Abstract
Background: The use of ultrasound to evaluate cervical anatomy and to guide tracheal puncture in real‐time has been advocated to improve safety and efficacy of percutaneous dilatational tracheostomy (PDT) in intensive care.
Objective: To review the potential role, attributed theoretical benefits and supporting literature for ultrasound during PDT.
Results: A significant number of mostly observational studies and case series support this modality. Real‐time guidance enables clear visualisation of anatomical landmarks and results in a consistently high success and low complication rate, with appropriate positioning of the tracheal puncture. Recognition of unconventional vascular anatomy enables selection of an appropriate alternative puncture site or an elective open surgical approach.
Conclusion: Current literature supports that using ultrasound for percutaneous tracheostomy is quick, safe, reliable and offers a plausible advantage over the traditional landmark guided procedure, especially in select patient groups, such as those who are morbidly obese or have difficult to palpate cervical anatomy.
Keywords: dilatational, percutaneous, tracheostomy, trachea
Introduction
With increasing availability of affordable mobile ultrasound systems, real‐time ultrasound guidance is now widely used during routine invasive procedures such as insertion of percutaneous drains and vascular access catheters. The role of ultrasound during various airway procedures has also been the focus of attention in recent years. This article reviews the potential role, attributed theoretical benefits and supporting literature for using ultrasound during percutaneous dilatational tracheostomy in the critical care patient population.
Background
Patients in intensive care frequently require tracheostomy for long‐term ventilator support and percutaneous dilatational tracheostomy (PDT) has become established as the preferred method over surgical tracheostomy, 1 which is now reserved for select cases. Although overall complication rate is low, PDT remains one of the few procedures routinely undertaken in intensive care where serious adverse events including death are still reported. Most serious bleeding complications in the immediate peri‐procedural setting are related to unrecognised and unanticipated anatomical variations of vascular anatomy. 2 , 3 In the case of serious late bleeding complications, positioning of the tracheostomy either too distally or too laterally is thought to be a significant factor contributing to eventual erosion of the tracheostomy tube into a blood vessel. 4 , 5 Tracheal tube malposition, resulting in the tube lying at an angle to the tracheal axis and abutting the lateral or posterior tracheal wall can cause partial tube lumen obstruction, which has been implicated in failure to wean from ventilation and otherwise unexplained episodes of desaturation. 6 , 7 Although the exact aspects of tracheostomy placement leading to this complication are not well understood, lateral (off midline) or axial (too caudal or cranial) malposition may play an important role. Placement too proximally – especially between the cricoid cartilage and the first tracheal ring – is associated with an increased rate of late tracheal stenosis. 8 Bronchoscopic guidance during the procedure has been widely used as an additional safety adjunct to confirm intra‐tracheal placement of the guide‐wire before the tract is dilated, this however provides little additional assistance in appropriately positioning the initial puncture or avoiding aberrant vessels. With the increasingly wide availability of bedside ultrasonography in the intensive care setting, pre‐procedural and real‐time intra procedural ultrasound guidance has been advocated as a potential tool to further improve safety and efficacy of the procedure. 9 – 11
Not uncommonly, patients requiring a tracheostomy will have anatomy that with regards to a PDT will be considered difficult. This is most often due to morbid obesity or anatomical deformity from chronic musculoskeletal pathology or prior injuries and surgical procedures. This can make palpation of the traditionally used anatomical features difficult or even impossible. 12 – 14 This in turn can lead to an increase in the rate of procedural complications, failed and multiple puncture attempts. Suboptimal position of the tracheal puncture can subsequently lead to tracheostomy tube malposition. The difficulties encountered in locating anatomical landmarks such as the crico‐thyroid membrane and difficulties with tracheal puncture when the tracheal anatomy is not readily palpable have been highlighted by recent publications. 15 Ultrasound in such a setting performs well in a simulated environment 16 , 17 and has been demonstrated to be useful in clinical practice. 18
Figure 1.
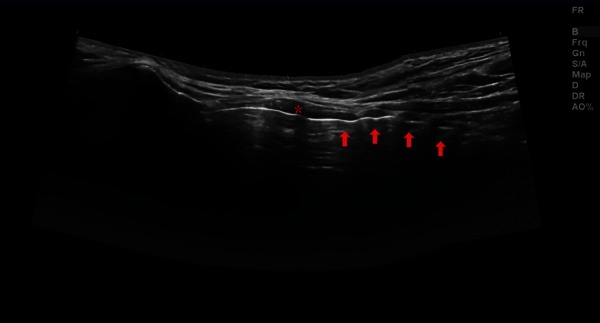
Panoramic longitudinal view. Asterisk = cricoid cartilage, arrows = tracheal rings.
Figure 2.
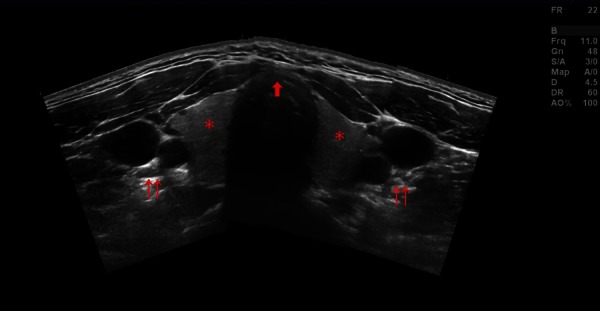
Panoramic transverse view. Asterisk = thyroid gland, double arrows = left and right carotid artery and jugular vein, single arrow = tracheal cartilage, ideal puncture site in the anterior midline.
Ultrasound use in percutaneous dilatational tracheostomy
The theoretical advantage of using pre‐procedural ultrasound lies with the ability to identify relevant cervical anatomy and aberrant pre‐tracheal vasculature in order to avoid immediate vascular complications. 19 It may also aid in proper selection of tracheostomy tube size and length, especially in patients with an increased pre‐tracheal soft tissue diameter or in children. 20 Infra‐procedural (i.e. real‐time) ultrasound may assist not only by revealing potentially aberrant vessels 21 but also with identifying the preferred puncture location and guiding the needle puncture of the trachea. The technique is not dissimilar to that routinely used during ultrasound guided vascular access.
Ultrasonographic anatomy of the anterior neck with consideration to the implications for tracheostomy was first described in 1995 22 and a report of real‐time ultrasound guided puncture for percutaneous tracheostomy was first published in 1999. 23 2D ultrasound using a linear array probe readily identifies the position and anatomical relation of all important landmarks. These include the thyroid and cricoid cartilage, the tracheal rings, the thyroid gland and the carotid and jugular vessels. Aberrant vascular structures crossing the midline should be noted and can further be evaluated by colour or spectral Doppler. Real time imaging can be used to identify the desired level of puncture in a sagittal plane in the midline over the trachea, while a ninety degree rotation of the probe allows for an out of plane approach to guiding the needle tip (represented by an acoustic shadow) towards the midline. Results using this technique have been described by Chacko, et al. 24
Figure 3a and 3b.
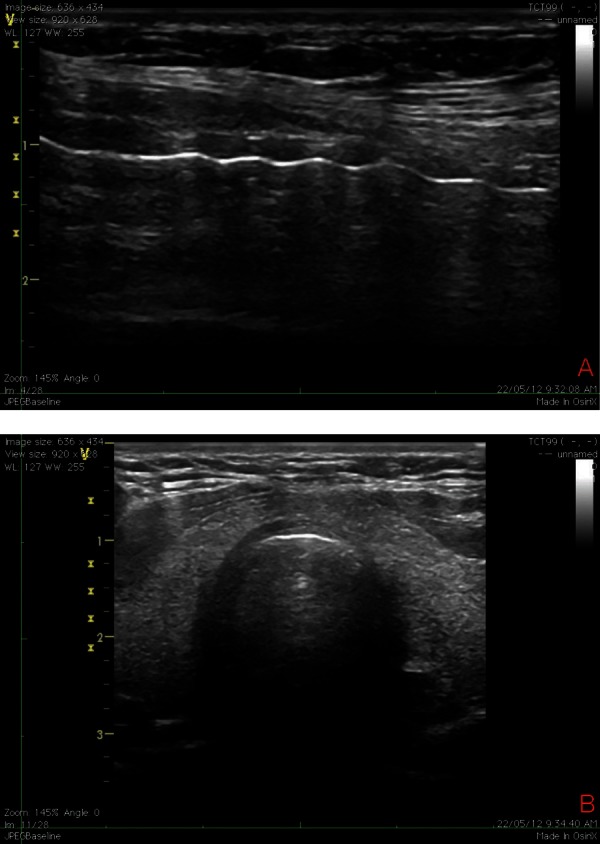
Actual longitudinal (A) and transverse (B) views obtained with a linear probe during bedside percutaneous tracheostomy.
Pre‐procedural ultrasound examination of the neck
The utility of pre‐procedural scanning of the neck has been investigated by a number of authors. Bonde, et al. describe 28 consecutive patients over a study period of one year who underwent PDT. 25 The authors report a change in the planned location of the tracheal puncture in nine of the 28 patients, mostly in an attempt to avoid puncture of the thyroid isthmus as well as three cases of electively ligating vessels during the procedure based on ultrasound findings. Complication rate is low with only two cases of minor bleeding reported. Patients with severe coagulopathy as well as those with short necks or those who were morbidly obese were excluded. Kollig, et al. reported 72 consecutive patients requiring PDT over a 22 month period. 26
Figure 4.
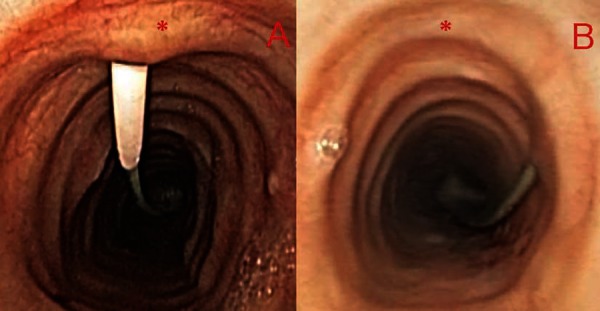
Bronchoscopic view of appropriately positioned tracheal puncture (A) and example of extremely lateral puncture with potential for complications (B). The asterisk marks the anterior tracheal midline.
Figure 5.
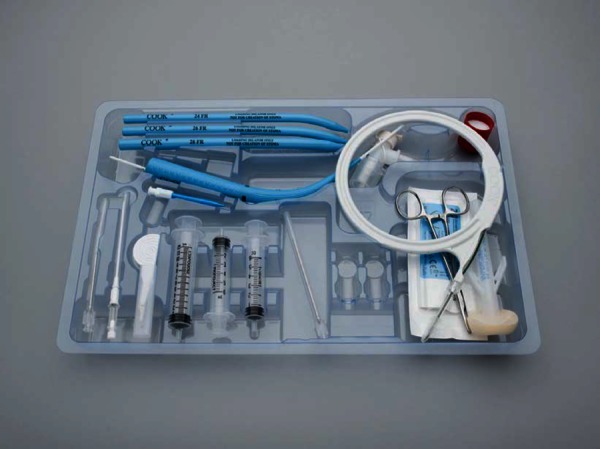
Percutaneous tracheostomy kit – Ciaglia single dilator method. Using the Seldinger technique, needle puncture of the trachea is followed by placement of the guide wire through the needle. Dilatation of the tract is carried out using the single tapered dilator followed by placement of the tracheostomy tube over the guide wire. Permission for use granted by Cook Medical Incorporated, Bloomington, Indiana.
All patients underwent pre‐procedure ultrasound evaluation of the neck followed by percutaneous tracheostomy, with bronchoscopic control. Ultrasound findings resulted in a change from the planned tracheal puncture site in 23.6% of patients. The reported complication rate is low with only one case of minor peri‐procedural bleeding in the group.
Real‐time ultrasound guidance for tracheal puncture during percutaneous tracheostomy
Šustić et al. retrospectively examined the en‐bloc resected tracheas of twenty‐six consecutive intensive care patients who underwent PDT but later died. 27 Fifteen patients had conventional landmark guided PDT and in 11 patients PDT was carried out using real‐time ultrasound guidance. The indication for using ultrasound was a not clearly palpable cricoid or otherwise challenging anatomy. Five patients (33%) in the landmark group had cranially displaced tracheostomy tubes – defined as being between the cricoid cartilage and the first tracheal ring – whereas no patient was found to have cranial misplacement in the real‐time ultrasound group (P < 0.05). Fractured tracheal rings were found in 43% vs. 36% of patients in the two groups respectively (P = NS). The same authors have also published their results from a 26–month period where they randomly assigned adult intensive care patients who had acute cervical cord injury with subsequent anterior cervical spinal fusion and required tracheostomy, to either surgical tracheostomy (ST) or real‐time ultrasound guided PDT 28 Complication rate was low with one case of minor peri‐procedural bleeding in each group. Two cases of wound infection were noted in the surgical group, no infections occurred with PDT. The average time required for performing the procedure was eight vs. 21 minutes (P < 0.05) in the PDT and ST groups respectively. Recently Rajajee, et al. demonstrated that real‐time ultrasound guidance was used to appropriately position the tracheal puncture as confirmed by bronchoscopy in thirteen patients. 29 The authors observed no significant peri‐procedural complication. Their results are mirrored by the findings of Chacko, et al. in a larger series of 62 patients. 24 In another recent publication Guinot, et al. prospectively evaluated the implications of obesity in ultrasound guided tracheostomy in intensive care patients. 30 Over an 18–month period, 50 consecutive patients who underwent the procedure were assessed in two groups based on body mass index (BMI). Median BMI was 34 vs. 25, (P < 0.001) respectively. The investigators utilised both pre‐procedure and real‐time intra procedure ultrasound. There was no difference in time required to perform the tracheostomy or complication rate, which involved only minor complications and was low in both groups. Patients with platelet counts below 80.000/mm–3 or INR above 1.2 were however excluded. Notably, the authors report that the location of the tracheal puncture, as compared to that determined by landmark technique prior to ultrasound examination, was changed based on the ultrasound findings in 50% of patients, and that this was due to aberrant vasculature in 32%.
Discussion
Ultrasound guidance, either peri‐procedurally or real‐time, has been proposed as an additional measure to improve safety and efficacy of percutaneous dilatational tracheostomy and receives mention in the recently published ANZICS Percutaneous Dilatational Tracheostomy Consensus Statement. 31
Although to date no study has compared pre– or intra‐procedural ultrasound guidance to the traditional landmark approach in a prospective randomised controlled fashion, a significant number of observational studies and case series appear to support the utility of this modality. Publications consistently report a low peri‐procedural complication and high success rate, which is on par or better than historical data. Plausible advantage arises from being able to avoid aberrant vascular structures and from easy visual localisation of important anatomical landmarks, which may at times be impossible to locate by palpation. Interestingly in the three studies that did report on this, the percentage of cases where the intended puncture site was changed based on ultrasound findings was quite high, 23.6%, 32.1% and 50% respectively. This implies that a large number of conventional tracheal punctures may not be performed at the perceived ideal anatomical location, though this may not translate directly to a similarly striking difference in complication rates. On the other hand, many of the postulated benefits relating to appropriate positioning of the tracheostomy tube relate to avoidance of mid to long‐term complications. As no study has undertaken long‐term follow‐up, the extent of this potential benefit cannot be adequately assessed based on current literature. It should also be noted that significant complications both immediate and longer term are relatively rare, therefore a large sample size would be required to detect and demonstrate a statistically significant effect.
Conclusion
Despite the lack of high quality supporting evidence, overall available data appear to suggest that using ultrasound for percutaneous tracheostomy is quick, safe, reliable and offers a plausible advantage over the traditional landmark guided procedure, especially in select patient groups. These include the morbidly obese and those who have difficult to palpate or unconventional cervical anatomy. A randomised controlled trial comparing the use of ultrasound to a traditional landmark guided approach during PDT would be required to provide more definitive information on the role and degree of potential benefit offered by adopting this modality in routine day‐to‐day intensive care practice.
Appendix A
Recommendations on tracheostomy position and technique
Percutaneous tracheostomy in intensive care is performed at the bedside under general plus local anaesthesia, using an aseptic seldinger technique.
The optimal position for the tracheal puncture is in the anterior midline between the 1st and 4th tracheal rings.
Recognising unconventional anatomy and avoiding large vascular structures is important.
Avoiding the thyroid isthmus is often not possible and is not felt to be necessary.
Tips on ultrasound technique
Pre‐procedure scanning is useful to identify aberrant vasculature and has also been used to mark the ideal puncture location. The marking method however carries inherent risks due to the mobility of the skin related to underlying structures especially in patients with a significant pre‐tracheal soft tissue diameter.
Real‐time guidance provides the most reliable information on puncture position. Both in and out of plane techniques have been described. The in‐plane approach is often not feasible due to lack of physical space especially in patients with short necks. The perpendicular angle of puncture during this procedure also makes in plane visualisation difficult. Once the planned level of puncture is identified in a longitudinal plane, an out of plane approach is recommended where a transverse probe position just above the level of the planned puncture and slight caudal angulation usually allows for good visualisation and direction of the needle tip towards the tracheal midline.
In evaluating vascular structures it is important to ensure that structures are not compressed by excessive pressure from the probe. In addition to 2D imaging, colour and spectral Doppler may also be of use. If clinically not contraindicated, a slight head down position may help to distend collapsed vessels, however this technique is only practical prior to the procedure.
References
- 1. Delaney A, Bagshaw SM, Nalos M. Percutaneous dilatational tracheostomy versus surgical tracheostomy in critically ill patients: a systematic review and meta‐analysis. Crit Care 2006; 10 (2): R55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McCormick B, Manara AR. Mortality from percutaneous dilatational tracheostomy. A report of three cases. Anaesthesia 2005; 60 (5): 490–95. [DOI] [PubMed] [Google Scholar]
- 3. Shlugman D. Acute fatal haemorrhage during percutaneous dilatational tracheostomy. Br J Anaesth 2003; 90 (4): 517–20. [DOI] [PubMed] [Google Scholar]
- 4. Ayoub OM, Griffiths MV. Aortic Arch laceration: a lethal complication after percutaneous tracheostomy. Laryngoscope 2007; 117 (1): 176–78. [DOI] [PubMed] [Google Scholar]
- 5. Grant CA. Tracheo‐innominate artery fistula after percutaneous tracheostomy: three case reports and a clinical review. Br J Anaesth 2006; 96(1): 127–31. [DOI] [PubMed] [Google Scholar]
- 6. Schmidt U, Hess D, Kwo J, Lagambina S, Gettings E, Khandwala F, et al. Tracheostomy tube malposition in patients admitted to a respiratory acute care unit following prolonged ventilation. Chest 2008; 134 (2): 288–94. [DOI] [PubMed] [Google Scholar]
- 7. Polderman KH, Spijkstra JJ, de Bree R, Christiaans HM, Gelissen HP, Wester JP, et al. Percutaneous dilatational tracheostomy in the ICU: optimal organization, low complication rates, and description of a new complication. Chest 2003; 123 (5): 1595–602. [DOI] [PubMed] [Google Scholar]
- 8. Sarper A, Ayten A, Eser I, Ozbudak O, Demircan A. Tracheal stenosis after tracheostomy or intubation: review with special regard to cause and management. Texas Heart Institute journal / from the Texas Heart Institute of St. Luke's Episcopal Hospital, Texas Children's Hospital, 2005; 32 (2), 154–58. [PMC free article] [PubMed] [Google Scholar]
- 9. Flint AC, Midde R, Rao VA, Lasman TE, Ho PT. Bedside ultrasound screening for pretracheal vascular structures may minimize the risks of percutaneous dilatational tracheostomy. Neurocrit Care 2009; 11 (3): 372–76. [DOI] [PubMed] [Google Scholar]
- 10. Hatfield A, Bodenham A. Portable ultrasonic scanning of the anterior neck before percutaneous dilatational tracheostomy. Anaesthesia 1999; 54 (7): 660–63. [DOI] [PubMed] [Google Scholar]
- 11. Tremblay LN, Scales DC. Ultrasound‐guided tracheostomy – not for the many, but perhaps the few… or the one. Crit Care 2011; 15 (2): 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kost KM. Endoscopic Percutaneous dilatational tracheotomy: a prospective evaluation of 500 consecutive cases. Laryngoscope 2005; 115 (S107). [DOI] [PubMed] [Google Scholar]
- 13. Husein OF, Massick DD. Cricoid palpability as a selection criterion for bedside tracheostomy. (2005). Otolaryngol Head Neck Surg 2005; 133 (6): 839–44. [DOI] [PubMed] [Google Scholar]
- 14. Byhahn C, Lischke V, Meininger D, Halbig S, Westphal K. Perioperative complications during percutaneous tracheostomy in obese patients. Anaesthesia 2005; 60 (1): 12–15. [DOI] [PubMed] [Google Scholar]
- 15. Elliott DS, Baker PA, Scott MR, Birch CW, Thompson JM. Accuracy of surface landmark identification for cannula cricothyroidotomy Anaesthesia 2010; 65 (9): 889–94. [DOI] [PubMed] [Google Scholar]
- 16. Dinsmore J, Heard AM, Green RJ. The use of ultrasound to guide time‐critical cannula tracheotomy when anterior neck airway anatomy is unidentifiable. Eur J Anaesthesiol 2011; 28 (7): 506–10. [DOI] [PubMed] [Google Scholar]
- 17. Pearson N, Huebner K. The use of ultrasound guidance for cricothyroidotomy in three simulated cadaveric difficult airway models. Ann Emerg Med 2012; 3: 159. [Google Scholar]
- 18. Šustić A. Ultrasound‐guided percutaneous dilatational tracheostomy with laryngeal mask airway control in a morbidly obese patient. J Clin Anesth 2004; 16 (2): 121–23. [DOI] [PubMed] [Google Scholar]
- 19. Flint AC, Midde R, Rao VA, Lasman TE, Ho PT. Bedside ultrasound screening for pretracheal vascular structures may minimize the risks of percutaneous dilatational tracheostomy. Neurocrit Care 2009; 11 (3): 372–76. [DOI] [PubMed] [Google Scholar]
- 20. Hardee PS, Ng SY, Cashman M. Ultrasound imaging in the preoperative estimation of the size of tracheostomy tube required in specialised operations in children. Br J Oral Maxillofac Surg 2003; 41 (5): 312–16. [DOI] [PubMed] [Google Scholar]
- 21. Baumber R. Neck ultrasound prior to percutaneous tracheostomy: should this now be a standard of practice? J Intens Care Soc 2011; 12 (4): 342. [Google Scholar]
- 22. Bertram S, Emshoff R, Norer B. Ultrasonographic anatomy of the anterior neck: implications for tracheostomy. J Oral Maxillofacial Surg 1995; 53 (12), 1420–24. [DOI] [PubMed] [Google Scholar]
- 23. Sustić A, Zupan Z, Eskinja N, Dirlić A, Bajek G. Ultrasonographically guided percutaneous dilatational tracheostomy after anterior cervical spine fixation. Acta Anaesthesiol Scand 1999; 43 (10): 1078–80. [DOI] [PubMed] [Google Scholar]
- 24. Chacko J, Nikahat J, Gagan B, Umesh K, Ramanathan M. Real‐time ultrasound‐guided percutaneous dilatational tracheostomy. Intensive Care Med 2012; 38 (5): 920–21. [DOI] [PubMed] [Google Scholar]
- 25. Bonde J, Nørgaard N, Antonsen K, Faber T. Implementation of percutaneous dilation tracheotomy – value of preincisional ultrasonic examination? Acta Anaesthesiol Scand 1999; 43 (2): 163–66. [DOI] [PubMed] [Google Scholar]
- 26. Kollig E, Heydenreich U, Roetman B, Hopf F, Muhr G. Ultrasound and bronchoscopic controlled percutaneous tracheostomy on trauma ICU. Injury 2000; 31 (9): 663–68. [DOI] [PubMed] [Google Scholar]
- 27. Šustić A, Kovac D, Zgaljardić Z, Zupan Z, Krstulović B. Ultrasound‐guided percutaneous dilatational tracheostomy: a safe method to avoid cranial misplacement of the tracheostomy tube. Intensive Care Med 2000; 26 (9): 1379–81. [DOI] [PubMed] [Google Scholar]
- 28. Šustić A, Krstulović B, Eskinja N, Zelić M, Ledić D, Turina D. Surgical tracheostomy versus percutaneous dilational tracheostomy in patients with anterior cervical spine fixation: preliminary report. Spine 2002; 27 (17): 1942–45. [DOI] [PubMed] [Google Scholar]
- 29. Rajajee V, Fletcher JJ, Rochlen LR, Jacobs TL. Real‐time ultrasound‐guided percutaneous dilatational tracheostomy: a feasibility study. Crit Care 2011; 15 (1): R67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Guinot PG, Zogheib E, Petiot S, Marienne JP, Guerin AM, Monet P, et al. Ultrasound‐guided percutaneous tracheostomy in critically ill obese patients. Crit Care 2012; 16 (2): R40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. ANZICS . Percutaneous Dilatational Tracheostomy. Consensus Statement 2010.


