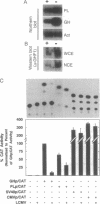
Abstract
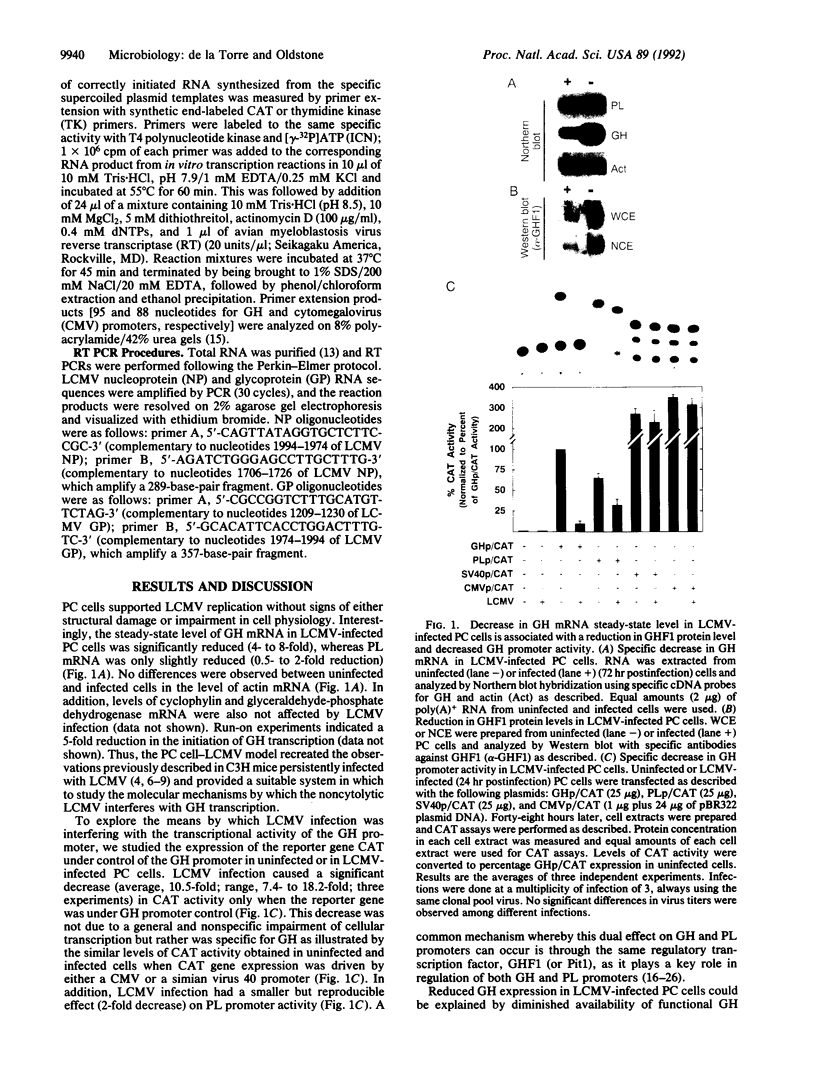
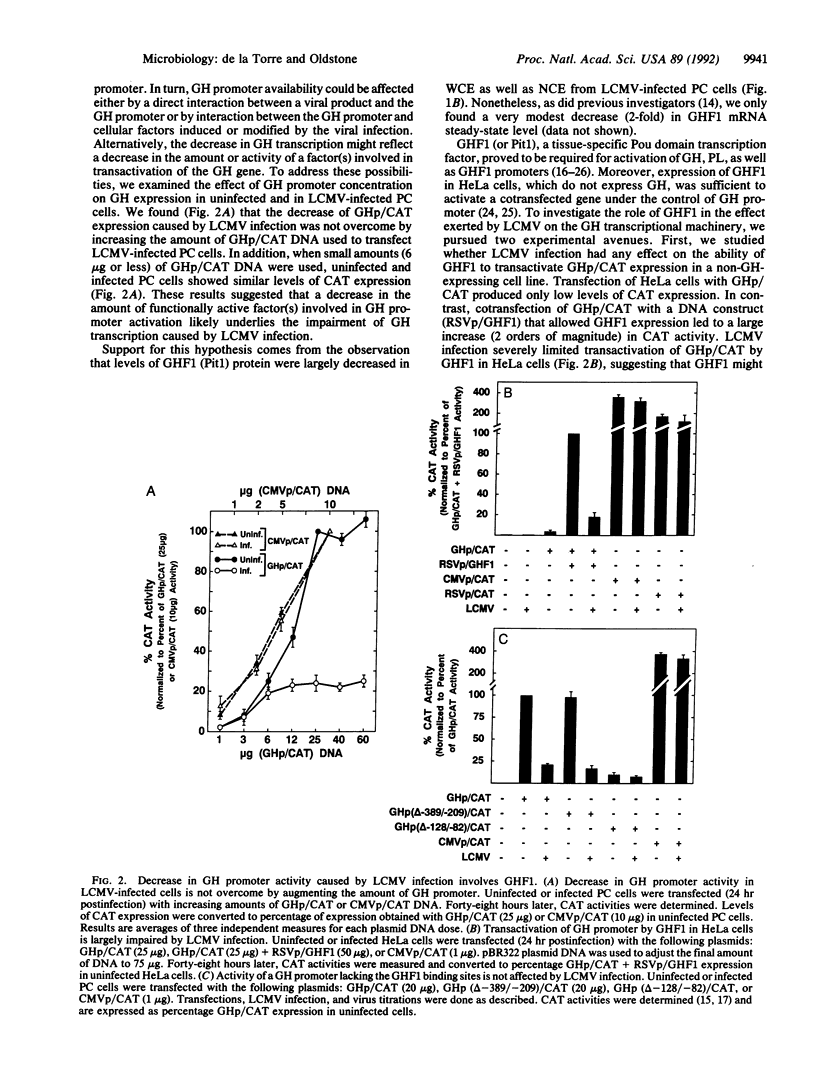
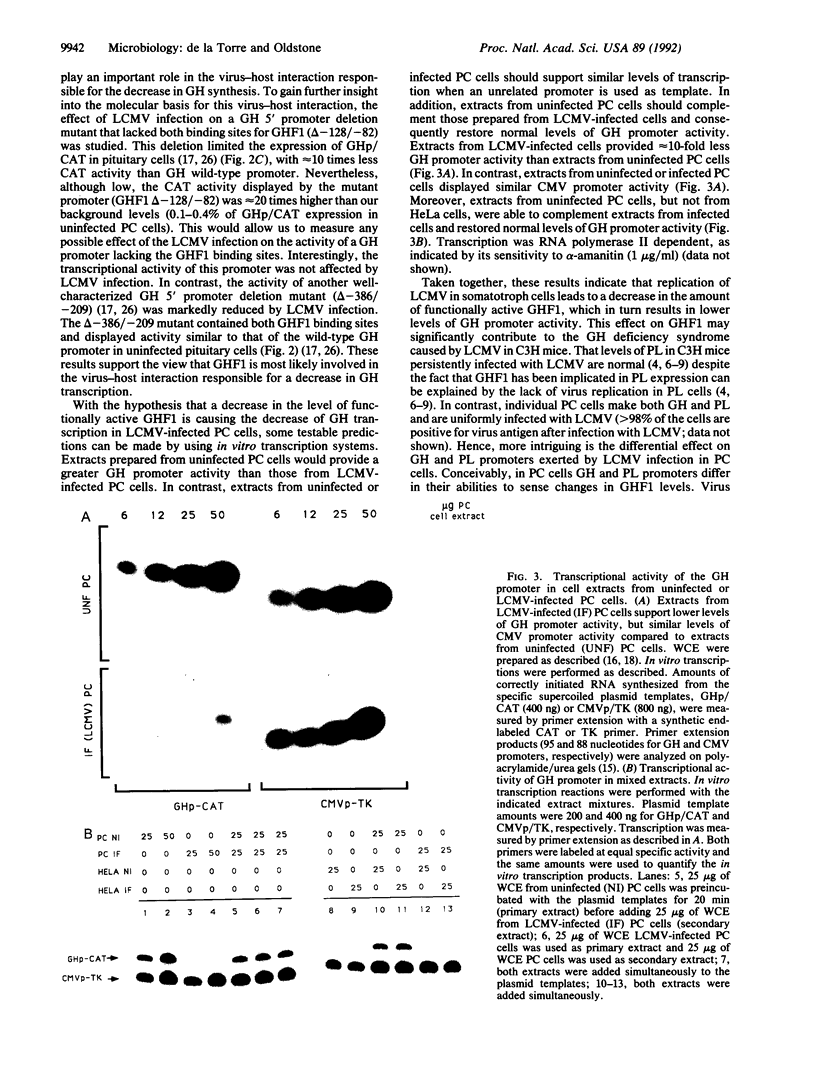
Viruses that establish persistent infections may show selective and unique effects on the host's transcriptional machinery. Lymphocytic choriomeningitis virus (LCMV), a noncytolytic virus, can persistently infect a rat pituitary cell line. Although the infected cells remain free of structural damage, virus markedly interferes with growth hormone (GH) but only minimally interferes with prolactin transcription. The study of GH promoter-chloramphenicol acetyltransferase-transfected cells and GH promoter deletion mutants demonstrates that the viral effect is at the level of GH promoter and is due to interference with GH transactivator factor GHF1 (Pit1). Treatment of LCMV-infected cells with the antiviral agent ribavirin cures the infection and restores normal GH mRNA levels. These results illustrate a molecular mechanism by which a virus infection can disrupt synthesis of a cell's differentiated product without perturbing vital cellular functions.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bateman A., Singh A., Kral T., Solomon S. The immune-hypothalamic-pituitary-adrenal axis. Endocr Rev. 1989 Feb;10(1):92–112. doi: 10.1210/edrv-10-1-92. [DOI] [PubMed] [Google Scholar]
- Bodner M., Castrillo J. L., Theill L. E., Deerinck T., Ellisman M., Karin M. The pituitary-specific transcription factor GHF-1 is a homeobox-containing protein. Cell. 1988 Nov 4;55(3):505–518. doi: 10.1016/0092-8674(88)90037-2. [DOI] [PubMed] [Google Scholar]
- Bodner M., Karin M. A pituitary-specific trans-acting factor can stimulate transcription from the growth hormone promoter in extracts of nonexpressing cells. Cell. 1987 Jul 17;50(2):267–275. doi: 10.1016/0092-8674(87)90222-4. [DOI] [PubMed] [Google Scholar]
- Castrillo J. L., Theill L. E., Karin M. Function of the homeodomain protein GHF1 in pituitary cell proliferation. Science. 1991 Jul 12;253(5016):197–199. doi: 10.1126/science.1677216. [DOI] [PubMed] [Google Scholar]
- Chen R. P., Ingraham H. A., Treacy M. N., Albert V. R., Wilson L., Rosenfeld M. G. Autoregulation of pit-1 gene expression mediated by two cis-active promoter elements. Nature. 1990 Aug 9;346(6284):583–586. doi: 10.1038/346583a0. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Brar A., Frohman L. A. Growth hormone synthesis and secretion by a somatomammotroph cell line derived from normal adult pituitary of the rat. Endocrinology. 1988 Nov;123(5):2276–2283. doi: 10.1210/endo-123-5-2276. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Dana S., Karin M. Induction of human growth hormone promoter activity by the adenosine 3',5'-monophosphate pathway involves a novel responsive element. Mol Endocrinol. 1989 May;3(5):815–821. doi: 10.1210/mend-3-5-815. [DOI] [PubMed] [Google Scholar]
- Dollé P., Castrillo J. L., Theill L. E., Deerinck T., Ellisman M., Karin M. Expression of GHF-1 protein in mouse pituitaries correlates both temporally and spatially with the onset of growth hormone gene activity. Cell. 1990 Mar 9;60(5):809–820. doi: 10.1016/0092-8674(90)90095-v. [DOI] [PubMed] [Google Scholar]
- Dutko F. J., Oldstone M. B. Genomic and biological variation among commonly used lymphocytic choriomeningitis virus strains. J Gen Virol. 1983 Aug;64(Pt 8):1689–1698. doi: 10.1099/0022-1317-64-8-1689. [DOI] [PubMed] [Google Scholar]
- Fox S. R., Jong M. T., Casanova J., Ye Z. S., Stanley F., Samuels H. H. The homeodomain protein, Pit-1/GHF-1, is capable of binding to and activating cell-specific elements of both the growth hormone and prolactin gene promoters. Mol Endocrinol. 1990 Jul;4(7):1069–1080. doi: 10.1210/mend-4-7-1069. [DOI] [PubMed] [Google Scholar]
- Ingraham H. A., Chen R. P., Mangalam H. J., Elsholtz H. P., Flynn S. E., Lin C. R., Simmons D. M., Swanson L., Rosenfeld M. G. A tissue-specific transcription factor containing a homeodomain specifies a pituitary phenotype. Cell. 1988 Nov 4;55(3):519–529. doi: 10.1016/0092-8674(88)90038-4. [DOI] [PubMed] [Google Scholar]
- Kapiloff M. S., Farkash Y., Wegner M., Rosenfeld M. G. Variable effects of phosphorylation of Pit-1 dictated by the DNA response elements. Science. 1991 Aug 16;253(5021):786–789. doi: 10.1126/science.1652153. [DOI] [PubMed] [Google Scholar]
- Klavinskis L. S., Oldstone M. B. Lymphocytic choriomeningitis virus selectively alters differentiated but not housekeeping functions: block in expression of growth hormone gene is at the level of transcriptional initiation. Virology. 1989 Feb;168(2):232–235. doi: 10.1016/0042-6822(89)90262-6. [DOI] [PubMed] [Google Scholar]
- Lefevre C., Imagawa M., Dana S., Grindlay J., Bodner M., Karin M. Tissue-specific expression of the human growth hormone gene is conferred in part by the binding of a specific trans-acting factor. EMBO J. 1987 Apr;6(4):971–981. doi: 10.1002/j.1460-2075.1987.tb04847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Crenshaw E. B., 3rd, Rawson E. J., Simmons D. M., Swanson L. W., Rosenfeld M. G. Dwarf locus mutants lacking three pituitary cell types result from mutations in the POU-domain gene pit-1. Nature. 1990 Oct 11;347(6293):528–533. doi: 10.1038/347528a0. [DOI] [PubMed] [Google Scholar]
- Mangalam H. J., Albert V. R., Ingraham H. A., Kapiloff M., Wilson L., Nelson C., Elsholtz H., Rosenfeld M. G. A pituitary POU domain protein, Pit-1, activates both growth hormone and prolactin promoters transcriptionally. Genes Dev. 1989 Jul;3(7):946–958. doi: 10.1101/gad.3.7.946. [DOI] [PubMed] [Google Scholar]
- McCormick A., Wu D., Castrillo J. L., Dana S., Strobl J., Thompson E. B., Karin M. Extinction of growth hormone expression in somatic cell hybrids involves repression of the specific trans-activator GHF-1. Cell. 1988 Oct 21;55(2):379–389. doi: 10.1016/0092-8674(88)90061-x. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B., Ahmed R., Buchmeier M. J., Blount P., Tishon A. Perturbation of differentiated functions during viral infection in vivo. I. Relationship of lymphocytic choriomeningitis virus and host strains to growth hormone deficiency. Virology. 1985 Apr 15;142(1):158–174. doi: 10.1016/0042-6822(85)90430-1. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B., Blount P., Southern P. J., Lampert P. W. Cytoimmunotherapy for persistent virus infection reveals a unique clearance pattern from the central nervous system. Nature. 1986 May 15;321(6067):239–243. doi: 10.1038/321239a0. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B., Rodriguez M., Daughaday W. H., Lampert P. W. Viral perturbation of endocrine function: disordered cell function leads to disturbed homeostasis and disease. Nature. 1984 Jan 19;307(5948):278–281. doi: 10.1038/307278a0. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B. Viral alteration of cell function. Sci Am. 1989 Aug;261(2):42–48. doi: 10.1038/scientificamerican0889-42. [DOI] [PubMed] [Google Scholar]
- Valsamakis A., Riviere Y., Oldstone M. B. Perturbation of differentiated functions in vivo during persistent viral infection. III. Decreased growth hormone mRNA. Virology. 1987 Feb;156(2):214–220. doi: 10.1016/0042-6822(87)90400-4. [DOI] [PubMed] [Google Scholar]
- de la Torre J. C., Borrow P., Oldstone M. B. Viral persistence and disease: cytopathology in the absence of cytolysis. Br Med Bull. 1991 Oct;47(4):838–851. doi: 10.1093/oxfordjournals.bmb.a072515. [DOI] [PubMed] [Google Scholar]