Abstract
Rationale and objectives: Several commercially available breast tissue markers are promoted as being sonographically visible, allowing for subsequent targeting using ultrasound. The aim of this study was to compare the visibility of selected sonographic markers with the use of tissue phantoms.
Materials and methods: Seven different markers were deployed into chicken and beef tissue phantoms, including a non‐sonographically enhanced marker used as a baseline. Six participants assessed their sonographic visibility and needle targeted the markers using ultrasound. The sonographic visibility of each marker was graded, with scores corrected for accuracy following mammographic review of needle targeting position.
Results: Only four of the six “ultrasound enhanced” markers demonstrated statistically significant greater visibility than the non‐sonographically designed marker (P range < 0.001 to 0.04). Marker size (P < 0.001) and composition (P < 0.004) were shown to be contributing factors, with the composition of the BiomarC™ (Carbon Medical Technologies Inc, St Paul, MN, USA) demonstrating the highest conspicuity adjusted for length.
Conclusion: There is significant variance in the visibility of breast tissue markers purported to be visible on ultrasound. Marker size, composition and possibly shape are contributory factors, with the utilisation of non‐metallic components associated with improved conspicuity. Our study provides a basis for further determination of optimal marker qualities, and we recommend evaluation with a larger sample size and an “in‐vivo” technique.
Keywords: absorbable implants, imaging, phantoms, mammary, surgical instruments, surgical clips, ultrasonography
Introduction
The deployment of breast tissue markers is well established to enhance the subsequent visibility of clinical and radiological regions of interest. Markers are commonly deployed when a lesion undergoing percutaneous biopsy is natively inconspicuous, predicted to become so following neoadjuvant therapy, or where the biopsy process itself has significantly reduced the lesion's visibility or removed it entirely. Marker placement facilitates accurate re‐localisation for repeat biopsy or preoperative hookwire placement with targeting traditionally performed stereotactically.
The recent availability of markers designed for sonographic visualisation has allowed for ultrasound targeting as an alternative to stereotaxis, with advantages including reduced time and personnel needs, greater access, increased patient comfort and absence of ionising radiation. Furthermore these markers in conjunction with intraoperative ultrasound may obviate the need for separate pre‐operative localisation procedures completely. 1
A secondary utility for markers is to facilitate cross‐modality lesion correlation. While alternative methods including observation of post‐biopsy changes, or the injection of negative (air) or positive (iodinated) contrast can be utilised with variable ease, success and financial burdens, there are instances where a more discrete or less transient marker is required. 2 The use of these markers now allow sonographic correlation after placement under stereotaxis or MRI.
In our experience however, the ease of sonographic visualisation of purportedly ultrasound‐enhanced markers is highly variable, potentially requiring the default utilisation of stereotactic targeting. Clearly, reliable localisation must be a key quality of marker design, in addition to other requirements such as clinical safety, ease of deployment, cost and resistance to immediate or delayed migration.
The purpose of this study was to assess and compare the sonographic visibility of several breast tissue markers, to evaluate their likely utility in future clinical situations. A secondary goal was to assess the relationship of marker detection to observer clinical experience.
Materials and methods
Ethics approval for the study was not deemed necessary by the chairperson of the Institutional Ethics Committee. The authors do not have any conflicts of interest to declare, and no funding was received, although all markers utilised were provided by the respective vendors upon request.
Materials–breast tissue markers and tissue phantoms
Seven breast tissue markers were evaluated (Table 1, Figure 1), recognising that these were not an exhaustive representation of all commercially available products, and that all size and shape variants were not tested for logistical reasons. Inclusion was based on markers that were either currently utilised in our multidisciplinary breast imaging centre, or that were promoted as sonographically enhanced and available upon contact with local vendor representatives.
Table 1.
Overview of Breast Tissue Markers (information as provided by the relevant vendors).
| Tissue marker Variant trialled (Company) | Composition Other variant(s) | Marker size: Radio‐opaque & sonographic components Other variant(s) | Shape of radio‐opaque component Other variant(s) | Available introduction methods (Including other marker variants) |
|---|---|---|---|---|
| BiomarC™ Tissue Marker 040306: Size 2 (Carbon Medical Technologies, Inc.) | Pyrolytic carbon coated Zirconium oxide | Size 1: 1 × 3 mm Size 2: 2 × 4 mm | Barbell | Side or end deployment MRI Stereotactic: Mammotome® 8G/11G; Suros® 9G/12G; Vacora® 14G Core: direct or coaxial |
| CeleroMark™/TriMark®* CeleroMark™: Shape 1 (Hologic, Inc.) | Shape 1: Titanium (with rough surface) Shape 2: Titanium | Shape 1: 3 mm ™ 1.4 mm Shape 2 (2S): 2 mm ™ 1.4 mm | Shape 1: Cylindrical Shape 2 (2S): Dumbbell | Celero®: End deployment Non‐Introducer/Introducer US 12G TriMark®: Rigid Side Deployment Non‐Introducer US 9G Introducer Stereo/US 9G/12G MRI 9G ATEC™ Handpiece Stereo 9G/12G |
| Gel Mark® Ultra † GMUEC–10GSS (SenoRx / Bard Australia Pty Ltd) | 11 resorbable PLA/PGA pellets‡ with 1 × radiopaque wireform: 316 stainless steel (SS) or titanium (GT) | Radiopaque: 2.54 × 1.14 × 0.13 mm Sonographic: 10×6.1mm pellets +1 × 3.8 mm marker pellet = “64.8 mm“ | SS: 316L stainless steel “BCAR” shape GT: Titanium “S” shape | Side deployment 7G/10G Encor®, Encor 360®, Mammotome® |
| SecurMark™ S‐Mark‐Eviva–2S–13 (Shape 2) (Hologic, Inc.) | Titanium surrounded by suture‐like bioabsorbable glycoprene material | Radiopaque:Shape 1: 1.78 × 0.914 mm Shape 2: 1.90 ™ 0.914 mm Sonographic: Netting–15 mm | Shape 1: Cylindrical Shape 2 (2S): “T‐shaped“ | End deployment Non‐Introducer US 9G/12G Introducer US 12G Celero®, ATEC™, Eviva® |
| UltraClip® II (non‐PVA variants) 865017 Ribbon for MR (Bard Australia Pty Ltd) | Ribbon (Titanium) Wing (Inconel 625 alloy) Coil (BioDur 108 alloy) | 3 mm (ribbon) | Ribbon Wing Coil | End deployment Direct or Coaxial US Stereotactic MRI |
| UltraClip® II with PVA 863017 Ribbon with PVA for US (Bard Australia Pty Ltd) | Titanium with nonabsorbable PVA polymer pellet | Radiopaque: 3 mm Sonographic: 8.89 mm | Ribbon | End deployment Direct or Coaxial US Stereotactic |
| V‐Mark™ V‐Mark™ for US 766914100SST (Angiotech Pharmaceuticals, Inc.) | Bioabsorbable plug (US visible up to 6 weeks) with Titanium hookwire | Radiopaque: 5 mm (0.2”) Sonographic: Average total length 13.33 mm (ranges from 12.45–14.22 mm) | Hookwire | Non‐introducer US Coaxial Introducer 14G Suros® 9G/12G; Mammotome® 11G |
Footnotes:
CeleroMark™ and TriMark® markers are identical
Other sonographically enhanced tissue markers from SenoRx include: Gel Mark® (Porcine gelatin + stainless steel “S” or “BCAR” wireform–US visible for 10–14 days); SenoMark™ (PGA microfiber pads + titanium “X”, “S” or “O” wireform–3 weeks); Gel Mark® UltraCor® (PLA/PGA pellet + stainless steel “BCAR” or titanium “S” wireform–4–6 weeks); UltraCor® (316 stainless steel coil shaped wireform); SenoMark™ UltraCor® MRI (PGA microfiber pad + stainless steel “M” or titanium “X” wireform–3 weeks)
PLA/PGA = polylactic acid / polyglycolic pellets (US visible for 4–6 weeks)
Figure 1.
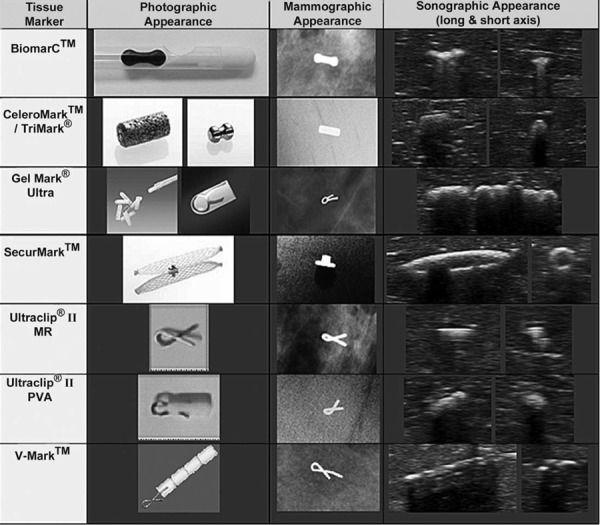
Photographic, mammographic and sonographic images of each marker. Images notto scale. Photographic images provided with permission from the respective vendors: Photographic images of the CeleroMark™/Trimark® and SecurMark™ courtesy of Hologic, Inc. © 2011. All rights reserved. Photographic image of the BiomarC™ courtesy of Carbon Medical Technologies, Inc. ©2011. All rights reserved. Photographic images of the Gel Mark® Ultra, Ultraclip® II MR and Ultraclip® II PVA courtesy of Bard Australia Pty Ltd ©2011. All rights reserved. Photographic image of the V‐Mark™ courtesy of Angiotech Pharmaceuticals, Inc. © 2011. All rights reserved.
All of the markers tested were designed for sonographic visibility, with the exception of the MR Conditional Ultraclip® II (reference no. 865017) (Bard Australia, North Ryde NSW), which was the only Ultraclip® variant able to be placed under MRI. Inclusion therefore allowed assessment of its utility if MR placement and subsequent US cross‐correlation was required, and it further served as a baseline reference for the sonographically enhanced markers.
For this phantom study we utilised fowl tissue, with prior studies suggesting it to be reasonably sonographically analogous to human breast tissue. 3 , 4 , 5 , 6 Chicken breasts were considered to be roughly comparable in size to a human breast quadrant, given that in a clinical setting the quadrant (if not the o'clock location) where the marker lies is likely to be known. The chicken sonographic appearance was felt to represent the more homogeneous spectrum of human breast tissue, and therefore we elected to also evaluate the markers in beef tissue to represent the more complex spectrum of human breast ultrasound patterns.
Methods–preparation of samples
Markers were deployed into eight similar sized skinless boneless chicken breasts and eight beef steaks with one phantom in each set designated as a control. Markers were deployed under ultrasound guidance utilising the provided introduction devices, except for those with flexible or blunt tipped introducers where a coaxial technique was utilised. To prevent participants from deducing marker location by the entry site or needle tract, markers were placed with:
-
i
Samples inverted
-
ii
Under saline to minimise air introduction into the tract
-
iii
Two additional false tracts were created in each sample (excluding the control).
To minimise bias from marker placement, all markers were positioned:
-
i
Within the central third of tissue depth
-
ii
Within the central half by area (i.e. avoiding edges)
-
iii
Deployed as parallel to the surface as possible.
Samples were labelled with non‐identifying alphanumeric characters, and randomised between the chicken and beef samples. Baseline sonographic images of all markers were taken at the time of placement, followed by baseline x‐rays.
Methods–participant assessment of samples
Six radiologists participated in the study: two registrars in training with three months of prior breast imaging experience; two Fellows with an interest in breast imaging; and two specialised breast imaging consultant radiologists. The study was performed over two separate days, one week apart (one registrar, one Fellow, one consultant each day), with the samples frozen in the intervening week. The first three participants assessed the markers in both the chicken and beef samples, however there was a marked increase in echogenicity and shadowing in the beef phantoms following freezing and defrosting precluding further use. As a result, the second three participants assessed the chicken samples alone, with the order reversed to compensate for any “learning curve” effect.
Each participant was given a brief study overview and reviewed photographic, mammographic and in‐situ sonographic images of each marker. Imaging was performed with a Toshiba, Aplio XG v5 (Toshiba, North Ryde, NSW) with a high frequency 12 MHz linear probe (PLT–1204BX). Participants were given one minute per sample to either localise the marker or to determine that no marker was present. If a marker was thought to be present, a sonographic image was obtained and participants then needle targeted the finding. Participants were required to grade their ease in locating the finding according to Table 2a.
Table 2a.
Grading System for participant ease in marker localisation
| Grade | Description |
|---|---|
| 0–Not visualized | The participant did not localise a marker in one minute |
| 1–Unsure of localisation | The participant was unsure if the targeted finding represented the marker |
| 2–Localised with difficulty | The participant had difficulty localising the finding but was confident it was the marker |
| 3–Localised easily | The participant easily localised the finding and was confident it was the marker |
Methods–scoring
Targeted samples were then x‐rayed, and the needles removed prior to the next participant, with note that the entry points of the targeting needles were not readily visible. The sample x‐rays for each participant were scored for accuracy as in Table 2b, taking into account the varying radiolucent and radiopaque components of each marker, with reference to the sonographic images at targeting if any uncertainty. If an incorrect localisation was readily attributable to another finding such as an air locule, this was recorded.
Table 2b.
Scoring system for accuracy of marker localisation
| Score | Description |
|---|---|
| NL–Not localized | No finding was needle targeted in a non‐control sample |
| IL–Incorrectly localised | The finding that was needle targeted did not correspond to the marker |
| CL–Correctly localised | The finding that was needle targeted corresponded to the marker |
| NL/CL | No marker was localised in the control sample (i.e. non localisation was a correct assessment) |
The participants' grades of ease of localisation were then corrected by two different methods. For comparative assessment of the markers (Table 2c), if an incorrect localisation occurred, the score was corrected to 0 as the marker was not seen, while correctly localised scores were preserved.
Table 2c.
Corrected scoring method 1–for comparative assessment of markers
| US Grading\XR Scoring | NL–Not Localised | IL–Incorrectly Localised | CL–Correctly Localised |
|---|---|---|---|
| 0–Not Visualised | 0 | 0 | 0 |
| 1–Unsure of localization | n/a | 0 | 1 |
| 2–Localised with difficulty | n/a | 0 | 2 |
| 3–Localised easily | n/a | 0 | 3 |
For comparative assessment of the participants (Table 2d), if an incorrect localisation occurred, the score was inverted (e.g. 2 corrected to −2), to progressively “penalise” the participant for the confidence in which they inaccurately identified a non‐marker finding as a marker. Correctly localised scores were again preserved, with non‐localisation of a marker in the control sample also considered correct with a score of 3.
Table 2d.
Corrected scoring method 2–for comparative assessment of participants
| US Scoring\XR Scoring | NL–Not Localised | IL–Incorrectly Localised | CL–Correctly Localised |
|---|---|---|---|
| 0–Not Visualised | 0(3 if control NL/CL) | 0 | 0 |
| 1–Unsure of localization | n/a | −1 | 1 |
| 2–Localised with difficulty | n/a | −2 | 2 |
| 3–Localised easily | n/a | −3 | 3 |
Methods–statistical analysis
The primary method of analysis to contrast the different markers and to examine the influence of the participant level of experience, and composition and size of the markers was an ordinal logistic regression analysis with corrected accuracy as the dependent variable. This method was chosen because the accuracy measure constitutes an ordinal scale of measurement. 7 , 8 As with any type of regression method, ordinal logistic regression assumes that the observations are independent, which is violated in this study because there are multiple observations for each participant and phantom as a result of the nested design. To overcome this problem the regression models were run with robust estimation of the standard error that accommodates the clustering of observations. Another assumption of the method is the proportional odds assumption, which assumes that the odds ratios are proportional to the ordered level of the outcome variable, accuracy. This assumption was tested using a maximum likelihood test and found to be satisfactory for the primary analysis of accuracy for each marker (P = 0.155).
Results
The full tabulated results for all participants have been included in Appendix A.
Comparative corrected marker visibility scores
Summated corrected scores for each marker are presented in Table 3. The non‐sonographically enhanced Ultraclip® II MR as expected had the lowest score, with odds ratios of the other markers relative to the Ultraclip® II MR calculated, adjusted for participant experience and phantom type, using an ordinal logistic regression analysis with odds ratio and P values corrected for clustering. Robust standard errors were utilised to estimate P values, to correct for the multiple observations made by each observer within the data clustering.
Table 3.
Summated corrected scores (scores corrected by method 1)
| Tissue Marker | Total Corrected Score Chicken | Total Corrected Score Beef | Total Corrected Score Both | Odds Ratio | Robust standard error | P value |
|---|---|---|---|---|---|---|
| Ultraclip® II MR | 0 | 5 | 5 | (1) | – | – |
| Ultraclip® II PVA | 11 | 3 | 14 | 6.13 | 5.98 | 0.063 |
| CeleroMark™ | 15 | 3 | 18 | 12.6 | 26.9 | 0.237 |
| V‐Mark™ | 14 | 5 | 19 | 11.7 | 12.0 | 0.017 |
| BiomarC™ | 12 | 8 | 20 | 19.2 | 15.8 | < 0 .001 |
| Gel Mark® Ultra | 18 | 5 | 23 | 38.9 | 69.2 | 0.039 |
| SecurMark™ | 18 | 6 | 24 | 42.5 | 71.5 | 0.026 |
The SecurMark™ (Hologic Inc Marlborough, MA, United States), Gel Mark® Ultra (Bard Australia, North Ryde, NSW), BiomarC™ (Carbon Medical Technologies Inc, St Paul, MN, USA) and V‐Mark™ (Angiotech Pharmaceuticals, Vancouver, BC, Canada) demonstrated significantly greater scores and therefore implied visibility than the non‐sonographically designed Ultraclip® II MR (P < 0.05), while the UltraClip® II PVA (designed to be sonographically visible) did not. The CeleroMark™ (Hologic Inc Marlborough, MA, United States), although having a score similar to the V‐Mark, was not significantly more visible than the baseline clip, due to its markedly greater standard error, which was predominantly due to poor performance in the beef phantom.
Effect of marker size
The effect of marker size on visibility was assessed by dividing the markers into groups lesser (Ultraclip® MR, Ultraclip® PVA, CeleroMark™, BiomarC™) or greater (V‐Mark™, SecurMark™) than 10 mm length. The Gel Mark® Ultra was excluded as an outlier, firstly due to its significantly greater total length (64.8 mm) than the other markers. Secondly it was difficult to estimate its true length/diameter in an in vivo setting given its composition of multiple (eleven) pellets, which presumably would conform to the biopsy cavity, resulting in a maximal diameter considerably less than the theoretical 64.8 mm.
Statistical evaluation by clustered ordinal logistic regression analysis demonstrated a statistically significant odds ratio of 1.28 (P < 0.001) with larger markers more accurately visible. Individual marker scores proportionate to their maximal length were also ranked in order (excluding the Gel Mark® Ultra) (Table 4), however it was felt the study was not adequately powered to evaluate whether these differences were statistically significant. Of note, while the CeleroMark™ had the greatest score/length ratio, the large standard error demonstrated on prior statistical evaluation may also influence the validity of this result.
Table 4.
Corrected marker scores relative to maximal length
| Tissue Marker | Total Corrected Marker Score | Marker Length | Calculated Marker Score/ Length |
|---|---|---|---|
| CeleroMark™∗ | 18 | 3 mm | 6.0 |
| BiomarC™ | 20 | 4 mm | 5.0 |
| Ultraclip® II MR | 5 | 3 mm | 1.67 |
| SecurMark™ | 24 | 15 mm | 1.6 |
| Ultraclip® II PVA† | 14 | 8.89 mm | 1.57 |
| V‐Mark™ | 19 | 13.3 mm | 1.43 |
Not statistically significantly nore visible than the baseline clip on the prior analysis †Non‐sonographically designed baseline clip
Effect of marker composition
Markers were grouped by composition as follows with the BiomarC™ categorised separately, being composed of a ceramic alloy and therefore containing no elemental metallic component.
-
i
Metal only: Ultraclip® II MR (titanium); CeleroMark™ (titanium)
-
ii
Combined (Non‐metallic + Metallic components): Ultraclip® II PVA (titanium + PVA); V‐Mark™ (titanium + bioabsorbable plug); Gel Mark® Ultra (stainless steel + PLA/PGA); SecurMark™ (titanium + glycoprene)
-
iii
Non‐Metal only: BiomarC™ (carbon coated zirconium oxide). The average scores of each of these groups (Metal only 11.5, Combined 20, Non‐Metal 20), appear to indicate that markers with either additional non‐metal components, or non‐metal only (BiomarC™), were substantially more visible than those composed of metal alone. However when marker size is factored in (again considering the Gel Mark® Ultra as an outlier), odds ratios relative to the BiomarC™ using a clustered ordinal logistic regression analysis (Table 5) imply that the composition of the BiomarC™ was substantially more visible than any of the other markers.
Table 5.
Odds ratios of size adjusted marker scores grouped by composition
| Composition Group | Odds Ratio of Size Adjusted Marker Scores (Calculated Relative to Group III) | P value |
|---|---|---|
| Group I: Metal Ultraclip® II MR; CeleroMark™ | 0.289 | 0.004 |
| Group II: Combined Ultraclip® II PVA; V‐Mark™; SecurMark™ (Gel Mark® Ultra excluded as an outlier) | 0.0905 | < 0.001 |
| Group III: Non‐Metal BiomarC™ | (1) | n/a |
Comparative corrected participant scores
Total corrected participant scores for consultants were 52, versus registrars 35 and fellows 27, without statistically significant differences between the groups elucidated in this study (Table 6).
Table 6.
Comparative Marker scores grouped by participant experience
| Level | Total Score | Odds Ratio | P value |
|---|---|---|---|
| Registrar | 35 | (1) | n/a |
| Fellow | 27 | 1.17 | 0.880 |
| Consultant | 52 | 1.57 | 0.081 |
Discussion
Our study confirms the considerable variability in the sonographic visibility of various breast tissue markers, with statistically significant differences elucidated in our relatively small sample size. Compared to the baseline non‐sonographically enhanced Ultraclip® II MR, the V‐Mark™, BiomarC™, Gel Mark® Ultra and SecurMark™ were significantly more accurately visualised, with a trend of increasing visibility in that order. In contrast our study was not able to demonstrate significant enhancement of visibility of the Ultraclip® II PVA and the CeleroMark™.
While it is not possible to elucidate all of the contributing factors resulting in these variances in visibility, we isolated two factors: marker size and composition, which were thought to be contributory.
Marker size
Three of the four markers that were significantly more visible than the baseline marker were greater than 10 mm in length, a finding confirmed to be statistically significant. Of interest, the remaining high scoring marker was the BiomarC™ whose length of 4 mm was minimally different to the baseline Ultraclip® II MR (3 mm), and as such factors apart from size would seem to be in effect. This observation was supported by the BiomarC™ having the highest “marker score/maximal length” ratio, excluding the CeleroMark™ for reasons discussed before.
While these findings imply that increased marker size equates to increased marker visibility, the same cannot necessarily be said in regard to accuracy of lesion localisation. Presumably a threshold is reached where an increase in size provides minimal additional visibility, but unnecessarily increases the size of the region marked, potentially confusing the precise lesion location especially for smaller lesions. In a similar sense, the accuracy of lesion localisation may also be influenced by the ratio of the marker size to the biopsy cavity size particularly in vacuum assisted biopsies.
Marker composition and shape
Analysis of the markers grouped by composition revealed that the non‐metallic BiomarC™ and combined metal/radiolucent markers scored more highly than the solely metallic markers. However once marker size was taken into account, the BiomarC™ was statistically significantly more visible than either of the other two categories, which may relate to its carbon coated zirconium oxide composition.
Marker shape also presumably influences visibility, however due to marked variability in this and the limited sample size, statistical assessment of this was not possible. However we postulate that markers with shapes that may be mimicked by physiological structures, such as those with linear sonographic profiles may be less conspicuous compared to those with non‐anatomical configurations. The barbell shape of the BiomarC™, beaded appearance of the V‐Mark™, or net‐like appearance of the SecurMark™, may potentially afford some advantage in this regard.
Participants
While there was no statistically significant difference between the registrars, fellows and consultants performance in regard to participant accuracy, the non‐significant trend to the consultants performing better, compared to the registrars and fellows would conform with expected results.
Limitations
We recognise the following potential limitations and confounding factors with our study, and discuss methods utilised to abate their effect:
-
1
Tissue phantoms: as with any study utilising imaging phantoms, results may not be fully transferable to a clinical setting, depending on the phantom's ability to mimic the original in vivo tissue. We utilised two different tissue phantoms (beef and chicken) to better represent a spectrum of variably echogenic human breast tissue, recognising that “ex vivo” testing does not reproduce the expected absorption of gas or absorbable marker components, and effects of bleeding or tissue healing in in‐vivo scenarios.
-
2
Inhomogeneity of samples: the presence of echogenic air locules in samples would presumably increase the potential for incorrect localisations. While markers were introduced under saline to prevent this, we nonetheless identified six instances where this occurred. However four of the six were in the control samples, suggesting the effect of air locules on comparative marker results was relatively minimal.
-
3
Variance in marker position: variability in marker placement position may create bias, however conformity to certain parameters of depth, location and angulation were undertaken to reduce this, though with enough variance to prevent predictability.
-
4
Visibility of entry sites and tracts: the deduction of marker position by observation of needle entry sites or tracts could be a confounding factor, however minimised by introduction via the inverted surface, and creation of false tracts. The tracts from prior participants' needle targeting were not readily visible and would not advantage one marker over another.
-
5
Sample size: we recognise the limited sample size, with each marker assessed in two samples (chicken and beef), with each sample assessed by six observers. Greater numbers of both samples and observers would increase the number of trends reaching statistical significance.
-
6
Learning curve: To reduce the effect of increased observer experience with evaluation of each progressive sample, the order of assessment (for the chicken samples) was reversed for the second set of participants.
-
7
Participant familiarity with markers: potentially markers that participants had prior experience with would be more readily identifiable. To reduce this, participants were shown images of all markers immediately prior to the study. Moreover the three markers that recorded the lowest total scores were those used previously in our institution.
-
8
Scoring: potential subjectivity in determining the accuracy of marker localisation for markers with considerable non‐radiopaque components was reduced by careful review of the sonographic images obtained at targeting.
We therefore consider that all potential sources of bias were sufficiently accounted for in the construction of the study, such that the results obtained were a true reflection of the comparative visibility of the evaluated markers.
Conclusion
The use of sonographically visible breast tissue markers for ultrasound inconspicuous breast lesions allows access to the benefits of ultrasound targeting over the traditional requirement for stereotactic marker localisation. Along with other issues such as patient safety, non‐migration and ease of use, reliable and accurate sonographic “in vivo” marker visualisation is a primary prerequisite for marker functionality.
We have demonstrated that there is considerable variability in the visibility of purported sonographically visible breast markers, and have reported comparative findings for six ultrasound markers. Specifically the SecurMark™, Gel Mark® Ultra, BiomarC™ and V‐Mark™ demonstrated a statistically significant advantage in visibility over the baseline non‐sonographically enhanced marker (Ultraclip® II MR), while no such advantage was conferred to the Ultraclip® II PVA and CeleroMark™ within the studied sample size. Our study confirms that marker size is an influential factor, however composition and possibly marker shape are also contributory, with the BiomarC™ performing very well for its small size. We have demonstrated that the addition of non‐metallic components and use of innovative materials and/or surface coatings is associated with improved marker visibility, and postulate that a distinctly non‐anatomic shape also enhances conspicuity.
While recognising that this trial has limitations related to a relatively small sample size and use of tissue phantoms, our results provide information that may assist in future selection of ultrasound enhanced markers by our institution and others. As such we believe our study provides a basis for further research to assess the optimal size, shape and composition of sonographically visible breast biopsy markers and advocate evaluation with a larger sample size using and in‐vivo technology.
Appendix A: Full tabulated participant results
| 1st set of participants | Registrar 1 | Fellow 1 | Consultant 1 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tissue | Marker | Ease US | Grade MG | Corr Marker | Corr Part | Ease US | Grade MG | Corr Marker | Corr Part | Ease US | Grade MG | Corr Marker | Corr Part |
| Chicken | BiomarC™ | 3 | CL | 3 | 3 | 3 | IL (air) | 0 | −3 | 3 | CL | 3 | 3 |
| Chicken | CeleroMark™ | 2 | IL (air) | 0 | −2 | 3 | CL | 3 | 3 | 3 | CL | 3 | 3 |
| Chicken | Control | 0 | NL/CL | – | 3 | 3 | IL (air) | – | −3 | 1 | IL (air) | – | −1 |
| Chicken | Gel Mark® Ultra | 3 | CL | 3 | 3 | 3 | CL | 3 | 3 | 3 | CL | 3 | 3 |
| Chicken | SecurMark™ | 3 | CL | 3 | 3 | 3 | CL | 3 | 3 | 3 | CL | 3 | 3 |
| Chicken | Ultraclip® II MR | 0 | NL | 0 | 0 | 0 | NL | 0 | 0 | 0 | NL | 0 | 0 |
| Chicken | Ultraclip® II PVA | 3 | CL | 3 | 3 | 0 | NL | 0 | 0 | 3 | CL | 3 | 3 |
| Chicken | V‐Mark™ | 3 | CL | 3 | 3 | 3 | CL | 3 | 3 | 1 | CL | 1 | 1 |
| Total Score | 17 | 16 | 18 | 6 | 17 | 15 | |||||||
| Beef | BiomarC™ | 3 | CL | 3 | 3 | 3 | CL | 3 | 3 | 2 | CL | 2 | 2 |
| Beef | CeleroMark™ | 1 | CL | 1 | 1 | 0 | NL | 0 | 0 | 2 | CL | 2 | 2 |
| Beef | Control | 0 | NL/CL | – | 3 | 2 | IL (air) | – | −2 | 0 | NL/CL | – | 3 |
| Beef | Gel Mark® Ultra | 1 | CL | 1 | 1 | 1 | CL | 1 | 1 | 3 | CL | 3 | 3 |
| Beef | SecurMark™ | 3 | CL | 3 | 3 | 2 | CL | 2 | 2 | 1 | CL | 1 | 1 |
| Beef | Ultraclip® II MR | 3 | CL | 3 | 3 | 2 | IL | 0 | −2 | 2 | CL | 2 | 2 |
| Beef | Ultraclip® II PVA | 3 | IL | 0 | −3 | 1 | IL | 0 | −1 | 3 | CL | 3 | 3 |
| Beef | V‐Mark™ | 0 | NL | 0 | 0 | 3 | CL | 3 | 3 | 2 | CL | 2 | 2 |
| Total Score | 14 | 11 | 14 | 4 | 15 | 18 | |||||||
| 2nd set of participants | Registrar 2 | Fellow 2 | Consultant 2 | ||||||||||
| Tissue | Marker | Ease US | Grade MG | Corr Marker | Corr Part | Ease US | Grade MG | Corr Marker | Corr Part | Ease US | Grade MG | Corr Marker | Corr Part |
| Chicken | BiomarC™ | 0 | NL | 0 | 0 | 3 | CL | 3 | 3 | 3 | CL | 3 | 3 |
| Chicken | CeleroMark™ | 3 | CL | 3 | 3 | 3 | CL | 3 | 3 | 3 | CL | 3 | 3 |
| Chicken | Control | 1 | IL (air) | – | −1 | 2 | IL | – | −1 | 0 | NL/CL | – | 3 |
| Chicken | Gel Mark® Ultra | 3 | CL | 3 | 3 | 3 | CL | 3 | 3 | 3 | CL | 3 | 3 |
| Chicken | SecurMark™ | 3 | CL | 3 | 3 | 3 | CL | 3 | 3 | 3 | CL | 3 | 3 |
| Chicken | Ultraclip® II MR | 2 | IL | 0 | −2 | 0 | NL | 0 | 0 | 0 | NL | 0 | 0 |
| Chicken | Ultraclip® II PVA | 0 | NL | 0 | 0 | 3 | CL | 3 | 3 | 2 | CL | 2 | 2 |
| Chicken | V‐Mark™ | 2 | CL | 2 | 2 | 3 | CL | 3 | 3 | 2 | CL | 2 | 2 |
| Total Score | 14 | 8 | 20 | 17 | 16 | 19 | |||||||
Ease US = Participant's ease in marker localisation on US Score (as per Table 2a)
Grade MG = Grading on mammogram of accuracy of marker localisation (as per Table 2b)
CL = Correct Localisation; IL = Incorrect Localisation; NL = Not Localised
NL/CL = No finding localised in the control appropriately
(air) = Incorrect localisation due to targeting of an air locule
Corr Marker = Corrected score for marker assessment (as per Table 2c)
Corr Part = Corrected score for participant assessment (as per Table 2d)
References
- 1. Eby PR, Calhoun KE, Kurland BF, DeMartini WB, Gutierrez RL, Peacock S, et al. Preoperative and intraoperative sonographic visibility of collagen‐based breast biopsy marker clips. Acad Radiol 2010; 17 (3): 340–47. [DOI] [PubMed] [Google Scholar]
- 2. Guenin MA. Clip Placement during sonographically guided large‐core breast biopsy for mammographic‐sonographic correlation. AJR Am J Roentgenol 2000; 175: 1053–55. [DOI] [PubMed] [Google Scholar]
- 3. Georgian‐Smith D, Shiels WE II. From the RSNA refresher courses. Freehand interventional sonography in the breast: principles and clinical applications. Radiographics 1996; 16 (1): 149–61. [DOI] [PubMed] [Google Scholar]
- 4. Smith WL, Surry KJ, Kumar A, McCurdy L, Downey DB, Fenster A. Comparison of core needle breast biopsy techniques: freehand versus three‐dimensional US guidance. Acad Radiol 2002; 9 (5): 541–50. [DOI] [PubMed] [Google Scholar]
- 5. Hassard MK, McCurdy LI, Williams JC, Downey DB. Training module to teach ultrasound‐guided breast biopsy skills to residents improves accuracy. Can Assoc Radiol J 2003; 54: 155–59. [PubMed] [Google Scholar]
- 6. Harvey JA, Moran RE, Hamer MM, DeAngelis GA, Omary RA. Evaluation of a turkey‐breast phantom for teaching freehand, US‐guided core‐needle breast biopsy. Acad Radiol 1997; 4 (8): 565–69. [DOI] [PubMed] [Google Scholar]
- 7. Stevens SS. On the theory of scales of measurement. Science 1946; 103: 677–80. [DOI] [PubMed] [Google Scholar]
- 8. Harrell FE Jr. Regression modelling strategies: with applications to linear models, logistic regression and survival analysis. New York: Springer, 2001. [Google Scholar]


