectively predictive. Because they may meet these criteria, the surface electrocardiogram (ECG) and ECG-related assessment techniques are being evaluated to determine if they can be used to identify patients at high risk for SCA. The surface ECG was first developed in 1902, using a string galvanometer to record the electrical potential difference between the extremities.12 It has become an essential tool for the initial evaluation of patients with cardiac complaints, specifically for identifying arrhythmias and ischemic heart disease.13 The ECG is an especially attractive screening tool because of its low cost and noninvasive application. We review the current knowledge base regarding the potential of surface ECG-based screening tools to predict SCA.
VENTRICULAR FIBRILLATION AND VENTRICULAR TACHYCARDIA IN SUDDEN CARDIAC ARREST
As mentioned above, SCA is most commonly caused by ventricular arrhythmias, particularly sustained monomorphic ventricular tachycardia and VF.2 Monomorphic ventricular tachycardia is often supported by a scar-based reentry circuit that can be triggered by a poorly timed premature ventricular contraction, variations in cardiac cycle length, and variations in heart rate.14 Considering this mechanism, ICD therapy (antitachycardia pacing and/or high-voltage shock) is effective in controlling this arrhythmia, and with pharmacologic control of triggers and catheter ablation, the freedom from future events can be lasting.15 In contrast, VF is thought to be caused by multiple rotors throughout the heart and is commonly caused by ischemia or primary VF syndromes.16 In this setting, ICD discharge can abort SCA acutely, but without control of causative factors (eg, ischemia), VF may recur.17
QT PROLONGATION
One of the most well-known ECG parameters potentially identifying SCA risk is QT prolongation. The surface ECG QT interval represents the duration of ventricular depolarization and repolarization. Slower ventricular repolarization manifests as QT prolongation and indicates an increase in the temporal dispersion of various ventricular regions' refractory periods.18 As the QT prolongs, the vulnerable period for arrhythmia induction also prolongs, resulting in increased susceptibility to ventricular tachyarrhythmias.19 Prolonged QT intervals found in congenital, acquired, and drug-induced conditions have been related to the development of complex and sometimes fatal arrhythmias—most often, the rhythm torsade de pointes.20
Studies of QT prolongation and its association with SCD have come to varying conclusions. For example, a study of 457 post–myocardial infarction (MI) patients showed no consistent relationship between QT interval and mortality.21 Subsequently, another study of 280 infarct survivors again showed that QT dispersion was unable to independently predict ventricular tachycardia, VF, and death.22 In contrast, a study comparing 17 patients who died of cardiac arrhythmia to 51 matched survivors of VT showed mean QRS duration and mean QT to be independent contributors to the risk of arrhythmia and ultimately to cardiac death.23 On the whole, the evidence is mixed regarding QT prolongation as an independent ECG predictor of SCA. It may be useful in some populations, but not in others.
QRS DURATION
The ECG QRS complex represents ventricular depolarization. QRS duration has been shown consistently to be associated with increased all-cause mortality,24,25 but the literature reports mixed results with regard to SCA specifically related to arrhythmia. In specific patient populations—for example, in the setting of aggressive antihypertensive therapy—prolonged QRS duration was directly correlated with the risk of SCD, independent of many other known risk factors.26 In contrast, the Multicenter UnSustained Tachycardia Trial (MUSTT) of patients with ischemic cardiomyopathy, nonsustained ventricular tachycardia, and LVEF ≤40% showed that despite the relationship between overall mortality and the wide QRS patterns of left bundle branch block and nonspecific intraventricular conduction delay, no significant association was seen between wide QRS patterns and inducibility of ventricular tachycardia with programmed ventricular stimulation.27 In addition, the Pacing Fast Ventricular Tachycardia Reduces Shock Therapies (PainFREE Rx II) trial of ICD recipients found that QRS duration had no utility for predicting appropriate ICD therapies for VT.28
FRAGMENTED QRS COMPLEXES
Fragmented QRS complexes (fQRS) are thought to represent conduction disarray caused by ventricular myocardial fibrosis and/or scarring.29 As shown in Figure 1, the fQRS may have multiple multicomponent morphological patterns, with QRS duration able to be greater than or less than the normal cutoff of 120 ms.30 Das et al described fQRS complexes as a marker of abnormal ventricular depolarization and a predictor of mortality and SCD.30 Subsequently, Strauss et al published a system of QRS scoring correlated with the presence of ventricular myocardial scar on cardiac magnetic resonance imaging in patients with either ischemic or nonischemic cardiomyopathy.31 This scoring scheme involved measurements of amplitude, duration, and notching of the Q, R, and S waves in 10 of 12 standard ECG leads (excluding leads III and aVR). An examination of 797 patients from the ICD arm of SCD-HeFT demonstrated that a higher fQRS score correlated with a higher incidence of subsequent ventricular arrhythmia.32 Based on these limited findings, the presence and severity of QRS fragmentation may be a promising tool to assist in identification of patients at highest risk for SCD but likely will not be powerful enough to independently risk stratify patients regarding SCA.
Figure 1.
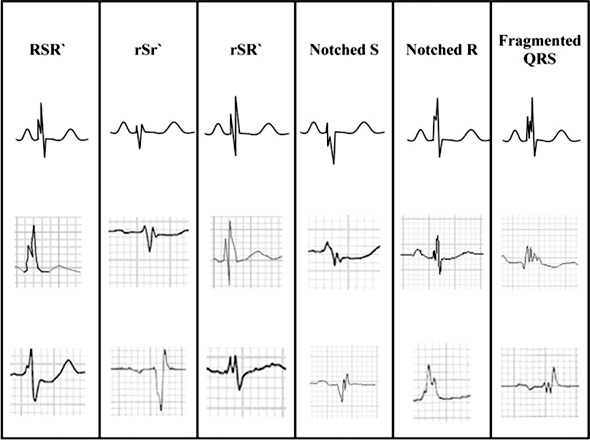
Different morphologies of the fragmented QRS. (From Das et al30 and reprinted with permission of Elsevier and the Heart Rhythm Society.)
EARLY REPOLARIZATION
The early repolarization (ER) pattern on surface ECG classically has been considered a normal variant, but the literature has described a potential role for this finding as a prognosticator of risk for ventricular arrhythmia. ER is defined as elevation of the J point (ie, the junction of the QRS complex and ST-segment) by 0.1 mV in 2 contiguous inferior or lateral ECG leads.33 Studies have shown an increased incidence of early repolarization in patients with idiopathic VF compared to control subjects (31% vs 5%, P<0.001).34 In addition, during follow-up, patients with ER in that population were found to have a higher incidence of recurrent episodes of VF. Importantly, the ER pattern may have an inherited component: Nunn et al found that first-degree relatives of patients who died from SCA were more likely to display findings of J point elevation on surface ECG compared to control subjects.35 Mutations noted in L-type calcium channel subunits are currently being investigated as potentially causative of this malignant phenotype.35 Whether early repolarization can be used to reliably predict SCD remains to be seen, but its ease of detection makes it an appealing target for future studies.
HOLTER MONITORING
The Holter monitor consists of a 2- or 3-lead ambulatory ECG that records the heart rate and rhythm, typically for a period of 24-48 hours. Basic parameters commonly assessed through Holter monitoring include heart rhythm(s), frequency of ectopy (ie, premature atrial or ventricular contractions), average heart rate, and heart rate range over time.
Previous studies have demonstrated an association between Holter-detected episodes of nonsustained ventricular tachycardia and/or significant premature ventricular contraction burden and subsequent significant arrhythmic events and mortality.36,37 In 2005, Mäkikallio et al demonstrated in a post-MI population that nonsustained ventricular tachycardia was an independent predictor of SCD, even after adjustment for age, diabetes, and LVEF.38
In a study of patients with or without known cardiac disease, data from 867 subjects who underwent 24-hour Holter monitoring were reviewed, and a high resting heart rate was found to be independently associated with ventricular arrhythmia, even after controlling for LVEF.39 In fact, a metaanalysis published in 2016 showed that high resting heart rate is an independent predictor not only of cardiovascular mortality but also of all-cause mortality in the general population.40
HEART RATE VARIABILITY
Healthy individuals demonstrate considerable heart rate variability over time. Heart rate variability has been shown to be an index of parasympathetic function, and lower heart rate variability has been linked to an increased risk for ventricular arrhythmia and mortality.41,42 For example, in a post-MI population, patients with low heart rate variability, as indicated by a standard deviation of all normal RR intervals (SDNN) of <50 ms, had a significantly higher risk of 1-year mortality compared to patients with an SDNN >50 ms (34% vs 9%, P<0.0001), independent of LVEF.43 As promising as these data appear, heart rate variability is influenced by numerous factors including age, sex, and medications, thus making it difficult to compare across broad patient populations. In support of this notion, a review from Murukesan et al in 2013 concluded that heart rate variability must be combined with other ECG markers (eg, T wave alternans and heart rate turbulence) to achieve substantial prognostic value.44 In addition, there are technical limitations; for example, heart rate variability cannot be evaluated in patients with active atrial fibrillation or frequent ectopy.45
HEART RATE TURBULENCE
Heart rate turbulence is defined by minute changes in ventricular cycle length following premature ventricular contractions. After a premature ventricular contraction, the normal response is a brief initial increase in heart rate, followed by a return to baseline. These changes are the result of premature ventricular contraction–induced hemodynamic disturbances, and the speed at which they happen ultimately provides information regarding cardiovascular autonomic function. Heart rate turbulence is qualified by 2 parameters: turbulence onset and turbulence slope. Turbulence onset is the relative change in the RR interval caused by a premature ventricular contraction, and turbulence slope is the rate of change of the RR interval back to baseline.46 Heart rate turbulence can also be induced through the use of intracardiac pacing performed in the electrophysiology laboratory or through an implanted pacemaker or an ICD.47 One contemporary protocol for measuring induced heart rate turbulence involves computing turbulence slope and turbulence onset following 10 ventricular extrastimuli with a coupling interval of 60%-70% of the sinus cycle length.47
With regard to SCA prediction, heart rate turbulence has been most extensively studied in the post-MI population and has been shown to be a strong independent predictor of SCA risk.38,48 For example, the REFINE (Risk Estimation Following Infarction Noninvasive Evaluation) study of patients with LVEF <50% showed that heart rate turbulence can be helpful for identifying such patients at high risk for cardiac arrest.49 Another study involving 2,343 patients with preserved LVEF in the post-MI period showed that severe autonomic failure (ie, decreased heart rate turbulence in combination with poor heart rate deceleration capacity) identifies a high-risk group equivalent in number and mortality risk to the population of post-MI patients with LVEF <30%.50 In opposition to this finding, a retrospective analysis of 884 patients from the MADIT-II trial of post-MI patients with LVEF ≤30% showed that heart rate turbulence parameters were not independently predictive of arrhythmia or SCA.51 However, this study was limited by a short recording period that may not have provided an adequate representation of average heart rate turbulence.
Of the information obtained from Holter monitoring, heart rate turbulence may be the most promising parameter for prediction of SCD but likely as part of a multifactor scoring system. Heart rate turbulence is limited by the fact that it has primarily been studied in the post-MI population, so generalization to other populations is not advised at present. In addition, heart rate turbulence is affected by several potential confounding variables such as age, medications, left ventricle function, and cardiac perfusion status.46 Furthermore, the need for an extended recording may decrease its attractiveness for routine use. In the future, a target for research may be the use of shorter recording periods, as well as expanded use of paced ventricular extrasystoles to simulate passive heart rate turbulence recordings.
SIGNAL-AVERAGED ELECTROCARDIOGRAM
The signal-averaged ECG (SAECG) is an amplified and processed ECG that can detect abnormal microvolt-level potentials not visible on a standard surface ECG. These microvolt potentials are known as late potentials because they occur during the terminal portion of the processed QRS complex. Late potentials arise from areas of slow conduction, indicating delayed depolarization of diseased myocardium that could indicate risk for reentrant ventricular arrhythmias. The presence of late potentials has been demonstrated to predict the inducibility of ventricular tachycardia at invasive electrophysiology study.52 In addition, a study analyzing a subset of the MUSTT found that the criteria of reduced ejection fraction combined with abnormal SAECG may have utility in identifying patients at particularly high risk for SCD.53 In contrast, however, the Coronary Artery Bypass Graft (CABG) Patch Trial in patients with coronary artery disease, depressed LVEF, and abnormal SAECG found no evidence of improved survival in patients who received ICD placement compared to patients who did not receive an ICD.54
Several studies have examined the utility of SAECG in the post-MI period, showing no significant difference in survival between patients with a positive SAECG and a negative SAECG.55,56 The negative predictive value of SAECG was noted to be high (>95%), but its positive predictive value was much lower (20%), therefore making it minimally useful for detecting patients at risk for SCA.57 The utility of SAECG has been questioned further in the post–percutaneous coronary intervention (PCI) era. In a series of 968 post-MI patients (91% of whom received PCI), Bauer et al demonstrated that the presence of late potentials had no significant association with arrhythmia or cardiac death during 34 months of follow-up.58
The acquisition of SAECG recordings can be technically challenging. Because the late potentials that define a positive SAECG are typically of low amplitude, particular attention to skin preparation prior to electrode placement is necessary to reduce signal noise. Electronic filters can further reduce signal noise by evening out baseline drift and by filtering out high-frequency signals such as skeletal muscle potentials. SAECG is then performed by collecting an extended period of ECG recording (often 5-20 minutes) and then averaging the QRS complexes to reduce the signal-to-noise ratio, revealing low-amplitude signals that otherwise are invisible.59 Most commonly, signals are averaged through the temporal technique that uses an automated computer algorithm to reduce random noise by averaging the QRS complexes over time.59 This technique has 3 distinct requirements. First, the ECG signal must be repetitive and without variation. Second, the entire signal needs to be referenced to a single point on the ECG. Third, the signal of interest and the noise must be independent and remain independent.59 Because of these requirements, variability in the signal (eg, premature beats, irregular rhythms, variable bundle branch block) would make the averaging and noise filtration inaccurate.59
With a high negative predictive value, low positive predictive value, and technical challenges, SAECG may provide some valuable information but at present is not a useful tool for routine screening for patients at risk for SCD. Rather, a positive SAECG is most often used today as one of the minor diagnostic criteria for the fairly rare inherited disorder arrhythmogenic right ventricular cardiomyopathy.60,61
T WAVE ALTERNANS
The T wave represents ventricular repolarization. T wave alternans (TWA) is beat-to-beat fluctuation in the amplitude of the T wave as shown in Figure 2. TWA may be detected in all patients at high heart rates, but it is abnormal when significant TWA exists at relatively slow heart rates (ie, <110 bpm).62 Abnormal TWA is associated with increased dispersion of repolarization of the left ventricle and therefore has been studied for its value in predicting SCD. TWA can be assessed during exercise, atrial pacing, or ambulatory Holter monitoring.63,64
Figure 2.
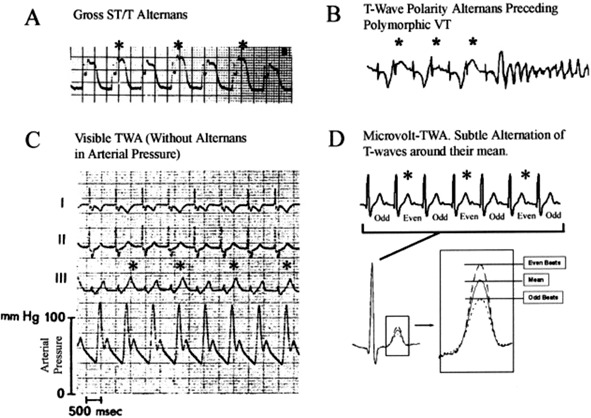
T wave alternans (TWA) of increasing subtlety. A. Gross alternans of elevated ST/T-segments in a patient with angina pectoris preceding ventricular tachycardia (VT). B. Visible alternans of T wave polarity in a woman without angina, heralding polymorphic VT. C. Subtle but visible TWA after tachycardia termination, without arterial pressure alternans (bottom). D. Visually inapparent microvolt-level TWA, uncovered by digital signal processing. *=The more positive T wave of each alternating pair. (From Narayan64 and reprinted with permission of Elsevier and the American College of Cardiology.)
As with many other ECG-based proposed risk stratification tools, studies of TWA have shown varying results. A study of a Holter-monitored subset of the Cardiovascular Health Study population demonstrated that increased amplitude of TWA was associated with SCD.65 In addition, a prospective study of 1,003 patients with coronary artery disease with a wide range of LVEFs demonstrated that assessment of TWA through ambulatory Holter monitoring could be valuable as an independent predictor of risk for SCD.66 Similarly, following MI, microvolt TWA was demonstrated to be a strong predictor of serious arrhythmias and SCD, regardless of left ventricle function.67-69 However, Chow et al found that microvolt TWA was unable to reliably predict ventricular arrhythmias in a post-ICD population with LVEF ≤30%, although microvolt TWA had a significant negative predictive value with regard to overall mortality.70 Supporting this finding, the Alternans Before Cardioverter Defibrillator (ABCD) trial concluded that the true value of microvolt TWA lies in identifying patients who are least likely to benefit from ICD placement.71
The use of TWA was further tailored by the Cornell group who demonstrated that neither TWA nor invasive electrophysiology study offered any negative predictive value in patients with a wide QRS complex (>120 ms).72 Thus, the use of TWA as a risk stratifier seems to have a significant additional limitation: patients can only be deemed truly low risk if they have both a negative TWA test and a narrow QRS. In summary, TWA could be useful in reclassifying patients who otherwise might be considered at high risk for SCA (eg, those with reduced LVEF) as actually being at lower risk, but it is less likely to be valuable as a tool for detecting high risk in otherwise low-risk patients.
T-PEAK TO T-END
The interval from the peak of the T wave to the end of the T wave on the surface ECG (T-peak to T-end, or Tpe) has been shown to be a measure of dispersion of ventricular repolarization (DVR).73-75 Prolongation of this interval signifies greater DVR and therefore increased susceptibility to reentrant VT.76,77
Longer Tpe has been shown to predict VT, sudden death, or both in many populations with diverse characteristics.78 Nearly every published trial has shown longer Tpe to be associated with higher risk. For example, in 2007, prolonged Tpe was shown to be associated with increased mortality in patients with congenital and acquired long QT syndrome,79 and similar findings were reported in 2009 in patients undergoing primary PCI for MI.80 The relationship of Tpe interval with VT also has been described extensively in patients with Brugada syndrome. In 2006, 29 subjects with confirmed Brugada syndrome were studied, together with a similar number of age- and sex-matched controls. A significant positive correlation was found between lengthened Tpe intervals and subsequent serious arrhythmic events.81 Another study of 23 individuals with Brugada syndrome correlated Tpe intervals with inducibility of VT/VF at programmed stimulation: there was a direct relationship between lengthened Tpe interval and inducibility of VT and VF.82 Of course, by virtue of their low numbers of subjects, both of these studies were limited by low power. However, these early findings made Tpe an appealing subject for researchers investigating ECG predictors of SCD.
In 2011, Panikkath et al described the relationship between Tpe and SCD.83 Using ECGs in sinus rhythm, this group compared the Tpe intervals of 353 patients who had experienced SCD to the Tpe intervals of a matched control group of 342 patients. They found that Tpe had a significant association with SCD, even after adjusting for age, sex, QTc, QRS duration, and left ventricle function. In 2012, the Ochsner group investigated the relationship of rate-corrected Tpe (Tpec) to VT and/or death in patients with left ventricle dysfunction and an ICD. The study showed that after a mean follow-up of 30 months, a longer Tpec correlated with a higher incidence of appropriate ICD therapy and/or death, even after correcting for other univariable predictors.84
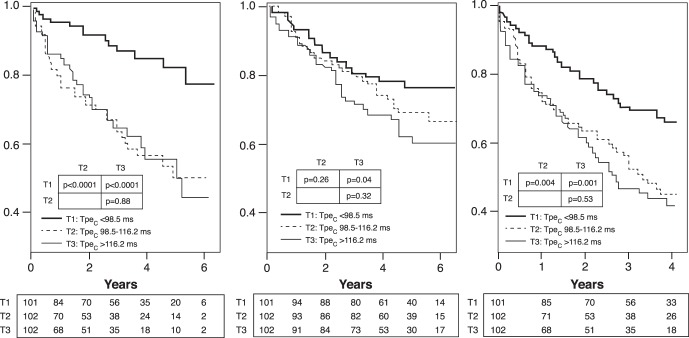
Considering the convincing relationship between the Tpec interval and the occurrence of VT and SCD in a variety of populations,80-83,85-88 the Ochsner cardiac electrophysiology group further examined Tpe in a population with the most common indication for primary prevention ICD placement: systolic cardiomyopathy without a prior history of VT. In this investigation, published in 2015, the authors studied 305 patients with LVEF ≤35% who had undergone ICD placement for primary prevention. The results are depicted in Figure 3. Even after controlling for a variety of univariable predictors, the data showed that the Tpec interval was independently predictive of VT, all-cause mortality, and the combined endpoint of VT/VF or death.78
Figure 3.
The utility of the T-peak to T-end (Tpe) interval for the prediction of ventricular tachyarrhythmia and death. A. Kaplan-Meier plots of freedom from appropriate implantable cardioverter-defibrillator therapy, stratified by tertiles (T1, T2, T3) of corrected Tpe (Tpec). B. Kaplan-Meier plots of all-cause mortality, stratified by tertiles of Tpec. C. Kaplan-Meier plots of arrhythmia-free survival, stratified by tertiles of Tpec. (From Rosenthal et al78 and reprinted with permission of Elsevier and the Heart Rhythm Society.)
In ongoing research, the Ochsner group is continuing to refine the use of Tpe through examination of the optimal measurement method for this interval, as well as the optimal method for heart rate correction. These studies, thus far published only in abstract form, have shown that the predictive ability of Tpe can vary depending on the method used to measure this interval and that prediction is enhanced by correction for heart rate.89 Further, among heart rate correction formulas, the Framingham and Hodges methods appear to improve prediction more than the methods of Bazett and Fridericia.90
Once the use of Tpe is standardized, it may become valuable as a part of future multicomponent risk prediction algorithms.
INHERITED SUDDEN DEATH SYNDROMES
Ion channels located in the cardiac cellular membrane are responsible for the cardiac action potential. When a genetic mutation results in abnormal functioning of these channels, a patient can be at risk for life-threatening arrhythmias.91 Considering that these abnormalities affect conduction, some can be detected on the resting ECG, such as the Brugada, long QT (LQT), and short QT (SQT) syndromes.
Brugada syndrome is an autosomal dominant sudden death syndrome related to mutations affecting the sodium channel that accounts for 4%-12% of cases of SCD.92 It is represented on the ECG as coved ST-segment elevation in the anterior precordial leads, although some variation in morphology does occur and the pattern can be variably present over time, even in the same individual.93 When Brugada syndrome manifests, it is usually as VT in young adults, but such events have also been described in children.92 These events can occur during sleep or rest and especially during times of fever, as some Brugada-causing mutations are thought to affect the Na+ channel in a temperature-dependent manner.93 Males have a higher frequency of arrhythmic events because of a sex-specific effect on gene expression.94
LQT syndrome is the most common channelopathy, estimated to occur in approximately 1 in 2,500 people, putting them at risk for torsade de pointes and SCD.92 LQT syndrome was first classified as the autosomal dominant Romano-Ward syndrome and the autosomal recessive Jervell and Lange-Nielsen syndrome that is associated with bilateral sensorineural deafness.95 The various subtypes of LQT syndrome are encoded by different mutant genes and are associated with different arrhythmic triggers, the most common being exercise such as swimming (LQT type 1), loud noises including alarms (LQT type 2), and sleeping (LQT type 3).96 The current mainstay of treatment for the most common types of LQT syndrome is beta blockade and avoidance of triggers.92 For patients who suffer VT events despite beta blockade and for patients with LQT types that tend not to respond as well to beta blockade, ICD placement is recommended.97
SQT syndrome is the most severe form of the major channelopathies and most commonly first presents as SCD occurring during rest, sleep, or exertion.92 It is diagnosed in the presence of a QTc <330 ms or with a QTc <360 ms with one of the following: documented causative gene mutation, family history of SQT syndrome, family history of sudden death at age ≤40, or prior VT/VF episode that is otherwise unexplained.3 It is related to autosomal dominant mutations in the K+ channel resulting in 3 syndromes, classified as SQTS1, SQTS2, and SQTS3.92 Considering the severity of SQT syndrome, the only recommended therapy is ICD placement, especially if the patient is a survivor of cardiac arrest or has documented spontaneous, sustained VT with or without syncope (Class I indication).3 ICD placement should also be considered in asymptomatic patients formally diagnosed with SQT syndrome who also have a family history of SQT syndrome.3
Wolff-Parkinson-White (WPW) syndrome is more common than the syndromes discussed above, affecting 2-4 persons per 1,000.98 WPW is not related to a known mutation but to the presence of an accessory atrioventricular conduction pathway located outside of the atrioventricular node. Because most such extranodal pathways lack the decremental conduction properties characteristic of the atrioventricular node, sudden death in WPW is most often related to the occurrence of atrial fibrillation, followed by rapid conduction to the ventricle over the accessory pathway, leading to VF.99 An asymptomatic patient with the classic findings of WPW on resting ECG (ie, a short PR interval and a delta wave, indicating ventricular preexcitation) has an annual SCA risk of 0.1%.100 Exercise testing and/or invasive programmed stimulation can be used to further risk stratify this population into those at low risk and those at high risk.101 Ablation of the accessory pathway in the asymptomatic patient is deemed reasonable in the current American College of Cardiology guidelines, but individualized decisions based on patient-provider discussion should guide therapy.102 When the accessory pathway is known to be high risk (ie, robustly conductive) or when it is associated with supraventricular tachycardia, ablation is indicated.
CONCLUSION
SCA is a common cause of death. While depressed LVEF is one of today's most useful indicators of risk, there is significant room for improvement in risk stratification, as LVEF is neither sensitive enough nor specific enough a predictor of SCA. When deciding whether to place an ICD, the clinician must weigh numerous factors including cardiac disease burden, noncardiac comorbidities, and patient preferences, as well as the possible adverse outcomes of the presence of an ICD, including inappropriate shocks and device-related infection. Although the surface ECG parameters described in this review may provide a considerable amount of useful information regarding arrhythmic substrate and SCA risk, any single test is unlikely to be powerful enough to adequately risk stratify patients regarding SCA. The identification of new tools for risk stratification of SCA is an active area of ongoing research. It seems possible that one or more of the candidate surface ECG parameters, including nonsustained ventricular tachycardia, high resting heart rate, heart rate turbulence, and Tpe, may become useful components of multifactorial risk stratification calculators in the future.
ACKNOWLEDGMENTS
The authors have no financial or proprietary interest in the subject matter of this article.
This article meets the Accreditation Council for Graduate Medical Education and the American Board of Medical Specialties Maintenance of Certification competencies for Patient Care, Medical Knowledge, and Practice-Based Learning and Improvement.
REFERENCES
- 1. Pagidipati NJ, Gaziano TA. Estimating deaths from cardiovascular disease: a review of global methodologies of mortality measurement. Circulation. 2013. February 12; 127 6: 749- 756. doi: 10.1161/CIRCULATIONAHA.112.128413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Writing Group Members , Mozaffarian D Benjamin EJ. et al. American Heart Association Statistics Committee; Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2016 Update: a report from the American Heart Association. Circulation. 2016. January 26; 133 4: e38- e60. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 3. Priori SG, Wilde AA, Horie M, et al. Executive summary: HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes. Heart Rhythm. 2013. December; 10 12: e85- e108. doi: 10.1016/j.hrthm.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 4. Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005. January 20; 352 3: 225- 237. [DOI] [PubMed] [Google Scholar]
- 5. Moss AJ, Zareba W, Hall WJ, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002. March 21; 346 12: 877- 883. [DOI] [PubMed] [Google Scholar]
- 6. Disertori M, Gulizia MM, Casolo G, et al. Improving the appropriateness of sudden arrhythmic death primary prevention by implantable cardioverter-defibrillator therapy in patients with low left ventricular ejection fraction. Point of view. J Cardiovasc Med (Hagerstown). 2016. April; 17 4: 245- 255. doi: 10.2459/JCM.0000000000000368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Myerberg RJ, Castellanos A. Cardiac arrest and sudden cardiac death. : Bonow RO, Mann DL, Zipes DP, Libby P. Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine. 5th ed. Philadelphia, PA: W. B. Saunders Co.; 1997: 845- 884. [Google Scholar]
- 8. Poole JE, Johnson GW, Hellkamp AS, et al. Prognostic importance of defibrillator shocks in patients with heart failure. N Engl J Med. 2008. September 4; 359 10: 1009- 1017. doi: 10.1056/NEJMoa071098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Reynolds MR, Cohen DJ, Kugelmass AD, et al. The frequency and incremental cost of major complications among medicare beneficiaries receiving implantable cardioverter-defibrillators. J Am Coll Cardiol. 2006; 47 12: 2493- 2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. van Rees JB, de Bie MK, Thijssen J, Borleffs CJ, Schalij MJ, van Erven L. Implantation-related complications of implantable cardioverter-defibrillators and cardiac resynchronization therapy devices: a systematic review of randomized clinical trials. J Am Coll Cardiol. 2011. August 30; 58 10: 995- 1000. doi: 10.1016/j.jacc.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 11. Biffi M. ICD programming. Indian Heart J. 2014. Jan-Feb; 66 Suppl 1: S88- S100. doi: 10.1016/j.ihj.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Howell JD. Diagnostic technologies: X-rays, electrocardiograms, and CAT scans. South Calif Law Rev. 1991. November; 65 1: 529- 564. [PubMed] [Google Scholar]
- 13. Fye WB. A history of the origin, evolution, and impact of electrocardiography. Am J Cardiol. 1994. May 15; 73 13: 937- 949. [DOI] [PubMed] [Google Scholar]
- 14. Das MK, Scott LR, Miller JM. Focal mechanism of ventricular tachycardia in coronary artery disease. Heart Rhythm. 2010. March; 7 3: 305- 311. doi: 10.1016/j.hrthm.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 15. Santangeli P, Muser D, Maeda S, et al. Comparative effectiveness of antiarrhythmic drugs and catheter ablation for the prevention of recurrent ventricular tachycardia in patients with implantable cardioverter-defibrillators: a systematic review and meta-analysis of randomized controlled trials. Heart Rhythm. 2016. July; 13 7: 1552- 1559. doi: 10.1016/j.hrthm.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 16. Jalife J. Ventricular fibrillation: mechanisms of initiation and maintenance. Annu Rev Physiol. 2000; 62: 25- 50. [DOI] [PubMed] [Google Scholar]
- 17. Stevenson WG, John RM. Ventricular arrhythmias in patients with implanted defibrillators. Circulation. 2011. October 18; 124 16: e411- e414. doi: 10.1161/CIRCULATIONAHA.111.064816. [DOI] [PubMed] [Google Scholar]
- 18. Abildskov JA. Adrenergic effects on the QT interval of the electrocardiogram. Am Heart J. 1976. August; 92 2: 210- 216. [DOI] [PubMed] [Google Scholar]
- 19. Han J, Goel BG. Electrophysiologic precursors of ventricular tachyarrhythmias. Arch Intern Med. 1972. May; 129 5: 749- 755. [PubMed] [Google Scholar]
- 20. Locati E, Schwartz PJ. Prognostic value of QT interval prolongation in post myocardial infarction patients. Eur Heart J. 1987. March; 8 Suppl A: 121- 126. [DOI] [PubMed] [Google Scholar]
- 21. Pohjola-Sintonen S, Siltanen P, Haapakoski J. Usefulness of QTc interval on the discharge electrocardiogram for predicting survival after acute myocardial infarction. Am J Cardiol. 1986. May 1; 57 13: 1066- 1068. [DOI] [PubMed] [Google Scholar]
- 22. Zabel M, Klingenheben T, Franz MR, Hohnloser SH. Assessment of QT dispersion for prediction of mortality or arrhythmic events after myocardial infarction: results of a prospective, long-term follow-up study. Circulation. 1998. June 30; 97 25: 2543- 2550. [DOI] [PubMed] [Google Scholar]
- 23. Zareba W, Moss AJ, le Cessie S. Dispersion of ventricular repolarization and arrhythmic cardiac death in coronary artery disease. Am J Cardiol. 1994. September 15; 74 6: 550- 553. [DOI] [PubMed] [Google Scholar]
- 24. Hathaway WR, Peterson ED, Wagner GS, et al. Prognostic significance of the initial electrocardiogram in patients with acute myocardial infarction. GUSTO-I Investigators. Global Utilization of Streptokinase and t-PA for Occluded Coronary Arteries. JAMA. 1998. February 4; 279 5: 387- 391. [DOI] [PubMed] [Google Scholar]
- 25. Petrina M, Goodman SG, Eagle KA. The 12-lead electrocardiogram as a predictive tool of mortality after acute myocardial infarction: current status in an era of revascularization and reperfusion. Am Heart J. 2006. July; 152 1: 11- 18. [DOI] [PubMed] [Google Scholar]
- 26. Morin DP, Oikarinen L, Viitasalo M, et al. QRS duration predicts sudden cardiac death in hypertensive patients undergoing intensive medical therapy: the LIFE study. Eur Heart J. 2009. December; 30 23: 2908- 2914. doi: 10.1093/eurheartj/ehp321. [DOI] [PubMed] [Google Scholar]
- 27. Zimetbaum PJ, Buxton AE, Batsford W, et al. Electrocardiographic predictors of arrhythmic death and total mortality in the multicenter unsustained tachycardia trial. Circulation. 2004. August 17; 110 7: 766- 769. [DOI] [PubMed] [Google Scholar]
- 28. Buxton AE, Sweeney MO, Wathen MS, et al. QRS duration does not predict occurrence of ventricular tachyarrhythmias in patients with implanted cardioverter-defibrillators. J Am Coll Cardiol. 2005. July 19; 46 2: 310- 316. [DOI] [PubMed] [Google Scholar]
- 29. Flowers NC, Horan LG, Thomas JR, Tolleson WJ. The anatomic basis for high-frequency components in the electrocardiogram. Circulation. 1969. April; 39 4: 531- 539. [DOI] [PubMed] [Google Scholar]
- 30. Das MK, Saha C, El Masry H, et al. Fragmented QRS on a 12-lead ECG: a predictor of mortality and cardiac events in patients with coronary artery disease. Heart Rhythm. 2007. November; 4 11: 1385- 1392. [DOI] [PubMed] [Google Scholar]
- 31. Strauss DG, Selvester RH, Lima JA, et al. ECG quantification of myocardial scar in cardiomyopathy patients with or without conduction defects: correlation with cardiac magnetic resonance and arrhythmogenesis. Circ Arrhythm Electrophysiol. 2008. December; 1 5: 327- 336. doi: 10.1161/CIRCEP.108.798660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Strauss DG, Poole JE, Wagner GS, et al. An ECG index of myocardial scar enhances prediction of defibrillator shocks: an analysis of the Sudden Cardiac Death in Heart Failure Trial. Heart Rhythm. 2011. January; 8 1: 38- 45. doi: 10.1016/j.hrthm.2010.09.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ali A, Butt N, Sheikh AS. Early repolarization syndrome: a cause of sudden cardiac death. World J Cardiol. 2015. August 26; 7 8: 466- 475. doi: 10.4330/wjc.v7.i8.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Haïssaguerre M, Derval N, Sacher F, et al. Sudden cardiac arrest associated with early repolarization. N Engl J Med. 2008. May 8; 358 19: 2016- 2023. doi: 10.1056/NEJMoa071968. [DOI] [PubMed] [Google Scholar]
- 35. Nunn LM, Bhar-Amato J, Lowe MD, et al. Prevalence of J-point elevation in sudden arrhythmic death syndrome families. J Am Coll Cardiol. 2011. July 12; 58 3: 286- 290. doi: 10.1016/j.jacc.2011.03.028. [DOI] [PubMed] [Google Scholar]
- 36. Bigger JT, Jr, Fleiss JL, Kleiger R, Miller JP, Rolnitzky LM. The relationships among ventricular arrhythmias, left ventricular dysfunction, and mortality in the 2 years after myocardial infarction. Circulation. 1984. February; 69 2: 250- 258. [DOI] [PubMed] [Google Scholar]
- 37. Kostis JB, Byington R, Friedman LM, Goldstein S, Furberg C. Prognostic significance of ventricular ectopic activity in survivors of acute myocardial infarction. J Am Coll Cardiol. 1987. August; 10 2: 231- 242. [DOI] [PubMed] [Google Scholar]
- 38. Mäkikallio TH, Barthel P, Schneider R, et al. Prediction of sudden cardiac death after acute myocardial infarction: role of Holter monitoring in the modern treatment era. Eur Hear J. 2005. April; 26 8: 762- 769. [DOI] [PubMed] [Google Scholar]
- 39. Soliman EZ, Elsalam MA, Li Y. The relationship between high resting heart rate and ventricular arrhythmogenesis in patients referred to ambulatory 24 h electrocardiographic recording. Europace. 2010. February; 12 2: 261- 265. doi: 10.1093/europace/eup344. [DOI] [PubMed] [Google Scholar]
- 40. Zhang D, Shen X, Qi X. Resting heart rate and all-cause and cardiovascular mortality in the general population: a meta-analysis. CMAJ. 2016. February 16; 188 3: E53- E63. doi: 10.1503/cmaj.150535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Farrell TG, Bashir Y, Cripps T, et al. Risk stratification for arrhythmic events in postinfarction patients based on heart rate variability, ambulatory electrocardiographic variables and the signal-averaged electrocardiogram. J Am Coll Cardiol. 1991. September; 18 3: 687- 697. [DOI] [PubMed] [Google Scholar]
- 42. La Rovere MT, Bigger JT, Jr, Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators. Lancet. 1998. February 14; 351 9101: 478- 484. [DOI] [PubMed] [Google Scholar]
- 43. Kleiger RE, Miller JP, Bigger JT, Jr, Moss AJ. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am J Cardiol. 1987. February 1; 59 4: 256- 262. [DOI] [PubMed] [Google Scholar]
- 44. Murukesan L, Murugappan M, Iqbal M. Sudden cardiac death prediction using ECG signal derivative (heart rate variability): a review. IEEE 9th International Colloquium on Signal Processing and Its Applications. 2013: 269-274. doi: 10.1109/CSPA.2013.6530054. [Google Scholar]
- 45. Malik M, Bigger JT, Camm AJ, et al. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur Heart J. 1996. March; 17 3: 354- 381. [PubMed] [Google Scholar]
- 46. Bauer A, Malik M, Schmidt G, et al. Heart rate turbulence: standards of measurement, physiological interpretation, and clinical use: International Society for Holter and Noninvasive Electrophysiology Consensus. J Am Coll Cardiol. 2008. October 21; 52 17: 1353- 1365. doi: 10.1016/j.jacc.2008.07.041. [DOI] [PubMed] [Google Scholar]
- 47. Watanabe MA. Heart rate turbulence: a review. Indian Pacing Electrophysiol J. 2003. January 1; 3 1: 10- 22. [PMC free article] [PubMed] [Google Scholar]
- 48. Francis J, Watanabe MA, Schmidt G. Heart rate turbulence: a new predictor for risk of sudden cardiac death. Ann Noninvasive Electrocardiol. 2005. January; 10 1: 102- 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Exner DV, Kavanagh KM, Slawnych MP, et al. REFINE Investigators. Noninvasive risk assessment early after a myocardial infarction the REFINE study. J Am Coll Cardiol. 2007. December 11; 50 24: 2275- 2284. [DOI] [PubMed] [Google Scholar]
- 50. Bauer A, Barthel P, Schneider R, et al. Improved Stratification of Autonomic Regulation for risk prediction in post-infarction patients with preserved left ventricular function (ISAR-Risk). Eur Heart J. 2009. March; 30 5: 576- 583. doi: 10.1093/eurheartj/ehn540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Berkowitsch A, Zareba W, Neumann T, et al. Risk stratification using heart rate turbulence and ventricular arrhythmia in MADIT II: usefulness and limitations of a 10-minute Holter recording. Ann Noninvasive Electrocardiol. 2004. July; 9 3: 270- 279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hammill SC, Tchou PJ, Kienzle MG, Haisty WK, Ozawa Y, Underwood DA. Establishment of signal-averaged electrocardiographic criteria with Frank XYZ leads and spectral filter used alone and in combination with ejection fraction to predict inducible ventricular tachycardia in coronary artery disease. Am J Cardiol. 1992. August 1; 70 3: 316- 320. [DOI] [PubMed] [Google Scholar]
- 53. Gomes JA, Cain ME, Buxton AE, Josephson ME, Lee KL, Hafley GE. Prediction of long-term outcomes by signal-averaged electrocardiography in patients with unsustained ventricular tachycardia, coronary artery disease, and left ventricular dysfunction. Circulation. 2001. July 24; 104 4: 436- 441. [DOI] [PubMed] [Google Scholar]
- 54. Bigger JT., Jr. Prophylactic use of implanted cardiac defibrillators in patients at high risk for ventricular arrhythmias after coronary-artery bypass graft surgery. Coronary Artery Bypass Graft (CABG) Patch Trial Investigators. N Engl J Med. 1997. November 27; 337 22: 1569- 1575. [DOI] [PubMed] [Google Scholar]
- 55. Grimm M, Billhardt RA, Mayerhofer KE, Denes P. Prognostic significance of signal-averaged ECGs during acute myocardial infarction: a preliminary report. J Electrocardiol. 1988. August; 21 3: 283- 288. [DOI] [PubMed] [Google Scholar]
- 56. Hartikainen JE, Malik M, Staunton A, Poloniecki J, Camm AJ. Distinction between arrhythmic and nonarrhythmic death after acute myocardial infarction based on heart rate variability, signal-averaged electrocardiogram, ventricular arrhythmias and left ventricular ejection fraction. J Am Coll Cardiol. 1996. August; 28 2: 296- 304. [DOI] [PubMed] [Google Scholar]
- 57. Pandey AK, Das A, Singwala AK, Bhatt KN. Prediction and stratification of the future cardiovascular arrhythmic events: signal averaged electrocardiography versus ejection fraction. Indian J Physiol Pharmacol. 2010. Apr-Jun; 54 2: 123- 132. [PubMed] [Google Scholar]
- 58. Bauer A, Guzik P, Barthel P, et al. Reduced prognostic power of ventricular late potentials in post-infarction patients of the reperfusion era. Eur Heart J. 2005. April; 26 8: 755- 761. [DOI] [PubMed] [Google Scholar]
- 59. Cain M, Anderson JL, Arnsdorf MF, et al. Signal-averaged electrocardiography. J Am Coll Cardiol. 1996. January; 27 1: 238- 249. [PubMed] [Google Scholar]
- 60. McKenna WJ, Thiene G, Nava A, et al. Diagnosis of arrhythmogenic right ventricular dysplasia/cardiomyopathy. Task Force of the Working Group Myocardial and Pericardial Disease of the European Society of Cardiology and of the Scientific Council on Cardiomyopathies of the International Society and Federation of Cardiology. Br Heart J. 1994. March; 71 3: 215- 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Marcus FI, McKenna WJ, Sherrill D, et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the Task Force Criteria. Eur Heart J. 2010. April; 31 7: 806- 814. doi: 10.1093/eurheartj/ehq025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kitamura H, Ohnishi Y, Okajima K, et al. Onset heart rate of microvolt-level T-wave alternans provides clinical and prognostic value in nonischemic dilated cardiomyopathy. J Am Coll Cardiol. 2002. January 16; 39 2: 295- 300. [DOI] [PubMed] [Google Scholar]
- 63. Nearing BD, Verrier RL. Modified moving average analysis of T-wave alternans to predict ventricular fibrillation with high accuracy. J Appl Physiol (1985). 2002. February; 92 2: 541- 549. [DOI] [PubMed] [Google Scholar]
- 64. Narayan SM. T-wave alternans and the susceptibility to ventricular arrhythmias. J Am Coll Cardiol. 2006. January 17; 47 2: 269- 281. [DOI] [PubMed] [Google Scholar]
- 65. Stein PK, Sanghavi D, Sotoodehnia N, Siscovick DS, Gottdiener J. Association of Holter-based measures including T-wave alternans with risk of sudden cardiac death in the community-dwelling elderly: the Cardiovascular Health Study. J Electrocardiol. 2010. May-Jun; 43 3: 251- 259. doi: 10.1016/j.jelectrocard.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Slawnych MP, Nieminen T, Kähönen M, et al. REFINE (Risk Estimation Following Infarction Noninvasive Evaluation); FINCAVAS (Finnish Cardiovascular Study) Investigators. Post-exercise assessment of cardiac repolarization alternans in patients with coronary artery disease using the modified moving average method. J Am Coll Cardiol. 2009. March 31; 53 13: 1130- 1137. doi: 10.1016/j.jacc.2008.12.026. [DOI] [PubMed] [Google Scholar]
- 67. Bloomfield DM, Steinman RC, Namerow PB, et al. Microvolt T-wave alternans distinguishes between patients likely and patients not likely to benefit from implanted cardiac defibrillator therapy: a solution to the Multicenter Automatic Defibrillator Implantation Trial (MADIT) II conundrum. Circulation. 2004. October 5; 110 14: 1885- 1889. [DOI] [PubMed] [Google Scholar]
- 68. Chow T, Kereiakes DJ, Bartone C, et al. Prognostic utility of microvolt T-wave alternans in risk stratification of patients with ischemic cardiomyopathy. J Am Coll Cardiol. 2006. May 2; 47 9: 1820- 1827. [DOI] [PubMed] [Google Scholar]
- 69. Ikeda T, Saito H, Tanno K, et al. T-wave alternans as a predictor for sudden cardiac death after myocardial infarction. Am J Cardiol. 2002. January 1; 89 1: 79- 82. [DOI] [PubMed] [Google Scholar]
- 70. Chow T, Kereiakes DJ, Onufer J, et al. MASTER Trial Investigators. Does microvolt T-wave alternans testing predict ventricular tachyarrhythmias in patients with ischemic cardiomyopathy and prophylactic defibrillators? The MASTER (Microvolt T Wave Alternans Testing for Risk Stratification of Post-Myocardial Infarction Patients) trial. J Am Coll Cardiol. 2008. November 11; 52 20: 1607- 1615. doi: 10.1016/j.jacc.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 71. Costantini O, Hohnloser SH, Kirk MM, et al. ABCD Trial Investigators. The ABCD (Alternans Before Cardioverter Defibrillator) Trial. Strategies using T-wave alternans to improve efficiency of sudden cardiac death prevention. J Am Coll Cardiol. 2009. February 10; 53 6: 471- 479. doi: 10.1016/j.jacc.2008.08.077. [DOI] [PubMed] [Google Scholar]
- 72. Morin DP, Zacks ES, Mauer AC, et al. Effect of bundle branch block on microvolt T-wave alternans and electrophysiologic testing in patients with ischemic cardiomyopathy. Heart Rhythm. 2007. July; 4 7: 904- 912. [DOI] [PubMed] [Google Scholar]
- 73. Antzelevitch C, Sicouri S, Litovsky SH, et al. Heterogeneity within the ventricular wall. Electrophysiology and pharmacology of epicardial, endocardial, and M cells. Circ Res. 1991. December; 69 6: 1427- 1449. [DOI] [PubMed] [Google Scholar]
- 74. Opthof T, Coronel R, Janse MJ. Is there a significant transmural gradient in repolarization time in the intact heart?: repolarization gradients in the intact heart. Circ Arrhythm Electrophysiol. 2009. February; 2 1: 89- 96. doi: 10.1161/CIRCEP.108.825356. [DOI] [PubMed] [Google Scholar]
- 75. Taggart P, Sutton PM, Opthof T, et al. Transmural repolarisation in the left ventricle in humans during normoxia and ischaemia. Cardiovasc Res. 2001. June; 50 3: 454- 462. [DOI] [PubMed] [Google Scholar]
- 76. Antzelevitch C, Shimizu W, Yan GX, Sicouri S. Cellular basis for QT dispersion. J Electrocardiol. 1998: 30 Suppl: 168- 175. [DOI] [PubMed] [Google Scholar]
- 77. Yan GX, Antzelevitch C. Cellular basis for the normal T wave and the electrocardiographic manifestations of the long-QT syndrome. Circulation. 1998. November 3; 98 18: 1928- 1936. [DOI] [PubMed] [Google Scholar]
- 78. Rosenthal TM, Stahls PF., 3rd AbiSamra FM, et al. T-peak to T-end interval for prediction of ventricular tachyarrhythmia and mortality in a primary prevention population with systolic cardiomyopathy. Heart Rhythm. 2015. August; 12 8: 1789- 1797. doi: 10.1016/j.hrthm.2015.04.035. [DOI] [PubMed] [Google Scholar]
- 79. Topilski I, Rogowski O, Rosso R, et al. The morphology of the QT interval predicts torsade de pointes during acquired bradyarrhythmias. J Am Coll Cardiol. 2007. January 23; 49 3: 320- 328. [DOI] [PubMed] [Google Scholar]
- 80. Haarmark C, Hansen PR, Vedel-Larsen E, et al. The prognostic value of the Tpeak-Tend interval in patients undergoing primary percutaneous coronary intervention for ST-segment elevation myocardial infarction. J Electrocardiol. 2009. Nov-Dec; 42 6: 555- 560. doi: 10.1016/j.jelectrocard.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 81. Castro Hevia J, Antzelevitch C. TornésBárzaga F, et al. Tpeak-Tend and Tpeak-Tend dispersion as risk factors for ventricular tachycardia/ventricular fibrillation in patients with the Brugada syndrome. J Am Coll Cardiol. 2006. May 2; 47 9: 1828- 1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Letsas KP, Weber R, Astheimer K, Kalusche D, Arentz T. Tpeak-Tend interval and Tpeak-Tend/QT ratio as markers of ventricular tachycardia inducibility in subjects with Brugada ECG phenotype. Europace. 2010. February; 12 2: 271- 274. doi: 10.1093/europace/eup357. [DOI] [PubMed] [Google Scholar]
- 83. Panikkath R, Reinier K, Uy-Evanado A, et al. Prolonged Tpeak-to-Tend interval on the resting ECG is associated with increased risk of sudden cardiac death. Circ Arrhythm Electrophysiol. 2011. August; 4 4: 441- 447. doi: 10.1161/CIRCEP.110.960658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Morin DP, Saad MN, Shams OF, et al. Relationships between the T-peak to T-end interval, ventricular tachyarrhythmia, and death in left ventricular systolic dysfunction. Europace. 2012. August; 14 8: 1172- 1179. doi: 10.1093/europace/eur426. [DOI] [PubMed] [Google Scholar]
- 85. Watanabe N, Kobayashi Y, Tanno K, et al. Transmural dispersion of repolarization and ventricular tachyarrhythmias. J Electrocardiol. 2004. July; 37 3: 191- 200. [DOI] [PubMed] [Google Scholar]
- 86. Zhao X, Xie Z, Chu Y, et al. Association between Tp-e/QT ratio and prognosis in patients undergoing primary percutaneous coronary intervention for ST-segment elevation myocardial infarction. Clin Cardiol. 2012. September; 35 9: 559- 564. doi: 10.1002/clc.22022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Porthan K, Viitasalo M, Toivonen L, et al. Predictive value of electrocardiographic T-wave morphology parameters and T-wave peak to T-wave end interval for sudden cardiac death in the general population. Circ Arrhythm Electrophysiol. 2013. August; 6 4: 690- 696. doi: 10.1161/CIRCEP.113.000356. [DOI] [PubMed] [Google Scholar]
- 88. Smetana P, Schmidt A, Zabel M, et al. Assessment of repolarization heterogeneity for prediction of mortality in cardiovascular disease: peak to the end of the T wave interval and nondipolar repolarization components. J Electrocardiol. 2011. May-Jun; 44 3: 301- 308. doi: 10.1016/j.jelectrocard.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 89. Stahls PF, Rosenthal TM. AbiSamra F, et al. The effect of measurement method on the T-peak to T-end interval's ability to stratify risk for ventricular tachyarrhythmia and mortality. Heart Rhythm. 2013. May; 10 5 Suppl: S427- S428. [Google Scholar]
- 90. Rosenthal TM, Stahls PF. AbiSamra F, et al. Effect of heart rate correction on the T-peak to T-end interval's risk-stratification ability. Heart Rhythm. 2016. May; 13 5 Suppl: S420. [Google Scholar]
- 91. Behere SP, Weindling SN. Inherited arrhythmias: the cardiac channelopathies. Ann Pediatr Cardiol. 2015. Sep-Dec; 8 3: 210- 220. doi: 10.4103/0974-2069.164695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Sarquella-Brugada G, Campuzano O, Iglesias A, et al. Genetics of sudden cardiac death in children and young athletes. Cardiol Young. 2013. April; 23 2: 159- 173. doi: 10.1017/S1047951112001138. [DOI] [PubMed] [Google Scholar]
- 93. Chockalingam P, Wilde A. The multifaceted cardiac sodium channel and its clinical implications. Heart. 2012. September; 98 17: 1318- 1324. doi: 10.1136/heartjnl-2012-301784. [DOI] [PubMed] [Google Scholar]
- 94. Amin AS, Asghari-Roodsari A, Tan HL. Cardiac sodium channelopathies. Pflugers Arch. 2010. July; 460 2: 223- 237. doi: 10.1007/s00424-009-0761-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Nakano Y, Shimizu W. Genetics of long-QT syndrome. J Hum Genet. 2016. January; 61 1: 51- 55. doi: 10.1038/jhg.2015.74. [DOI] [PubMed] [Google Scholar]
- 96. Tester DJ, Ackerman MJ. The molecular autopsy: should the evaluation continue after the funeral? Pediatr Cardiol. 2012. March; 33 3: 461- 470. doi: 10.1007/s00246-012-0160-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Tracy CM, Epstein AE, Darbar D, et al. American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines; Heart Rhythm Society. 2012. ACCF/AHA/HRS focused update of the 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. [corrected] Circulation. 2012 Oct 2; 126 14: 1784- 1800. doi: 10.1161/CIR.0b013e3182618569. [DOI] [PubMed] [Google Scholar]
- 98. Averill KH, Fosmoe RJ, Lamb LE. Electrocardiographic findings in 67,375 asymptomatic subjects. IV. Wolff-Parkinson-White syndrome. Am J Cardiol. 1960. July; 6: 108- 129. [DOI] [PubMed] [Google Scholar]
- 99. Klein GJ, Bashore TM, Sellers TD, Pritchett EL, Smith WM, Gallagher JJ. Ventricular fibrillation in the Wolff-Parkinson-White syndrome. N Engl J Med. 1979. November 15; 301 20: 1080- 1085. [DOI] [PubMed] [Google Scholar]
- 100. Klein GJ, Prystowsky EN, Yee R, Sharma AD, Laupacis A. Asymptomatic Wolff-Parkinson-White. Should we intervene? Circulation. 1989. December; 80 6: 1902- 1905. [DOI] [PubMed] [Google Scholar]
- 101. Pediatric and Congenital Electrophysiology Society (PACES); Heart Rhythm Society (HRS); American College of Cardiology Foundation (ACCF); American Heart Association (AHA); American Academy of Pediatrics (AAP); Canadian Heart Rhythm Society (CHRS) , Cohen MI Triedman JK Cannon BC. et al. PACES/HRS expert consensus statement on the management of the asymptomatic young patient with a Wolff-Parkinson-White (WPW, ventricular preexcitation) electrocardiographic pattern: developed in partnership between the Pediatric and Congenital Electrophysiology Society (PACES) and the Heart Rhythm Society (HRS). Endorsed by the governing bodies of PACES, HRS, the American College of Cardiology Foundation (ACCF), the American Heart Association (AHA), the American Academy of Pediatrics (AAP), and the Canadian Heart Rhythm Society (CHRS). Heart Rhythm. 2012. June; 9 6: 1006- 1024. doi: 10.1016/j.hrthm.2012.03.050. [DOI] [PubMed] [Google Scholar]
- 102. Page RL, Joglar JA, Caldwell MA, et al. 2015. ACC/AHA/HRS Guideline for the Management of Adult Patients With Supraventricular Tachycardia: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2016 Apr 5; 67 13: e27- e115. doi: 10.1016/j.jacc.2015.08.856. [DOI] [PubMed] [Google Scholar]