Abstract
Hip pain is a common paediatric presentation and is potentially serious. While hip pain can be attributed to primary hip pathology, the hip area is also a common site for referred pain. This often poses a diagnostic challenge particularly in the young child who may not verbalise the point of pain and may not report an injury.
Differential diagnoses for paediatric hip joint pain range from fracture, transient synovitis, septic arthritis with or without osteomyelitis, juvenile idiopathic arthritis (JIA, previously juvenile rheumatoid arthritis JRA), Legg‐Calve‐Perthes' disease (LCP), slipped capital femoral epiphysis (SCFE) to haemarthrosis in patient with a clotting disorder. Referred pain from abdominal pathology, for example; appendicitis, psoas abscess or haematoma should also be considered.
The evaluation and management of hip pain requires a thorough history and physical examination. A radiograph is usually indicated to rule out any bony injury. Septic arthritis is a medical emergency requiring urgent surgical as well as medical treatment. Ultrasound of the hip joint plays a role in helping to differentiate which hips require early intervention and to guide a needle aspiration of joint fluid if indicated.
In this article, we aim to review the anatomy of the hip, techniques in ultrasonographic examination and some common pathologies in the paediatric hip.
The child with a limp
The child with a limp is a common presentation and may be attributed to primary hip pathology or other pathologies that cause referred pain to the hip and groin region. Accordingly, the presentation may be non‐specific. It is not always easy to assess the paediatric patient clinically.
Imaging has become an integral part of the evaluation of a child with a limp. Any imaging evaluation should be preceded and guided by a detailed history and physical examination. The age of patient, localisation of pain where possible, length of time of symptoms, antecedent history of trauma, signs of systemic illness such as fever and tachycardia, recent history of infections and laboratory findings all help narrow the focus of the imaging. The differential diagnoses of certain developmental problems are age‐specific.
Ultrasound is safe and readily available in the acute setting. Ultrasound is superior to plain radiography in identifying a hip joint effusion and can assist in joint fluid aspiration.
Anatomy of the hip joint and the anterior hip joint capsule
The hip joint (acetabulofemoral joint) is a “ball‐and‐socket” synovial joint surrounded by a strong joint capsule. The maximal intracapsular volume of the hip is achieved when the hip is in a flexed, abducted and externally rotated orientation. This is the position of comfort in a child with a hip effusion.
The proximal femur and acetabulum are predominantly cartilaginous at birth. Hence, ultrasound allows direct visualisation of the morphology of the acetabular roof and its relationship with the femoral head 1 .
In children with a limp, the focus of ultrasound is placed on imaging the anterior joint capsule. Correlation between ultrasound and cadaveric study shows there is a fold of joint capsule in the space between the iliopsoas muscle and the femoral neck. The fold is made up of two separate layers (anterior and posterior) each measuring up to 2–4 mm in thickness. The anterior recess of the joint space separates these layers, which may contain a small amount of fluid in healthy individuals. These structures are easily identified with modern ultrasound equipment (Fig. 1) 2 .
Fig. 1.
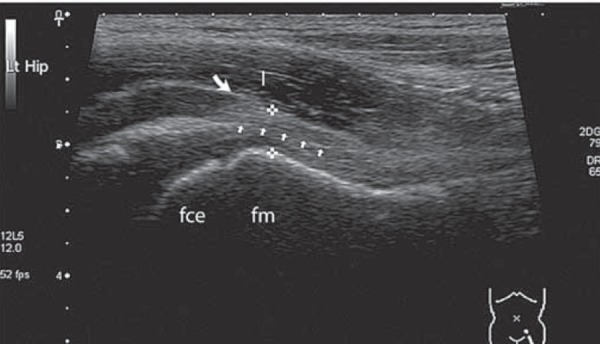
Normal anatomy of the anterior hip joint capsule. fce = femoral capital epiphysis; fm = femoral metaphysis; between cursors = both layers of joint capsule (hyperechoeic to muscle); I = iliopsoas muscle; small arrows = echogenic interface between joint capsule layers.
Scanning technique
Ultrasound in the coronal plane is well established as the preferred imaging plane to assess developmental dysplasia of the hip in infants 1 . The anterior approach is ideal to assess the presence of joint fluid in a child of any age.
The hip joint is examined with the transducer positioned obliquely along the long axis of the femoral neck (Fig. 2). A high frequency linear array transducer is used, with the frequency and focal points adjusted to allow for the wide range in size of paediatric patients with hip pain.
Fig. 2.
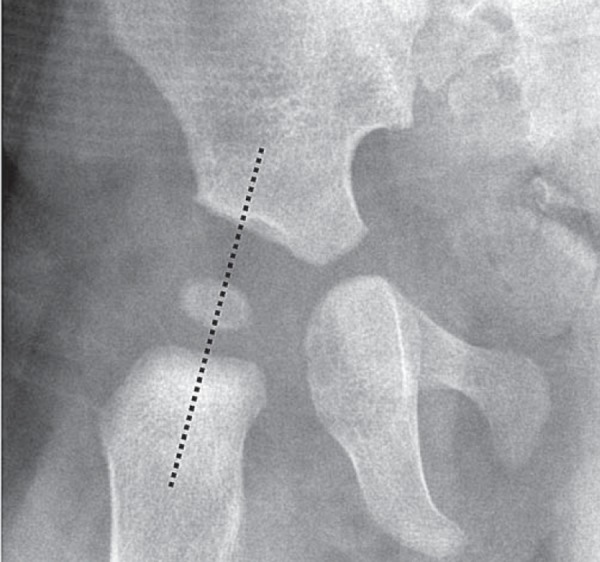
Plane of probe placement. Probe should be placed along dotted line (femoral neck) for imaging the anterior hip joint capsule.
Table 1.
Common causes for hip pain in the paediatric patient.
| Mechanical | Vascular | Infectious | Autoimmune | Oncologic | Referred |
|---|---|---|---|---|---|
| Fracture/dislocation | Legg‐Calve‐Perthes disease (LCP) | Septic arthritis | Transient synovitis | Benign – fibrous dysplasia, simple/aneurysmal bone cyst | Appendicitis |
| Slipped femoral capital epiphysis (scfe) | Avascular necrosis (avn) | Osteomyelitis | Juvenile idiopathic arthritis (jia) | Malignant – osteosarcoma, Ewing's | Psoas abscess Pyomyositis |
| Haemarthrosis | Sickle cell disease | Psoas haemorrhage |
Both hip joints should be imaged for comparison. The patient is positioned in a neutral position with the legs straight and the feet slightly externally rotated. The leg may be gently rotated internally and externally to ensure imaging of the whole of the head of the femur.
If pain prohibits a neutral position, the contralateral leg should be positioned in a mirror image to the affected side, thus allowing a true comparison of anatomy and pathology.
The ultrasound image should include the joint space, head of femur, neck of femur, joint capsule and overlying iliopsoas muscle. Ultrasound is very useful for identifying joint effusion in the hip. As little as 1 cm3 of joint effusion can be identified on ultrasound 3 , 4 .
A wide footprint linear transducer should be used if available, with additional width obtained with the trapezoid or expanded field of view setting. The normal joint capsule follows the contour of the neck of femur in a concave shape. The normal anterior and posterior joint capsule layers abut each other. When an effusion is present, the two layers of joint capsule are separated by fluid, causing the anterior layer to become convex in relation to the neck of the femur 2 . Imaging within the first 24 hours of symptoms may not reveal an effusion. If symptoms persist a repeat scan is warranted 5 .
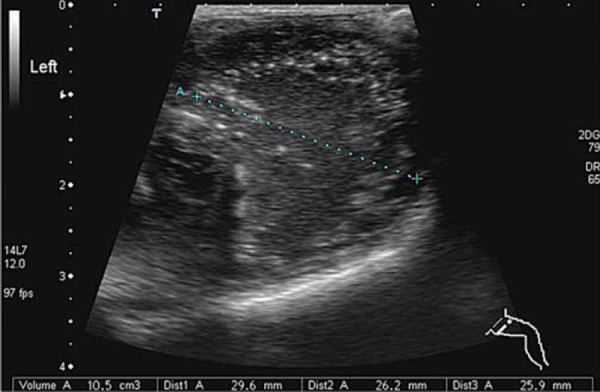
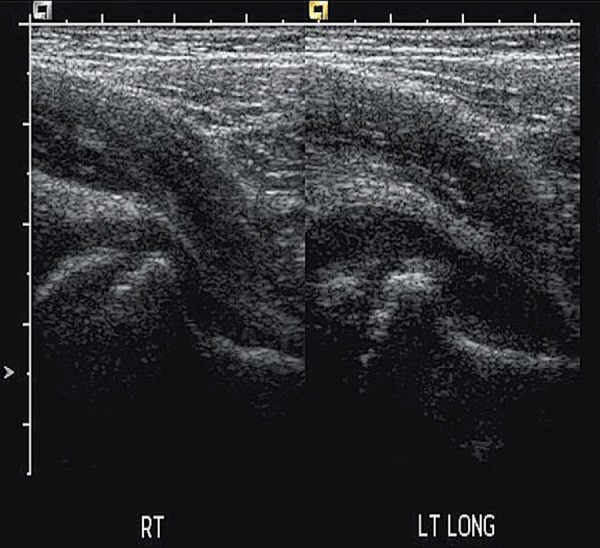
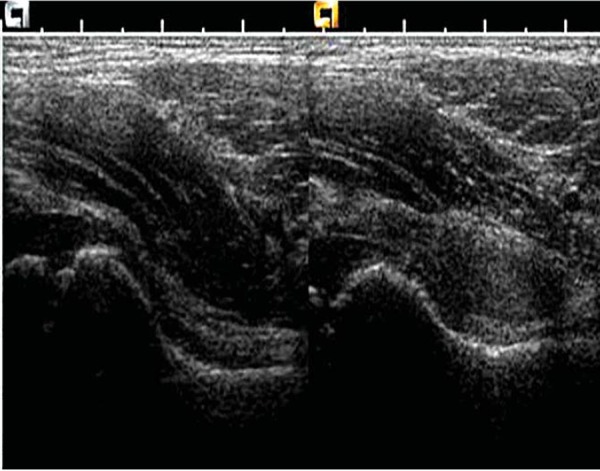
If only a small footprint transducer is available (Fig. 3a) the area of interest may be imaged utilising a split screen or panoramic function, ensuring the entire region of interest is imaged 5 (Figs. 3b, 3c).
Fig. 3a.
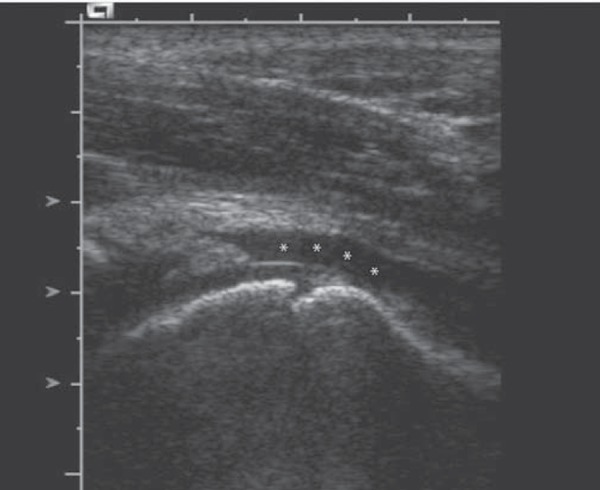
Single screen image.∗ = effusion.
Fig. 3b.
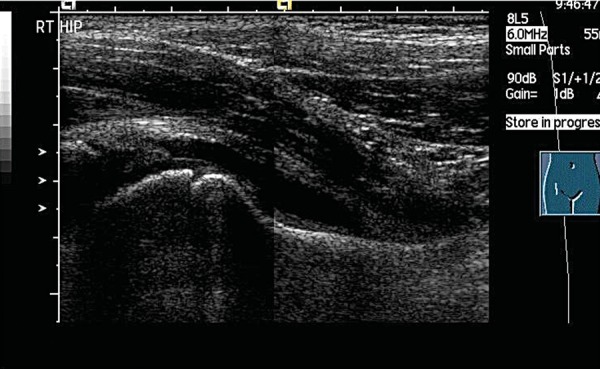
Split screen compilation image.
Fig. 3c.
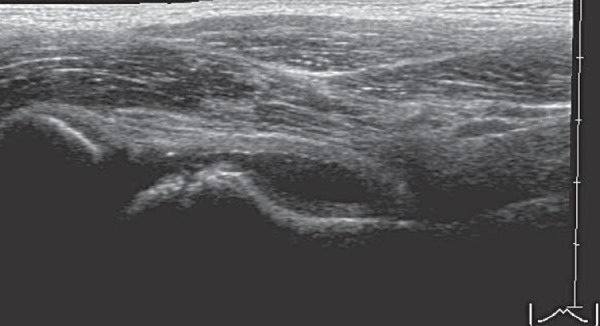
Panoramic view of hip.
An effusion may be a primary condition or secondary to underlying pathology. Examination of the head of femur is required to determine if a primary pathology such as Legg‐Calve‐Perthes'disease (LCP) or slipped femoral capital epiphysis (SCFE) is present or whether an infective process such as osteomylitis causing bone irregularity is evident.
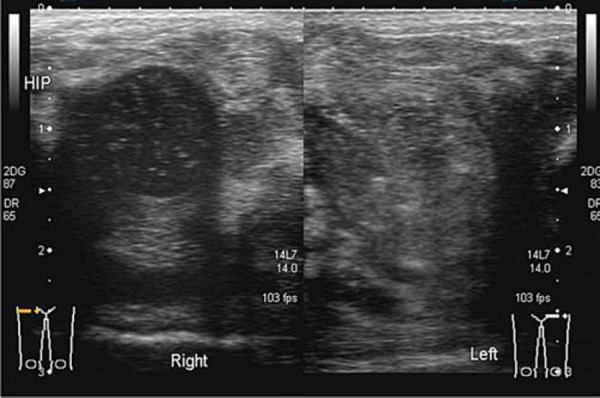
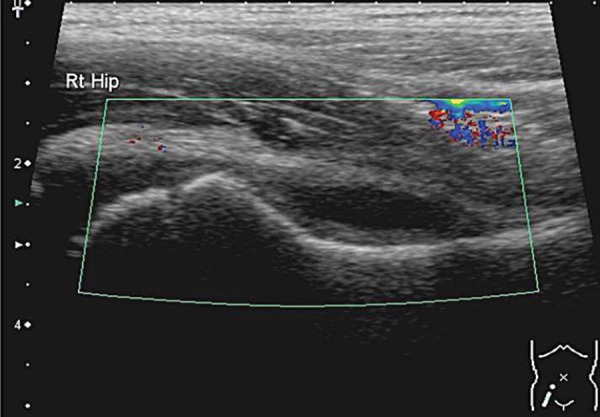
An infective process may display hyperaemia of the tissues around the area of infection evident on colour Doppler (Fig. 4). This is less reliable if the patient has been started on antibiotic therapy.
Fig. 4.
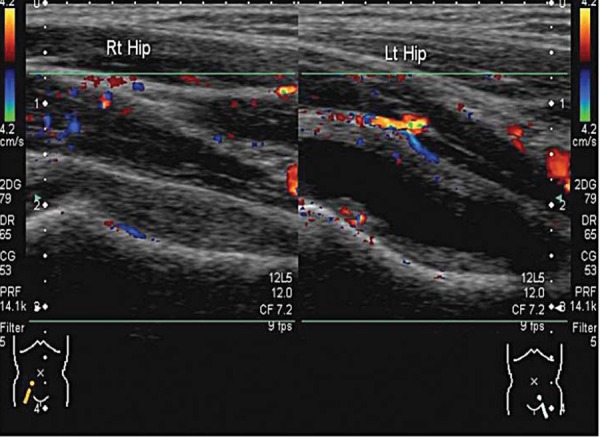
Normal right hip compared to the left hip with an effusion. Hyperaemia of the soft tissues is evident on the left side. The machine settings are kept the same for comparison.
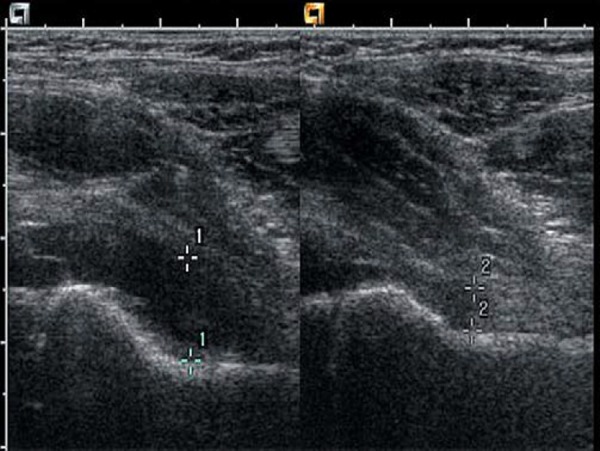
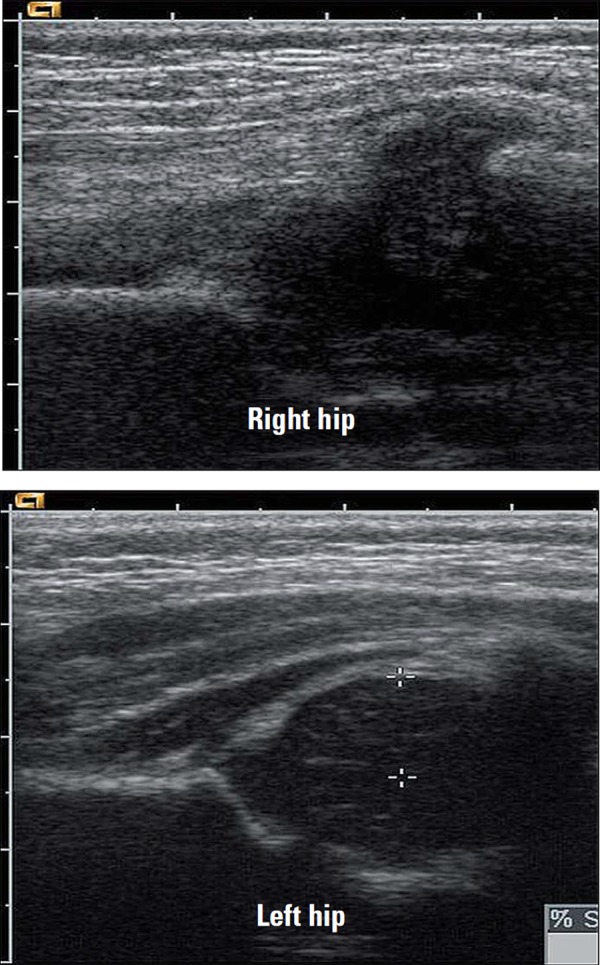
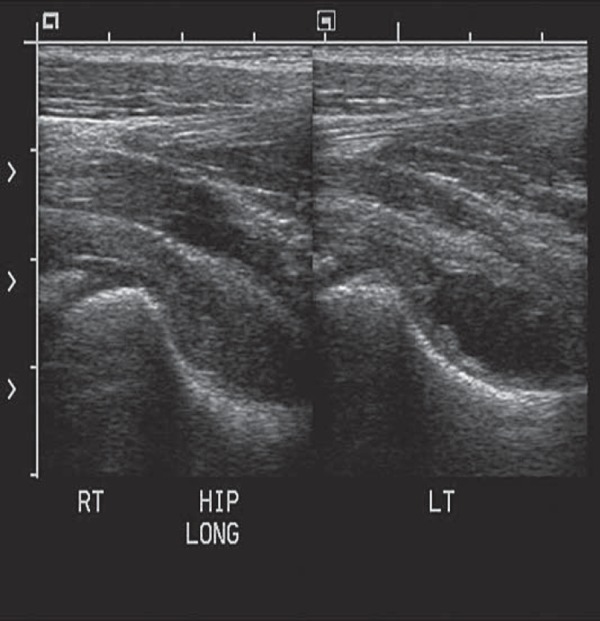
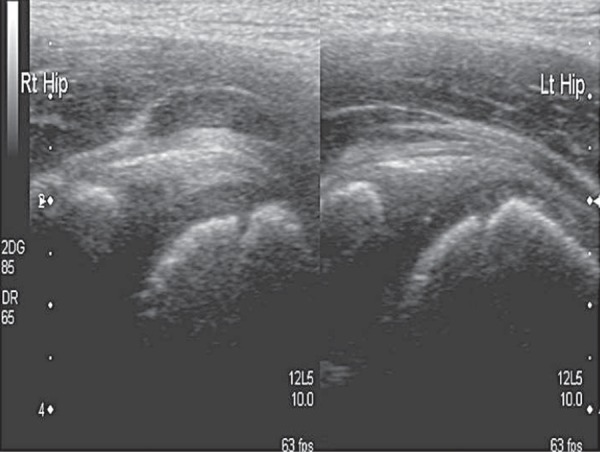
The gain setting should be adjusted to allow good penetration without losing definition of overlying muscle fibres and to ensure that any fluid seen is displayed correctly. Comparison images of the affected and unaffected hip are taken in the longitudinal and the transverse planes (Fig. 5), and include the head of femur (Fig. 6). Pathology is often present in both hips, particularly in systemic conditions such as juvenile idiopathic arthritis (JIA).
Fig. 5.
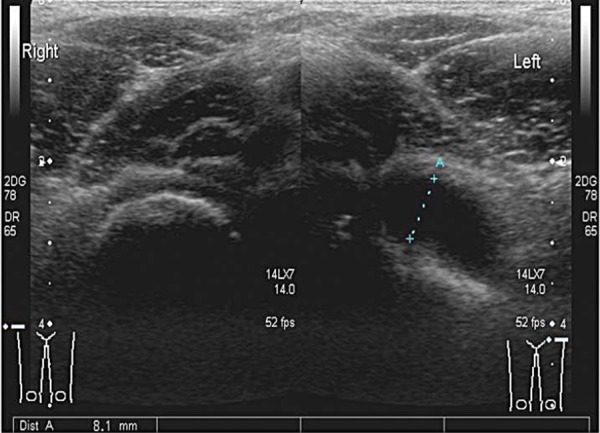
Transverse image of both hips at the level of the neck of femur. Fluid distends the joint capsule on the left side (as marked by calipers).
Fig. 6.
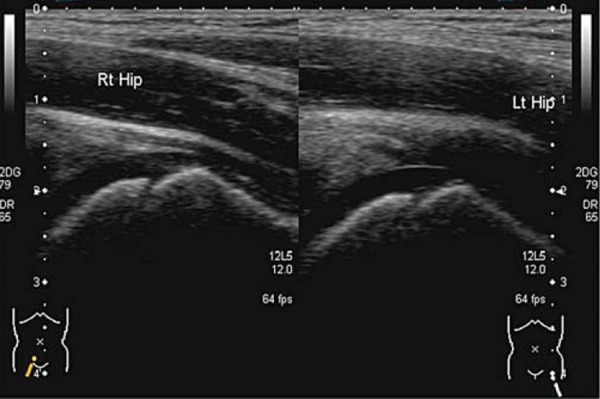
Comparison of the head of femur.
Pathology
Irritable hip – transient synovitis and reactive arthritis
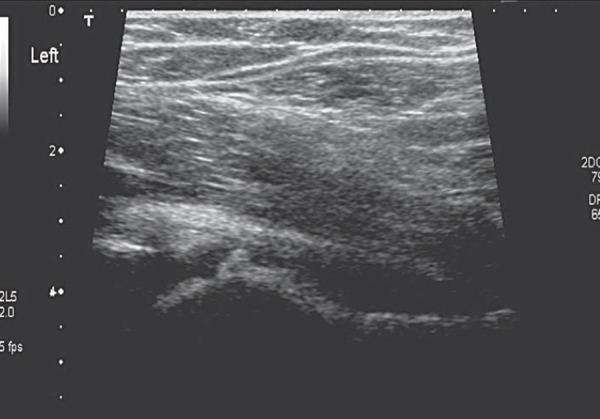
Irritable hip or transient synovitis (TS) is the most common cause of hip pain in the paediatric patient 6 . TS (Fig. 7) and reactive arthritis (Figs. 8a, 8b) are both benign, self limiting conditions. The temperature is usually normal or slightly raised, white cell count (WCC) and erythrocyte sedimentation rate (ESR) are normal or close to normal. Treatment is rest and analgesia. The addition of an anti‐inflammatory may speed recovery 7 . Usually these conditions follow a recent viral illness in particular an upper respiratory tract infection (URTI) 6 .
Fig. 7.
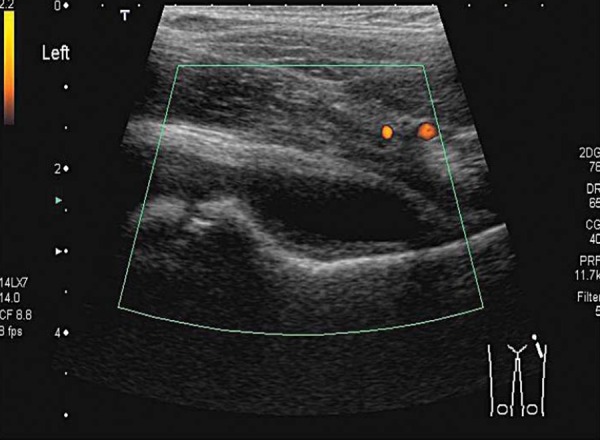
Transient synovitis; hypoechoic effusion without hyperaemia.
Fig. 8a.
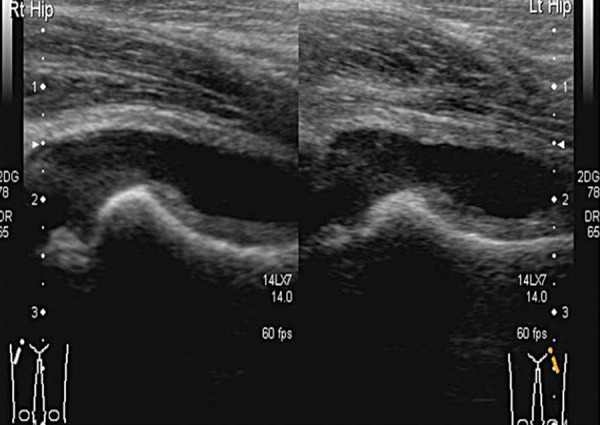
Reactive arthritis, often bilateral.
Fig. 8b.
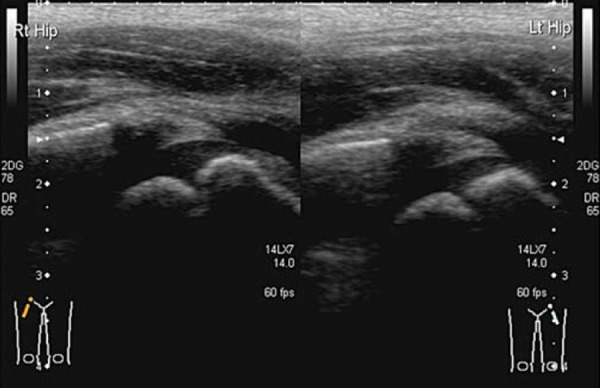
Reactive arthritis; normal head of femur and joint space.
Septic arthritis
Septic arthritis most often affects the hip and knee in the paediatric population 8 .
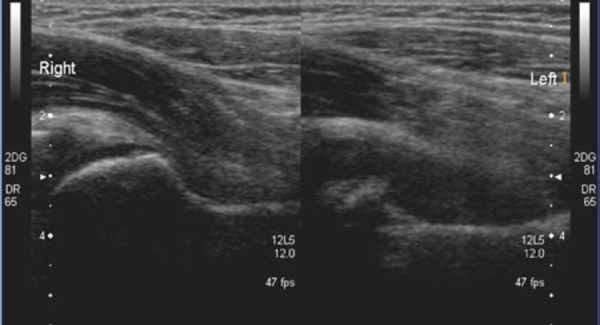
The patient presents with severe pain, the leg is usually held in flexion, abduction and external rotation 9 . Scanning may be difficult without pain relief. The temperature is raised and the child can quickly become septicaemic. The WCC and ESR are both raised. Systemic sepsis leading to multiorgan failure can occur rapidly 10 . A delay in treatment may lead to leg length discrepancy, due to femoral growth arrest and joint deformity, with a high incidence of late osteoarthritis 8 . Osteomyelitis may be a cause of the septic arthritis or secondary to it 8 , 11 (Figs. 9a, 9b). The classic ultrasound findings in septic arthritis of the hip joint are an effusion, which may be turbid in nature and hyperaemia of the adjacent soft tissues (Figs. 4, 10, 11).
Fig. 9a.
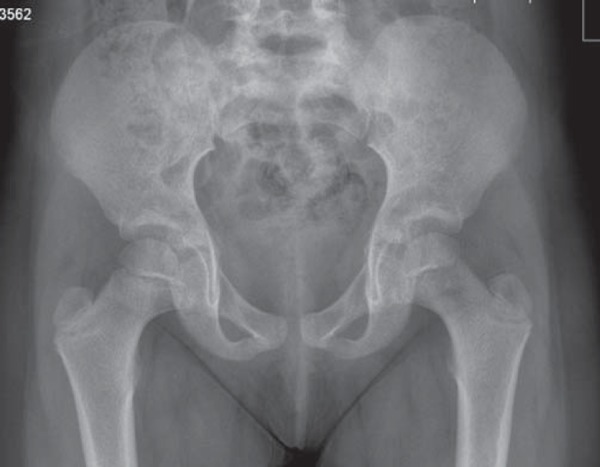
X‐Ray of Osteomyelitis left neck of femur.
Fig. 9b.
Lytic lesion in the metaphysis of emoral neck with effusion..
Fig. 10.
Septic Arthritis of the right hip joint. Septations are seen within the fluid.
Fig. 11.
Pyarthritis of left hip joint.
Blood borne pathogens are directly deposited in the joint. The most common pathogen is Staphylococcus Aureus, followed by a small number with Methicillin‐Resistant Staphyloccocus Aureus (MRSA). Previously seen haemophilus influenza has been very rarely seen since the introduction of the HIB vaccination 12 .
Treatment is early antibiotic cover and either aspiration or open lavage and drainage. Ultrasound guided aspiration allows decompression of the joint and provides a sample of fluid so antibiotic treatment can be tailored to the specific pathogen. At least three weeks of antibiotics, primarily intravenous is required. Givon, et al. advocate treatment by repeated daily ultrasound guided needle aspirations until the fluid is clear 13 .
Septic arthritis in the neonate
Premature infants are at risk of infection. Regular intervention, in particular vascular access lines increases the risk of introduced infection 14 (Fig. 11). Premature babies have an immature immune system and do not exhibit obvious signs of sepsis. The prevalence of MRSA is increasing in hospital intensive care units 15 . Neonates in intensive care units with septic arthritis are more likely to have MRSA infection than non‐MRSA. Bony destruction can occur quickly (Figs. 12, 13), with a worse outcome than those with non‐MRSA infection, due to the limited range of antibiotics effective in treating an MRSA infection and the relatively late presentation.
Fig. 12.
Collection in upper thigh.
Fig. 13a.
Destruction of head of femur of left hip due to MRSA septic arthritis
Fig. 13b.
Destruction of head of femur of right hip.
Disruption of the epiphysis leads to arrest of growth of the upper femur with resultant leg length discrepencies Destruction of the head of femur leads to life long joint deformity. Early identification of joint sepsis aids early aggressive management, which increases the chance of retaining the use of the joint and limb 15 .
Juvenile idiopathic arthritis
Hip involvement in juvenile idiopathic arthritis (JIA) tends to be bilateral and develops in 30–50% of children suffering from JIA. It is uncommon to have hip monoarthritis. Clinical examination and history usually suggest the diagnosis.
Ultrasound may show a joint effusion and thickened synovium (Fig. 14). These changes and other findings of joint destruction are often better seen on MRI or plain radiographs. Ultrasound can be used for image‐guided intra‐articular steroid injections.
Fig. 14.
Bilateral JIA.
Legg‐Calve Perthes' disease
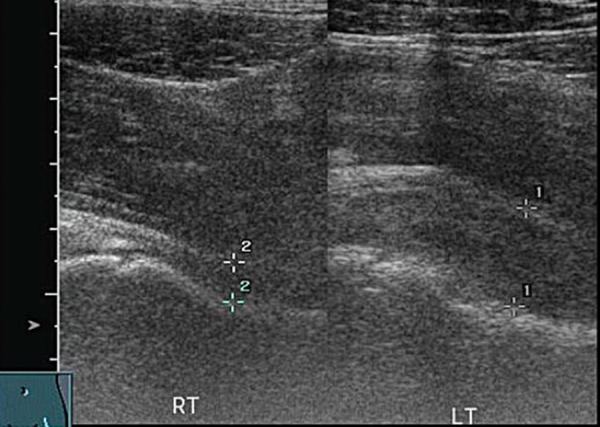
Legg‐Calve Perthes' disease is an idiopathic avascular necrosis (AVN) of the hip joint. The peak incidence of LCP is between four and eight years of age with boys being effected more than girls 16 . Recently LCP disease has been reported in children younger than two years of age 17 , 18 and must be a differential diagnosis in any hip pain investigation of young children. Trauma to the hip joint can cause increased intraosseus pressure and compression of blood vessels, this may lead to avascular necrosis 18 . In advanced disease, radiographs will display the classic picture of a reduced height head of femur with fragmentation. In early LCP the radiograph may be normal. A joint effusion may accompany LCP. An underlying pathology should be suspected when a sustained effusion is present (Figs. 15a, 15b).
Fig. 15a.
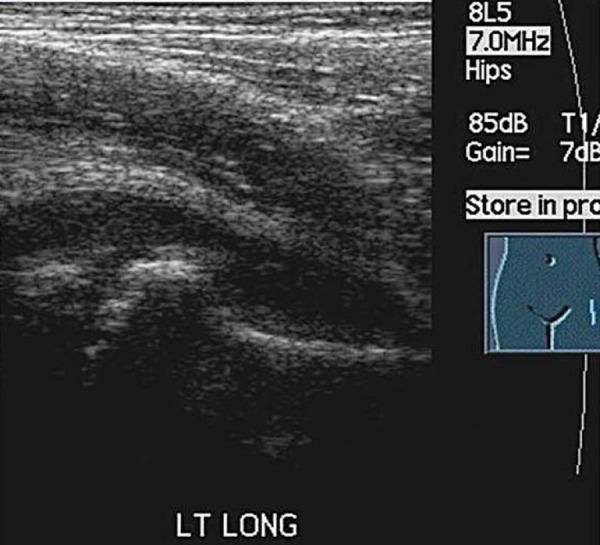
Hip effusion.
Fig. 15b.
Comparison of both heads of femur demonstrates irregularity of left head of femur (LCP) as well as a joint effusion.
In early LCP the head of femur may exhibit slight irregularity (Figs. 16a, 16b). The treatment for LCP, especially when diagnosed early, is rest.
Fig. 16a.
Hip effusion.
Fig. 16b.
Appreciation of possible head of femur irregularity more obvious in comparison to normal hip.
Slipped capital femoral epiphysis
Slipped capital femoral epiphysis (SCFE) occurs in early adolescence, predominately in obese boys, before fusion of the physis. Male to female ratio is approximately 3:1, average age is 12 years, over half are initially unilateral, the contralateral side may be affected at a later stage. Endocrinopathies such as thyroid dysfunction are common in children with SCFE 19 .
Untreated SCFE may result in avascular necrosis, growth deformity of the femur, abnormally positioned leg and early osteoarthritis.
Diagnosis of SCFE is generally radiological, with a minor slip most evident in a frog leg projection. MRI has been used to identify “pre‐slip” hips in at risk patients. Widening of the physis is an indication of imminent slippage and a prophylactic pinning operation warranted 20 .
Ultrasound does not usually play a role in the diagnosis of SCFE, although its use in diagnosis and follow‐up had been described in the past 21 . Nevertheless, the relationship between the head of femur and physis should be established when scanning a patient in the “at risk” group. A reactive effusion may be the presenting symptom (Figs. 17a, b). When a normal head of femur can not be established, the patient should always proceed to having an X‐ray (Fig. 17c & 17d).
Fig. 17a.
Joint effusion with stepped appearance of the head of femur.
Fig. 17b.
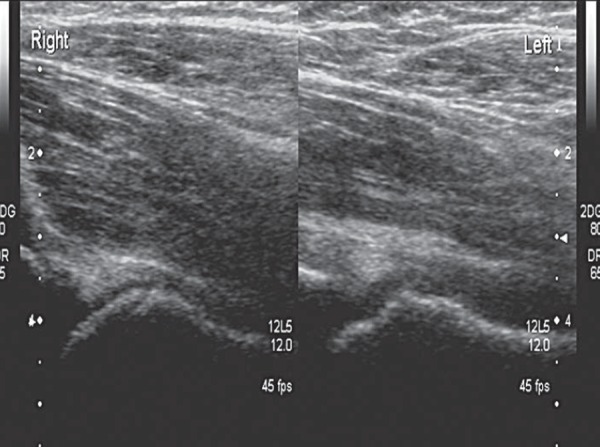
SCFE of left hip joint. Almost fused physis on the normal right side and the stepped appearance of physis on left.
Fig. 17c.
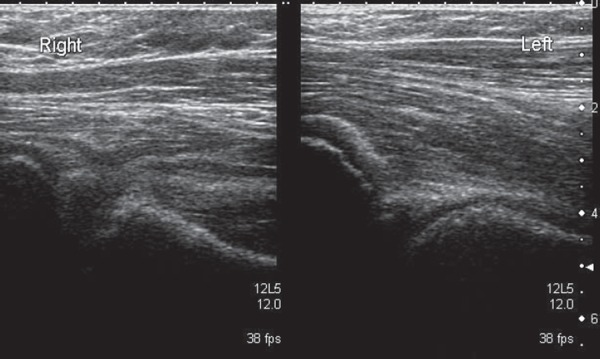
Non‐visualisation of right head of femur
Fig. 17d.
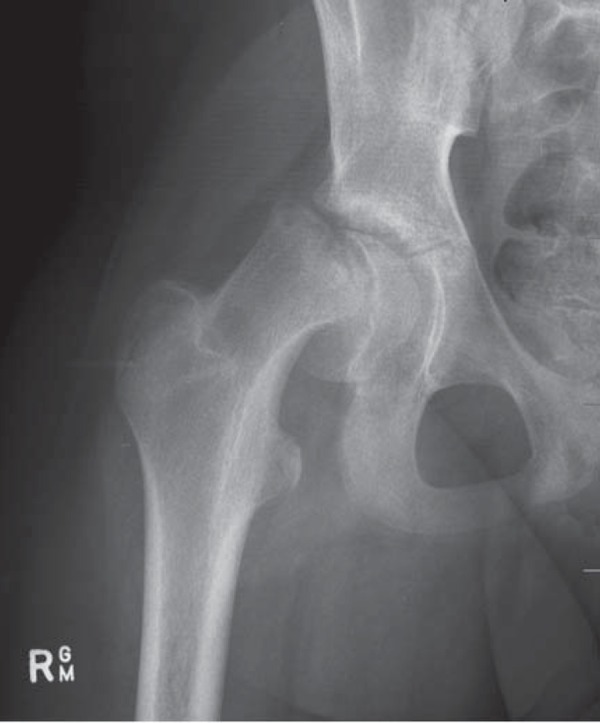
Xray of right hip with SCFE.
Haemarthrosis
Patients with haemophilia are prone to bleeding into joints and muscles with little or no injury. Recombinant factor VIII will alleviate symptoms quickly if the injury is muscular in nature. If the symptoms persist a haemarthrosis of the hip joint may be implicated. The joint capsule is distended by blood (Fig. 18). An ultrasound may differentiate between a bleed into the joint space or overlying muscle, such as the iliopsoas muscle. A haemarthrosis may cause increased pressure within the joint capsule, which can lead to vascular compromise and avascular necrosis.
Fig. 18.
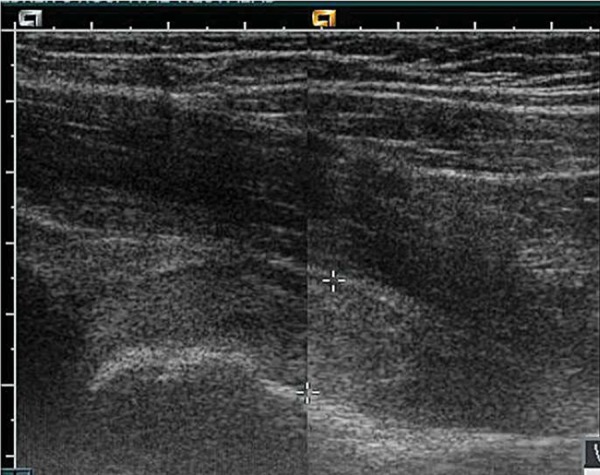
Haemarthrosis of left hip joint.
If dangerously increased pressure is suspected ultrasound‐guided aspiration of the joint space can alleviate pain and reduce pressure within the joint space 22 .
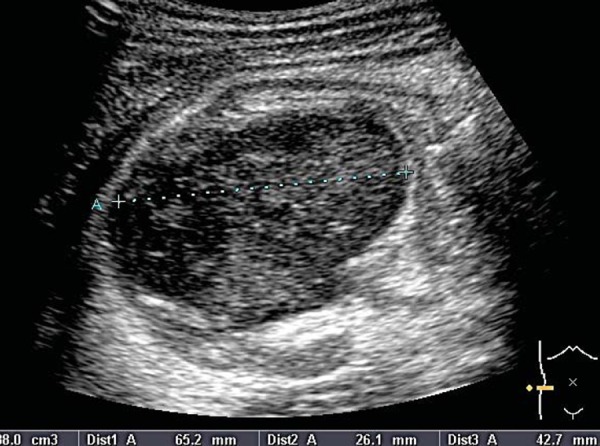
There is difficulty differentiating the joint capsule from the haemorrhage in Fig. 18 as they are almost isoechoic. This case demonstrates the value of imaging the contralateral hip to appreciate the difference (Fig. 19). The history will differentiate haemarthrosis from septic arthritis.
Fig. 19.
Haemarthrosis of left hip joint.
Condition simulating hip pathology
Abdominal pathology such as appendicitis, may cause hip pain, particularly when a pelvic abscess is present (Fig. 20). Iliopsoas haematoma or abscess (Fig. 21) may cause referred pain and refusal to weight bear. Pyomyositis of surrounding muscles of the hip will result in hip pain.
Fig. 20.
Abdominal abscess.
Fig. 21.
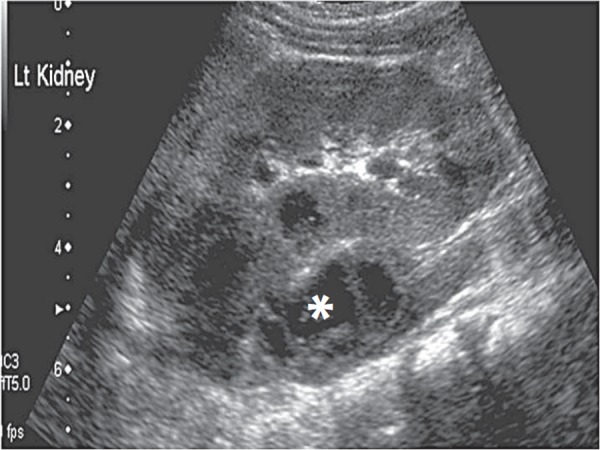
Left psoas abscess (∗).
Bone tumours such as Ewings sarcoma and systemic oncological causes such as recent onset leukaemia may present as isolated hip pain 10 .
Conclusion
We have reviewed the anatomy, ultrasonographic technique and findings in some of the more common conditions that may present when investigating a child with a limp.
While the ultrasonographic findings in many of these conditions are non‐specific, ultrasound is quick and easy to perform in an acute setting and is very sensitive in identifying hip effusions; it also allows for image‐guided paracentesis of hip effusion if indicated.
Imaging must include the head of femur as well as joint capsule. A joint effusion may be secondary to underlying pathology. Hyperaemia may not be present in an infectious process, especially if antibiotic cover has commenced.
Ultrasound remains an indispensable tool in the diagnosis and management of conditions common in a child with a limp.
References
- 1. Graf R. The diagnosis of congenital hip joint dislocation by the ultrasonic compound treatment. Arch Orthop Traumat 1980; 97: 117–33. [DOI] [PubMed] [Google Scholar]
- 2. Robben SGF, Lequin MH, Diepstraten FM, den Hollander JC, Entius CAC, Meradji M. Anterior joint capsule of the normal hip and in children with transient synovitis: US study with anatomical histological correlation. Radiology 1999; 210: 499–507. [DOI] [PubMed] [Google Scholar]
- 3. Zieger MM, Dorr U, Schulz RD. Ultrasonography of hip joint effusions. Skeletal Radiol 1987; 16: 607–11. [DOI] [PubMed] [Google Scholar]
- 4. Grisson LE, Harcke HT. Pediatric Musculoskeletal Ultrasound. In: Rumack CM, Wilson SR, Charboneau JW, editors. Johnson JAM, Assoc ed. Diagnostic Ultrasound (3rd ed). St Louis: Mosby; 2005. pp 2035–59. [Google Scholar]
- 5. Gordon JE, Huang M, Dobbs M, Luhmann SJ, Szymanski DA, Schoenecker PL. Causes of false‐negative ultrasound scans in the diagnosis of septic arthritis of the hip in children. J Pediatr Orthop 2002; 22: 312–6. [PubMed] [Google Scholar]
- 6. Skinner J, Glancy S, Beattie TF, Hendry GM. Transient synovitis: is there a need to aspirate hip joint effusions? Eur J Emergency Med 2002; 9: 15–8. [DOI] [PubMed] [Google Scholar]
- 7. Kermond, S , Barnett P, Graham HK. The use of Ibuprofen in the treatment of transient synovitis of the hip. J Paediatr Child Health 1999; 35 (5): A10. [Google Scholar]
- 8. Belthur, MV , Palazzi DL, Miller JA, Phillips WA, Weinberg J. A clinical analysis of shoulder and hip joint infections in children. J Pediatr Orthop 2009; 29: 828–33. [DOI] [PubMed] [Google Scholar]
- 9. Dennis, L . A 5‐Year old female child with groin, thigh and knee pain. Physician Assist 2001; 25 (8): 32–4. [Google Scholar]
- 10. Frick SL. Evaluation of the child who has hip pain. Orthoped Clin Nth Am 2006; 37: 133–40. [DOI] [PubMed] [Google Scholar]
- 11. Wagner‐Weiner L. Pediatric rheumatology for adult rheumatologist. J Clin Rheumatol 2008; 14 (2): 109–19. [DOI] [PubMed] [Google Scholar]
- 12. Goergens ED, McEvoy A, Watson M, Barrett IR. Acute osteomyelitis and septic arthritis in children. J Peadiatr Child Health 2005; 41 (1–2): 59–62. [DOI] [PubMed] [Google Scholar]
- 13. Givon U, Liberman, Schindler A, Blankstein A, Ganel A. Treatment of septic arthritis of the hip joint by repeated ultrasound‐guided aspirations. J Pediatr Orthop 2004; 15: 266–70. [DOI] [PubMed] [Google Scholar]
- 14. Song HR, Oh CW, Guille JT, Song K‐W, Kyung H‐S, Kim S‐Y, Park B‐C. lateral growth disturbance of the proximal femur in premature infants who had Neonatal Sepsis. J Pediatr Orthop B 2006; 15: 178–82. [DOI] [PubMed] [Google Scholar]
- 15. Mortia, M , Nakamura H, Kitano T. Comparison of clinical outcomes after treatment of hip arthritis caused by MRSA with that caused by non‐MRSA in infants. J Pediatr Orthop B 2009; 18: 1–5. [DOI] [PubMed] [Google Scholar]
- 16. Flynn JM, Mahta S. An Evidence‐based Approach to the Evaluation and Management of Hip Pain In Children. Pediatr Case Rev 2002; 2 (1): 26–32. [DOI] [PubMed] [Google Scholar]
- 17. Gent E, Antapur P, Fairhurst J, Taylor GR, Clarke NMP. Perthes' disease in the very young child. J Pediatr Orthop B 2006; 15: 16–22. [DOI] [PubMed] [Google Scholar]
- 18. Maus U, Andereya NI, Gravius S, Niedhart C, Ohnsorge JAK, Niehard FU. Perthes' or Perthes' resembling disease in 13‐month‐old boy. J Pediatr Orthop 2008; 17: 120–4. [DOI] [PubMed] [Google Scholar]
- 19. Shank CF, Thiel EJ, Klingele KE. Valgus Slipped Capital Femoral Epiphysis: Pervalence. Presentation and Treatment Options. J Pediatr Orthop 2010; 30 (2): 140–6. [DOI] [PubMed] [Google Scholar]
- 20. Nattrass GR, Pirpiris M. Pediatric Hip disorders. Curr Opin Orthopaed 2002; 13 (6): 393–400. [Google Scholar]
- 21. Kallio P, Lequesne G, Paterson D, Foster B, Jones J. Ultrasonography in Slipped Capital Femoral Epiphysis. J Bone Joint Surg 1991; 73‐B: 884–9. [DOI] [PubMed] [Google Scholar]
- 22. Robertson J, Connolly B, Hilliard P, Wedge J, Babyn P, Carcao M. Acute hamarthrosis of the hip joint: rapid convalelscence following ultrasound guided aspiration: 12PO76. Haemophilia 2008; 14 (2): 81. [DOI] [PubMed] [Google Scholar]


