Abstract
Ectopic pregnancy that implants within the scar tissue of a previous caesarean scar is a situation that is seldom encountered and is almost invariably incompatible with a successful pregnancy. Caesarean scar ectopic pregnancies are fraught with life threatening complications such as scar rupture, significant haemorrhage, disseminated intravascular coagulation and the need for emergency life saving hysterectomy. The clinical diagnosis can be elusive, particularly in the early stages; therefore clinicians should be familiar with the condition's sonographic hallmarks. Early diagnosis and management is the key to preventing these complications. We describe a case of caesarean scar pregnancy which was initially misdiagnosed as “a spontaneous miscarriage in progress”, resulting in uncontrollable bleeding, necessitating an emergency abdominal hysterectomy. We also endeavour to review the literature with regards to the use of ultrasound in its management, treatment and follow up.
Keywords: caesarean, ectopic, hysterectomy, scar, ultrasound
Case report
A 31‐year‐old multiparous female presented to the emergency department with a history of vaginal bleeding, cramping lower abdominal pain and dizziness. This was on a background of an earlier presentation to the emergency department four weeks prior, when a diagnosis of a “spontaneous miscarriage in progress” was made. This was managed expectantly due to patient preference. Transvaginal (TV) ultrasound on initial presentation had revealed a non‐viable intrauterine pregnancy in the lower part of the uterus with an estimated gestational age of 7 weeks (Figures 1, 2 and 3). She had five pregnancies in total; three miscarriages and two live children born by lower segment caesarean section. The most recent caesarean was performed four years prior, which was complicated by a post‐operative wound infection.
Figure 1.
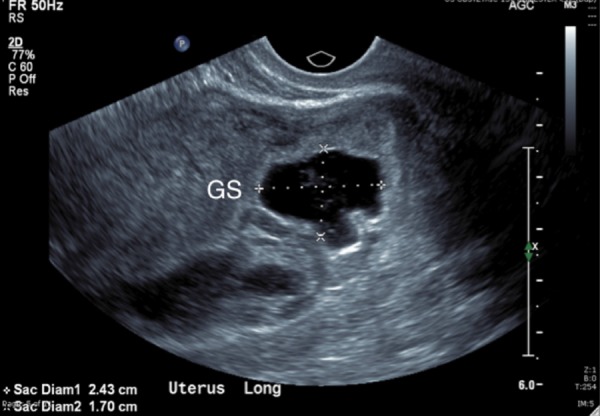
Trans‐vaginal longitudinal ultrasound scan showing a gestational sac implanted anteriorly in the lower uterus, encroaching on to the cervix. Note is made of decidual reaction around the gestational sac.
Figure 2.
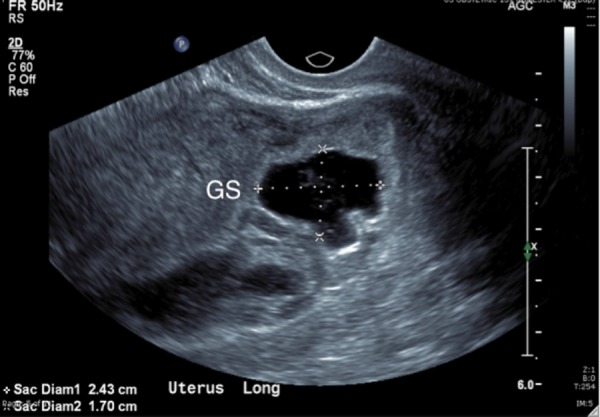
Trans‐vaginal transverse ultrasound image demonstrating an irregular gestational sac in the lower uterine and upper cervical region of the uterus.
Figure 3.
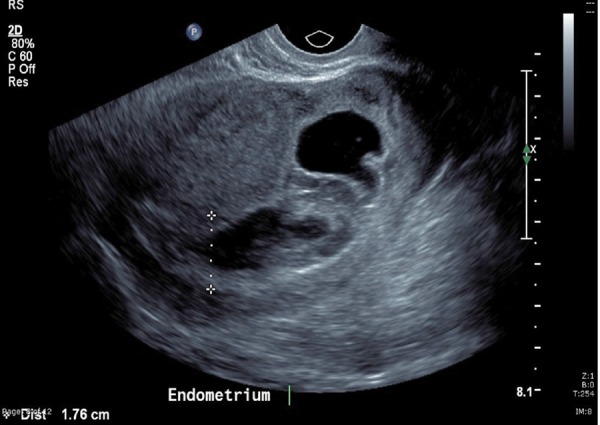
TV Ultrasound image showing the endometrial cavity dilated with bleeding superior to the sac.
In the emergency department, it was noted that the bleeding had been significant, resulting in hemodynamic compromise. The speculum examination confirmed the cervix partially dilated with blood clots. Estimated blood loss initially was 1 litre resulting in a drop of the haemoglobin to 109g/L, platelets to 73×109/L, and an increase in INR to 1.4IU. She underwent an emergency dilatation and curettage with significant ongoing bleeding of a further 2 litres. Post‐operatively, she was admitted to the intensive care unit (ICU) where she continued to be hypotensive with a blood pressure of 65/30 mm Hg and ongoing bleeding. She was returned to the operating theatre for an urgent total abdominal hysterectomy.
During the hysterectomy, it was noted that the lower part of the uterus and the cervix appeared quite abnormal and fragile with a blue‐black discoloration (Figure 4). After completion of the surgical procedure, gross inspection of the uterus revealed a necrotic and friable lower segment of the uterus and the cervix (Figure 5). She was admitted to the ICU again post operatively and required inotropic support to maintain her blood pressure. The total estimated blood loss was 6.5 litres. Recovery was gradual, and she was discharged 6 days post hysterectomy.
Figure 4.
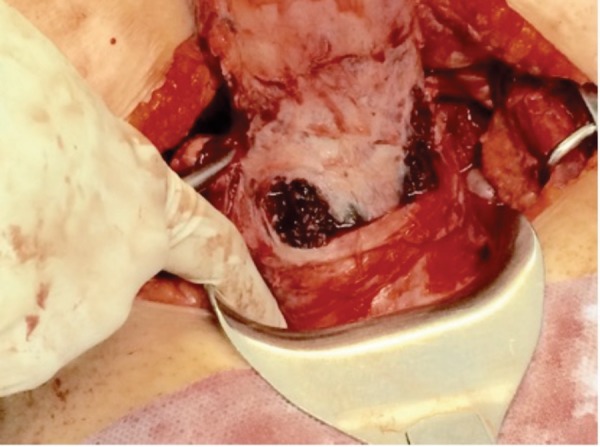
Intra‐operative image of the lower segment of uterus, demonstrating blue‐ black discoloration of the cervix.
Figure 5.
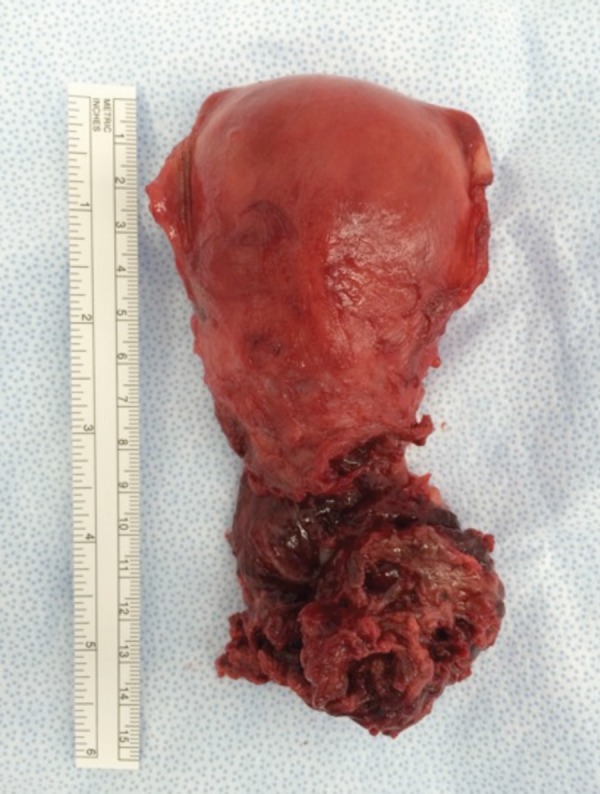
Post hysterectomy specimen demonstrating a friable, necrotic appearing lower part of the uterus and cervix.
While histopathology of the uterine curettings showed blood with no chorionic villi or fetal parts, histopathology of the uterus confirmed the diagnosis of a caesarean scar pregnancy (CSP). There was evidence of a 10 mm gestational sac in the lateral cervix, closely related to the disrupted caesarean scar. Chorionic villi were noted throughout the cervix, almost obliterating it entirely (Figure 6). There was no evidence of invasive gestational trophoblastic disease.
Figure 6.
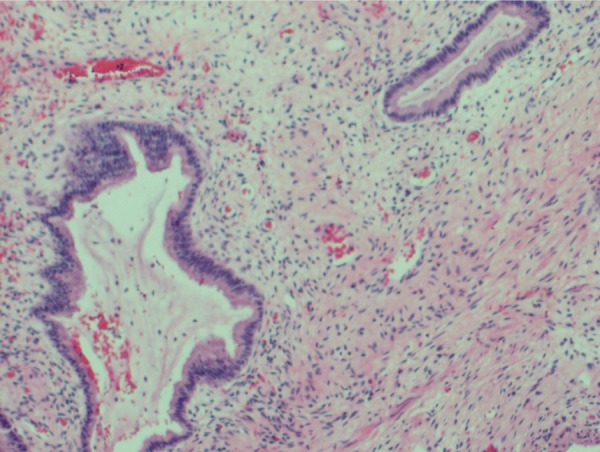
Histological appearance of the gestational sac with chorionic villi in cervical tissue.
Discussion
CSP, where the ovum implants in the scar of a previous caesarean delivery, is one of the rare forms of ectopic pregnancy, with an incidence of 1 in 1800–2200 pregnancies, and comprises 6.1% of all ectopic pregnancies. 1 – 4 In recent years, the rate of diagnosis of CSP has risen and is believed to be, in part, due to improvements in imaging technology. 3 CSP is histologically very similar to placenta accreta, whereby scarring and dehiscence of the uterine wall may allow deep penetration of the trophoblastic tissue during implantation. 5 , 6 This is analogous to the blastocyst implanting in the fibrous tissue of a caesarean scar.
Risk factors for the condition, other than a history of caesarean sections, have not been established, however multiple caesareans, a past history of scar ectopic pregnancy, trauma, previous myomectomy or curette, poor wound healing, manual removal of the placenta, and assisted reproductive therapy may be implicated. 1 , 7 – 9
Ultrasound is highly useful for diagnosing CSP in the early stages of pregnancy and the diagnosis is usually made at 5–6 weeks gestation on TV examination. Late diagnosis does occasionally occur, with an increase in the incidence of complications such as scar rupture and major haemorrhage. 10 , 11 Early intervention has been shown to be important in minimising the risk of such complications 12 and facilitation of conservative management. 1 , 13
Ultrasonography is often the first step in detecting a CSP. Transabdominal (TA) ultrasound may be used to obtain a panoramic view of the uterus, prior to more close inspection with the TV probe. 14 The CSP usually presents as a heterogeneous mass, containing cystic‐solid, or mixed echoes within the caesarean scar or lower uterine segment. 12 , 15 Ultrasound is highly useful for diagnosing CSP in the early stages of pregnancy, with a sensitivity of 86.4% and a specificity of 92.3%. 16 – 18
Adherence to a standard set of diagnostic criteria may help reduce the risk of misdiagnosis and potentially catastrophic consequences of this condition. 19 Based on ultrasound findings, CSPs may be classified into two types: Type 1 characterised by an amniotic sac that protrudes toward the cervico‐isthmic space and uterine cavity and Type 2, an implantation that bulges toward the serosa, less than 4 mm from the bladder wall. Clinically, a Type 1 CSP may progress to term, though with a high risk of massive bleeding. 20
In addition, one set of diagnostic criteria for CSP is well described in the literature:
1 An empty uterus and cervical canal
2 Development of a gestational sac or mixed echogenic mass in the anterior isthmic portion
3 The presence of very thin myometrium between the bladder wall and the sac, or discontinuity of the anterior uterine wall on a sagittal view of the uterus. 21
Other sonographic markers include peri‐trophoblastic color Doppler with low impedance, high velocity flow, resistive index of < 0.5 and peak systolic‐diastolic ratio of < 3. 14
The differential diagnosis of CSP includes a spontaneous miscarriage in process, a vascular tumour or a molar pregnancy. 7 , 22 – 24 CSP has been reported to be misdiagnosed in 36% of cases as reported by Li, et al. 12 which may be predominantly due to CSPs often presenting in a manner very similar to an early miscarriage, with pain and vaginal bleeding with an elevated beta HCG. In addition to this, the ultrasound findings may resemble a gestational sac in the cervico‐isthmic space, where one would expect to see a sac in the process of being expelled. Adequate visualisation of a scar pregnancy depends on factors such as the gestational age, equipment quality and the skill and technique of the examiner, maternal factors including BMI, presence of fibroids or ovarian pathology. 25 , 26
Failed pregnancies and cervical ectopics may be distinguished from cesarean scar ectopics by features such as location in the cervical canal, thickness of the overlying myometrium, colour flow, and the ‘sliding organ sign’ where displacement of the sac in the cervico‐isthmic space is possible with gentle probe pressure. 3 , 27 A decidual reaction around the gestational sac is usually present in a scar ectopic, which is suggestive of the pregnancy being implanted in the scar area rather than retained products. 28
If, rather than retained products (Figures 1, 2, 3), CSP is suspected or equivocal, and the patient is hemodynamically stable, interval ultrasound or magnetic resonance imaging (MRI) may be useful in making the diagnosis. 29 – 32 One study showed that contrast‐enhanced MRI resulted in the accurate diagnosis of CSP in 95.5% of cases, as opposed to 88.6% with regular ultrasound. 33 However, the use of MRI should best be limited to equivocal cases on ultrasound, due to the prolonged acquisition time. 3 , 10 , 34
The early diagnosis of a scar ectopic would permit a greater range of management options that are less invasive, have a lower rate of complications and a higher chance of preserving fertility. 13 , 35 , 36 Additionally, ultrasound can serve as a powerful decision making tool, allowing the clinician to form an individual management plan based on gestational age, the precise location of the sac, vascularity and thickness of the myometrium. 37
Treatment
A CSP may frequently culminate in a hysterectomy, with the prevalence ranging from 2–12.5%. 7 , 16 , 34 , 38 Reasons for performing hysterectomy include massive or persistent vaginal bleeding, 39 delay in diagnosis, 25 , 40 failure of fertility preserving measures 41 , 42 and occasionally patient preference. 43 It has been shown that management with bilateral uterine artery ablation (UAA) results in less haemorrhage and fewer hysterectomies. 41 , 44 However, UAA may not be readily available or be an appropriate option in the event of torrential bleeding. In these instances, hysterectomy may be a life saving measure. Additionally, ultrasound has been found to be a valuable tool in the management of CSP allowing the administration of embryocidal agents directly to the sac under sonographic guidance. 45 The use of local treatment is widely documented and has proven to be very safe and effective in appropriately selected patients, while also reducing the need for systemic therapy. 46 , 47 TV and TA methods have both been described in the literature, however a TV approach is preferred, as it allows for better visualisation, the use of a shorter needle, and confers a lower risk of damage to local structures. 37 One case report describes using transrectal sonography for local treatment with an embryocide, which has benefits over a TV route as it allows more room for instruments in the vagina and better visualisation of the uterine cavity. 48 Sonographic signs of a successful embryocide instillation include slowing of embryonic heart movement and the contents of the gestational sac becoming more echogenic and less well defined. 23
Surgical termination of pregnancy has also been undertaken under ultrasound guidance, permitting a more directed surgical approach, also with a potentially decreased risk of injury to local structures. 49 , 50 One study describes a technique where the ultrasound itself is the embryocidal agent‐ high intensity focussed ultrasound (HIFU), where by the heat energy is directed at the gestation, stopping cardiac activity and resulting in rapidly falling HCG levels. 51 This technique was found to be effective for larger gestations with strong peripheral colour Doppler signals.
Another way in which ultrasound may be beneficial is in the diagnosis of sequelae related to the CSP, such as the development of arteriovenous malformations, 52 persistent gestational sac 53 and gestational trophoblastic disease, 54 as well as post operative complications such as hematoma formation. 6 Ultrasound has also been found to be valuable in the follow up of patients treated for CSP, to ensure the success of treatment and subsequent resolution of the gestational sac. 55 The literature suggests that time to resolution on ultrasound scan is highly variable, and the sac remnants may be visible on ultrasound long after the beta human chorionic gonadotrophin (hCG) becomes undetectable and can range from 2 to 12 months. 26 , 56 , 57 There do not seem to be any universal protocols for follow up of these patients, but most centres perform weekly hCG and weekly to monthly ultrasounds until resolution. 19 Furthermore, several studies have been published looking at the power of ultrasound in assessing caesarean scars in the non‐pregnant state. It has been proposed that a CSP may develop from the seeding of a blastocyst within scar defects. Several studies have demonstrated that these defects can be identified in the non‐pregnant state, using this method. 45 TV ultrasound has been found to be up to 100% sensitive and specific for detecting caesarean scars defects with the use of saline sonohysterography. 58 – 60 The use of ultrasound in the evaluation of pregnant patients with previous caesarean deliveries has been recommended. 16
In summary, CSP is a very rare occurrence that deserves further attention due to its increasing incidence and risk of significant complications. It may present, as it did in this case, with vaginal bleeding or abdominal pain, or it may be an incidental finding on a routine antenatal ultrasound. It is a diagnosis that must be considered by clinicians and sonographers alike, due to the serious consequences of a missed diagnosis and delayed treatment.
Consent
Verbal consent was obtained from the patient for this publication and for the accompanying images.
Competing interests
The authors declare that they have no competing interests
Authors' contributions
AK was involved in the surgical management of the case. AK also contributed to the interpretation of the ultrasound scans. AK and KC compiled and approved the final manuscript and ultrasound images.
References
- 1. Winder SR, Condous G. Ultrasound Diagnosis of Ectopic Pregnancy. AJUM 2011; 14 (2): 29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ushakov FB, Aceman PJ, Schenker JG. Cervical pregnancy: past and future. Obstet Gynecol Surv 1997; 52 (1): 45–59. [DOI] [PubMed] [Google Scholar]
- 3. Ash A, Smith A, Maxwell D. Caesarean scar pregnancy. BJOG 2007; 114 (3): 253–263. Epub 23 February, 2007. [DOI] [PubMed] [Google Scholar]
- 4. Seow KM, Huang LW, Lin YH, Lin MY, Tsai YL, Hwang JL. Cesarean scar pregnancy: issues in management. Ultrasound Obstet Gynecol 2004; 23 (3): 247–253. Epub 18 March, 2004. [DOI] [PubMed] [Google Scholar]
- 5. Tantbirojn P, Crum CP, Parast MM. Pathophysiology of placenta creta: the role of decidua and extravillous trophoblast. Placenta 2008; 29 (7): 639–645. Epub 03 June, 2008. [DOI] [PubMed] [Google Scholar]
- 6. Timor‐Tritsch IE, Monteagudo A, Cali G, Palacios‐Jaraquemada JM, Maymon R, Arslan AA, et al. Cesarean scar pregnancy and early placenta accreta share common histology. Ultrasound Obstet Gynecol 2014; 43 (4): 383–395. Epub 2013/12/21. [DOI] [PubMed] [Google Scholar]
- 7. Qian Z‐D. Li‐Li Huang, Identifying risk factors for recurrent cesarean scar pregnancy: a case‐control study. Fertil Steril 2014; 102 (1): 129–34. [DOI] [PubMed] [Google Scholar]
- 8. Bij de Vaate AJ, Naji O, Witmer M, Veersema S, Brölmann HA, Bourne T, Huirne JA. Prevalence, potential risk factors for development and symptoms related to the presence of uterine niches following Cesarean section: systematic review. Ultrasound Obstet Gynecol 2014; 43 (4): 372–82. [DOI] [PubMed] [Google Scholar]
- 9. Marcus SC, Goff B. Extrauterine pregnancy resulting from early uterine rupture. Obstet Gynecol 1999; 94 (5 Pt 2): 804–05. [DOI] [PubMed] [Google Scholar]
- 10. Smith A, Ash A, Maxwell D. Sonographic diagnosis of cesarean scar pregnancy at 16 weeks. J Clin Ultrasound: JCU. 2007; 35 (4): 212–215. Epub 17 March, 2007. [DOI] [PubMed] [Google Scholar]
- 11. Overcash RT, Khackician ZH. Late‐first‐trimester cesarean section scar ectopic pregnancy with placenta increta: a case report. J Clin Ultrasound 2012; 57 (1–2): 61–664. Epub 14 February, 2012. [PubMed] [Google Scholar]
- 12. Li Y, Xiang Y, Wan X, Feng F, Ren T. (Clinical study on 39 cases with caesarean scar pregnancy with sonographic mass). Chinese. 2014; 49 (1): 10–13. Epub 04 April, 2014. [PubMed] [Google Scholar]
- 13. Chueh HY, Cheng PJ, Wang CW, Shaw SW, Lee CL, Soong YK. Ectopic twin pregnancy in cesarean scar after in vitro fertilization/embryo transfer: case report. Fertil Steril. 2008; 90 (5): 2009 19–21. Epub 2008/02/19. [DOI] [PubMed] [Google Scholar]
- 14. Osborn DA, Williams TR, Craig BM. Cesarean scar pregnancy: sonographic and magnetic resonance imaging findings, complications, and treatment. J Ultrasound Med 2012; 31 (9): 1449–1456. Epub 28 August, 2012. [DOI] [PubMed] [Google Scholar]
- 15. Ko JK, Cheung VY. Caesarean scar pregnancy: A 10 year experience. Aust N Z J Obstet Gynaecol 2014; 55 (1): 64–9. [DOI] [PubMed] [Google Scholar]
- 16. Polat I, Alkis I, Sahbaz A, Sahin O, Ekiz A, Gulac B, et al. Diagnosis and management of cesarean scar pregnancy. Clin Exp Obstet Gynecol 2012; 39 (3): 365–368. Epub 20 November, 2012. [PubMed] [Google Scholar]
- 17. Rotas MA, Haberman S, Levgur M. Cesarean scar ectopic pregnancies: etiology, diagnosis, and management. Obstet Gynecol 2006; 107 (6): 1373–1381. Epub 02 June, 2006. [DOI] [PubMed] [Google Scholar]
- 18. Wu QY, Xue Q, Huang XZ, Tan J. Clinical analysis of diagnosis and treatment of 13 cases with cesarean scar pregnancy. Clin Exp Obstet Gynecol 2014; 41 (2): 128–31. [PubMed] [Google Scholar]
- 19. Shih JC. Cesarean scar pregnancy: diagnosis with three‐dimensional (3D) ultrasound and 3D power Doppler. Ultrasound Obstet Gynecol 2004; 23 (3): 306–307. Epub 18 March, 2004. [DOI] [PubMed] [Google Scholar]
- 20. Vial Y, Petignat P, Hohlfeld P. Pregnancy in a cesarean scar. Ultrasound Obstet Gynecol 2000; 16 (6): 592–593. Epub 13 February, 2001. [DOI] [PubMed] [Google Scholar]
- 21. Godin PA, Bassil S, Donnez J. An ectopic pregnancy developing in a previous caesarian section scar. Fertil Steril 1997; 67 (2): 398–400. Epub 01 February, 1997. [DOI] [PubMed] [Google Scholar]
- 22. Tan G, Chong YS, Biswas A. Caesarean scar pregnancy: a diagnosis to consider carefully in patients with risk factors. Ann Acad Med Singapore, Singapore. 2005; 34 (2): 216–219. Epub 14 April, 2005. [PubMed] [Google Scholar]
- 23. Kochhar PK, Sarangal M, Gupta U. Conservative management of cesarean scar pregnancy with uterine arteriovenous malformation: a case report. J Reprod Med 2013; 58 (1–2): 81–84. Epub 02 March, 2013. [PubMed] [Google Scholar]
- 24. Soydinc HE, Evsen MS, Sak ME, Gul T. Cesarean scar pregnancy mimicking malignant tumor: a case report. J Reprod Med 2011; 56 (11–12): 518–520. Epub 27 December, 2011. [PubMed] [Google Scholar]
- 25. Hong SC, Lau MS, Yam PK. Ectopic pregnancy in previous Caesarean section scar. Singapore Med J 2011; 52 (6): e115–e117. Epub 07 July, 2011. [PubMed] [Google Scholar]
- 26. Haimov‐Kochman R, Sciaky‐Tamir Y, Yanai N, Yagel S. Conservative management of two ectopic pregnancies implanted in previous uterine scars. Ultrasound Obstet Gynecol 2002; 19 (6): 616–619. Epub 06 June, 2002. [DOI] [PubMed] [Google Scholar]
- 27. Jurkovic DH, Woelfer B, Lawrence A, Salim R, Elson CJ. First trimester diagnosis and management of pregnancies implanted in the lower uterine segment cesarean segment scar. Ultrasound Obstet Gynecol 2003; 21 (3): 220–27. [DOI] [PubMed] [Google Scholar]
- 28. Simons ME, Cooperberg PL, Graham MF. Ultrasound findings in ectopic gestation. Can Assoc Radiol J 1986; 37 (1): 9–12. Epub 01 March, 1986. [PubMed] [Google Scholar]
- 29. Holland MG, Bienstock JL. Recurrent ectopic pregnancy in a cesarean scar. Obstet Gynecol 2008; 111 (2 Pt 2): 541–545. Epub 02 February, 2008. [DOI] [PubMed] [Google Scholar]
- 30. Yazicioglu HF, Turgut S, Madazli R, Aygun M, Cebi Z, Sonmez S. An unusual case of heterotopic twin pregnancy managed successfully with selective feticide. Ultrasound Obstet Gynecol 2004; 23 (6): 626–627. Epub 02 June, 2004. [DOI] [PubMed] [Google Scholar]
- 31. Wu R, Klein MA, Mahboob S, Gupta M, Katz DS. Magnetic resonance imaging as an adjunct to ultrasound in evaluating cesarean scar ectopic pregnancy. J Clin Imaging Sci. 2013; 3: 16 Epub 03 July, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. de Vaate AJ, Brolmann HA, van der Slikke JW, Wouters MG, Schats R, Huirne JA. Therapeutic options of caesarean scar pregnancy: case series and literature review. J Clin Ultrasound: JCU. 2010; 38 (2): 75–84. Epub 11 November, 2009. [DOI] [PubMed] [Google Scholar]
- 33. Huang QZM, Zhai RY. The use of contrast‐ enhanced magnetic resonance imaging to diagnose cesarean scar pregnancies. Int J Gynaecol Obstet. 2014; 127 (2): 144–146. Epub Jun 30. [DOI] [PubMed] [Google Scholar]
- 34. Zhang Y, Gu Y, Wang JM, Li Y. Analysis of cases with cesarean scar pregnancy. J Obstet Gynaecol Res 2013; 39 (1): 195–202. Epub 30 May, 2012. [DOI] [PubMed] [Google Scholar]
- 35. Yu XL, Zhang N, Zuo WL. (Cesarean scar pregnancy: an analysis of 100 cases). Chinese. 2011; 91 (45): 3186–3189. Epub 16 February, 2012. [PubMed] [Google Scholar]
- 36. Liang F, He J. Methotrexate‐based bilateral uterine arterial chemoembolization for treatment of cesarean scar pregnancy. AOGS 2010; 89 (12): 1592–1594. Epub 24 August, 2010. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Y DH, Cheng JM, Guo YS. Treatment options to terminate persistent caesarean scar pregnancy. Gynaecol Obstet Invest. 2013; 75 (2): 115–119. Epub Dec 29. [DOI] [PubMed] [Google Scholar]
- 38. Timor‐Tritsch IE, Monteagudo A. Unforeseen consequences of the increasing rate of cesarean deliveries: early placenta accreta and cesarean scar pregnancy. A review. Am J Obstet Gynecol 2012; 207 (1): 14–29. Epub 21 April, 2012. [DOI] [PubMed] [Google Scholar]
- 39. Chen ZY, Zhang XM, Xu H, Zhang J, Huang XF. (Management of cesarean scar pregnancy by hysteroscopy combined with uterine artery embolism). Chinese. 2011; 46 (8): 591–594. Epub 16 December, 2011. [PubMed] [Google Scholar]
- 40. Hanif S, Hanif H, Sharif S. Acute abdomen at 12 weeks secondary to placenta percreta. JCPSP‐Pakistan. J Coll Physicians Surg Pak 2011; 21 (9): 572–573. Epub 15 September, 2011. [PubMed] [Google Scholar]
- 41. Shen L, Tan A, Zhu H, Guo C, Liu D, Huang W. Bilateral uterine artery chemoembolization with methotrexate for cesarean scar pregnancy. Am J Obstet Gynecol 2012; 207 (5): 386e1–6 Epub 31 October 2012. [DOI] [PubMed] [Google Scholar]
- 42. Zhang B, Jiang ZB, Huang MS, Guan SH, Zhu KS, Qian JS, et al. Uterine artery embolization combined with methotrexate in the treatment of cesarean scar pregnancy: results of a case series and review of the literature. J Vasc Interv Radiol 2012; 23 (12): 1582–1588. Epub 28 November, 2012. [DOI] [PubMed] [Google Scholar]
- 43. Sekiguchi A, Okuda N, Kawabata I, Nakai A, Takeshita T. Ultrasound detection of lacunae‐like image of a cesarean scar pregnancy in the first trimester. J Nippon Med Sch 2013; 80 (1): 70–73. Epub 09 March, 2013. [DOI] [PubMed] [Google Scholar]
- 44. Wu X, Zhang X, Zhu J, Di W. Caesarean scar pregnancy: comparative efficacy and safety of treatment by uterine artery chemoembolization and systemic methotrexate injection. EJOG 2012; 161 (1): 75–79. Epub 14 December, 2011. [DOI] [PubMed] [Google Scholar]
- 45. Li N, Zhu F, Fu S, Shi X. Transvaginal ultrasound‐guided embryo aspiration plus local administration of low‐dose methotrexate for caesarean scar pregnancy. UMB 2012; 38 (2): 209–213. Epub 20 December, 2011. [DOI] [PubMed] [Google Scholar]
- 46. Smorgick N, Vaknin Z, Pansky M, Halperin R, Herman A, Maymon R. Combined local and systemic methotrexate treatment of viable ectopic pregnancy: outcomes of 31 cases. J Clin Ultrasound: JCU. 2008; 36 (9): 545–550. Epub 16 July, 2008. [DOI] [PubMed] [Google Scholar]
- 47. Doubilet PM, Benson CB, Frates MC, Ginsburg E. Sonographically guided minimally invasive treatment of unusual ectopic pregnancies. J Ultrasound Med 2004; 23 (3): 359–370. Epub 02 April, 2004. [DOI] [PubMed] [Google Scholar]
- 48. Bignardi T, Condous G. Transrectal ultrasound‐guided surgical evacuation of Cesarean scar ectopic pregnancy. UOG 2010; 35 (4): 481–85. Epub 26 February, 2010. [DOI] [PubMed] [Google Scholar]
- 49. Demirel LC, Bodur H, Selam B, Lembet A, Ergin T. Laparoscopic management of heterotopic cesarean scar pregnancy with preservation of intrauterine gestation and delivery at term: case report. Fertil Steril. 2009; 91 (4): 1293 e5–1293 e–7. Epub 2008/03/21. [DOI] [PubMed] [Google Scholar]
- 50. Arslan M, Pata O, Dilek TU, Aktas A, Aban M, Dilek S. Treatment of viable cesarean scar ectopic pregnancy with suction curettage. Int J Gynaecol Obstet 2005; 89 (2): 163–166. Epub 26 April, 2005. [DOI] [PubMed] [Google Scholar]
- 51. Huang L, Du Y, Zhao C. High‐intensity focused ultrasound combined with dilatation and curettage for Cesarean scar pregnancy. UOG 2014; 43 (1): 98–101. Epub 10 July, 2013. [DOI] [PubMed] [Google Scholar]
- 52. Kim D, Moon NR, Lee SR, Won YD, Lee HJ, Park TC, et al. Acquired uterine arteriovenous malformation in a cesarean scar pregnancy. TJOG 2013; 52 (4): 590–592. Epub 15 January, 2014. [DOI] [PubMed] [Google Scholar]
- 53. Zhang Y, Duan H, Cheng JM, Guo YS. Treatment options to terminate persistent cesarean scar pregnancy. Gynecol Obstet Invest 2013; 75 (2): 115–119. Epub 09 January, 2013. [DOI] [PubMed] [Google Scholar]
- 54. Wu CF, Hsu CY, Chen CP. Ectopic molar pregnancy in a cesarean scar. TJOG 2006; 45 (4): 343–345. Epub 19 December, 2006. [DOI] [PubMed] [Google Scholar]
- 55. Deb S, Clewes J, Hewer C, Raine‐Fenning N. The management of Cesarean scar ectopic pregnancy following treatment with methotrexate – a clinical challenge. UOG 2007; 30 (6): 889–892. Epub 04 October, 2007. [DOI] [PubMed] [Google Scholar]
- 56. Zhang XB, Zhong YC, Chi JC, Shen JL, Qiu XX, Xu JR, et al. Caesarean scar pregnancy: treatment with bilateral uterine artery chemoembolization combined with dilation and curettage. J Int Med Res 2012; 40 (5): 1919–1930. Epub 05 December, 2012. [DOI] [PubMed] [Google Scholar]
- 57. Muraji M, Mabuchi S, Hisamoto K, Muranishi M, Kanagawa T, Nishio Y, et al. Cesarean scar pregnancies successfully treated with methotrexate. Acta Obstet Gynecol Scand 2009; 88 (6): 720–723. Epub 31 March, 2009. [DOI] [PubMed] [Google Scholar]
- 58. Armstrong V, Hansen WF, Van Voorhis BJ, Syrop CH. Detection of cesarean scars by transvaginal ultrasound. Obstet Gynecol 2003; 101 (1): 61–65. Epub 09 January, 2003. [DOI] [PubMed] [Google Scholar]
- 59. Monteagudo A, Carreno C, Timor‐Tritsch IE. Saline infusion sonohysterography in nonpregnant women with previous cesarean delivery: the “niche” in the scar. J Ultrasound Med 2001; 20 (10): 1105–1115. Epub 06 October, 2001. [DOI] [PubMed] [Google Scholar]
- 60. Roberge SB, Chaillet N, Moore L, Jastrow N, Demers S, Bujold E. Systematic review of cesarean scar assessment in the nonpregnant state: imaging techniques and uterine scar defect. Am J Perinatol 2012; 29 (6): 465–471. [DOI] [PubMed] [Google Scholar]


