Abstract
Office sonovaginography (SVG) challenges the concept of the “normal” pelvic ultrasound in women with chronic pelvic pain and suspected endometriosis. When positive for posterior compartment deep infiltrating endometriosis, with or without Pouch of Douglas obliteration, office SVG allows for triaging of women to the appropriately trained advanced laparoscopic gynaecological and colorectal surgical team. We advocate that all women with suspected endometriosis undergo office SVG prior to laparoscopy to ensure an accurate pre‐operative mapping of endometriosis disease location and extension.
Introduction
Traditional transvaginal (TVS) evaluation of the pelvis in women with chronic pelvic pain is an excellent diagnostic tool when unilateral or bilateral endometriomata are present 1 , 2 . This TVS hard marker correlates well with laparoscopic histological confirmation of endometriomata. In fact, TVS predicts endometriomata with a sensitivity and specificity of 90% and 96%, respectively 3 . Nearly 50% of these ovarian lesions are unilocular cystic lesions containing ground glass material (Fig. 1) 4 . Hyperechogenic wall foci can be seen in up to a third of endometriomas and are quite distinctive, as they are rarely found in other benign non‐resolving ovarian cysts (Fig. 2) 5 .
Fig. 1.
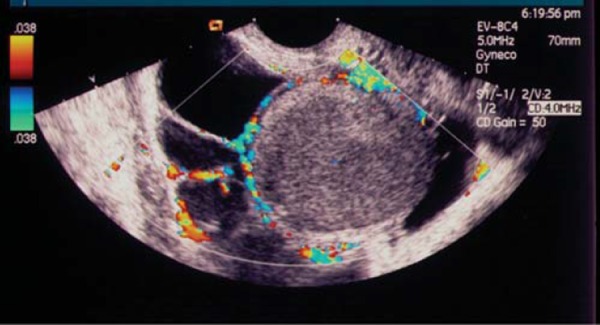
Endometrioma – unilocular cystic lesion with ground glass contents.
Fig. 2.
Endometrioma – unilocular cystic lesion with ground glass contents. Note the hyperechoic focus or “sludge” which is avascular on power Doppler assessment.
In the absence of endometriomata on ultrasound scan, the pelvis is more often than not reported to be radiologically “normal”. Despite this report of a “normal” pelvis on TVS, a significant proportion of these women with chronic pelvic pain will have endometriosis present at subsequent laparoscopy. Up to 60% of women with chronic pelvic pain will have an abnormal laparoscopy. At laparoscopy, endometriosis will be seen in 28% of cases and adhesions will be present in 25% of cases 6 . Superficial implants of endometriosis can be found in up to 15% of normal healthy women but are not visible with imaging 7 .
A report which reads “normal pelvis” can be misleading and does not assist the laparoscopic surgeon in preoperative mapping and planning in women with suspected endometriosis. We believe that pre‐operative staging of pelvic endometriosis is essential in not only determining the extension of disease, but also modulating the laparoscopic surgical approach 8 . In the hands of an experienced sonological operator, real‐time TVS can be a useful pre‐operative tool for the prediction of Pouch of Douglas (POD) obliteration and posterior compartment deep infiltrating endometriosis (DIE). Both these ultrasound features are almost always overlooked by sonographers and sonologists, thus resulting in a high proportion of women with chronic pelvic pain being potentially wrongfully classified as having a “normal” pelvis on pre‐operative ultrasound.
What can be done to improve the performance of preoperative ultrasound to predict endometriosis? How can we improve our ability to exclude POD obliteration and posterior DIE and in turn redefine a “normal” pelvic ultrasound when these ultrasound features are indeed absent?
These questions are important not only for the sonographers and sonologists who perform the ultrasound scans but more importantly for the laparoscopic surgeon.
In our study population, we have made a concerted effort to actively look for additional ultrasound features such as POD obliteration and posterior compartment DIE in an otherwise conventionally reported “normal” pelvis. There is no doubt a learning curve to this relatively new approach in Australia but the results are very encouraging indeed. In our unit, our provisional results have demonstrated a sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) for TVS in the prediction of POD obliteration prior to laparoscopy of 90.9%, 94.1%, 83.3% and 97%, respectively 10 . Bowel nodules are often associated POD obliteration 9 , and therefore, women with POD obliteration at pre‐operative TVS require tertiary referral to advanced laparoscopic centres, with the possible need for colorectal input (Fig. 3). Pre‐operative diagnosis is absolutely essential in these women, and the use of office sonovaginography (SVG) can bridge this gap. Interestingly, at this year's Australian Gynaecological Endoscopic Society (AGES) Annual Scientific Meeting in Melbourne, our data were recognised and awarded the prize for best presentation. The laparoscopic surgeons believe in this relatively new technique; it is time for the ultrasound community of Australia to follow suit.
Fig. 3.
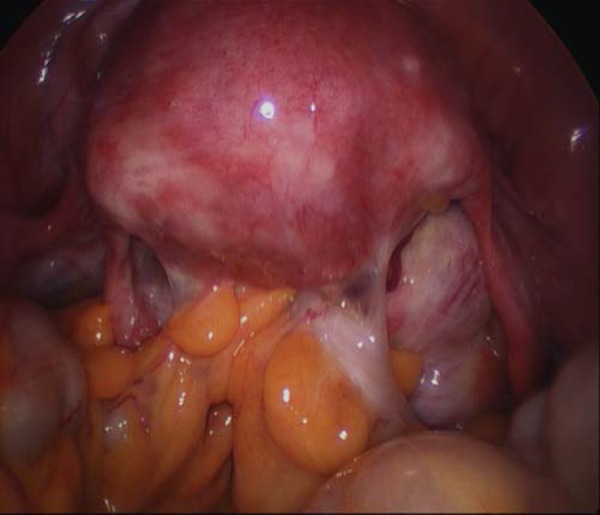
Obliterated Pouch of Douglas at laparoscopy (with permission from Elsevier, Khong, et al. JMIG, 2011, in press).
An estimated 5–10% of women with endometriosis will have bowel involvement, and it can be very difficult to predict this group pre‐operatively. DIE is usually found in the following locations: the uterosacral ligaments, the rectosigmoid junction, the anterior wall of the rectum, the vaginal wall and fournices, and the bladder. A systematic approach to evaluating the posterior compartment for DIE is imperative in all women with suspected endometriosis. Several imaging techniques have been used to predict of posterior compartment DIE prior to laparoscopy. These include TVS 11 – 14 , TVS with water contrast in the rectum 15 , 16 , SVG 17 , transrectal ultrasound (TRUS) 13 , sonorectovaginography 18 and MRI 19 , 20 . TVS is currently the recommended first‐line imaging technique for the prediction of DIE, which is due to its cost‐effectiveness, low level of patient discomfort, and high diagnostic accuracy (provided the scan is performed by an experienced operator).
During TVS, the posterior compartment can be assessed for bowel endometriosis by moving the probe up and down along the posterior vaginal wall from the lower vagina at level of anal canal to the posterior fornix. In 2009, Piketty, et al. used this technique to predict bowel infiltration with a sensitivity and specificity of 90.7% and 96.5%, respectively. In the same study, TRUS was compared with TVS. TRUS was found to have a slightly higher sensitivity and specificity than TVS for predicting DIE, but TRUS was not as well tolerated and was not as accurate in detecting USL and bladder endometriosis 13 .
A recent systematic review and meta‐analysis of 10 studies further confirmed the accuracy of TVS in the preoperative diagnosis of bowel endometriosis, with pooled estimates of sensitivities and specificities being 91% and 98%, and positive and negative predictive values being 98% and 95%, respectively 21 .
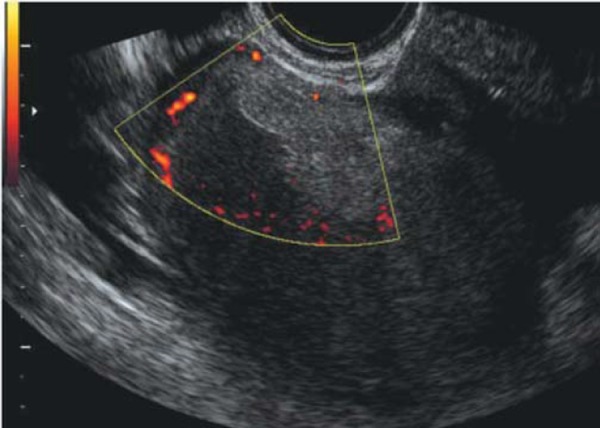
One of the weaknesses of TVS is the low sensitivity (29%) in the detection of nodules in the rectovaginal septum (RVS) and vaginal wall 22 . SVG can improve the detection of the deep infiltrating endometriosis involving the RVS by creating an acoustic window between the TV probe and the surrounding vaginal structures (Fig. 4). SVG was first described by Dessole, et al. in 2003, where saline was infused into the vagina to create an acoustic window, thereby improving visualisation of the rectovaginal septum. In this study SVG predicted rectovaginal endometriosis (Figs. 5a and 5b) more accurately than TVS, with a sensitivity and specificity of 90.6% and 85.7%, respectively 17 . We have used this originally described technique, however we found that as there is a need for a second operator (to keep the labia majora closed) and as the procedure is “messy” due to fluid overflow, its applicability in an office setting is less than desirable. More recently, Guirrera, et al. modified the SVG technique by placing gel within the probe cover to create a “stand‐off” effect (instead of infiltrating the vagina with saline), which also improved in the sensitivity and specificity for DIE in these locations. 23 We have also used this newer technique, however, the main limitation is that the vaginal fournices (both anterior and posterior) tend to adopt the outline of the filled condom, thus negating anatomical contours of the inner vagina.
Fig. 4.
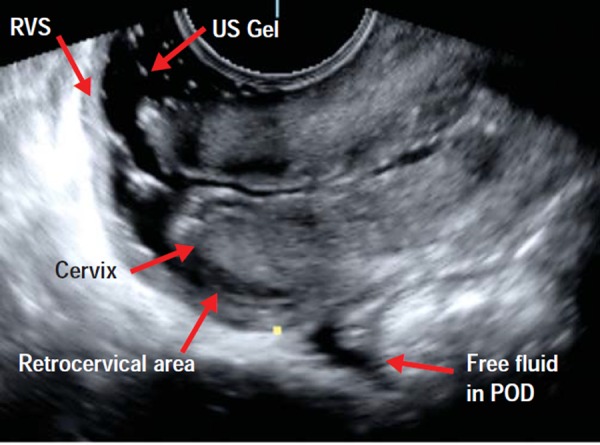
Normal posterior compartment as demonstrated with office gel sonovaginography. This image demonstrates a normal rectovaginal septum (RVS), normal retrocervical area, normal posterior vaginal fournix and a small amount of free fluid in the POD.
Fig. 5a.
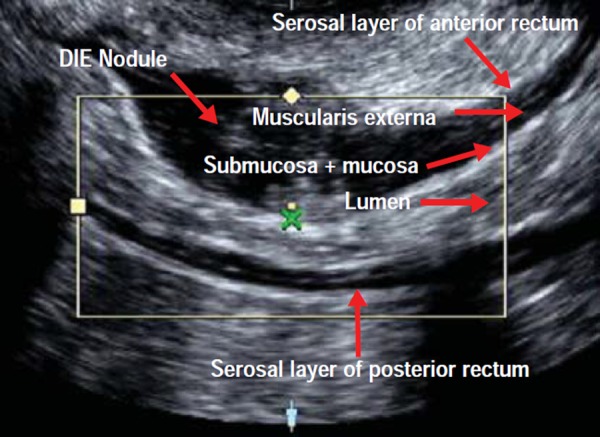
Posterior compartment deep infiltrating endometriosis as demonstrated with office gel sonovaginography. This image demonstrates a hypoechoic lesion which is located in the anterior rectal wall (this endometriotic nodule is extra‐mucosal, i.e. not full thickness).
Fig. 5b.
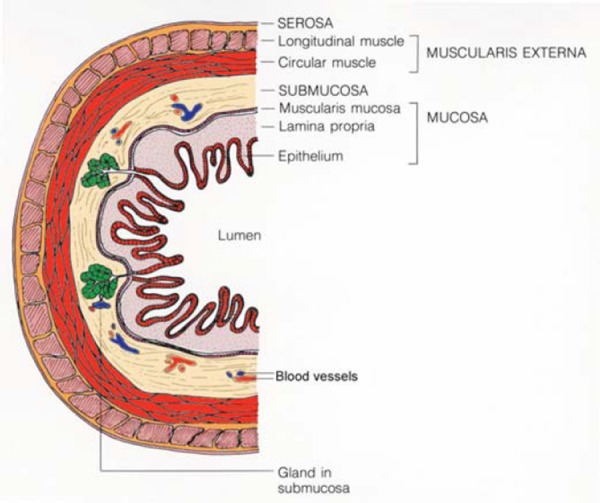
Cross‐sectional schematic representation of histological layers of the rectum. Each layer is clearly represented in the ultrasound image of Fig. 5a.
Our approach to office SVG, which has shown promising results, is the introduction of 10–20 mL of ultrasound gel into the posterior fornix of the vagina prior to imaging. This newly described technique does not require a second operator to keep the labia majora closed and the ultrasound gel distends the vagina and allows the anatomical contours of the inner vagina to be clearly visualised. Importantly, the ultrasound gel forms an acoustic window between the transvaginal probe and the surrounding vaginal structures, namely the anterior and posterior vaginal fournices, anterior and posterior vaginal walls, retrocervical area, uterosacral ligaments, Pouch of Douglas, rectovaginal septum, and rectosigmoid colon. We currently use this method of office SVG in our centre to predict midline posterior compartment DIE (i.e. rectovaginal, retrocervical, and rectosigmoid nodules) in women prior to endometriosis surgery (Figs. 5a and 5b).
Our preliminary results have found the sensitivity, specificity, NPV and PPV to be 100%, 91.7%, 70% and 100%, respectively 24 . It is important to note that the women included in our ongoing study are referred via tertiary endoscopic surgical units with a special interest in endometriosis surgery.
By including both TVS and office SVG in the pre‐operative assessment of women with suspected endometriosis, we can improve the prediction of POD obliteration and DIE prior to surgery. These imaging techniques demonstrate high specificity and NPV, and this additional pre‐operative imaging information is critical for the mapping/planning of advanced laparoscopic endometriosis surgery.
Office SVG challenges the concept of the “normal” pelvic ultrasound in women with chronic pelvic pain and suspected endometriosis, and allows for triaging of women to the appropriately trained advanced laparoscopic surgeon. We advocate that all women with suspected endometriosis undergo office SVG prior to laparoscopy to ensure an accurate pre‐operative mapping of endometriosis disease location and extension. This novel approach aims to provide additional information over and above a regular TVS, which in turn facilitates the planning of combined laparoscopic gynaecological and colo‐rectal procedures.
By performing a more detailed pre‐operative ultrasound assessment for severe endometriosis, we hope that in the future this will negate the need for women to undergo consecutive laparoscopies at different sites: one by the “general” gynaecologist and the second by the appropriately trained advanced laparoscopic endometriosis surgeon.
References
- 1. Mais V, Guerriero, Ajossa S, Angiolucci M, Paoletti AM, Melis GB. The efficiency of transvaginal ultrasound in the diagnosis of endometrioma. Fert Steril 1993; 60; 776–80. [DOI] [PubMed] [Google Scholar]
- 2. Guerriero, Mais V , Ajossa S, Melis GB. Predictive value of colour Doppler for ovarian endometrioma. Fert Steril 1995; 63: 1136–7. [DOI] [PubMed] [Google Scholar]
- 3. Jermy K, Luise C, Bourne T. The characterization of common ovarian cysts in premenopausal women. Ultrasound Obstet Gynecol 2001; 17: 140–4. [DOI] [PubMed] [Google Scholar]
- 4. Timmerman D, Valentin L, Bourne T, Collins WP, Verrelst H, Vergote I. International Ovarian Tumor Analysis (IOTA) Group . Terms, definitions and measurements to describe the sonographic features of adnexal tumors: a consensus opinionfrom the International Ovarian Tumor Analysis (IOTA) Group. Ultrasound Obstet Gynecol 2000: 16: 500–5. [DOI] [PubMed] [Google Scholar]
- 5. Patel MD, Feldstein VA, Chen DC, Lipson SD, Filly RA. Endometriomas: diagnostic performance of US. Radiology 1999; 213 (3): 930. [DOI] [PubMed] [Google Scholar]
- 6. Howard FM. The role of laparoscopy in chronic pelvic pain: promise and pitfalls. Obstet Gynecol Surv 1993; 48 (6): 357–87. [DOI] [PubMed] [Google Scholar]
- 7. Okaro E, Condous G, Khalid A, Timmerman D, Ameye L, VanHuffel S, Bourne T. The use of ultrasound‐based “soft markers” for the prediction of pelvic pathology in women with chronic pelvic pain, can we reduce the need for laparoscopy? BJOG 2006; 113: 251–6. [DOI] [PubMed] [Google Scholar]
- 8. Exacoustos C, Zupi E, Carusotti C, Rinaldo D, Marconi D, Lanzi G, et al. Staging of pelvic endometriosis: role of sonographic appearance in determining extension of disease and modulating surgical approach. J Am Assoc Gynecol Laparosc 2003; 10: 378–82. [DOI] [PubMed] [Google Scholar]
- 9. Khong SY, Bignardi T, Luscombe G, Lam A. Is Pouch of Douglas obliteration a marker of bowel endometriosis? J Minim Invasive Gynecol 2011. (In press). [DOI] [PubMed]
- 10. Reid S, Winder S, Lu C, Reid G, Abbott J, Cario G, Chou D, Condous G. Can we predict Pouch of Douglas obliteration in women with suspected endometriosis using a new real‐time transvaginal ultrasound technique? The “Sliding Sign”. World Congress on Endometriosis. Montpellier: 2011. (In press). [DOI] [PubMed] [Google Scholar]
- 11. Abrao MS, Goncalves MOC, Dias JA Jr, Podgaec S, Chamie LP, Blasbalg R. Comparison between clinical examination, transvaginal sonography and magnetic resonance imaging for the diagnosis of deep endometriosis. Hum Reprod 2007; 22: 3092–7. [DOI] [PubMed] [Google Scholar]
- 12. Bazot M, Malzy P, Cortez A. Roseau G, Amouyal P, Darai E. Accuracy of transvaginal sonography and rectal endoscopicsonography in the diagnosis of deep infiltrating endometriosis. Ultrasound Obstet Gynecol 2007; 30: 994–1001. [DOI] [PubMed] [Google Scholar]
- 13. Piketty M, Chopin N, Dousset B, Millischer‐Bellaische AE, Roseau G, Leconte M, Borghese B, Chapron C. Preoperative work‐up for patients with deeply infiltrating endometriosis: transvaginal ultrasonography must definitely be the first‐line imaging examination. Hum Reprod 2009; 24: 602–7. [DOI] [PubMed] [Google Scholar]
- 14. Hudelist G, Oberwinkler KH, Singer CF, Tuttlies F, Rauter G, Riter O, Keckstein J. Combination of transvaginal sonography and clinical examination for preoperative diagnosis of pelvic endometriosis. Hum Reprod 2009; 1: 1–7. [DOI] [PubMed] [Google Scholar]
- 15. Menada M Valenzano, Remorgida V, Abbamonte LH, Nicoletti A, Ragni N, Ferrero S. Does transvaginal ultrasonography combined with water‐contrast in the rectum aid in the diagnosis of rectovaginal endometriosis infiltrating the bowel? Hum Reprod 2008: 23 (5); 1069–75. [DOI] [PubMed] [Google Scholar]
- 16. Morotti M, Ferrero S, Bogliolo S, Venturini PL, Remorgida V, Menada M Valenzano. Transvaginal ultrasonography with water‐contrast in the rectum in the diagnosis of bowel endometriosis. Minerva Ginecol 2010; 62 (3): 179–85. [PubMed] [Google Scholar]
- 17. Dessole S, Farina M, Rubattu G, Cosmi E, Ambrosini G, Nardelli GB. Sonovaginography is a new technique for assessing rectovaginal endometriosis. Fertil Steril 2003; 79 (4): 1023–7. [DOI] [PubMed] [Google Scholar]
- 18. Bignardi T and Condous G. Sonorectovaginography: A new sonographic technique for imaging of the posterior compartment of the pelvis. J Ultrasound Med 2008; 27: 1479–83. [DOI] [PubMed] [Google Scholar]
- 19. Grasso RF, Di Giacomo V, Sedati P, Sizzi O, Florio G, Faiella E, Rossetti A, Del Vescovo R, Zobel B Beomonte. Diagnosis of deep infiltrating endometriosis: accuracy of magnetic resonance imaging and transvaginal 3D ultrasonography. Abdom Imaging 2010; 35 (6): 716–25. [DOI] [PubMed] [Google Scholar]
- 20. Hottat N, Larrousse C, Anaf V, Noël JC, Matos C, Absil J, Metens T. Endometriosis: Contribution of 3.0‐T pelvic MR imaging in preoperative assessment – initial results. Radiology 2009: 253: 126–34. [DOI] [PubMed] [Google Scholar]
- 21. Hudelist G, English J, Thomas AE, Tinelli A, Singer CF, and Keckstein J. Diagnostic accuracy of transvaginal ultrasound for noninvasive diagnosis of bowel endometriosis: systematic review and meta‐analysis. Ultrasound Obstet Gynecol 2011; 37: 257–63. [DOI] [PubMed] [Google Scholar]
- 22. Bazot M, Thomassin I, Hourani R, Cortez A, Darai E. Diagnostic accuracy of transvaginal sonography for deep pelvic endometriosis. Ultrasound Obstet Gynecol 2004; 24 (2): 180–5. [DOI] [PubMed] [Google Scholar]
- 23. Guerriero S, Ajossa S, Gerada M, D'Aquila M, Piras B, Melis GB. “Tenderness‐guided” transvaginal ultrasonography: a new method for the detection of deep endometriosis in patients with chronic pelvic pain. Fertil Steril. 2007; 88 (5): 1293–7. [DOI] [PubMed] [Google Scholar]
- 24. Reid S, Winder S, Lu C, Reid G, Abbott J, Cario G, Chou D, Condous G. Can we predict posterior compartment deep infiltrative endometriosis (DIE) using office sonovaginography (SVG) in women undergoing laparoscopy for chronic pelvic pain? World Congress on Endometriosis. Montpellier: 2011. (In press). [Google Scholar]


