Introduction
Endoscopic ultrasound (EUS) combines endoscopic visualisation of the gastrointestinal tract with high frequency ultrasound within the structure of a modified endoscope. The merging of these two technologies enables precise imaging of the layers of the gastrointestinal wall as well as accurate assessment of extraluminal structures, thereby facilitating therapeutic intervention. This review outlines the diagnostic and therapeutic applications of EUS, with comparisons to conventional techniques.
Equipment
Echoendoscopes are designed using either a radial or curvilinear array system. The format may be mechanical or electronic. The electronic echoendoscope is now more favoured as it contains no moving parts and is thus more durable. The design is essentially that of a modified gastroscope, having both optical video views as well as ultrasound capability.
Radial
Mechanical radial echoendoscopes were available commercially in the late 1980s. A rotating ultrasound transducer with a range of frequencies between 5–20 MHz 1 is situated distal to an oblique‐viewing lens at the tip of the endoscope. A water filled balloon allows for acoustic coupling (Fig. 1). The images obtained are cross‐sectional in nature, perpendicular to the endoscope shaft, akin to ‘slices’ obtained via CT scanning (Fig. 2a). Electronic radial echoendoscopes provide Doppler capabilities.
Fig. 1.

The electronic radial echoendoscope (Olympus Corporation, Tokyo, Japan).
Fig. 2a.
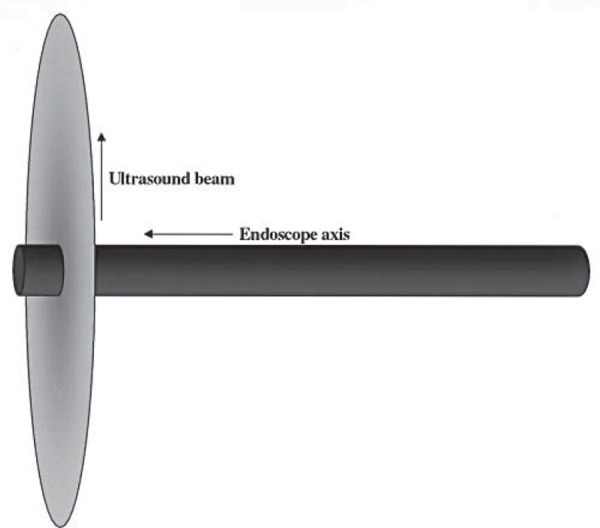
The radial echoendoscope scans at an axis perpendicular to the endoscope shaft.
Linear
The scanning plane of electronic linear echoendoscopes is oriented in the same plane as the scope shaft and accessory channel with the field of view ranging between 120° to 180°. The most important difference between radial and linear echoendoscopes is the ability to perform fine needle aspiration using the linear echoendoscope. Fine needle aspiration (FNA) cannot be performed using the radial echoendoscope because the ultrasound beam would pass through the needle at right angles and the needle would appear as a ‘dot’. With the linear echoendoscope, however, the needle passes in the same axis as the ultrasound beam, thus it is visible in its entirety as it is passed into the targeted lesion (Fig. 2b).
Fig. 2b.
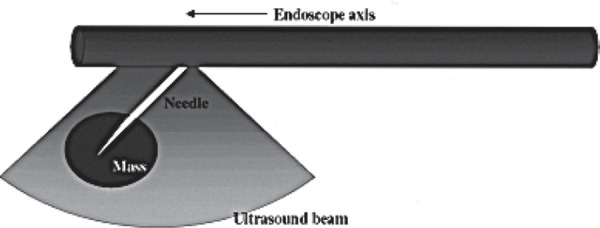
The linear echoendoscope scans in a plane parallel to the endoscope shaft. Instruments inserted through the accessory channel are visualised as they pass through the ultrasound beam.
FNA needles
FNA needles for EUS applications range in size from 25G to 19G. Larger needles may increase trauma and result in a more bloody sample but are required for therapeutic EUS procedures where guidewires must be passed through the needle interior. Needles may have beveled or ball‐tips (the latter reduces the risk of scope channel injury during inadvertent deployment) and contain stylets, which prevent obstruction of the needle with “contamination” by normal gut wall mucosa as it is advanced through this layer into the lesion. Suction may be applied to aid aspiration of tissue. An EUS nylon cytologic brush is useful in sampling pancreatic lesions, where needle aspirates are often acellular 2 .
19G Trucut biopsy needles are cutting needles that obtain core specimens, being potentially more accurate than EUS‐FNA for the evaluation of submucosal lesions and lymphomas 3 . These devices are technically demanding however, and do not function well when the echoendoscope is angulated, particularly in the second part of the duodenum 4 .
Technique
EUS procedures are performed in the same fashion as standard endoscopic examinations. The majority of cases are performed on an outpatient basis and intravenous sedation is usually employed. Procedure duration varies according to the complexity of the region being imaged and whether or not FNA is performed.
The echoendoscope is passed through the mouth until the tip reaches the target region. If the lesion of interest lies within the gut wall, water can be instilled into the gut lumen and the echoendoscope “floated” next to the lesion so that high quality images can be obtained using water as a conductive medium. Alternatively, acoustic coupling with the mucosa is achieved using a water‐filled balloon at the tip of the echoendoscope.
Extraluminal lesions are assessed using anatomical “stations”. The upper retroperitoneum (pancreatic body and tail, spleen, retroperitoneal lymph nodes, left adrenal gland and left lobe of the liver) is viewed through the gastric wall (Fig. 3). To assess the lower retroperitoneum (pancreatic head, common bile duct), the echoendoscope is positioned in the proximal duodenum (Fig. 4). Structures within the posterior mediastinum (heart, pleura, spine, vascular structures and posterior mediastinal lymph nodes) are visualised through the oesophageal wall (Fig. 5).
Fig. 3.
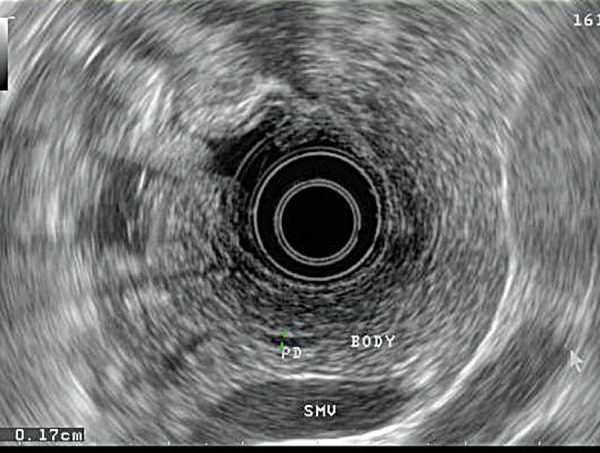
Visualisation of the pancreatic body and tail via the gastric wall (PD = pancreatic duct, BODY = pancreatic body parenchyma, SMV = superior mesenteric vein).
Fig. 4.
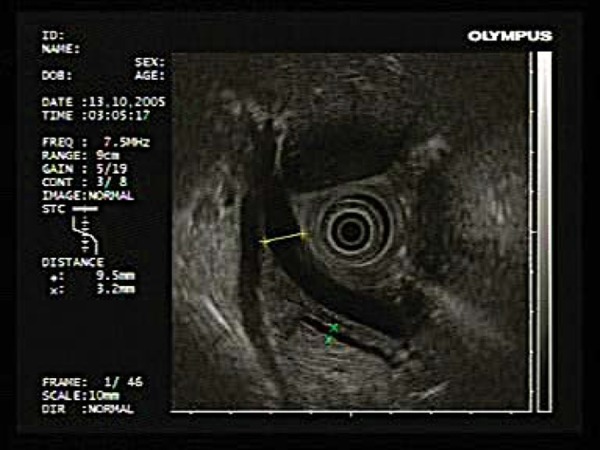
Views obtained through the duodenal cap: common bile duct closest to the transducer and pancreatic duct below this.
Fig. 5.
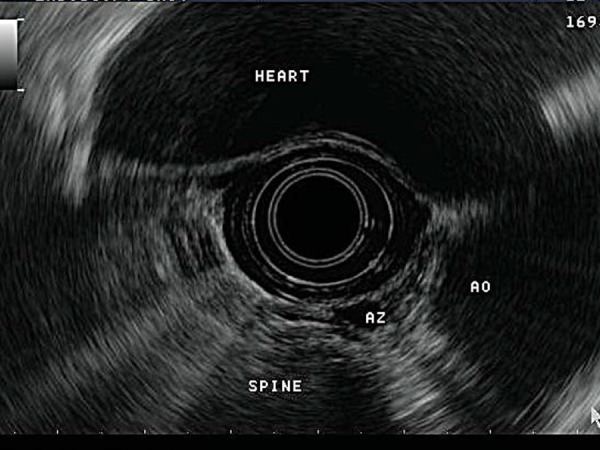
Mediastinal structures visualised via the oesophagus. (Ao = aorta, Az = azygous vein).
EUS‐FNA of mass lesions lying outside the gut wall is often performed by using the radial echoendoscope initially to identify the lesion and then using the linear echoendoscope to execute the actual biopsy. Colour Doppler enables the recognition of blood flow within vascular structures to ensure that no blood vessels lie between the needle and the targeted lesion. The needle is passed through the gut wall into the target lesion under real‐time ultrasound guidance (Fig. 6). The internal stylet is removed and the needle is passed back and forth with suction applied via a syringe. After withdrawal of the needle, the aspirated contents are expressed onto a slide or transport medium for cell block or flow cytometry. The presence of an on‐site cytopathologist to give instant feedback regarding specimen quality improves diagnostic certainty 5 .
Fig. 6.
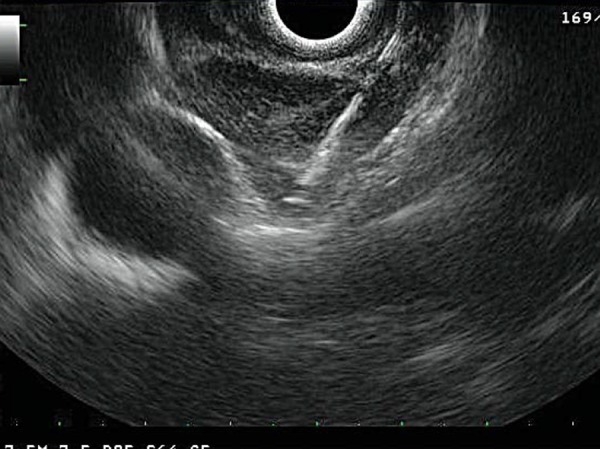
Fine needle aspiration of a mediastinal lymph node.
Complications
As most echoendoscopes are oblique viewing and have a longer, more rigid tip than conventional endoscopes, passage of the instrument through the oropharynx should be made with due care. Despite this, the incidence of perforation does not appear to be more frequent than during standard endoscopy 6 . Most complications with EUS occur during therapeutic applications where the overall complication rate for EUS‐FNA is between 1–2% 7 and include infection (particularly for EUS‐FNA of pancreatic cystic lesions), haemorrhage, pancreatitis and duodenal perforation. Infectious complications following EUS‐FNA of solid lesions or lymph nodes are rare and prophylactic antibiotics are not recommended 8 .
EUS training
The Australian Conjoint Committee for the Recognition of Training in Gastrointestinal Endoscopy requires that candidates complete a minimum of 200 EUS examinations unassisted under supervision including 100 examinations for gastro‐oesophageal lesions/tumours and 100 examinations for pancreatico‐biliary investigations. A minimum of 50 FNA examinations (25 or more of which must be pancreatico‐biliary) must be performed unassisted under supervision 9 .
Indications
Diagnostic/staging
EUS has an established role in a wide variety of applications, including the assessment and staging of malignancy, evaluation of submucosal abnormalities, mediastinal lymphadenopathy and pancreatico‐biliary disease. EUS‐FNA compares favourably to US or CT guided percutaneous biopsy techniques particularly for smaller lesions 10 .
Malignancy
The American Joint Committee on Cancer stages luminal GI malignancies according to the TNM classification 11 . A grade is given to depth of invasion (T), presence of locoregional lymph nodes (N) and presence of distant metastases (M). EUS is most beneficial in locoregional T and N staging, providing an accuracy of approximately 85% in GI luminal cancers 12 .
In the assessment of cancers arising from within the gastrointestinal tract wall, EUS is of benefit as it can depict the five histologic layers of the gut wall in fine detail (Fig. 7), permitting accurate T staging. Extraluminal tumours that lie in close proximity to the gut lumen, such as pancreatic tumours, can also be staged with regards to invasion into nearby vasculature and other adjacent structures. EUS also allows visualisation of regional lymph nodes that lie adjacent to tumours and FNA can be performed where appropriate. During lymph node staging, the biopsy needle should not traverse the primary tumour as this may lead to false positive results. The impact of EUS‐FNA is significant in that it changes the management of patients with gastro‐intestinal, pancreatic and pulmonary malignancy, often resulting in the avoidance of unnecessary surgery 13 – 15 .
Fig. 7.
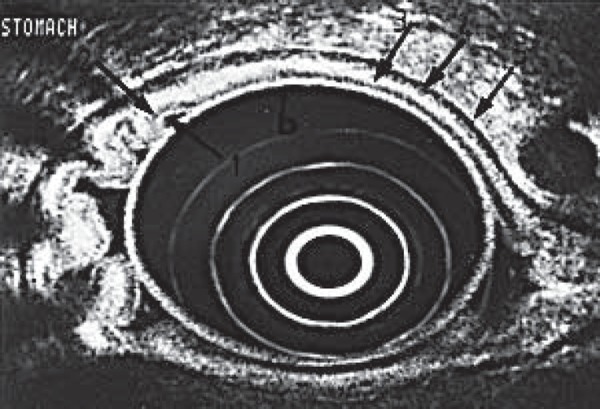
The five layers of the gastric wall at EUS, from inner to outer: innermost two layers (white and black) = mucosa, third layer (white) = submucosa, fourth layer (black) = muscularis propria, fifth layer (white) = serosa.
Oesophageal cancer
EUS has been demonstrated to have higher sensitivity in detecting oesophageal cancer when compared to CT and positron emission tomography (PET) with 18F‐Fluorodeoxyglucose 16 (Fig. 8) and represents an important complementary test in this setting. Using EUS with high frequency miniprobes, patients identified to have tumour limited to the lamina propria of the mucosal layer are unlikely to have lymph node involvement and may be candidates for endoscopic mucosal resection rather than oesophagectomy 17 . The accuracy of EUS‐FNA in detecting involvement of locoregional lymph nodes is >85% when compared to surgical specimens 18 . Most importantly, the presence of coeliac axis lymphadenopathy represents metastatic disease and EUS has been demonstrated to have superior sensitivity to CT and PET scanning in this setting 16 .
Fig. 8.
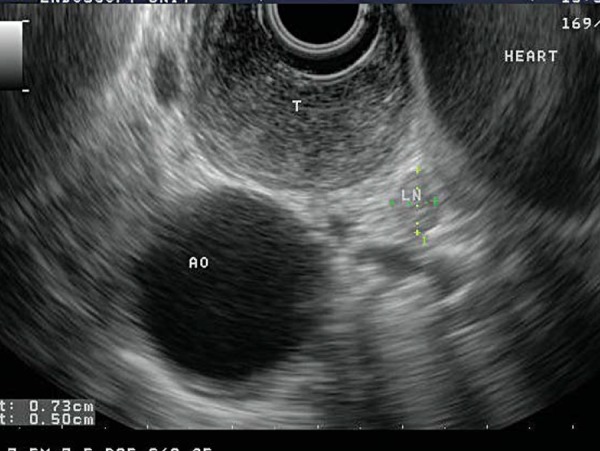
Staging of oesophageal cancer: the hypoechoic expansion of the oesophageal wall with tumour (T) does not invade the muscularis propria (black layer), hence is staged at T2. There is a regional lymph node (LN) measuring 0.73 × 0.5 cm that is round and hypoechoic, suggestive of malignant involvement. The aorta (AO) and heart can be seen adjacent.
In patients with Barrett's oesophagus and high‐grade dysplasia, the role of EUS for the detection of occult cancer and malignant lymphadenopathy is yet to be clearly defined 19 .
Gastric cancer
EUS is beneficial in the assessment of early gastric cancer. Those patients without submucosal invasion (T1) can be considered for endoscopic mucosal resection rather than gastrectomy. For established gastric cancer, EUS is superior to CT in assessing locoregional stage 20 , although three dimensional multidetector row CT techniques have improved accuracy 21 . In patients with gastric lymphoma, EUS is particularly accurate in assessing T stage 22 . EUS‐FNA is valuable for lymph node sampling and biopsying the gastric wall when the EUS appearance is abnormal but mucosal biopsies are non‐diagnostic.
Rectal cancer
In patients with rectal cancer, neoadjuvant and adjuvant chemoradiation therapy is indicated for advanced locoregional disease. EUS is between 80–95% accurate and is superior to CT for T staging 23 . Staging using MRI with rectal coils has similar efficacy to EUS, except in the differentiation between T1 (invading submucosa) and T2 tumours (invading muscularis propria), where EUS may be superior 24 . Accuracy of EUS is reduced following radiotherapy, due to the presence of inflammation and fibrosis 25 . EUS accuracy in N staging is similar to CT and MRI as benign inflammatory lymphadenopathy may accompany rectal cancer 26 .
Pancreatic cancer
The sensitivity of EUS for the detection of a pancreatic mass was 96% when results from 22 studies were combined 27 . However, when benign lesions and ampullary tumours were excluded, sensitivity decreased. Comparisons with helical CT, angiography, MRI and PET suggest that EUS is more sensitive for the detection of tumours and vascular invasion (Fig. 9) but that CT, MRI and PET are complementary for the determination of resectability of the tumour 28 – 31 . The sensitivity and specificity of EUS‐FNA for the diagnosis of pancreatic tumours is 85% and 98% respectively 27 . EUS‐FNA of pancreatic cancer has gained favour due to the risk of needle track seeding with percutaneous biopsies 32 . The tissue planes that are passed when performing transduodenal EUS‐FNA for a pancreatic head cancer will be resected in any subsequent surgery, thus the risk of seeding via this technique is inconsequential.
Fig. 9.
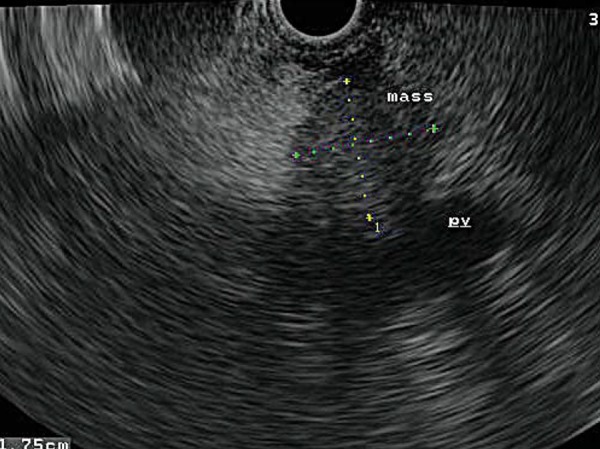
A 1.75 × 1.78 cm tumour (‘mass’) in the head of pancreas impinges upon, but does not invade, the portal vein (‘pv’).
In the localisation of pancreatic neuroendocrine tumours, which are often <1 cm, EUS is superior to CT, MRI and somatostatin receptor scintigraphy 33 (Fig. 10). EUS is also superior to CT, MRI and transabdominal US for the staging of periampullary carcinomas 34 .
Fig. 10.
A neuroendocrine tumour in the body of the pancreas, FNA being performed. Note the sharp bordered, small, rounded configuration with a combination of cystic and solid elements – this is typical of the EUS appearance of neuroendocrine tumours.
Pancreatic cystic tumours may be benign, malignant or have malignant potential and differentiation between these types using conventional imaging is difficult. EUS can be considered complementary for distinguishing such lesions 35 , 36 (Fig. 11) although one study found little interobserver agreement 37 . EUS‐FNA sampling of cystic fluid (Fig. 12) distinguishes mucinous from non‐mucinous cysts by measurement of cyst fluid CEA levels with high specificity but does not predict malignancy 38 .
Fig. 11.
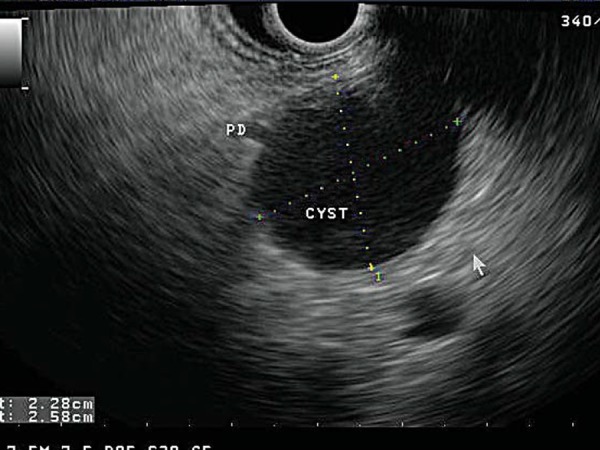
A unilocular cystic lesion (‘CYST’) measuring 2.28 × 2.58 cm. Note that it communicates with the main pancreatic duct (‘PD’), suggesting a diagnosis of intraductal papillary mucinous tumour.
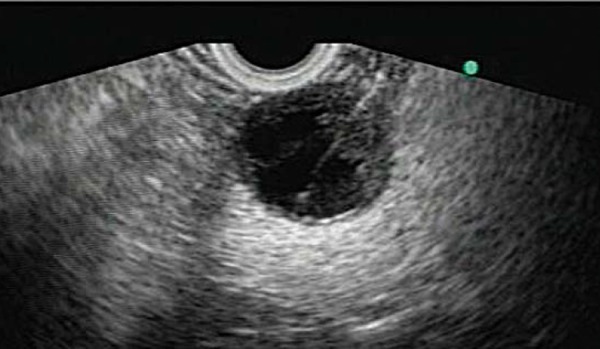
Fig. 12.
The clear, non‐viscous fluid aspirated at EUS from a serous cystadenoma.
Submucosal GIT lesions
Submucosal lesions are often encountered during routine endoscopy, mucosal biopsies of which are often non‐diagnostic. EUS determines the layer of origin and detects characteristic appearances of cysts, lipomas, leiomyomas and gastrointestinal stromal tumours (Figs. 13,14). EUS‐FNA of such lesions has a diagnostic yield of up to 91% 44 .
Fig. 13.
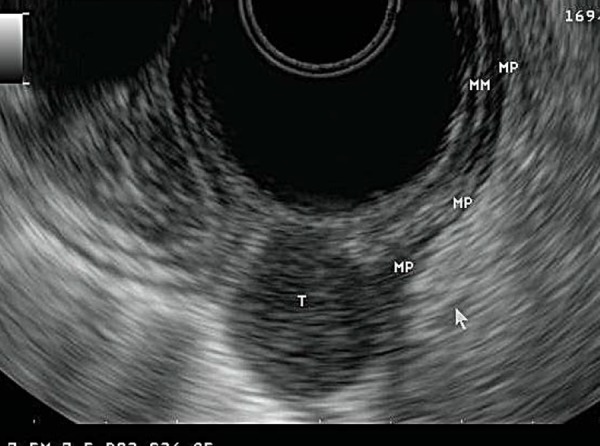
A gastrointestinal stromal tumour (GIST). It has a characteristic hypoechoic appearance (T) and arises from the fourth layer of the gastric wall, the muscularis propria (MP).
Fig. 14.
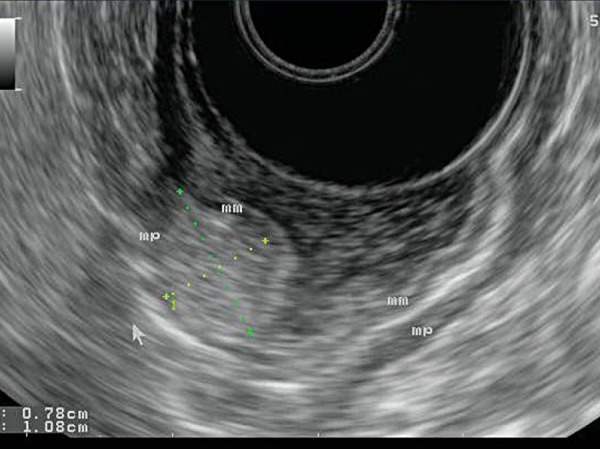
A lipoma arising from within the gastric wall. Endoscopically, this would look the same as the GIST seen in Fig. 13. However, EUS allows differentiation between the two: the lipoma is brightly hyperechoic.
Lung cancer
EUS‐FNA is >90% accurate in nodal staging of non small cell lung cancer (NSCLC) 39 , being more sensitive than CT 40 , 41 and more specific than PET 40 – 42 . This investigation also has efficacy in assessing tumour stage, biopsy of tumour adjacent to the oesophagus and assessment of metastatic disease in the left liver lobe and left adrenal gland. EUS‐FNA reduces the need for mediastinoscopy/thoracotomy by up to 50% 43 and should be considered the first line investigation for tissue sampling of nodes in the posterior mediastinum (Fig. 15).
Fig. 15.
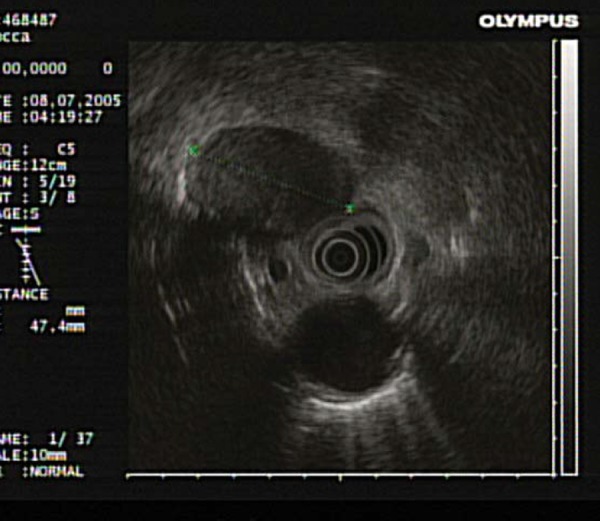
A large malignant lymph node seen at the 11 o'clock position within the mediastinum of a patient with a known primary lung carcinoma.
Mediastinal lymphadenopathy of uncertain aetiology
Posterior mediastinal masses (aortopulmonary window, subcarinal and perioesophageal stations) are usually initially detected on CT and have a wide differential, including infective and granulomatous disease, lymphoma, primary pulmonary and metastatic malignancy. These masses in the posterior mediastinum are readily amenable to EUS‐FNA via a transoesophageal route. EUS visualisation of anterior mediastinal lymphadenopathy is disrupted by air interference from the trachea; these groups are better assessed with endobronchial ultrasound.
When all four features: abnormal size >1cm, hypoechoic appearance, round shape and distinct margin are present, there is a high specificity, but low sensitivity for malignant infiltration 45 , 46 . EUS‐FNA provides a specimen sufficient for interpretation in over 95% of cases 47 with a sensitivity of 96% for the detection of nodal malignancy in patients with known malignant disease 48 . The American Society for Gastrointestinal Endoscopy recommends that FNA be performed during EUS evaluation of mediastinal lymphadenopathy, when the result will alter management 49 .
Benign pancreatic disease
The diagnosis of chronic pancreatitis is often difficult to establish on conventional imaging with CT, abdominal US and ERCP. EUS can be used to detect characteristic alterations of the pancreatic parenchyma and duct although there exists strong operator dependence. Hence, the role of EUS is complementary to other modalities in this setting. Autoimmune pancreatitis has characteristic EUS appearances and use of EUS‐FNA increases the diagnostic yield 50 .
Biliary stones
EUS has the advantage of visualising the biliary tree from within the duodenum without interference from abdominal gas or fat (Fig. 16). When compared to ERCP or intraoperative cholangiogram findings in a population of patients clinically suspected to have choledocholithiasis, EUS has a sensitivity between 89–94% and a specificity of 94% 51 , 52 . A meta‐analysis of five randomised, prospective, blinded trials comparing EUS and MRCP found them to be of comparable sensitivity and specificity 53 . A cost‐benefit analysis found EUS to be of greatest value in the setting of intermediate (11%–55%) risk for choledocholithiasis. However, ERCP remains preferable for patients whose pre‐test probability is high (>55% risk) because therapeutic intervention can be performed simultaneously 54 .
Fig. 16.
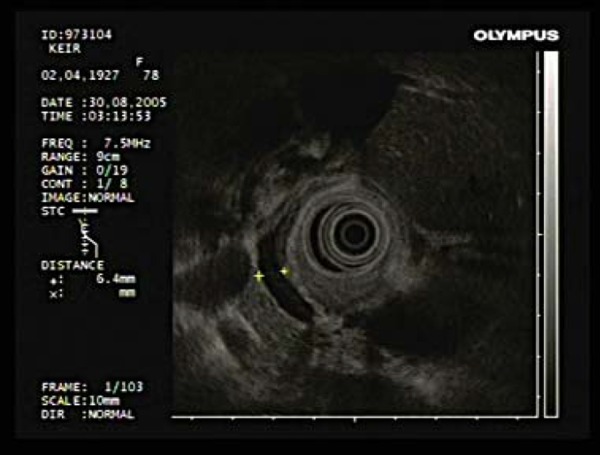
The CBD can be viewed in its entirety from the duodenal cap.
Perianal disease
Rectal EUS has been found to be effective in the assessment of perianal diseases. In the assessment of perianal Crohn's disease, EUS has a 91% accuracy, comparable and complementary to MRI and evaluation under general anaesthetic 55 . EUS also has >90% sensitivity for the detection of anal sphincter defects in faecal incontinence 56 , 57 however studies comparing EUS with MRI are conflicting 58 , 59 .
Therapeutic EUS
The echoendoscope can be sited adjacent to extraluminal structures, facilitating therapeutic injection and drainage procedures with great accuracy.
EUS‐guided injection therapy
Coeliac plexus block is achieved by the injection of bupivacaine and neurolysis with the injection of absolute alcohol. A linear echoendoscope is directed towards the coeliac ganglia at the origin of the coeliac trunk with a 22–19G FNA needle. Durable analgesia is obtained in up to 91% of patients with pancreatic cancer 60 , 61 . Complications are rare (1% 62 ) and the EUS technique is safer than the CT guided percutaneous approach 63 . The procedure is less efficacious in chronic pancreatitis however; Gress, et al. demonstrated that neurolysis with corticosteroids (triamcinolone) reduced pain scores beyond 12 weeks in 26% of subjects 64 .
The poor prognosis of pancreatic malignancy has prompted the use of EUS for intratumoural injection of chemotherapeutic agents 65 . EUS guided fine needle injection (FNI) of adenoviral vectors targeting tumour cells has been described 66 , 67 . Animal studies have assessed the efficacy of paclitaxel injection via EUS‐FNI into the porcine pancreas 68 . This modality of treatment remains in its experimental phases, however.
Attempts have been made at ablating the epithelial lining of cystic tumours of the pancreas with ethanol lavage via EUS‐FNI but with limited success 69 . A preliminary study found that the combination of ethanol lavage and paclitaxel injection was safe and effective 70 .
EUS‐guided drainage procedures
Surgical and percutaneous approaches to pancreatic pseudocyst drainage are associated with significant morbidity and mortality 71 . EUS‐guided transmural drainage of pancreatic pseudocysts is minimally invasive and does not result in problems such as cutaneous fistulae. It is performed by transgastric/transduodenal puncture under EUS guidance, followed by the insertion of double pigtail stents through the gut wall to create a cystogastrostomy/cystoenterostomy (Figs. 17,18). The procedure was successful in 94% of cases with no mortality in one series of 51 patients 72 .
Fig. 17.
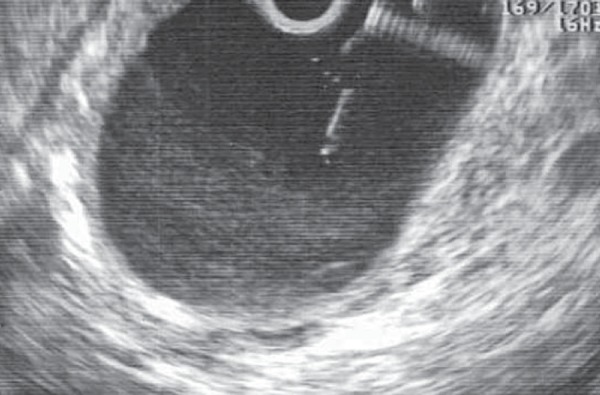
A pancreatic pseudocyst. The FNA needle is seen passing from the gastric wall into the cyst under EUS guidance. Fluid can be aspirated and sent for culture. The tract between the stomach and cyst is then dilated and stents inserted.
Fig. 18.
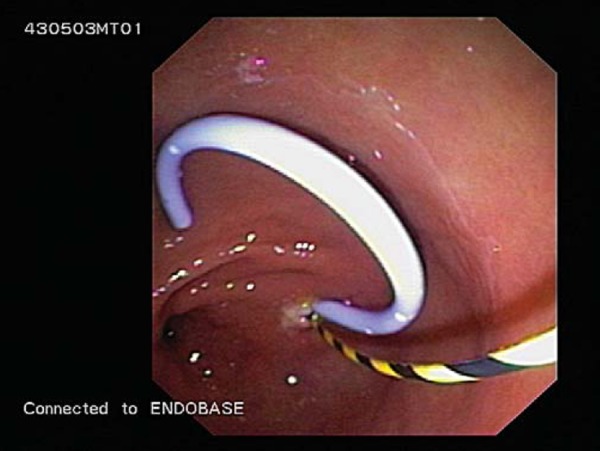
The insertion of double pigtail stents over guidewires from the stomach into the pseudocyst to allow drainage of the cyst into the stomach.
Similar techniques have been reported for the drainage of mediastinal 73 , hepatic 74 , splenic 75 , subphrenic 76 and pelvic 77 abscesses. EUS‐guided transmural cholecystenterostomy has been described in patients at high risk for surgical intervention 78 , 79 .
EUS‐guided cholangio‐pancreatic drainage following failed ERCP involves EUS‐guided puncture of a dilated pancreatic or biliary system, passage of a guidewire and insertion of a trans‐duodenal or trans‐gastric stent 80 and can obviate the need for percutaneous transhepatic drainage in suitable patients.
Conclusion
EUS facilitates the diagnosis of GI luminal and extraluminal masses and allows staging of a variety of malignancies, resulting in the avoidance of unnecessary surgery. Such indications, in combination with an expanding number of therapeutic applications, have established the role of EUS as a safe, accurate and cost‐effective tool.
References
- 1. Meenan J and Vu C. Basics of EUS: Equipment. In: Hawes R, and Fockens P. editors. Endosonography. Philadelphia: Saunders; 2006. pp. 17–26. [Google Scholar]
- 2. Al‐Haddad M, Raimondo M, Woodward T, Al‐Haddad M, Raimondo M, Woodward T, et al. Safety and efficacy of cytology brushings versus standard FNA in evaluating cystic lesions of the pancreas: a pilot study. Gastrointest Endosc 2007; 65 (6): 894–8. [DOI] [PubMed] [Google Scholar]
- 3. Levy MJ, Wiersema M. Comparison of endoscopic ultrasound‐guided trucut biopsy (EUS‐TCB) to endoscopic ultrasound‐guided fine needle aspiration (EUS‐FNA) (abstract W1228). Gastrointest Endosc 2003; 57: AB244. [Google Scholar]
- 4. Jones DB. Role of endoscopic ultrasound in staging upper gastrointestinal cancers. A NZ J Surg 2007; 77 (3): 166–72. [DOI] [PubMed] [Google Scholar]
- 5. Klapman JB, Logrono R, Dye CE, Waxman I. Clinical impact of on‐site cytopathology interpretation on endoscopic ultrasound‐guided fine needle aspiration. Am J Gastroenterol 2003; 98 (6): 1289–94. [DOI] [PubMed] [Google Scholar]
- 6. Adler DG, Jacobson BC, Davila RE, Hirota WK, Leighton JA, Qureshi WA, et al. ASGE guideline: complications of EUS. Gastrointest Endosc 2005; 61 (1): 8–12. [DOI] [PubMed] [Google Scholar]
- 7. Yamao K, Bhatia V, Mizuno N, Sawaki A, Shimizu Y, Irisawa A. Interventional endoscopic ultrasonography. J Gastroenterol Hepatol 2009. [Epub ahead of print]. [DOI] [PubMed]
- 8. Hirota WK, Petersen K, Baron TH, Goldstein JL, Jacobson BC, Leighton JA, et al. Guidelines for antibiotic prophylaxis for GI endoscopy. Gastrointest Endosc 2003; 58 (4): 475–82. [DOI] [PubMed] [Google Scholar]
- 9. Conjoint Committee for the Recognition of Training in Gastrointestinal Endoscopy . Procedural Requirements 2009. Available online at:www.conjoint.org.au/information.html#procedural [verified April 2009].
- 10. Erickson RA. EUS‐guided FNA. Gastrointest Endosc 2004; 60 (2): 267–79. [DOI] [PubMed] [Google Scholar]
- 11. American Joint Committee on Cancer , AJCC Cancer Staging Manual 6th ed. New York: Springer; 2002. [Google Scholar]
- 12. Grimm H, Binmoeller KF, Hamper K, Koch J, Henne‐Bruns D, Soehendra N. Endosonography for preoperative locoregional staging of esophageal and gastric cancer. Endoscopy 1993; 25 (3): 224–30. [DOI] [PubMed] [Google Scholar]
- 13. Mortensen MB, Pless T, Durup J, Ainsworth AP, Plagborg GJ, Hovendal C. Clinical impact of endoscopic ultrasound‐guided fine needle aspiration biopsy in patients with upper gastrointestinal tract malignancies. A prospective study. Endoscopy 2001; 33 (6): 478–83. [DOI] [PubMed] [Google Scholar]
- 14. LeBlanc JK, Devereaux BM, Imperiale TF, Kesler K, DeWitt JM, Cummings O, et al. Endoscopic ultrasound in non‐small cell lung cancer and negative mediastinum on computed tomography. Am J Respir Crit Care Med 2005; 171 (2): 177–82. [DOI] [PubMed] [Google Scholar]
- 15. Wallace MB, Ravenel J, Block MI, Fraig M, Silvestri G, Wildi S, et al. Endoscopic ultrasound in lung cancer patients with a normal mediastinum on computed tomography. Ann Thorac Surg 2004; 77 (5): 1763–8. [DOI] [PubMed] [Google Scholar]
- 16. van Vliet EP, Heijenbrok‐Kal MH, Hunink MG, Kuipers EJ, Siersema PD. Staging investigations for oesophageal cancer: a meta‐analysis. Br J Cancer 2008; 98 (3): 547–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Norton ID, Jones DB. Endoscopic ultrasound: diagnostic and therapeutic applications. Intern Med J 2003; 33 (1–2): 26–32. [DOI] [PubMed] [Google Scholar]
- 18. Wiersema MJ, Vilmann P, Giovannini M, Chang KJ, Wiersema LM. Endosonography‐guided fine‐needle aspiration biopsy: diagnostic accuracy and complication assessment. Gastroenterology 1997; 112 (4): 1087–95. [DOI] [PubMed] [Google Scholar]
- 19. ASGE Standards of Practice Committee , Gan SI, Rajan E, Adler DG, Baron TH, Anderson MA, Cash BD, et al. Role of EUS. Gastrointest Endosc 2007; 66 (3): 425–34. [DOI] [PubMed] [Google Scholar]
- 20. Botet JF, Lightdale CJ, Zauber AG, Gerdes H, Winawer SJ, Urmacher C, Brennan MF. Preoperative staging of gastric cancer: comparison of endoscopic US and dynamic CT. Radiology 1991; 181 (2): 426–32. [DOI] [PubMed] [Google Scholar]
- 21. Bhandari S, Shim CS, Kim JH, Jung IS, Cho JY, Lee JS, et al. Usefulness of three‐dimensional, multidetector row CT (virtual gastroscopy and multiplanar reconstruction) in the evaluation of gastric cancer: a comparison with conventional endoscopy, EUS, and histopathology. Gastrointest Endosc 2004; 59 (6): 619–26. [DOI] [PubMed] [Google Scholar]
- 22. Palazzo L, Roseau G, Ruskone‐Fourmestraux A, Rougier P, Chaussade S, Rambaud JC, et al. Endoscopic ultrasonography in the local staging of primary gastric lymphoma. Endoscopy 1993; 25 (8): 502–8. [DOI] [PubMed] [Google Scholar]
- 23. Davila RE, Rajan E, Adler D, Hirota WK, Jacobson BC, Leighton JA, et al. ASGE guideline: the role of endoscopy in the diagnosis, staging, and management of colorectal cancer. Gastrointest Endosc 2005; 61 (1): 1–7. [DOI] [PubMed] [Google Scholar]
- 24. Meyenberger C, Boni RA Huch, Bertschinger P, Zala GF, Klotz HP, Krestin GP. Endoscopic ultrasound and endorectal magnetic resonance imaging: a prospective, comparative study for preoperative staging and follow‐up of rectal cancer. Endoscopy 1995; 27 (7): 469–79. [DOI] [PubMed] [Google Scholar]
- 25. Oh YS, Early DS, Azar RR. Clinical applications of endoscopic ultrasound to oncology. Oncology 2005; 68 (4–6): 526–37. [DOI] [PubMed] [Google Scholar]
- 26. Kwok H, Bissett IP, Hill GL. Preoperative staging of rectal cancer. Int J Colorectal Dis 2000; 15 (1): 9–20. [DOI] [PubMed] [Google Scholar]
- 27. DeWitt J. EUS in Pancreatic Neoplasms. In, Hawes R. and Fockens P, editors. Endosonography. Philadelphia: Saunders; 2006. pp. 177–204. [Google Scholar]
- 28. Hunt GC and Faigel DO. Assessment of EUS for diagnosing, staging, and determining resectability of pancreatic cancer: a review. Gastrointest Endosc 2002; 55 (2): 232–7. [DOI] [PubMed] [Google Scholar]
- 29. Brugge WR, Lee MJ, Kelsey PB, Schapiro RH, Warshaw AL. The use of EUS to diagnose malignant portal venous system invasion by pancreatic cancer. Gastrointest Endosc 1996; 43 (6): 561–7. [DOI] [PubMed] [Google Scholar]
- 30. Ahmad NA, Lewis JD, Siegelman ES, Rosato EF, Ginsberg GG, Kochman ML. Role of endoscopic ultrasound and magnetic resonance imaging in the preoperative staging of pancreatic adenocarcinoma. Am J Gastroenterol 2000; 95 (8): 1926–31. [DOI] [PubMed] [Google Scholar]
- 31. Mertz HR, Sechopoulos P, Delbeke D, Leach SD. EUS, PET, and CT scanning for evaluation of pancreatic adenocarcinoma. Gastrointest Endosc 2000; 52 (3): 367–71. [DOI] [PubMed] [Google Scholar]
- 32. Caturelli E, Rapaccini GL, Anti M, Fabiano A, Fedeli G. Malignant seeding after fine‐needle aspiration biopsy of the pancreas. Diagn Imaging Clin Med 1985; 54 (2): 88–91. [PubMed] [Google Scholar]
- 33. Zimmer T, Ziegler K, Bäder M, Fett U, Hamm B, Riecken EO, Wiedenmann B. Localisation of neuroendocrine tumours of the upper gastrointestinal tract. Gut 1994; 35 (4): 471–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cannon ME, Carpenter SL, Elta GH, Nostrant TT, Kochman ML, Ginsberg GG, et al. EUS compared with CT, magnetic resonance imaging, and angiography and the influence of biliary stenting on staging accuracy of ampullary neoplasms. Gastrointest Endosc 1999; 50 (1): 27–33. [DOI] [PubMed] [Google Scholar]
- 35. Ahmad NA, Kochman ML, Lewis JD, Ginsberg GG. Can EUS alone differentiate between malignant and benign cystic lesions of the pancreas? Am J Gastroenterol 2001; 96 (12): 3295–300. [DOI] [PubMed] [Google Scholar]
- 36. Sedlack R, Affi A, Vazquez‐Sequeiros E, Norton ID, Clain JE, Wiersema MJ. Utility of EUS in the evaluation of cystic pancreatic lesions. Gastrointest Endosc 2002; 56 (4): 543–7. [DOI] [PubMed] [Google Scholar]
- 37. Ahmad NA, Kochman ML, Brensinger C, Brugge WR, Faigel DO, Gress FG, et al. Interobserver agreement among endosonographers for the diagnosis of neoplastic versus non‐neoplastic pancreatic cystic lesions. Gastrointest Endosc 2003; 58 (1): 59–64. [DOI] [PubMed] [Google Scholar]
- 38. Khalid A, Brugge W. ACG practice guidelines for the diagnosis and management of neoplastic pancreatic cysts. Am J Gastroenterol 2007; 102 (10): 2339–49. [DOI] [PubMed] [Google Scholar]
- 39. Eloubeidi MA, Cerfolio RJ, Chen VK, Desmond R, Syed S, Ojha B. Endoscopic ultrasound‐guided fine needle aspiration of mediastinal lymph node in patients with suspected lung cancer after positron emission tomography and computed tomography scans. Ann Thorac Surg 2005; 79 (1): 263–8. [DOI] [PubMed] [Google Scholar]
- 40. Toloza EM, Harpole L, McCrory DC. Noninvasive staging of non‐small cell lung cancer: a review of the current evidence. Chest 2003; 123 (1 Suppl): 137S–146S. [DOI] [PubMed] [Google Scholar]
- 41. Toloza EM, Harpole L, Detterbeck F, McCrory DC. Invasive staging of non‐small cell lung cancer: a review of the current evidence. Chest 2003; 123 (1 Suppl): 157S–166S. [DOI] [PubMed] [Google Scholar]
- 42. Fritscher‐Ravens A, Davidson BL, Hauber HP, Bohuslavizki KH, Bobrowski C, Lund C, et al. Endoscopic ultrasound, positron emission tomography, and computerized tomography for lung cancer. Am J Respir Crit Care Med 2003; 168 (11): 1293–7. [DOI] [PubMed] [Google Scholar]
- 43. Larsen SS, Krasnik M, Vilmann P, Jacobsen GK, Pedersen JH, Faurschou P, Folke K. Endoscopic ultrasound guided biopsy of mediastinal lesions has a major impact on patient management. Thorax 2002; 57 (2): 98–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ando N, Goto H, Niwa Y, Hirooka Y, Ohmiya N, Nagasaka T, Hayakawa T. The diagnosis of GI stromal tumors with EUS‐guided fine needle aspiration with immunohistochemical analysis. Gastrointest Endosc 2002; 55 (1): 37–43. [DOI] [PubMed] [Google Scholar]
- 45. Catalano MF, Sivak MV Jr., Rice T, Gragg LA, Van Dam J. Endosonographic features predictive of lymph node metastasis. Gastrointest Endosc 1994; 40 (4): 442–6. [DOI] [PubMed] [Google Scholar]
- 46. Bhutani, MS , Hawes RH, Hoffman BJ, A comparison of the accuracy of echo features during endoscopic ultrasound (EUS) and EUS‐guided fine‐needle aspiration for diagnosis of malignant lymph node invasion. Gastrointest Endosc 1997; 45 (6): 474–9. [DOI] [PubMed] [Google Scholar]
- 47. Fritscher‐Ravens A, Sriram PV, Bobrowski C, Pforte A, Topalidis T, Krause C. Mediastinal lymphadenopathy in patients with or without previous malignancy: EUS‐FNA‐based differential cytodiagnosis in 153 patients. Am J Gastroenterol 2000; 95 (9): 2278–84. [DOI] [PubMed] [Google Scholar]
- 48. Wiersema MJ, Vazquez‐Sequeiros E, Wiersema LM. Evaluation of mediastinal lymphadenopathy with endoscopic US‐guided fine‐needle aspiration biopsy. Radiology 2001; 219 (1): 252–7. [DOI] [PubMed] [Google Scholar]
- 49. Jacobson BC, Hirota WK, Goldstein JL, Leighton JA, Mallery JS, Waring JP, et al. The role of EUS for evaluation of mediastinal adenopathy. Gastrointest Endosc 2003; 58 (6): 819–21. [DOI] [PubMed] [Google Scholar]
- 50. Farrell JJ, Garber J, Sahani D, Brugge WR. EUS findings in patients with autoimmune pancreatitis. Gastrointest Endosc 2004; 60 (6): 927–36. [DOI] [PubMed] [Google Scholar]
- 51. Garrow D, Miller S, Sinha D, Conway J, Hoffman BJ, Hawes RH, Romagnuolo J. Endoscopic ultrasound: a meta‐analysis of test performance in suspected biliary obstruction. Clin Gastroenterol Hepatol 2007; 5 (5): 616–23. [DOI] [PubMed] [Google Scholar]
- 52. Tse F, Liu L, Barkun AN, Armstrong D, Moayyedi P. EUS: a meta‐analysis of test performance in suspected choledocholithiasis. Gastrointest Endosc 2008; 67 (2): 235–44. [DOI] [PubMed] [Google Scholar]
- 53. Verma D, Kapadia A, Eisen GM, Adler DG. EUS vs MRCP for detection of choledocholithiasis. Gastrointest Endosc 2006; 64 (2): 248–54. [DOI] [PubMed] [Google Scholar]
- 54. Sahai AV, Mauldin PD, Marsi V, Hawes RH, Hoffman BJ. Bile duct stones and laparoscopic cholecystectomy: a decision analysis to assess the roles of intraoperative cholangiography, EUS, and ERCP. Gastrointest Endosc 1999; 49 (3 Pt 1): 334–43. [DOI] [PubMed] [Google Scholar]
- 55. Schwartz DA, Wiersema MJ, Dudiak KM, Fletcher JG, Clain JE, Tremaine WJ, et al. A comparison of endoscopic ultrasound, magnetic resonance imaging, and exam under anesthesia for evaluation of Crohn's perianal fistulas. Gastroenterology 2001; 121 (5): 1064–72. [DOI] [PubMed] [Google Scholar]
- 56. Schäfer R, Heyer T, Gantke B, Schäfer A, Frieling T, Häussinger D, Enck P. Anal endosonography and manometry: comparison in patients with defecation problems. Dis Colon Rectum 1997; 40 (3): 293–7. [DOI] [PubMed] [Google Scholar]
- 57. Savoye‐Collet C, Savoye G, Koning E, Thoumas D, Michot F, Denis P, Benozio M. Anal endosonography after sphincter repair: specific patterns related to clinical outcome. Abdom Imaging 1999; 24 (6): 569–73. [DOI] [PubMed] [Google Scholar]
- 58. Rociu E, Stoker J, Eijkemans MJ, Schouten WR, Lameris JS. Fecal incontinence: endoanal US versus endoanal MR imaging. Radiology 1999; 212 (2): 453–8. [DOI] [PubMed] [Google Scholar]
- 59. Malouf AJ, Williams AB, Halligan S, Bartram CI, Dhillon S, Kamm MA. Prospective assessment of accuracy of endoanal MR imaging and endosonography in patients with fecal incontinence. Am J Roentgenol 2000; 175 (3): 741–5. [DOI] [PubMed] [Google Scholar]
- 60. Wiersema MJ, Wiersema LM. Endosonography‐guided celiac plexus neurolysis. Gastrointest Endosc 1996; 44 (6): 656–62. [DOI] [PubMed] [Google Scholar]
- 61. Gunaratnam NT, Sarma AV, Norton ID, Wiersema MJ. A prospective study of EUS‐guided celiac plexus neurolysis for pancreatic cancer pain. Gastrointest Endosc 2001; 54 (3): 316–24. [DOI] [PubMed] [Google Scholar]
- 62. Arcidiacono PG, Rossi M. Celiac plexus neurolysis. JOP 2004; 5 (4): 315–21. [PubMed] [Google Scholar]
- 63. Ang TL. Endoscopic ultrasound: moving from diagnostics to therapeutics. J Dig Dis 2008; 9 (3): 117–28. [DOI] [PubMed] [Google Scholar]
- 64. Gress F, Schmitt C, Sherman S, Ciaccia D, Ikenberry S, Lehman G. Endoscopic ultrasound‐guided celiac plexus block for managing abdominal pain associated with chronic pancreatitis: a prospective single center experience. Am J Gastroenterol 2001; 96 (2): 409–16. [DOI] [PubMed] [Google Scholar]
- 65. Micames CG, Gress FG. Local EUS‐guided injection of chemotherapeutic agents as adjuvant to systemic treatment: the first steps are made. Gastrointest Endosc 2007; 65 (3): 454–6. [DOI] [PubMed] [Google Scholar]
- 66. Chang KJ, Nguyen PT, Thompson JA, Kurosaki TT, Casey LR, Leung EC, Granger GA. Phase I clinical trial of allogeneic mixed lymphocyte culture (cytoimplant) delivered by endoscopic ultrasound‐guided fine‐needle injection in patients with advanced pancreatic carcinoma. Cancer 2000; 88 (6): 1325–35. [DOI] [PubMed] [Google Scholar]
- 67. Sangro B, Mazzolini G, Ruiz J, Herraiz M, Quiroga J, Herrero I, et al. Phase I trial of intratumoral injection of an adenovirus encoding interleukin–12 for advanced digestive tumors. J Clin Oncol 2004; 22 (8): 1389–97. [DOI] [PubMed] [Google Scholar]
- 68. Matthes K, Mino‐Kenudson M, Sahani DV, Holalkere N, Fowers KD, Rathi R, Brugge WR. EUS‐guided injection of paclitaxel (OncoGel) provides therapeutic drug concentrations in the porcine pancreas (with video). Gastrointest Endosc 2007; 65 (3): 448–53. [DOI] [PubMed] [Google Scholar]
- 69. Gan SI, Thompson CC, Lauwers GY, Bounds BC, Brugge WR. Ethanol lavage of pancreatic cystic lesions: initial pilot study. Gastrointest Endosc 2005; 61 (6): 746–52. [DOI] [PubMed] [Google Scholar]
- 70. Oh HC, Seo DW, Lee TY, Kim JY, Lee SS, Lee SK, Kim MH. New treatment for cystic tumors of the pancreas: EUS‐guided ethanol lavage with paclitaxel injection. Gastrointest Endosc 2008; 67 (4): 636–42. [DOI] [PubMed] [Google Scholar]
- 71. Vosoghi M, Sial S, Garrett B, Feng J, Lee T, Stabile BE, Eysselein VE. EUS‐guided pancreatic pseudocyst drainage: review and experience at Harbor‐UCLA Medical Center. MedGenMed 2002; 4 (3): 2. [PubMed] [Google Scholar]
- 72. Lopes CV, Pesenti C, Bories E, Caillol F, Giovannini M. Endoscopic‐ultrasound‐guided endoscopic transmural drainage of pancreatic pseudocysts and abscesses. Scand J Gastroenterol 2007; 42 (4): 524–9. [DOI] [PubMed] [Google Scholar]
- 73. Wehrmann T, Stergiou N, Vogel B, Riphaus A, Kockerling F, Frenz MB. Endoscopic debridement of paraesophageal, mediastinal abscesses: a prospective case series. Gastrointest Endosc 2005; 62 (3): 344–9. [DOI] [PubMed] [Google Scholar]
- 74. Seewald S, Imazu H, Omar S, Groth S, Seitz U, Brand B, et al. EUS‐guided drainage of hepatic abscess. Gastrointest Endosc 2005; 61 (3): 495–8. [DOI] [PubMed] [Google Scholar]
- 75. Lee DH, Cash BD, Womeldorph CM, Horwhat JD. Endoscopic therapy of a splenic abscess: definitive treatment via EUS‐guided transgastric drainage. Gastrointest Endosc 2006; 64 (4): 631–4. [DOI] [PubMed] [Google Scholar]
- 76. Seewald S, Brand B, Omar S, Yasuda I, Seitz U, Mendoza G, et al. EUS‐guided drainage of subphrenic abscess. Gastrointest Endosc 2004; 59 (4): 578–80. [DOI] [PubMed] [Google Scholar]
- 77. Trevino JM, Drelichman ER and Varadarajulu S. Modified technique for EUS‐guided drainage of pelvic abscess (with video). Gastrointest Endosc 2008; 68 (6): 1215–9. [DOI] [PubMed] [Google Scholar]
- 78. Kwan V, Kwan V, Eisendrath P, Antaki F, Le Moine O, Deviere J. EUS‐guided cholecystenterostomy: a new technique (with videos). Gastrointest Endosc 2007; 66 (3): 582–6. [DOI] [PubMed] [Google Scholar]
- 79. Lee SS, Do H Park, Hwang CY, Ahn CS, Lee TY, Seo DW, Lee SK, Kim MW. EUS‐guided transmural cholecystostomy as rescue management for acute cholecystitis in elderly or high‐risk patients: a prospective feasibility study. Gastrointest Endosc 2007; 66 (5): 1008–12. [DOI] [PubMed] [Google Scholar]
- 80. Shami VM and Kahaleh M. Endoscopic ultrasonography (EUS)‐guided access and therapy of pancreatico‐biliary disorders: EUS‐guided cholangio and pancreatic drainage. Gastrointest Endosc Clin N Am 2007; 17 (3): 581–93, vii–viii. [DOI] [PubMed] [Google Scholar]


