Abstract
Despite mu opioid receptor agonists are the cornerstones of moderate-to-severe acute pain treatment, their effectiveness in chronic pain conditions is controversial. In contrast to mu opioid receptor agonists, a number of studies have reported the effectiveness of delta opioid receptor agonists on neuropathic pain strengthening the idea that delta opioid receptors gain importance when chronic pain develops. Among other effects, it has been shown that delta opioid receptor activation in optic nerve astrocytes inhibits tumor necrosis factor-α-mediated inflammation in response to severe hypoxia. Considering the involvement of tumor necrosis factor-α in the development and maintenance of neuropathic pain, with this study we sought to correlate the effect of delta opioid receptor agonist on the development of mechanical allodynia to tumor necrosis factor-α expression at the site of nerve injury in rats subjected to chronic constriction injury of the sciatic nerve. To this aim, we measured the levels of tumor necrosis factor-α in the sciatic nerve of rats with neuropathic pain after repeated injections with a delta opioid receptor agonist. Results obtained demonstrated that repeated administrations of the delta opioid receptor agonist SNC80 (10 mg/kg, i.p. for seven consecutive days) significantly inhibited the development of mechanical allodynia in rats with neuropathic pain and that the improvement of neuropathic symptom was timely related to the reduced expression of tumor necrosis factor-α in the rat sciatic nerve. We demonstrated also that when treatment with the delta opioid receptor agonist was suspended both allodynia and tumor necrosis factor-α up-regulation in the sciatic nerve of rats with neuropathic pain were restored. These results show that persistent delta opioid receptor activation significantly attenuates neuropathic pain and negatively regulates sciatic nerve tumor necrosis factor-α expression in chronic constriction injury rats.
Keywords: Neuropathic pain, delta opioid receptor, tumor necrosis factor-α, mechanical allodynia
Background
Opioid receptors, known as MOR, DOR, and KOR (mu, delta and kappa opioid receptor), play a key role in pain control.1–3 They are expressed along nociceptive pathways from the first-order primary afferent neurons to descending inhibitory system. Each opioid receptor constitutes a distinct target for pain treatment and selectively controls nociceptive transmission.4 Despite MOR opioid agonists are the cornerstones of treatment of moderate-to-severe acute pain, their effectiveness for chronic pain management is controversial.5,6 MOR activation indeed produces not only analgesic effects but also serious side effects, including constipation, nausea, and sedation. Also, the development of tolerance and dependence might occur.2,4,7 Lately, due to the availability of highly selective non-peptidic agonist, DOR has become an attractive target for pain treatment8–10 and a number of studies indicate a promising role of DOR in chronic pain conditions.11–14 In contrast to MOR agonists, DOR activation weakly influences acute pain perception but efficiently decreases persistent pain.15 Also, in animals with neuropathic pain subjected to peripheral nerve injury (PNI), DOR protein levels increase within the ipsilateral sciatic nerve indicating the occurrence of DOR trafficking in the site of injury.11,12,16 Interestingly, DOR knockout animals exhibit enhanced neuropathic and inflammatory nociceptive response, suggesting the existence of an endogenous DOR tone under inflammatory and neuropathic pain conditions.8,12
The pathogenic role of neuroinflammation in the development of neuropathic pain has recently gained more attention.17 Pro-inflammatory cytokines, such as tumor necrosis factor-α (TNF-α), are considered key modulators in the cross-talk among immune cells, neurons, and glia, and their involvement in the development and maintenance of inflammatory and neuropathic pain conditions18,19 has been clearly demonstrated. At the site of nerve injury, TNF-α protein levels are rapidly up-regulated and increased levels are detected until day 14 after nerve injury.20 Also, microinjections of TNF-α directly into normal (uninjured) nerves produces a reduction of pain threshold with development of both thermal and mechanical hyperalgesia,21 whereas TNF-α-neutralizing antibodies attenuate thermal hyperalgesia and mechanical allodynia in animal models of neuropathic pain.22,23
Literature data supports a correlation between DOR and TNF-α.24–26 In a sepsis rat model, DOR activation was associated with a significant decrease in the serum levels of early and late pro-inflammatory citokines.24 Wang et al.25 demonstrated that DOR activation inhibits TNF-α-mediated inflammation in response to severe hypoxia in both glial and neuron-like cells.
However, to date no studies indicate whether TNF-α expression is under the control of DOR activation in neuropathic pain; thus, the aim of this study was to investigate whether the effect of the DOR agonist as an analgesic agent in rats with neuropathic pain could be related to the TNF-α expression at the site of nerve injury. To address this issue, we evaluated the effect of repeated administrations of the DOR agonist, SNC80, for seven consecutive days starting from the day of injury, on (a) the development of mechanical allodynia in rats underwent to chronic constriction injury (CCI) of the sciatic nerve and (b) changes in the expression of TNF-α protein level in the rat sciatic nerve at different time points from CCI by using Western blot analysis.
Materials and methods
Animals
Experiments were performed on male Sprague–Dawley rats (Harlan Laboratories, S.Pietro al Natisone (UD)) weighing 180–200 g. Animals were kept at a constant room temperature (25 ± 1℃) under a 12:12 h light and dark cycle with free access to food and water. Each rat was used for only one experiment. All tests were performed at room temperature (22–24℃) between 08:00 and 15:00. Experimental procedures were approved by the Local Ethical Committee and the Institutional Animal Care And Use Committee (IACUC), and all experiments were conducted in accordance with International Guidelines as well as European Communities Council Directive and National Regulations (EEC Council 86/609 and DL 116/92).
CCI model of neuropathic pain
The CCI model was used to induce neuropathic pain in rats. CCI was performed according to Bennett and Xie27 with minor modifications.5 Briefly, animals were anaesthetized with 2–4% isoflurane inhalation anesthesia, and an incision was made just below the hipbone, parallel to the left common sciatic nerve. The sciatic nerve was exposed and four ligatures (4/0 chromic silk, Ethicon) were loosely tied around the nerve at the level of the mid-thigh and proximal to the trifurcation of the nerve at about 1 mm spacing, until a brief twitch in the respective hind limb was observed. For sham operation, the sciatic nerve was exposed but not manipulated. The rat body temperature was maintained by using a warm blanket kept at constant temperature during surgical procedures until the rats recovered from anesthesia.
Assessment of mechanical allodynia
The assessment of tactile allodynia was performed by measuring the withdrawal threshold of the hind paw in response to a series of calibrated von Frey’s filaments.28 Rats were placed in a clear plastic testing chamber with a wire mesh bottom and allowed to acclimatize for 20 min. The ventral surface of the hind paw was mechanically stimulated from below with an ascending series of graded von Frey’s filaments with bending forces ranging from 0.02 to 30 g. The paw withdrawal threshold (PWT) was determined by the “up-down” method29 of sequentially increasing and decreasing the stimulus strength and was expressed as the mean withdrawal threshold.
Western blot analysis
Both right and left sciatic nerves were rapidly removed, frozen in liquid nitrogen, and stored at 80℃ until proteins extraction. Tissue samples were homogenized in lysis buffer (Tris-HCl pH = 7.4, 1% Triton-X100, NaCl 150 mmol/L, and EDTA 1 mmol/L), 10 µL buffer/1 mg tissue, and a cocktail of protease inhibitors (1:100, Sigma Aldrich). The homogenate was centrifuged at 15,000 r/min for 15 min at 4℃ and supernatant was collected. For Western blotting, protein samples containing an equal amount of protein (50 µg) were electrophoresed on 12% SDS-PAGE gels and transferred to nitrocellulose membranes blocked with 5% non-fat milk powder in TBST buffer. Membranes were incubated overnight at 4℃ with primary antibodies, mouse TNF-α (1:1000, Novex) and rabbit β-tubulin (1:1000, Cell signalling) used 1:1000, for proteins detection. After three washing in TBST (Tris 50 mM, NaCl 150 mM, 0.1% tween 20, adjust pH with HCl to pH 7.6), membranes were incubated with anti-mouse (1:20000, Jackson) and anti-rabbit HRP-conjugated (1:50000, Jackson) secondary antibody, for 1 h at room temperature. Proteins bands were visualized with Luminata Forte Western HRP substrate according to the manufacturer's instructions and revealed with Uvitec Cambridge Imaging System. The density of each band was quantified using ImageJ analysis software. All values are shown as the means, and each value represents the average of three independent experiments. Western blot quantification data were analyzed by one-way ANOVA and post hoc test (Bonferroni test). The level of significance was set at p < 0.05.
Experimental protocol
Study protocol
Animals were randomly assigned into three groups: (1) rats underwent to CCI (CCI animals); (2) sham operated mice underwent to similar surgery but with no ligatures placed around the sciatic nerve (SO animals); and (3) un-manipulated mice (naïve animals). Animals were treated as following:
CCI animals treated with normal saline (NS) (CCI-NS) (i.p. for 14 consecutive days);
SO animals treated with NS (SO-NS) (i.p. for 14 consecutive days);
CCI animals treated with SNC80 (CCI-SNC80) (10 mg/kg, i.p., for seven consecutive days plus NS for seven more consecutive days).
The behavioral measurements were carried out 1 day before, and 3, 7, 14 days after CCI. For Western blotting analysis, four rats per group were sacrificed on days 1, 3, 7 and 14 (Figure 1).
Figure 1.
Experimental design.
Drugs
SNC80 was purchased by Santa Cruz Biotechnology and was dissolved in normal saline before the administration.
Results
Effects of SNC80 on the development of mechanical allodynia
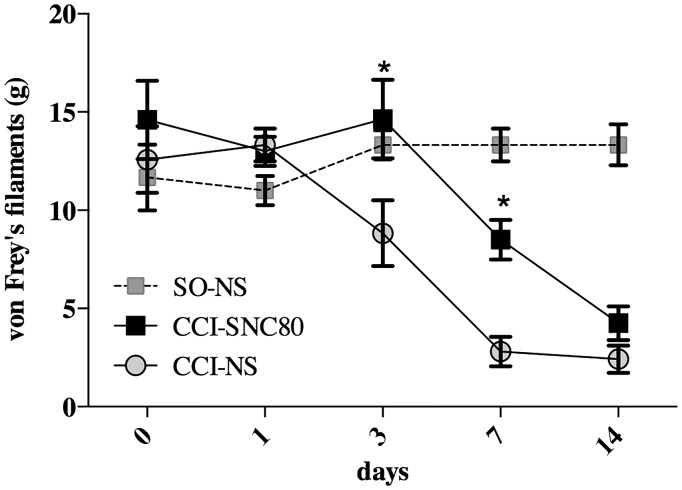
To investigate the effect of persistent DOR activation on CCI-induced mechanical allodynia, rats were injected with the DOR agonist, SNC80 (10 mg/kg, i.p.) for seven consecutive days after the induction of CCI. Basal mechanical threshold was evaluated one day before surgery and then on day 1, 3, 7, and 14 after CCI and compared to vehicle injected CCI and SO rats (Figure 2).
Figure 2.
Effect of the DOR agonist SNC80 on CCI-induced mechanical allodynia. CCI rats were treated either with SNC80 (10 mg/kg, i.p.) (CCI-SNC80 group) or normal saline (CCI-NS group), respectively, from 1 to 7 and 8 to 14 days after surgery. SO rats (SO-NS group) received normal saline for 14 consecutive days. Mechanical thresholds, measured with von Frey’s filaments, were evaluated before (day 0) and 1, 3, 7, and 14 days after surgery. Results are expressed in grams (g) and represent the means ± SEM from 8 to 10 rats. *p < 0.05 vs. NS-treated CCI rats (CCI-SNC80 vs. CCI-NS) (Two-way ANOVA + Bonferroni post hoc test).
After surgery, CCI rats developed progressive behavioral signs of mechanical sensitization, quantized as a decrease in the PWT in response to stimulation with von Frey’s filaments starting from postoperative day 3. No changes in mechanical threshold were observed in SO rats. In CCI rats, the decrease in PWT persisted up to day 14 in comparison to SO animals (Figure 2). I.p. administration of the DOR agonist, SNC80, immediately after the ligature, and for seven consecutive days, significantly reduced the development of mechanical allodynia as measured at day 3 and 7 after CCI when compared to vehicle-treated CCI rats (CCI-NS). However, the antiallodynic effect disappeared when the treatment with SNC80 was stopped.
Effect of SNC80 on sciatic nerve TNF-α protein level
To test the hypothesis that repeated systemic injections of the DOR agonist, SNC80, might regulate TNF-α expression in the sciatic nerve, we performed Western blot analysis in both ipsi- and contralateral sciatic nerves of CCI and SO rats. Also, a naïve group of rats was included in this experiment. Ipsi- and contralateral sciatic nerves of all rats were removed on day 1, 3, 7, and 14 after surgery and processed for TNF-α analysis.
Western blotting analysis shows no significant differences in TNF-α between groups at day 1 after surgery (Figure 3(a)). On the contrary, on day 3 after surgery, a significant increase of TNF-α expression was observed in the sciatic nerve of CCI rats ipsilateral to the nerve ligature compared to both contralateral sciatic nerve and SO or naïve animals (Figure 3(b)). Interestingly enough, a three-day treatment with the DOR agonist, SNC80, was able to significantly reduce the ipsilateral TNF-α up-regulation in CCI rats, suggesting that DOR activation is able to reduce CCI-induced TNF-α up-regulation in the sciatic nerve. Strikingly, SNC80 completely blocked CCI-induced TNF-α expression in the ligated sciatic nerve after a seven-day treatment (Figure 3(c)).
Figure 3.
Effect of SNC80 on TNF-α expression in the sciatic nerve of CCI rats. Quantification of TNF-α levels was performed in the ipsilateral and contralateral sciatic nerve (ipsi and contra, respectively) at day 1 (a), day 3 (b), day7 (c), and day 14 (d) from surgery of naïve, SO-NS, CCI-NS, and CCI-SNC80-treated rats. A representative Western blot analysis is shown in lower panels. Data are means ± SEM of three different analysis. *p < 0.05 versus ipsi naïve; **p < 0.01 versus ipsi naïve; ***p < 0.001 versus ipsi naïve; #p < 0.05 versus ipsi sham; ###p < 0.001 versus ipsi sham; §§p < 0.01 versus ipsi vehicle. One-way ANOVA + Bonferroni post hoc test.
In a different set of experiments, we investigated whether the effect of SNC80, administered for seven days after surgery, on TNF-α persists until day 14 from CCI. With this in mind, CCI rats were injected for seven consecutive days with SNC80 from surgery and for seven more consecutive days with NS. The sciatic nerve was removed at day 14 from the ligature and compared to both SO and naïve rats. Western blotting analysis clearly shows that SNC80 effect on TNF-α disappeared at day 14 from surgery (seven days from last administration) (Figure 3(d)) suggesting that a continuous DOR activation is needed to block TNF-α up-regulation in the sciatic nerve induced by CCI.
Discussion
Results obtained in this study demonstrated that a continuous administration of the DOR agonist SNC80 significantly inhibited the development (onset and maintenance) of mechanical allodynia in rats with neuropathic pain underwent to CCI. We have shown that the improvement of neuropathic pain symptoms by repeated DOR agonist administration is strictly related to the reduced expression of the pro-inflammatory cytokine TNF-α in the sciatic nerve. In addition, we demonstrated that when DOR agonist treatment was suspended, both allodynia and TNF-α up-regulation were restored.
Neuropathic pain is characterized by an altered transmission and modulation of nociception.3,30 Unfortunately its pharmacological treatment remains challenging due to ineffective therapies and resistance to classical analgesics including opioids.5,6 A number of studies have reported the effectiveness of DOR agonists on the inhibition of inflammatory31 and neuropathic pain induced by diabetes14 and sciatic nerve injury,11,18 strengthening the idea that DORs gain importance when chronic pain develops. DOR density and activity are up-regulated in chronic pain models and enhancement of thermal hyperalgesia, mechanical allodynia, and thermal allodynia was observed in male DOR knockout mice exposed to a partial sciatic nerve ligation.8,12 These data indicate the possible existence of a tonic activation of DOR during inflammatory and neuropathic pain that could counteract the manifestations of these pathological processes.
Consistently, our results demonstrated that a continuous DOR activation inhibited the development of mechanical allodynia and that the DOR antiallodynic effect disappeared when the treatment with the DOR agonist, SNC80, was stopped. Moreover, results from this study confirm our hypothesis that DOR agonists could have a critical role in the development of neuropathic pain by reducing TNF-α up-regulation in the injured nerve. Several studies showed the TNF-α overexpression in different states of chronic pain.20,32 Consistent with data from literature,20 in this study we observed a significant up-regulation of TNF-α in the ligated sciatic nerve on day 3, 7, 14 after surgery, in vehicle-treated CCI rats (Figure 3). The correlation between TNF-α overexpression and CCI symptoms is slightly different from what observed by other authors that described a fast TNF recovery in sciatic nerves after CCI.32 However, differences in the species (mouse vs. rat) and in the experimental procedures might account for these differences in the timing of TNF-α expression. In parallel with antiallodynic effect, SNC80 reduced TNF-α expression until the suspension of DOR agonist chronic treatment providing an explanation of the possible mechanism underlying the reduction of allodynia. Thus, in addition to its role in persistent pain relief,8,11–15 a chronic DOR activation may regulate TNF-α to inhibit the development of mechanical allodynia.
TNF-α is known to be one of the most prominent pro-inflammatory cytokines whose overexpression is involved in the inflammatory response and the onset of neuropathic pain consequent to PNI.33 Neuropathic pain induces broad molecular and cellular adaptations, including neuronal and glial changes that together contribute to consolidate persistent pain.12 Activated Schwann cells up-regulate TNF-α, which at the site of nerve injury begins the cascade of chemotropic and macrophage-mediated pathologic events associated with Wallerian degeneration.17,34,35
It was shown that TNF-α by itself produced hyperalgesia when injected into nerve.21 Vogel et al.36 indicate that mechanical allodynia is a complex phenomenon based on TNF-α-induced sensitization of several ion channels; this TNF-α modulation of allodynia is mediated by the activation of TNF-α receptor 1 and 2. Also, TNF-α inhibition causes significant reduction in degenerative tissue remodeling, as well as CCI-induced nociceptive behaviors17,37 and application of neutralizing antibodies to TNF-α attenuates pain-related behavior in CCI mice.23
The correlation between DOR activation and sciatic nerve TNF-α expression in CCI rats is supported by some literature data.25,26,38–40 DOR activation inhibits TNF-α-mediated inflammatory processes following exposure to severe hypoxia,25 and it has been demonstrated that stimulation of the DOR receptor suppresses activation of p38 MAPK and reduces the production of pro-inflammatory mediators including TNF-α in macrophages providing new clues to the potential mechanisms by which DOR agonists may protect against post-ischemic tissue injury. DOR decreases TNF-α levels in the hypoxic cortex.26 Moreover, SNC-121, a DOR agonist, attenuated TNF-α-induced MMP-2 secretion from human optic nerve head astrocytes39 and DOR activation opposes the production of TNF-α during early phases of glaucomatous injury.40
In summary, our data further support the hypothesis that DOR activation plays a key role in neuropathic pain, and provide novel information that the expression of sciatic nerve TNF-α is negatively regulated by this opioid receptor in neuropathic pain conditions.
Authors’ Contributions
CP, NV, RP, and SC designed and performed in vitro experiments. CP and GA designed and performed in vivo experiments. CP, SC, RT, GA, and GMS participated in the interpretation of data and wrote the manuscript. All the authors read and approved the final manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by University of Catania (FIR 2014) to CP.
References
- 1.Dickenson AH, Kieffer BL. Opiates: basic mechanisms. In: McMahon SB, Koltzenburg M. (eds). Textbook of pain, Churchill Livingstone, London: Elsevier, New York, 2013, pp. 413–428. [Google Scholar]
- 2.Nagase H, Fujii H. Opioids in preclinical and clinical trials. Top Curr Chem 2011; 299: 29–62. [DOI] [PubMed] [Google Scholar]
- 3.Tibbs GR, Posson DJ, Goldstein PA. Voltage-gated ion channels in the PNS: Novel therapies for neuropathic pain. Trends Pharmacol Sci 2016; 37: 522–542. [DOI] [PubMed] [Google Scholar]
- 4.Turnaturi R, Aricò G, Ronsisvalle G, et al. Multitarget opioid ligands in pain relief: new players in an old game. Eur J Med Chem 2016; 108: 211–228. [DOI] [PubMed] [Google Scholar]
- 5.Parenti C, Turnaturi R, Aricò G, et al. The multitarget opioid ligand LP1's effects in persistent pain and in primary cell neuronal cultures. Neuropharmacology 2013; 71: 70–82. [DOI] [PubMed] [Google Scholar]
- 6.Pasternak G, Pan YX. Mu opioid receptors in pain management. Acta Anaesthesiol 2011; 49: 21–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McDonald J, Lambert DG. Opioid mechanisms and opioid drugs. Anaesth Intensive Care Med 2008; 9: 33–37. [Google Scholar]
- 8.Gaveriaux-Ruff C, Nozaki C, Nadal X, et al. Genetic ablation of delta opioid receptors in nociceptive sensory neurons increases chronic pain and abolishes opioid analgesia. Pain 2011; 152: 1238–1248. [DOI] [PubMed] [Google Scholar]
- 9.Fujii H, Takahashi T, Nagase H. Non-peptidic δ opioid receptor agonists and antagonists (2000–2012). Expert Opin Ther Pat 2013; 23: 1181–1208. [DOI] [PubMed] [Google Scholar]
- 10.Pradhan AA, Befort K, Nozaki C, et al. The delta opioid receptor: an evolving target for the treatment of brain disorders. Trends Pharmacol Sci 201; 32: 581–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gavériaux-Ruff C, Kieffer BL. Delta opioid receptor analgesia: recent contributions from pharmacology and molecular approaches. Behav Pharmacol 2011; 22: 405–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nadal X, Baños JE, Kieffer BL, et al. Neuropathic pain is enhanced in delta-opioid receptor knockout mice. Eur J Neurosci 2006; 23: 830–834. [DOI] [PubMed] [Google Scholar]
- 13.Peppin JF, Raffa RB. Delta opioid agonists: a concise update on potential therapeutic applications. J Clin Pharm Ther 2015; 40: 155–166. [DOI] [PubMed] [Google Scholar]
- 14.Castany S, Carcolé M, Leánez S, et al. The antinociceptive effects of a δ-opioid receptor agonist in mice with painful diabetic neuropathy: Involvement of heme oxygenase 1. Neurosci Lett 2016; 614: 49–54. [DOI] [PubMed] [Google Scholar]
- 15.Nozaki C, Le Bourdonnec B, Reiss D, et al. δ-Opioid Mechanisms for ADL5747 and ADL5859 effects in mice: analgesia, locomotion, and receptor internalization. J Pharmacol Exp Ther 2012; 342: 799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kabli N, Cahill CM. Anti-allodynic effects of peripheral delta opioid receptors in neuropathic pain. Pain 2007; 127: 84–93. [DOI] [PubMed] [Google Scholar]
- 17.Myers RR, Shubayev VI. The ology of neuropathy: an integrative review of the role of neuroinflammation and TNF-α axonal transport in neuropathic pain. J Peripher Nerv Syst 2011; 16: 277–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hervera A, Negrete R, Leánez S, et al. The role of nitric oxide in the local antiallodynic and antihyperalgesic effects and expression of delta-opioid and cannabinoid-2 receptors during neuropathic pain in mice. J Pharmacol Exp Ther 2010; 334: 887–896. [DOI] [PubMed] [Google Scholar]
- 19.Leung L, Cahill CM. TNF-alpha and neuropathic pain-a review. J Neuroinflammation 2010; 7: 27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shubayev VI, Myers RR. Endoneurial remodeling by TNF alph- and TNF alpha-releasing proteases. A spatial and temporal co-localization study in painful neuropathy. J Peripher Nerv Syst 2002; 7: 28–36. [DOI] [PubMed] [Google Scholar]
- 21.Wagner R, Myers RR. Endoneurial injection of TNF-alpha produces neuropathic pain behaviors. Neuroreport 1996; 7: 2897–2901. [DOI] [PubMed] [Google Scholar]
- 22.Lindenlaub T, Teuteberg P, Hartung T, et al. Effects of neutralizing antibodies to TNF-alpha on pain-related behavior and nerve regeneration in mice with chronic constriction injury. Brain Res 2000; 866: 15–22. [DOI] [PubMed] [Google Scholar]
- 23.Sommer C, Lindenlaub T, Teuteberg P, et al. Anti-TNF-neutralizing antibodies reduce pain-related behavior in two different mouse models of painful mononeuropathy. Brain Res 2001; 913: 86–89. [DOI] [PubMed] [Google Scholar]
- 24.Tang CW, Feng WM, Du HM, et al. Delayed administration of D-Ala2-D-Leu5 enkephalin, a delta-opioid receptor agonist, improves survival in a rat model of sepsis. Tohoku J Exp Med 2011; 224: 69–76. [DOI] [PubMed] [Google Scholar]
- 25.Wang Q, Chao D, Chen T, et al. δ-Opioid receptors and inflammatory cytokines in hypoxia: differential regulation between glial and neuron-like cells. Transl Stroke Res 2014; 5: 476–483. [DOI] [PubMed] [Google Scholar]
- 26.Tian X, Hua F, Sandhu HK, et al. Effect of δ-opioid receptor activation on BDNF-TrkB vs. TNF-α in the mouse cortex exposed to prolonged hypoxia. Int J Mol Sci 2013; 14: 15959–15976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 1988; 33: 87–107. [DOI] [PubMed] [Google Scholar]
- 28.Parenti C, Marrazzo A, Aricò G, et al. The antagonistic effect of the sigma 1 receptor ligand (+)-MR200 on persistent pain induced by inflammation. Inflamm Res 2014; 63: 231–237. [DOI] [PubMed] [Google Scholar]
- 29.Dixon WJ. Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol 1980; 20: 441–462. [DOI] [PubMed] [Google Scholar]
- 30.Xu Q, Yaksh TL. A brief comparison of the pathophysiology of inflammatory versus neuropathic pain. Curr Opin Anaesthesiol 2011; 24: 400–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carcolé M, Castany S, Leánez S, et al. Treatment with a heme oxygenase 1inducer enhances the antinociceptive effects of µ-opioid, δ-opioid, and cannabinoid 2 receptors during inflammatory pain. J Pharmacol Exp Ther 2014; 351: 224–232. [DOI] [PubMed] [Google Scholar]
- 32.Sacerdote P, Franchi S, Trovato AE, et al. Transient early expression of TNF-alpha in sciatic nerve and dorsal root ganglia in a mouse model of painful peripheral neuropathy. Neurosci Lett 2008; 436: 210–213. [DOI] [PubMed] [Google Scholar]
- 33.Chen YW, Tzeng JI, Liu KS, et al. Systemic diphenidol reduces neuropathic allodynia and TNF-alpha overexpression in rats after chronic constriction injury. Neurosci Lett 2013; 552: 62–65. [DOI] [PubMed] [Google Scholar]
- 34.Myers RR, Campana WM, Shubayev VI. The role of neuroinflammation in neuropathic pain: mechanisms and therapeutic targets. Drug Discov Today 2006; 11: 8–20. [DOI] [PubMed] [Google Scholar]
- 35.Gaudet AD, Popovich PG, Ramer MS. Wallerian degeneration: gaining perspective on inflammatory events after peripheral nerve injury. J Neuroinflammation 2011; 8: 110–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vogel C, Stallforth S, Sommer C. Altered pain behavior and regeneration after nerve injury in TNF receptor deficient mice. J Peripher Nerv Syst 2006; 11: 294–303. [DOI] [PubMed] [Google Scholar]
- 37.Shubayev VI, Myers RR. Upregulation and interaction of TNFalpha and gelatinases A and B in painful peripheral nerve injury. Brain Res 2000; 855: 83–89. [DOI] [PubMed] [Google Scholar]
- 38.Husted TL, Govindaswami M, Oeltgen PR, et al. A delta2-opioid agonist inhibits p38 MAPK and suppresses activation of murine macrophages. J Surg Res 2005; 128: 45–49. [DOI] [PubMed] [Google Scholar]
- 39.Akhter N, Nix M, Abdul Y, et al. Delta-opioid receptors attenuate TNF-α-induced MMP-2 secretion from human ONH astrocytes. Invest Ophthalmol Vis Sci 2013; 54: 6605–6611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abdul Y, Akhter N, Husain S. Delta-opioid agonist SNC-121 protects retinal ganglion cell function in a chronic ocular hypertensive rat model. Invest Ophthalmol Vis Sci 2013; 54: 1816–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]