Introduction
It is said that “if you are born small, you keep dying for the rest of your life”. Small babies are more likely to suffer antenatal and intrapartum stillbirth 1 . Of pregnancy survivors, early neonatal adaptation is impaired and complications such as necrotising enterocolitis, respiratory distress, neurodevelopmental handicap and neonatal death are more common among small for gestational age (SGA) infants 2 . Many of these problems leave a legacy in childhood, and the fetal origins of adult disease are now well described by the Barker hypothesis. Coronary artery disease, obesity, hypertension, type 2 diabetes, the metabolic syndrome and premature death are more likely to occur in adults who were born small for gestational age 3 , 4 . This is the long‐term legacy of the adaptive behaviours of growth restricted infants in utero to ensure survival.
In contrast to SGA (birthweight less than the 10th centile) the American College of Obstetricians and Gynecologists (ACOG) describes intra‐uterine growth restriction (IUGR) as “a fetus that fails to reach its potential growth” 5 . This highlights the importance of recognising that some fetuses are small but not growth restricted. In accurately dated pregnancies, fetuses identified as being SGA may be, a) constitutionally small but healthy, b) truly growth restricted due to uteroplacental insufficiency, or c) affected by chromosomal/structural anomalies or chronic intrauterine infection (Table 1). Equally importantly, some fetuses are growth restricted but not small. This subset of IUGR can go undetected with the use of standardised population charts. If the reference ranges for fetal biometric data and birth weight account for maternal height, weight, ethnicity, parity and fetal gender (thus better identifying fetal growth potential), the detection of IUGR is enhanced 6 . Growth potential percentiles have been shown to be superior to conventional reference ranges for the prediction of adverse perinatal outcome 7 , 8 . The GROW (gestation related optimal weight) software has customised data for different countries (including Australia), and is able to be downloaded from www.gestation.net.
Table 1.
Non placental causes for small for gestational age fetuses.
| Genetic | Aneuploidy (T21, T18, T13) |
| Uniparental disomy (particularly 6,14,16) | |
| Single gene disorders / genetic syndromes affecting growth | |
| Skeletal dysplasias | |
| Infective | Toxoplasmosis |
| Cytomegalovirus | |
| Herpes Simplex (primary) | |
| Rubella | |
| Syphilis | |
| Parvovirus | |
| Structural Malformation | Cardiac defects |
| Anterior abdominal wall defects, particularly gastroschisis | |
| Neurological abnormalities | |
| Placental / cord abnormalities | Confined placental mosaicism |
| Single umbilical artery | |
| Velamentous cord insertion |
While acknowledging the importance of other causes for SGA, the remainder of this review will focus on disordered fetal growth associated with uteroplacental insufficiency. A variety of maternal conditions may be associated with placental insufficiency (Table 2), but will not be reviewed further here.
Table 2.
Maternal conditions associated with IUGR.
| Hypertension |
| Pre existing diabetes |
| Connective tissue disease (such as SLE) |
| Significant cardiac or respiratory disease, anemia |
| Inflammatory bowel disease |
| Thrombophilia (particularly antiphospholipid syndrome) |
| Malnutrition |
| Smoking, alcohol, drug use |
Pathophysiology of IUGR due to uteroplacental insufficiency
In normal pregnancy, the trophoblast invades into the myometrium, remodelling the end arteries (spiral arterioles)and replacing their muscular coat in order to create a “high flow, low resistance” circuit to facilitate exchange of nutrients and oxygen from the maternal to fetal compartment across the placental bed. This is manifest with a fall in uterine artery resistance with advancing gestation through the mid‐trimester. In pregnancies affected by IUGR, this normal transformation doesn't occur and the “high resistance, low flow” circuit persists. Placental floor infarcts and fetal villous obliteration further increase placental blood flow resistance, and nutrient exchange decreases or is outgrown. Uterine artery Doppler examinations at 22–24 weeks demonstrating increasing resistance to flow are associated with disorders of abnormal placentation, such as pre‐eclampsia, IUGR, placental abruption, preterm birth and perinatal death (Fig. 1).
Fig. 1a.
Normal “low resistance” uterine artery waveform in the midtrimester.
Fig. 1b.

Abnormal uterine artery vascular resistance, showing increased pulsatility and the presence of an early diastolic notch.
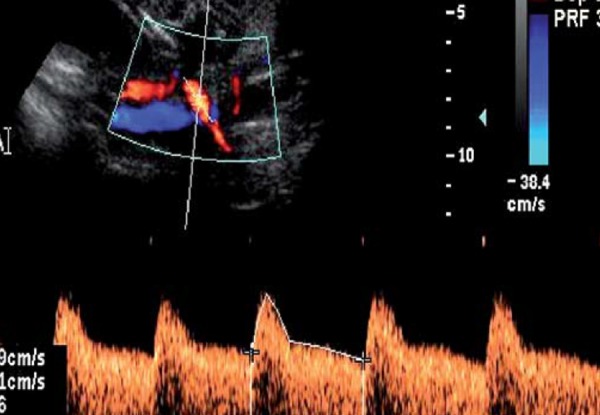
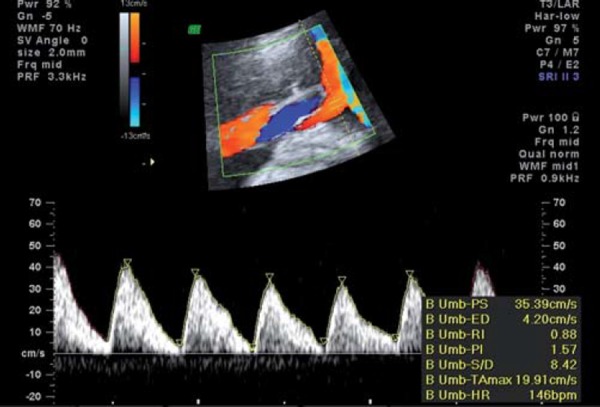
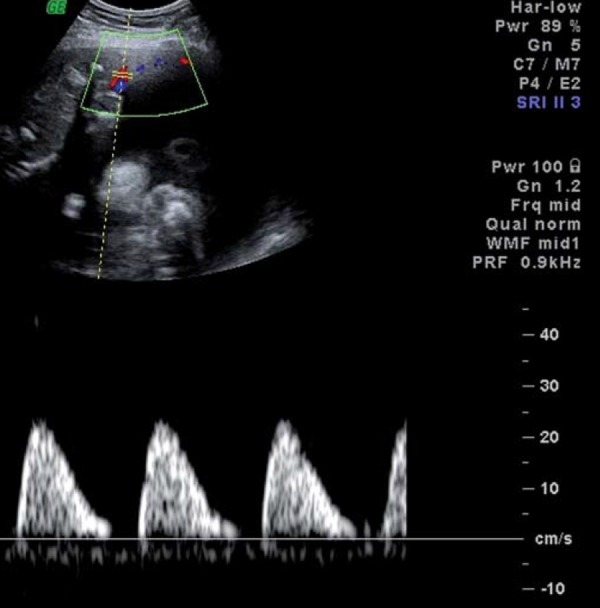
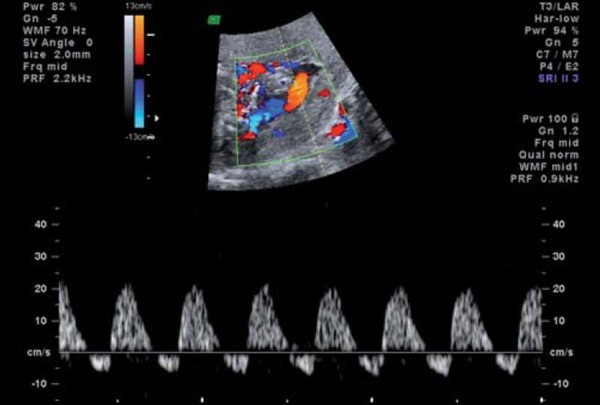
From the fetal side, umbilical artery waveforms correlate with both the tertiary villous architecture and blood flow resistance. When more than 30% of the fetal villous vasculature is abnormal, umbilical artery end‐diastolic velocities are reduced and Doppler resistance indices (SD ratio and PI) are elevated (Fig. 2a). When absent or reversed umbilical artery end‐diastolic velocity is observed (Figs. 2b, 2c), this suggests that 60–70% of the villous vascular tree is damaged 9 . In the face of increasing oxygen demand and decreasing supply, highly oxygenated blood is shunted preferentially through the ductus venosus, and streamed towards the left ventricle, favouring the cerebral and coronoary circulations. In the face of hypoxia and hypercapnia, cerebral autoregulation results in a fall in cerebral vascular resistance to maximise oxygen delivery to the brain (the so‐called “brain sparing” effect) (Fig. 3). The torso is relatively vasoconstricted, resulting in growth asymmetry and echogenic bowel. Redistribution away from the kidneys results in reduced urine output and amniotic fluid volume. All these features are suggestive of “fetal compensation” in the face of hypoxia.
Fig. 2a.
Increased SDR in the umbilical artery.
Fig. 2b.
Absent end‐diastolic flow in the umbilical artery.
Fig. 2c.
Reversed end diastolic flow in the umbilical artery.
Fig. 3a.
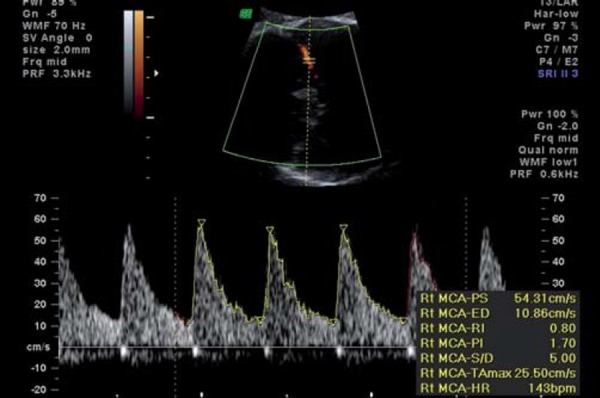
Middle cerebral artery normal waveform.
Fig. 3b.
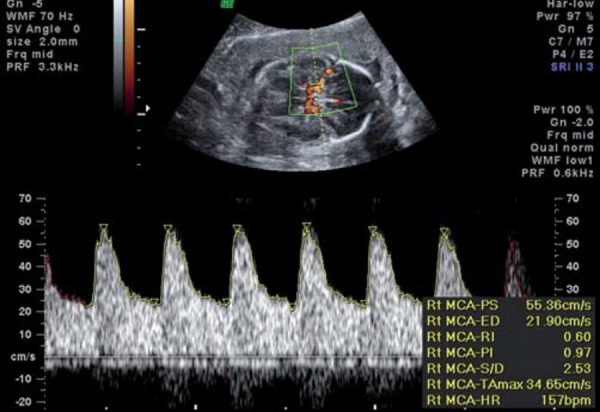
Middle cerebral artery waveform showing reduced resistance (lowered PI), known as “brain sparing”.
With increasing hypoxia, acidosis and, finally, asphyxia may result. Features of decompensation include worsening of fetal cardiac performance. Abnormal blood flow in the precordial veins is observed. In the ductus venosus (DV), increased pulsatility in the a wave (Fig. 4a) is followed by absence or reversal of the a wave, signifying impaired cardiac emptying during atrial systole (Figs. 4b, c). Neurologically, decompensation is evidenced by an impaired biophysical profile with deterioration in fetal breathing, body movements and tone. Reduced or absent fetal heart rate variability and late decelerations on computerised cardiotocograph are among the last signs to develop and are commonly associated with severe fetal acidemia. Final cardiac decompensation is seen with a pulsatile umbilical vein (Fig. 5). These “late” signs are associated with increasing academia, asphyxia and risk of perinatal death.
Fig. 4a.
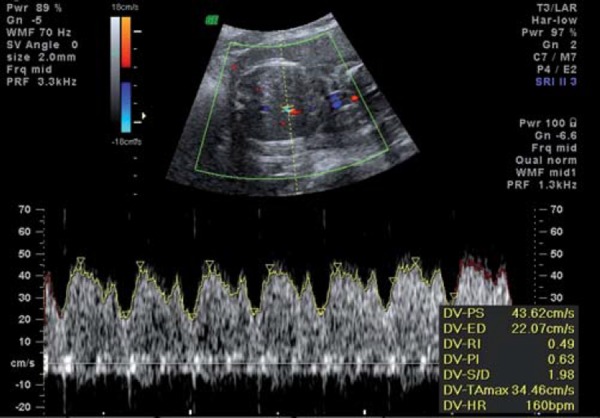
Normal ductus venosus waveform.
Fig. 4b.
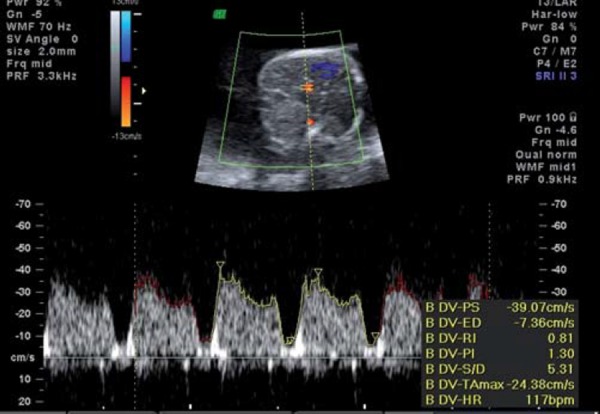
Ductus venosus showing increased pulsatility.
Fig. 4c.
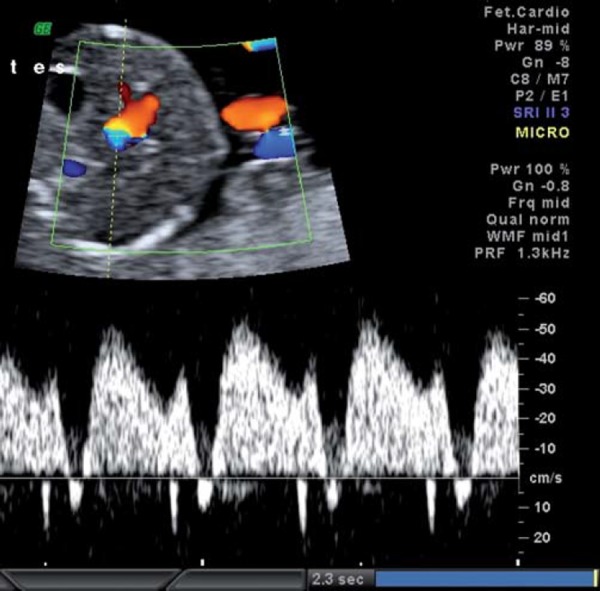
Ductus venosus showing reversal of the wave.
Fig. 5.
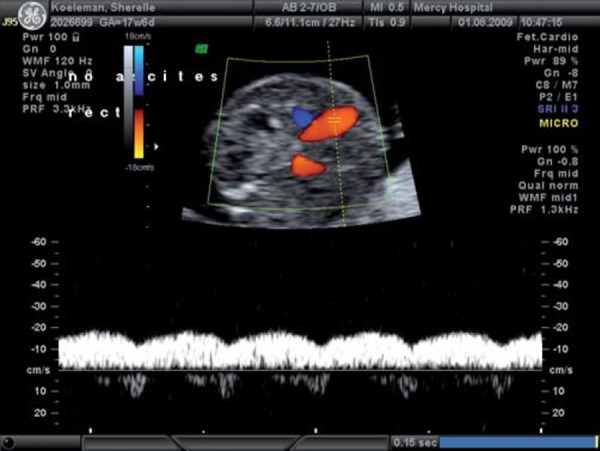
Pulsatile umbilical vein.
While most fetuses display this sequential series of events, a recent study has highlighted that where the pregnancy is complicated by maternal pre‐eclampsia, the growth restricted fetus follows a much less predictable course, with the sequential changes described above sometimes occurring rapidly and in a disorganised sequence 10 .
The role of ultrasound in screening for IUGR
Simple history taking can detect women at increased risk of fetal IUGR. Women with past obstetric history of an infant with SGA in their first pregnancy are at significantly increased risk of stillbirth 11 . Maternal medical conditions are significant risk factors for IUGR (Table 2). Clinically, the measurement of the symphyseal fundal height (SFH) has a reported sensitivity that ranges from 56% and 86% 12 , 13 . Nevertheless, the predictive ability of these clinical tools is limited.
Deficient placentation as indicated by enhanced vascular resistance in uterine artery Doppler studies in the second trimester is associated with an increased risk of pre‐eclampsia, IUGR and perinatal death. An increase in uterine artery resistance may be evidenced by an increase in resistance index (RI), pulsatility index (PI) or early diastolic notching. Uterine artery Doppler abnormalities are better for identifying severe, early onset IUGR or pre‐eclampsia than late or mild disease. In a recent meta‐analysis, abnormal uterine artery Doppler studies (increased uterine artery PI and/or notching) in the second trimester carried a positive likelihood ratio (LR) of 7.5 for pre‐eclampsia, 15.6 for severe pre‐eclampsia, 9.1 for IUGR and 14.6 for severe IUGR. In the setting of normal Doppler studies, the corresponding negative likelihood ratios were 0.6, 0.2, 0.9 and 0.8, respectively 14 . The sensitivity of abnormal uterine artery Doppler examinations in predicting pre‐eclampsia (47% and 74% for overall and severe pre‐eclampsia, respectively) is better than it is for IUGR (12% and 23% for overall and severe IUGR) 14 . Among women with abnormal uterine artery Doppler examinations in the second trimester, several randomised controlled trials have been performed to see if secondary prevention of IUGR or pre‐eclampsia with aspirin or Vitamin C and E is possible. Unfortunately, none of these treatments have been shown to be effective 15 . Second trimester uterine artery Doppler examination may be beneficial in stratifying risk for subsequent antenatal care, and may be of further value in reassuring high risk women in the setting of normal findings. Nevertheless, the clinical predictive value in low risk women is poor, and abnormalities in the second trimester appear to be too late for useful intervention. This has prompted investigators to consider the role of first trimester uterine artery evaluation.
Doppler velocimetry of uterine artery blood flow is technically feasible between 10 and 14 weeks of gestation, and the resistance index (RI) appears to be the most repeatable and reproducible measurement at this gestational age 16 . Although increased uterine artery resistance has been shown to be useful in detecting preterm pre‐eclampsia and IUGR, with sensitivities of 60–70%, the corresponding positive predictive values are low 14 , 17 . Pilalis, et al. have reported that the predictive value of first trimester uterine artery Doppler results can be improved with the addition of maternal serum PAPP‐A, suggesting that both markers in combination may be better than either alone 18 . If the predictive value of single or combination markers in the first trimester can be improved, then therapeutic initiatives are more likely to be successful. A recent systematic review and meta‐analysis has confirmed that ASA treatment started before 16 weeks gestation was linked with a significant reduction in IUGR of 49% 19 .
Ultrasound diagnosis of IUGR
The detection of IUGR is maximised when gestational age is well established, preferably with first trimester ultrasound. When gestational age is uncertain, the transcerebellar diameter is useful because it is one of the few soft tissue measurements that correlates well with gestational age even in the face of IUGR 20 . Having established gestational age, a multiparameter regression equation is used to calculate an estimated fetal weight, and a centile can be calculated using population based or – ideally – customised charts. As a single parameter, the abdominal circumference (AC) has good sensitivity and the highest positive predictive value for IUGR because it reflects both decreased subcutaneous fat and depleted liver glycogen stores 21 . The abdominal circumference percentile has the highest sensitivity and negative predictive value for the sonographic diagnosis of IUGR, defined postnatally by birth weight percentile 22 . Having identified the small fetus, it is important to do a targeted ultrasound examination for non‐placental causes (Table 1).
When the estimated fetal weight falls below the 10th percentile, umbilical artery Doppler examination is the most useful test for confirming the diagnosis of uteroplacental insufficiency 23 . Oligohydramnios further supports this diagnosis. It should be noted that the near term growth restricted fetus is much less likely to have an abnormal umbilical artery Doppler. In these fetuses, Doppler changes are likely to be more subtle, such as MCA evidence of redistribution 24 , and/or abnormal uterine artery waveforms 25 . Ghosh, et al. have recently reported that among women with a growth restricted fetus but normal umbilical artery Doppler examination, an abnormal uterine artery Doppler was similarly predictive for adverse perinatal outcome 26 .
Ultrasound surveillance of the growth restricted fetus
In the absence of any clearly effective therapy, the mainstay of management of IUGR is appropriate surveillance and timely delivery. In term IUGR, the prospective risk of stillbirth undoubtedly favours delivery. Preterm IUGR – particularly extreme preterm IUGR (< 32 weeks) – involves balancing the risks of awaiting organ maturation, (accepting an increasing risk of acidemia, asphyxia and stillbirth) and delivery, (accepting the risk of permanent injury from prematurity). The Growth Restriction Intervention Trial found that – among fetuses affected with severe early onset IUGR – long term neurological morbidity was improved among pregnancies where delivery was deferred as long as possible, even though perinatal mortality was unaltered 27 . Despite the limitations of this data, it suggests that delayed delivery is preferable, particularly at extremely early gestation (24–28 weeks) where survival may improve by up to 2% for every day that the fetus remains undelivered. Antenatal surveillance modalities include cardiotocography, biophysical profile score (tone, movement, breathing and amniotic fluid index) and arterial and venous fetal Doppler studies. These modalities used in combination, known as integrated fetal testing, allow the most precise assessment of fetal wellbeing 28 . During this time of surveillance, maternal contributors should be minimised and medical condition optimised. When delivery is anticipated before 34 weeks gestation, antenatal corticosteroids should be administered, and if delivery is to occur at less than 30 weeks gestation, magnesium sulphate should be administered for protection against cerebral palsy 29 .
The biophysical profile
A reactive cardiotocograph virtually excludes hypoxaemia, but a non‐reactive cardiotocograph is associated with a wide range of pH values. The biophysical profile score (BPS) applies categorical cutoffs for fetal tone, breathing, gross motor movement, and amniotic fluid index. It infers that if the peripheral biophysical variable is normal, then its corresponding central neuroregulatory centre is functional and not affected by hypoxia or acidosis. Consistent with this, a normal biophysical profile is associated with reduced rates of fetal death within the week 30 . In compromised fetuses, loss of fetal breathing movements occurs first, but this may be seen over a wide range of fetal arterial pH. Loss of fetal tone and movements are more reliably associated with pre‐labour acidemia (median pH of 7.1) 31 . The difficulty with using these biophysical variables alone is their limited ability to detect longitudinal deterioration. In addition, a recent study has demonstrated that the BPP performs poorly as a predictor of acidemia and fetal death in infants < 1000 g32. This study involved preterm growth restricted infants with abnormal umbilical artery Doppler examinations, who were subsequently delivered at a median gestational age of 27.6 weeks gestation and birth weight of 632 g. Among 27 fetuses with a BPP of 8/10 within 24 hours of delivery, there were three intrauterine fetal deaths, and 12 fetuses were born acidemic. Among 13 fetuses with a BPP of 6/10, there were three deaths, and seven were born acidemic. This suggests a more rapid and unpredictable deterioration in these fetuses than previously thought, and that increased frequency of multi‐parameter surveillance is necessary in pregnancies complicated by severe preterm growth restriction.
Fetal Doppler examination
Brain‐sparing, as evidenced by a decrease in the pulsatility index of the middle cerebral artery (MCA), reflects fetal cerebral autoregulation in the face of presumed hypoxia. The MCA PSV (peak systolic velocity) has been reported to provide additional information in fetuses with an abnormal MCA PI 33 . This study reported a better perinatal outcome in fetuses with an abnormal PI but normal PSV, compared to those with an abnormal PI accompanied by an elevated PSV. In addition, this study reported that the PSV remains elevated until death or delivery, where the MCA PI may rise in a preterminal phase of failed cerebral autoregulation 33 .
In the fetus with impaired growth and mildly abnormal umbilical artery Doppler studies, the appearance of brain sparing and oligohydramnios indicates an acceleration of the disease, with features now of fetal compensation. The frequency of surveillance needs to be increased, and in the moderately preterm fetus (e.g. 32–34 weeks gestation), consideration may be given to delivery. With progressive placental dysfunction, the fetus is at risk of decompensation and deterioration of venous indices. This reflects the cardiac dysfunction associated with increasing hypoxia and acidemia, with the right heart affected more than the left, and diastolic function worse than systolic function 34 . While elevated umbilical artery and brain sparing with normal venous studies is generally associated with a normal pH, elevated venous indices predicts fetal academia with 70–90% sensitivity and specificity. In view of the real risk of fetal acidosis and asphyxia, full multiparameter testing may be necessary up to several times per day if delivery is not planned. Absence or reversal of the DV a wave, or umbilical vein pulsations predict stillbirth with 65% sensitivity and 95% specificity 28 , and delivery – if at a viable gestation and weight – should be expedited. Interestingly, while absent / reversed a wave is a good predictor of fetal death, it does not seem to confer a worse long term neurological prognosis among survivors 35 , and others have challenged the notion that the DV is reliably associated with fetal acidosis 36 . The final trigger for delivery remains to be determined in the extremely preterm growth restricted fetus. A multicentre, randomised controlled trial (TRUFFLE Study) is currently investigating whether measurement of the fetal ductus venosus is the best parameter to guide timing of delivery of early preterm IUGR fetuses by comparing severe and mild DV abnormalities with traditional monitoring based on cardiotocography, and these data are eagerly awaited.
Future advances
Given the increased risks of late changes in the fetus with IUGR, it would be helpful to have a longitudinal parameter to follow during the phase between the early changes of compensation and the late changes of decompensation. It has been proposed that Doppler studies of blood flow in the aortic isthmus may be such a parameter. The aortic isthmus (AI) represents the only arterial connection between the right ventricle, (supplying the systemic and placental circulations), and the left ventricle, which preferentially supplies the cerebral circulation. The blood flow pattern of the aortic isthmus thus reflects the differences in vascular resistance between these systems. A recent study has found that AI‐PI consistently became abnormal (PI > 95th centile or retrograde flow) 1–2 weeks after abnormal UA and MCA flows were observed, and one week earlier than abnormal DV flow (> 95th percentile) 37 . AI Doppler studies could potentially be used to improve current algorithms for the prediction of poor perinatal outcome in preterm IUGR fetuses, and provide further guidance on optimising the time of delivery.
Conclusion
Fetal growth restriction is a complex multi‐system syndrome. It imposes a significant short and long‐term health burden on the affected fetus. Screening for growth restriction is important because it identifies the at‐risk population, and perinatal outcomes are improved in pregnancies with recognised, rather than unrecognised, growth restriction 38 . Accurate diagnosis of IUGR is enhanced by the use of customised growth charts and fetal biometry that places more emphasis on abdominal circumference than other biometric parameters. Once a fetus is found to be SGA, the diagnosis of uteroplacental IUGR can be achieved by excluding causes such as aneuploidy and infection, and the observation of umbilical and fetal Doppler abnormalities. Although there are continuing efforts to find effective therapies for established IUGR, the mainstay of management is appropriate multiparameter fetal surveillance, (including cardiotocography, biophysical variables and Doppler studies of the umbilical artery, middle cerebral artery, ductus venosus and umbilical vein) and optimal timing of delivery in order to maximise gestation in the severely preterm growth restricted infant.
References
- 1. Vashevnik S, Walker SP and Permezel M. Stillbirths and neonatal deaths in appropriate, small and large for birthweight for gestational age infants. A NZ J Obstet Gynaecol 2007; 47: 302–6. [DOI] [PubMed] [Google Scholar]
- 2. Rosenberg A. The IUGR Newborn. Sem Perinatol 2008; 32: 219–24. [DOI] [PubMed] [Google Scholar]
- 3. Barker DJ, Osmond C, Golding J, Kuh D, Wadsworth ME. Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. Br Med J 1989; 298: 564–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ross MG, Beall MH. Adult sequelae of intrauterine growth restriction. Semin Perinatol 2008; 32: 213–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. ACOG practice bulletin . Intrauterine growth restriction. N.12 January 2000. Int J Gynecol Obstet 2001; 72: 85–96. [Google Scholar]
- 6. Gardosi J, Chang A, Kalyan B, Sahota D, Symonds EM. Customised antenatal growth charts. Lancet 1992; 339: 283–7. [DOI] [PubMed] [Google Scholar]
- 7. Gardosi J, Clausson B, Francis A. The value of customised centiles in assessing perinatal mortality risk associated with parity and maternal size. BJOG 2009; 116 (10): 1356–63. [DOI] [PubMed] [Google Scholar]
- 8. Gardosi J, Francis A. Adverse pregnancy outcome and association with small for gestational age birthweight by customized and population‐based percentiles. Am J Obstet Gynecol 2009; 201 (1): 28.e1–8. [DOI] [PubMed] [Google Scholar]
- 9. Morrow RJ, Adamson SL, Bull SB, Ritchie JW: Effect of placental embolization on the umbilical artery velocity waveform in fetal sheep. Am J Obstet Gynecol 1989; 161: 1055–60. [DOI] [PubMed] [Google Scholar]
- 10. Mari G, Hanif F, Kruger M. Sequence of cardiovascular changes occurring in IUGR in pregnancies with and without pre‐eclampsia. Prenatal Diagnosis 2008; 28: 377–83. [DOI] [PubMed] [Google Scholar]
- 11. Surkan PS, Stephansson O, Dickman PW, Cnattingius S. Previous preterm and small‐for‐gestational‐age births and the subsequent risk of stillbirth. N Eng J Med 2004; 350: 777–85. [DOI] [PubMed] [Google Scholar]
- 12. Rosenberg K, Grant JM, Hepbrun M. Antenatal detection of growth retardation: actual practice in a large maternity hospital. Br J Obstet Gynaecol 1982; 89: 12–5. [DOI] [PubMed] [Google Scholar]
- 13. Belizan JM, Villar J, Nardin JC, Malamud J, De Vicurna LS. Diagnosis of intrauterine growth retardation by a simple clinical method: measurement of uterine height. Am J Obstet Gynecol 1978; 131: 643–6. [DOI] [PubMed] [Google Scholar]
- 14. Cnossen JS, Morris RK, ter Riet G, Mol BWJ, van der Post, JAM , Coomarasamy A, et al. Use of uterine artery Doppler ultrasonography to predict pre‐eclampsia and intrauterine growth restriction: a systematic review and bivariable meta‐analysis. CMAJ 2008; 178: 701–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sciscione AC, Hayes EJ. Uterine artery Doppler flow studies in obstetric practice. Am J Obstet Gynecol 2009; 201 (2): 121–6. [DOI] [PubMed] [Google Scholar]
- 16. Hollis B, Mavrides E, Campbell S, Tekay A, Thilaganathan B. Reproducibility and repeatability of transabdominal uterine artery Doppler velocimetry between 10 and 14 weeks of gestation. Ultrasound Obstet Gynecol 2001; 18: 593–7. [DOI] [PubMed] [Google Scholar]
- 17. Zhong Y, Tuuli M, Odibo AO. First‐trimester assessment of placenta function and the prediction of preeclampsia and intrauterine growth restriction. Prenat Diagn 2010; ePub ahead of print. [DOI] [PubMed]
- 18. Pilalis A, Souka P, Antsakalis P, Daskalakis G, Papntoniou N, Mesogitis S, Antsakalis A. Screening for pre‐eclampsia and fetal growth restriction by uterine artery Doppler and PAPP‐A at 11–14 weeks' gestation. Ultrasound Obstet Gynecol 2007; 29: 135–40. [DOI] [PubMed] [Google Scholar]
- 19. Bujold E, Morency AM, Roberge S, Lacasse Y, Forest JC, Giguère Y: Acetylsalicylic acid for the prevention of preeclampsia and intrauterine growth restriction in women with abnormal uterine artery Doppler: a systematic review and meta‐analysis. J Obstet Gynaecol Can 2009; 31: 818–26. [DOI] [PubMed] [Google Scholar]
- 20. Chavez MR, Ananth CV, Smulian JC, Vintzileos AM. Fetal transcerebellar diameter measurement for prediction of gestational age at the extremes of fetal growth. J Ultrasound Med 2007; 26: 1167–71. [DOI] [PubMed] [Google Scholar]
- 21. Benson CB, Doubilet PM, Saltzmann DH. Intrauterine growth retardation: predictive value of ultrasound criteria for antenatal diagnosis. Radiology 1986; 160: 415–7. [DOI] [PubMed] [Google Scholar]
- 22. Weiner CP, Robinson D: The sonographic diagnosis of intrauterine growth retardation using the postanatal ponderal index and the crown heel length as standards of diagnosis. Am J Perinatol 1989; 6: 380–3. [DOI] [PubMed] [Google Scholar]
- 23. Baschat AA, Weiner CP. Umbilical artery Doppler screening for detection of the small fetus in need of antepartum surveillance. Am J Obstet Gynecol 2000; 182: 154–8. [DOI] [PubMed] [Google Scholar]
- 24. Hershovitz R, Kingdom JC, Geary M, Rodeck CH. Fetal cerebral blood flow redistribution in late gestation: identification of compromise in small fetuses with normal umbilical artery Doppler. Ultrasound Obstet Gynecol 2000; 15: 209–12. [DOI] [PubMed] [Google Scholar]
- 25. Severi FM, Bocchi C, Visentin A, Falco P, Cobellis L, Florio P, Zagonari S, PIlu G. Uterine and fetal cerebral doppler predict the outcome of third‐trimester small‐for‐gestational age fetuses with normal umbilical artery Doppler. Ultrasound Obstet Gynecol 2002; 19: 225–8. [DOI] [PubMed] [Google Scholar]
- 26. Ghosh GS, Gudmundsson S. Uterine and umbilical artery Doppler are comparable in predicting perinatal outcome of growth‐restriced fetuses. BJOG 2009; 116: 424–30. [DOI] [PubMed] [Google Scholar]
- 27. Thornton JG, Hornbuckle J, Vail A, Spiegelhalter DJ, Levene M; GRIT study group : Infant wellbeing at 2 years of age in the Growth Restriction Intervention Trial (GRIT): multicentred randomised controlled trial. Lancet 2004; 364: 513–20. [DOI] [PubMed] [Google Scholar]
- 28. Turan S, Miller J, Baschat AA. Integrated testing and management in fetal growth restriction. Semin Perinatol 2008; 32: 194–200. [DOI] [PubMed] [Google Scholar]
- 29. Doyle LW, Crowther CA, Middleton P, Marret S. Antenatal magnesium sulfate and neurologic outcome in preterm infants; a systematic review. Obstet Gynecol 2009; 11 (6): 1327–33. [DOI] [PubMed] [Google Scholar]
- 30. Manning FA. Antepartum fetal testing: a critical appraisal. Curr Opin Obstet Gynecol 2009; 21: 348–52. [DOI] [PubMed] [Google Scholar]
- 31. Vintzileos AM, Fleming AD, Scorza WE, Wolf EJ, Balducci J, Campbell WA, Rodis JF. Relationship between fetal biophysical activities and umbilical cord blood gas values. Am J Obstet Gynecol 1991; 165: 707–13. [DOI] [PubMed] [Google Scholar]
- 32. Kaur S, Picconi JL, Chadha R, Kruger M, Mari G. Biophysical profile in the treatment of intrauterine growth‐restricted infants who weigh < 1000 g. Am J Obstet Gynecol 2008; 199: 264.e1–4. [DOI] [PubMed] [Google Scholar]
- 33. Mari G, Hanif F, Kruger M, Cosmi E, Santolaya‐Forgas J, Treadwell MC. Middle cerebral artery peak systolic velocity: a new Doppler parameter in the assessment of growth‐restricted fetuses. Ultrasound Obstet Gynecol 2007; 29: 310–16. [DOI] [PubMed] [Google Scholar]
- 34. Bahtiyar MO, Copel JA. Cardiac changes in the intrauterine growth restricted fetus. Semin Perinatol 2008; 32: 190–3. [DOI] [PubMed] [Google Scholar]
- 35. Baschat AA, Viscardi RM, Hussey‐Gardner B, Hashmi N, Harman C. Infant neurodevelopment following fetal growth restriction: relationship with antepartum surveillance parameters. Ultrasound Obstet Gynecol 2009; 33: 44–50. [DOI] [PubMed] [Google Scholar]
- 36. Picconi J, Hanif F, Mari G. Ductus venous reversed flow: is it an indication for delivery? Am J Perinatol 2008; 25: 199–204. [DOI] [PubMed] [Google Scholar]
- 37. Figueras F, Benavides A, Del Rio M, Crispi F, Eixarch E, Martinez JM, Hernandez‐Andrade E, Gratacós E. Monitoring of fetuses with intrauterine growth restriction: longitudinal changes in ductus venosus and aortic isthmus flow. Ultrasound Obstet Gynecol 2009; 33: 39–43. [DOI] [PubMed] [Google Scholar]
- 38. Lindqvist PG, Molin J. Does antenatal identification of small‐for‐gestational age fetuses significantly improve their outcome? Ultrasound Obstet Gynecol 2005; 25 (3): 258–64. [DOI] [PubMed] [Google Scholar]


