Abstract
Mycobacterium bovis is the causative agent of bovine tuberculosis (BTB), the pathogen responsible for serious economic impact on the livestock sector. In order to obtain data on isolated M. bovis strains and assist in the control and eradication program for BTB, a cross sectional descriptive molecular epidemiology study in the Brazilian Midwest was conducted. Through spoligotyping and 24-loci MIRU-VNTR methods, 37 clinical isolates of M. bovis circulating in the region were analyzed, 10 isolated from the state of Mato Grosso, 12 from the state of Mato Grosso do Sul and 15 from the state of Goiás. The spoligotyping analysis identified 10 distinct M. bovis profiles (SB0121 n = 14, SB0295 n = 6, SB0140 n = 6, SB0881 n = 3, SB1144 n = 2, SB1145 n = 2, SB0134 n = 1, SB1050 n = 1, SB1055 n = 1, SB1136 n = 1) grouped in six clusters and four orphan patterns. The MIRU-VNTR 24-loci grouped the same isolates in six clusters and 22 unique orphan patterns, showing higher discriminatory power than spoligotyping. When associating the results of both techniques, the isolates were grouped in five clusters and 24 unique M. bovis profiles. Among the 24-loci MIRU-VNTR evaluated, two, ETR-A and QUB 11b loci, showed high discriminatory ability (h = ≥ 0.50), while MIRU 16, MIRU 27, ETR-B, ETR-C, Mtub21 and QUB 26 loci showed moderate ability (h = 0.33 or h = 0.49) and were the most effective in evaluating the genotypic similarities among the clinical M. bovis isolate samples. Herein, the 29 patterns found amongst the 37 isolates of M. bovis circulating in the Brazilian Midwest can be due to the animal movement between regions, municipalities and farms, thus causing the spread of various M. bovis strains in herds from Midwest Brazil.
Introduction
Mycobacterium bovis is a bacteria belonging to the Mycobacterium tuberculosis complex (MTC), which, in addition to causing tuberculosis in cattle and buffaloes (BTB), can cause disease in several species of mammals, including humans, thus being considered a zoonosis [1,2].
BTB is a worldwide-distributed disease with striking prevalence in developing countries. This disease has socio-economic impacts by reducing livestock productivity due to early disposal of high zootechnical value animals, reduction in weight gain of affected animals and loss in the export of products from the cattle industry, mainly meat [3,4].
Infection by M. bovis in humans is typically caused by the consumption of animal food products contaminated by the bovine bacillus, usually unpasteurized milk and milk derivatives [5], leading to the development of tuberculosis in its extrapulmonary form [6]. Another route for M. bovis infection in humans is through airborne transmission [7,8]. These infections are clinically and pathologically indistinguishable from tuberculosis (TB) caused by M. tuberculosis [9,6]. It is suspected that infections caused by M. bovis are responsible for more than 4000 cases among the 100,000 cases of human tuberculosis described annually in Brazil [10,11]. However, according to the World Organization for Animal Health (OIE), the number of human TB cases caused by M. bovis in Brazil cannot be estimated [12], since bacteriological culture followed by biochemical identification tests to diagnose whether the infective agent was M. bovis or M. tuberculosis are not performed in most tuberculosis cases [13].
Cattle raising is very important for the Brazilian economy. Currently, the cattle herd in the country is over 212 million heads, and the Midwestern region, formed by the states of Mato Grosso, Mato Grosso do Sul and Goiás, is the main cattle-producing region [14] and the largest beef exporting region in the country [15]. Although livestock sanitary risks could impact the agribusiness on Brazilian economy, there is still a lack of updated data on the distribution and prevalence of BTB in the country and in the different producing regions. The latest official national prevalence data of the disease was in 2004, reporting a rate of 1.3% [8]. On the other hand, the estimated prevalence of the disease in the Midwest was of 0.37%, as described by Roxo, in 2004 [16]. In a recent study, the estimated prevalence of BTB for the state of Mato Grosso, which is part of the Midwest region, was estimated at 0.007% [17]. It is believed that, currently, the prevalence of BTB in the whole Midwestern region may be lower than that described in 2004 [16].
In order to reduce the prevalence and incidence of new BTB outbreaks in herds, to certify properties as free or monitored for the disease, and to offer consumers lower health risk products, Brazil, the Ministry of Agriculture Livestock and Supply (MAP) launched the National Program for Control and Eradication of Bovine Brucellosis and Tuberculosis (PNCEBT) [8] in 2001, which was regulated in 2004. This animal health program recommends performing the intradermal tuberculin test, followed by the slaughter of positive cattle, surveillance in slaughterhouses, tracing the origin of the outbreak and sanitation, as established by the International Organization for Animal Health [18].
The molecular identification of strains involved in BTB infection may contribute to an increased efficiency of disease control programs, since the identification of M. bovis genotypes prevalent in a particular area, allows to track and control the occurrence of multiple foci of disease [19,20], especially in areas with low prevalence of the disease, as is the case of the Brazilian Midwestern region.
Spacer oligotyping (spoligotyping) and variable number tandem repeat (VNTR) are amply used techniques in human tuberculosis epidemiological studies, as well as molecular typing of MTC species, which includes M. bovis [21]. When combined, spoligotyping and VNTR are able to distinguish the bacteria lineages more effectively [22,23,24], with a good cost/benefit relationship, due to speed, reproducibility and reliability of the performed genotyping [25,26,27,28].
The MIRU-VNTR is based on the size analysis of amplified fragments from multiple loci, determining the number of repetitions of each locus [29,30,31,32]. The analysis of the amplified fragment can be done manually by agarose gel electrophoresis [33] or automatically by capillary electrophoresis [34]. Each technique has its advantages and disadvantages that must be considered when choosing which to implement in the laboratory. Spoligotyping in combination with MIRU-VNTR analysis seems to be the best choice, since both have the advantage of being PCR-based, and, when combined, discriminatory power is improved [19].
In this context, a cross sectional study of molecular epidemiology was conducted for the characterization of M. bovis isolates circulating in the Brazilian Midwest and the comparison with M. bovis strains from other regions of Brazil and the world was performed.
Materials and Methods
Bacterial isolates and DNA extraction
The present study was based on a convenience sampling of BTB diagnosed between 2010 to 2013, at the National Agricultural Laboratory (LANAGRO/MAPA/BRASIL). A total of 37 M. bovis isolates were obtained from clinical samples taken from suspected BTB lesions from 37 animals that scored positive in the intradermal tuberculin test in the Brazilian Midwest region (Mato Grosso, Mato Grosso do Sul and Goiás). These isolates were previously identified by biochemical [26] and molecular tests [4]. DNA templates were extracted by the thermal lysis method [35] and purified using the commercial kit ChargeSwitch® PCR Clean-up kit (Invitrogen, CA, USA). DNA templates from M. bovis BCG and M. tuberculosis H37Rv were used as positive controls in the spoligotyping and MIRU-VNTR assays.
Spoligotyping
The spoligotyping method was conducted as described by Kamerbeek et al. (1997) [28]. Hybridisation of the PCR product to the spoligo-membrane was performed according to the manufacturer’s instructions (Ocimum Biosolutions, Telangana, IN). Bound fragments were detected by chemiluminescence after incubation with peroxidase-labelled streptavidin (1:4000). Only patterns with 100% similarity were considered as clusters. Those strains clustered by spoligotyping were analyzed by MIRU-VNTR to confirm their clonal relationships. M. bovis profiles were compared to those available at the Mbovis.org website (http://www.mbovis.org/) [36] and SITVIT-WEB (http://www.pasteur-guadeloupe.fr:8081/SITVIT_ONLINE/) databases.
MIRU-VNTR typing
M. bovis strain typing was carried out by MIRU-VNTR automated in-house technique, according to De-Beer et al. (2012) [37] with modifications. The detection of 24-loci MIRU-VNTR labeled with fluorophores (6FAM™/green, VIC®/blue and NED™/yellow) was performed, as recommended by Supply et al. (2006) [32]. For each sample, eight PCRs were carried out, using three primer pairs (triplex-PCR) each for the simultaneous amplification of three distinct loci [32].
Tríplex-PCR was performed using 0.4 μl of each primer (Applied Biosystem, CA, USA), at the concentrations described by Supply et al. (2006) [32], 1X KAPA2G Fast HotStar ReadMix PCR Kit® (Kapabiosystems, MA, USA), 1.87 μl of DMSO [p.a.] and 2 μl of purified DNA (about 20 ng) in a final volume of 20 μl. PCR assay conditions were 3 min at 95°C, followed by 30 cycles for 15 sec at 95°C, 15 sec at 59°C, 30 sec at 72°C and a final extension step at 72°C for 10 min.
PCR products (1 μl) were prepared for automated fragment reading on an optical plate—MicroAmp® Optical 96-well Reaction (Applied Biosystem, CA, USA) by adding 0.4 μl of the molecular marker GeneScan™ 1200 LIZ® Size Standard (Applied Biosystem), 8.6 μl Hidi formamide (Applied Biosystems) in a final volume of 10 μl. All mixtures were denatured at 95°C for 2 min and immediately cooled on ice. The fragment size of the amplicons was analyzed on a ABI 3130xl DNA sequence analyzer (Applied Biosystems) and the number of copies of each locus was determined by automated assignment using the GeneMapper® 4.0 software (Applied Biosystems). In case of doubtful results, the length of the repeats was double checked by size fragment estimation as compared to a DNA ladder (50 and 100 bp). Aplicons from M. bovis BCG and H37Rv strains were compared with the reference table described by Supply et al. (2000) [31].
The sample profiles were compared to those available at the database MIRU-VNTR plus (http://www.miru-vntrplus.org/MIRU/index.faces) and analyzed by BioNumerics software 6.6 (Applied Maths, Sint-Martens-Latem, BE).
Allelic and genotypic diversity calculations
The Hunter-Gaston discriminatory index (HGDI) [38] was used to calculate the allelic diversity within each MIRU-VNTR locus and the genotypic diversities (discriminatory power) of the spoligotyping assays, 24-MIRU-VNTR and the combination of both methodologies.
Clustering analysis
The number and fragment length of the genotype clusters were introduced as numerical data into an Excel spreadsheet template and different criteria for definition of the clusters were used, such as the analysis of individual spoligotyping or combination of results from spoligotyping and MIRU-VNTR. Data were analyzed by the BioNumerics software 6.6 (Applied Maths, East Flanders, BE) in order to construct the similarity matrices and the dendrogram (unweighted pair-grouping method analysis algorithm—UPGMA).
Results and Discussion
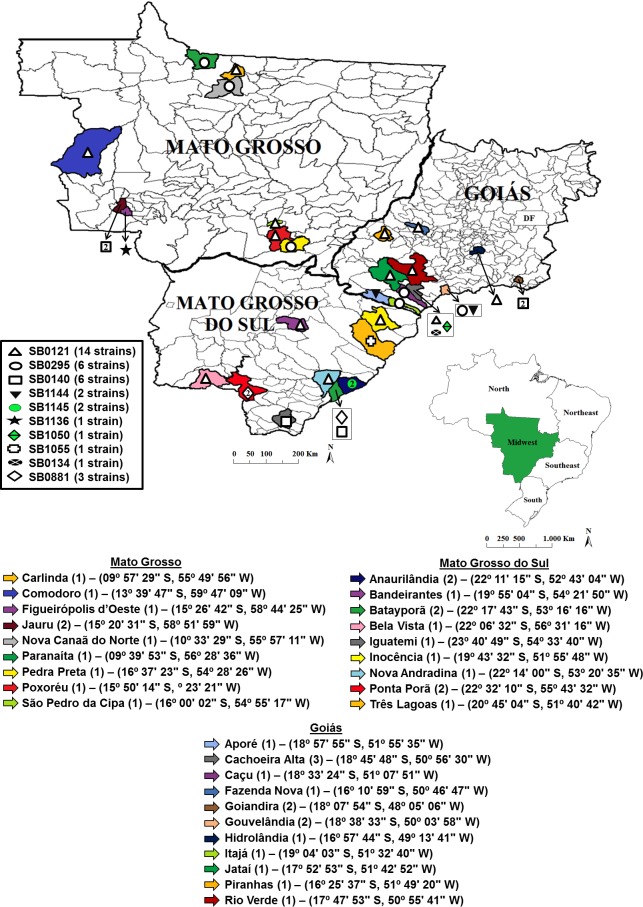
After the spoligotyping, the 37 M. bovis isolates were classified as (Table 1) SB0121 (n = 14; 37.8%), SB0295 (n = 6, 16.2%), SB0140 (n = 6), SB0881 (n = 3, 8.1%), SB1144 (n = 2, 5.4%) and SB1145 (n = 2). In addition, four strains (10.8%), SB0134, SB1050, SB1055 and SB1136, showed orphan patterns. The geographic distribution of the spoligotypes is presented in Fig 1.
Table 1. Molecular characterization of the 37 M. bovis isolates by spoligotyping method.
| Sample | Spoligotype | Spoligotype pattern |
|---|---|---|
| 44 | 1100000101111110111101111000011111111100000 | SB1145 |
| 45 | 1100000101111110111101111000011111111100000 | SB1145 |
| 49 | 1101111101111110111101111000000111111100000 | SB0881 |
| 52 | 1101111101111110111101111000000111111100000 | SB0881 |
| 10 | 1101111101111110111101111000000111111100000 | SB0881 |
| 35 | 1101111101111110111101111111111111111100000 | SB0121 |
| 36 | 1101111101111110111101111111111111111100000 | SB0121 |
| 11 | 1101111101111110111101111111111111111100000 | SB0121 |
| 22 | 1101111101111110111101111111111111111100000 | SB0121 |
| 23 | 1101111101111110111101111111111111111100000 | SB0121 |
| 30 | 1101111101111110111101111111111111111100000 | SB0121 |
| 37 | 1101111101111110111101111111111111111100000 | SB0121 |
| 33 | 1101111101111110111101111111111111111100000 | SB0121 |
| 39 | 1101111101111110111101111111111111111100000 | SB0121 |
| 48 | 1101111101111110111101111111111111111100000 | SB0121 |
| 17 | 1101111101111110111101111111111111111100000 | SB0121 |
| 5 | 1101111101111110111101111111111111111100000 | SB0121 |
| 4 | 1101111101111110111101111111111111111100000 | SB0121 |
| 38 | 1101111101111110111101111111111111111100000 | SB0121 |
| 15 | 1101111101111110111101111111111111110100000 | SB0295 |
| 16 | 1101111101111110111101111111111111110100000 | SB0295 |
| 13 | 1101111101111110111101111111111111110100000 | SB0295 |
| 12 | 1101111101111110111101111111111111110100000 | SB0295 |
| 25 | 1101111101111110111101111111111111110100000 | SB0295 |
| 18 | 1101111101111110111101111111111111110100000 | SB0295 |
| 1 | 1101111101111110111101111111100000001100000 | SB1144 |
| 21 | 1101111101111110111101111111100000001100000 | SB1144 |
| 19 | 1101101000001110111111111111111111111100000 | SB0140 |
| 20 | 1101101000001110111111111111111111111100000 | SB0140 |
| 27 | 1101101000001110111111111111111111111100000 | SB0140 |
| 28 | 1101101000001110111111111111111111111100000 | SB0140 |
| 46 | 1101101000001110111111111111111111111100000 | SB0140 |
| 29 | 1101101000001110111111111111111111111100000 | SB0140 |
| 14 | 1100011101111110111111111111111111111100000 | SB0134 |
| 24 | 0000000000011110111111111111111111111100000 | SB1136 |
| 3 | 1101111101111110111101111111100000111100000 | SB1050 |
| 9 | 1100011101111110111111111111111111110100000 | SB1055 |
| M. bovis BCG | 1101111101111110111111111111111111111100000 | Reference strains |
| M. tuberculosis H37Rv | 1111111111111111111001111111111100001111111 | Reference strains |
Fig 1. Geographic origin of each M. bovis spoligotype found in strains isolated at municipalities of Midwest Brazil.
The predominant spoligotype SB0121 was widespread in the three states of the Brazilian Midwest, and has also been described as the most prevalent in other Brazilian regions, including in the states of Rio Grande do Sul (92.9%), in the Southern region of the country [19], São Paulo (32.7%) [39] and Minas Gerais (16.4%) [40], both in the Southeastern region, in the state of Bahia (36%), in the Northeast, [3] and in the state of Mato Grosso do Sul (30.7%), in the Midwest [20]. Outside Brazil, SB0121 has been described in the Netherlands [41], France [41,42], Italy [43], Belgium [41], Portugal [44], Spain [45], Algeria [46], South Africa [47], Mexico [48,49] and Venezuela [49]. Interestingly, the SB0121 spoligotype has not yet described in Argentina, a country that borders Brazil and where animal movement between the countries frequently occurs [49].
The second most frequent spoligotype, SB0295, found in Mato Grosso and Goiás has been described in the states of São Paulo (35%) [39] and, Bahia (14%) [3], consistent with the national prevalence of 24% [49]. The SB0295 spoligotype has also been described in Spain [50], Portugal [44], France [42] and Mexico [51].
Spoligotypes SB0121 and SB0295 differ by one spacer only in the DR (direct repeat) region (Table 1) and were presently responsible for 54% genotypes of the strains isolated from Midwestern Brazil. The small discrepancy in these spoligotypes may be associated with strains that have undergone genetic mutation, which may cause difficulties in BTB diagnostics through the conventional tuberculin test, adopted throughout the country for BTB control in cattle herds [19,52,53]. Infections caused by strains classified as SB0121 and SB0295 spoligotypes occurred in municipalities very near to each other and suggests a selection of these lineages in these geographic locations (Fig 1).
Although spoligotype SB0140 was observed at a lower frequency (16.2%), it occurred in the three investigated states and was found with similar a frequency in São Paulo [54]. It has also been described throughout the four continents, in several countries, including Mexico [48,49,51], Argentina [49,55], Paraguay [55], Uruguay [55] Chile [49], France [41], Italy [43], Ireland [56,57], United Kingdom [58,59], South Africa [47] and Australia [56].
The SB0881 spoligotype was identified only in Mato Grosso do Sul (Fig 1) and is the third most prevalent in Brazil [49], having previously been reported in the country [20,39,40], and having also been shown to occur in Spain [45] and in France [41].
The SB1144 and SB1145 spoligotypes were identified in only two isolates each, the former in Goiás and the latter in Mato Grosso do Sul (Fig 1). These spolygotypes have only been found in Brazil. The spoligotype SB1145 is the most widely-distributed, being previously reported in São Paulo [54], Minas Gerais [40], Bahia [3] and Mato Grosso do Sul [20].
The less frequent spoligotyping profiles identified in this study were SB0134, SB1136, SB1050 and SB1055, with a single isolate each. SB1136 has been described only in Brazil (Mbovis.org) [40] while SB0134 has been reported in Brazil [40,60], in Italy [43,61], in Spain [45], in France [42,62], in Algeria [46] and in the United Kingdom [58,59]. SB1050 and SB1055 were reported in the Central and Latin Americas, particularly in Argentina, Paraguay, Uruguay, Mexico, Costa Rica (Mbovis.org) and Brazil [3,40,63].
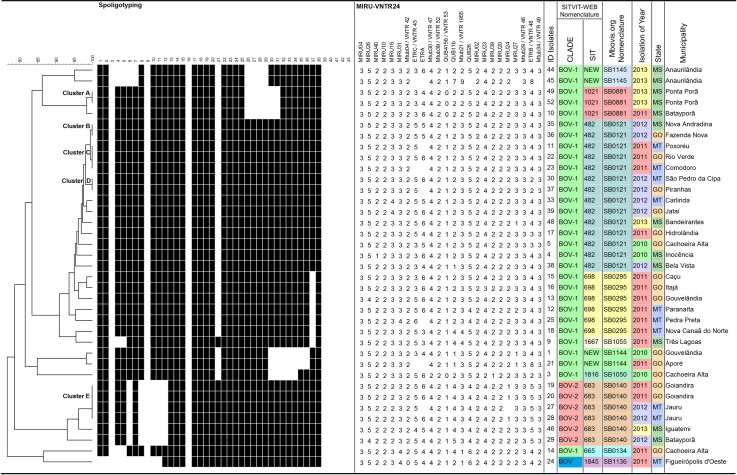
The 24-loci MIRU-VNTR patterns and the combined genotyping results are displayed in Table 2 and in Fig 2. The UPGMA based similarity of the combined genotypes are also shown.
Table 2. Molecular characterization of M. bovis isolates from cattle in Midwest Brazil.
| Sample | Spoligotype pattern | Spoligotype Cluster | 24-MIRU-VNTR profile | MIRU-VNTR cluster | Combined analyses cluster | State within Midwest Brazilian geographic region |
|---|---|---|---|---|---|---|
| 44 | SB1145 | Cluster S1 | 352233236421225242223343 | Orphan pattern | Orphan pattern | MS |
| 45 | SB1145 | S1 | 3522332**42179*24222*38* | Orphan pattern | Orphan pattern | MS |
| 49 | SB0881 | Cluster S2 | 352233234420225242223343 | Cluster M1 | Cluster A | MS |
| 52 | SB0881 | S2 | 352233234420225242223343 | M1 | A | MS |
| 10 | SB0881 | S2 | 352233236420225242223343 | Orphan pattern | Orphan pattern | MS |
| 35 | SB0121 | Cluster S3 | 352233255421235242223343 | Cluster M2 | Cluster B | MS |
| 36 | SB0121 | S3 | 352233255421235242223343 | M2 | B | GO |
| 11 | SB0121 | S3 | 35223325*421235242223343 | Cluster M3 | Cluster C | MT |
| 22 | SB0121 | S3 | 352233256421235242223343 | M3 | C | GO |
| 23 | SB0121 | S3 | 3522332**421235242223343 | M3 | C | MT |
| 30 | SB0121 | S3 | 352233256421235242223323 | Cluster M4 | Cluster D | MT |
| 37 | SB0121 | S3 | 35223325*421235242223323 | M4 | D | GO |
| 33 | SB0121 | S3 | 352243255421235242223343 | Orphan pattern | Orphan pattern | MT |
| 39 | SB0121 | S3 | 352243256421235242223343 | Orphan pattern | Orphan pattern | GO |
| 48 | SB0121 | S3 | 352233256421235242221343 | Orphan pattern | Orphan pattern | MS |
| 17 | SB0121 | S3 | 352233254421235242223333 | Orphan pattern | Orphan pattern | GO |
| 05 | SB0121 | S3 | 352233235421235242223333 | Orphan pattern | Orphan pattern | GO |
| 04 | SB0121 | S3 | 351233236421235242223343 | Orphan pattern | Orphan pattern | MS |
| 38 | SB0121 | S3 | 352233234421135242223343 | Orphan pattern | Orphan pattern | MS |
| 15 | SB0295 | Cluster S4 | 352233236421235242223343 | Orphan pattern | Orphan pattern | GO |
| 16 | SB0295 | S4 | 352233236421235232223343 | Orphan pattern | Orphan pattern | GO |
| 13 | SB0295 | S4 | 342233256421235242223343 | Orphan pattern | Orphan pattern | GO |
| 12 | SB0295 | S4 | 352233256421234242222343 | Orphan pattern | Orphan pattern | MT |
| 25 | SB0295 | S4 | 35223426*421234242223343 | Orphan pattern | Orphan pattern | MT |
| 18 | SB0295 | S4 | 352233253421445242223343 | Cluster M5 | Orphan pattern | MT |
| 09 | SB1055 | Orphan pattern | 352233253421445242223343 | M5 | Orphan pattern | MS |
| 01 | SB1144 | Cluster S5 | 352233234421135242221343 | Orphan pattern | Orphan pattern | GO |
| 21 | SB1144 | S5 | 3522332**421135242221383 | Orphan pattern | Orphan pattern | GO |
| 19 | SB0140 | Cluster S6 | 352223256421434242213353 | Cluster M6 | Cluster E | GO |
| 20 | SB0140 | S6 | 352223256421434242213353 | M6 | E | GO |
| 27 | SB0140 | S6 | 35222325*4214342422*3353 | M6 | E | MT |
| 28 | SB0140 | S6 | 352223256421434242213353 | M6 | E | MT |
| 46 | SB0140 | S6 | 352223245421434242223353 | Orphan pattern | Orphan pattern | MS |
| 29 | SB0140 | S6 | 342223254421534242222353 | Orphan pattern | Orphan pattern | MS |
| 14 | SB0134 | Orphan pattern | 352234254421416242222342 | Orphan pattern | Orphan pattern | GO |
| 24 | SB1136 | Orphan pattern | 352234054421216242222342 | Orphan pattern | Orphan pattern | MT |
| 03 | SB1050 | Orphan pattern | 352233256220235221223323 | Orphan pattern | Orphan pattern | GO |
*or**failed to amplify; MT—Mato Grosso state; MS—Mato Grosso do Sul state; GO—Goiás state.
Fig 2. Dendrogram generated by the BioNumerics 6.6 software (Applied Maths) based on the combination of spoligotyping and MIRU-VNTR analyses applied to the 37 M. bovis isolates, using the categorical index and unweighted pair-grouping method analysis algorithm (UPGMA).
While the spoligotyping resulted in six clusters containing 89.2% (33/37) of the isolates, the 24 MIRU-VNTR typing also resulted in six cluster, albeit containing only 40.5% (15/37) of the M. bovis isolates and 22 orphan patterns, demonstrating higher discriminatory power of 24 MIRU-VNTR for typing of M. bovis strains circulating in the Midwest region (Tables 2 and 3 and Fig 2).
Table 3. Discriminatory ability comparison among the spoligotyping and 24 MIRU-VNTR methods and the combination of both in detecting genetic similarities.
| Variability | Genotyping methods | ||
|---|---|---|---|
| Spoligotyping | 24-loci MIRU-VNTR | Combination of spoligotyping and 24-loci MIRU-VNTR | |
| Total profiles (n) | 10 | 28 | 29 |
| Orphan patterns (n) | 4 | 22 | 24 |
| Number of isolates by clusters | 2–14 | 2–4 | 2–4 |
| Number of grouped isolateds (n) (%) | 33 (89.2%) | 15 (40.5%) | 13 (35.1%) |
| Discriminatory index (HGDI) | 0.810 | 0.980 | 0.982 |
The allele diversity of each of the 24 MIRU-VNTR loci is presented in Table 4. Two loci (ETR-A and QUB 11b) were the most discriminatory (h = ≥ 0.50), while six presented moderate allelic diversity (MIRU 16, MIRU 27, ETR-B, ETR-C, Mtub21, QUB 26; h index between 0.33 to 0.49). Low allele diversity (h = ≤ 0.15) was observed for eight MIRUs and no diversity at all in another eight markers (Table 4). This means that eight MIRUs should be sufficient for the genotyping study of the M. bovis isolates from the Brazilian Midwest.
Table 4. Allele diversity of the 24-loci MIRU-VNTR.
| Number of repetitions | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Locus | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | Allele diversity (HGDI) (h index) |
| MIRU 02 | 37 | 0.00 | |||||||||
| MIRU 04 | 37 | 0.00 | |||||||||
| MIRU 10 | 37 | 0.00 | |||||||||
| MIRU 16 | 6 | 29 | 2 | 0.36 | |||||||
| MIRU 20 | 37 | 0.00 | |||||||||
| MIRU 23 | 1 | 1 | 35 | 0.10 | |||||||
| MIRU 24 | 3 | 33 | 0.15 | ||||||||
| MIRU 26 | 2 | 35 | 0.10 | ||||||||
| MIRU 27 | 3 | 4 | 29 | 0.33 | |||||||
| MIRU 31 | 34 | 3 | 0.15 | ||||||||
| MIRU 39 | 1 | 36 | 0.05 | ||||||||
| MIRU 40 | 1 | 36 | 0.05 | ||||||||
| ETR-A | 2 | 8 | 5 | 15 | 0.65 | ||||||
| ETR-B | 3 | 2 | 25 | 6 | 1 | 0.48 | |||||
| ETR-C | 10 | 1 | 22 | 1 | 0.49 | ||||||
| Mtub 04 | 1 | 36 | 0.00 | ||||||||
| Mtub 21 | 2 | 4 | 28 | 2 | 1 | 0.38 | |||||
| Mtub 29 | 37 | 0.00 | |||||||||
| Mtub 30 | 1 | 36 | 0.05 | ||||||||
| Mtub 34 | 2 | 34 | 0.11 | ||||||||
| Mtub 39 | 37 | 0.00 | |||||||||
| QUB 11b | 3 | 24 | 8 | 1 | 1 | 0.52 | |||||
| QUB 26 | 8 | 26 | 2 | 0.43 | |||||||
| QUB 4156 | 4 | 33 | 0.00 | ||||||||
These results corroborate with earlier data, which showed high resolution of ETR-A, ETR-B and ETR-C in the genotyping of M. bovis isolates from the state of Rio de Janeiro [64]. High resolution of ETR-A and ETR-B was also observed in Chad [23], Belgium [26] and Italy [43], proving their ample discriminatory power for M. bovis isolates, epidemiologically related or not [26,43].
Previous studies described the resolving power of the aforementioned ETRs (ETR A-F) [65] and QUBs (Queen's University Belfast VNTRs) [53], which are part of the 24-loci MIRU-VNTR set. Campos et al. (2013) [66] found a similar discrimination of M. bovis strains in Spain by evaluating the QUB 26 locus, but lower allelic discrimination by QUB 11b. Both loci were highly discriminative in Belgium [26], but only moderately discriminative in Italy [43].
Both MIRU 16 and MIRU 26 loci were highly discriminative in the study by Parreiras et al. (2012) [40], different from the present study. The MIRU 16 locus was considered inefficient for the differentiation of M. bovis strains in Ireland [67], Italy [43] and Portugal [27]. While Hilty et al. (2005) [23] and Allix et al. (2006) [26] described the MIRU 27 locus as highly discriminatory for M. bovis strains isolated in Chad and Belgium, Boniotti et al. (2009) [43] claimed this locus to be ineffective to characterize M. bovis isolates from Italy.
The lack of discriminatory power of MIRU 02, MIRU 10, MIRU 20, MIRU 23, MIRU 24, MIRU 31 and MIRU 39 was also demonstrated by Figueiredo et al. (2011) [64] and Parreiras et al. (2012) [40], both in Brazil, and by Roring et al. (2004) [67], in Ireland. Lack of differentiation by MIRU 02, MIRU 04, MIRU 10, MIRU 20, MIRU 23, MIRU 31, MIRU 39 and MIRU 40 loci was observed in Belgium [26] and Italy [43].
Although some individual loci show great discriminatory power, both for M. tuberculosis and M. bovis isolates, in general the loci are less polymorphic in M. bovis. Thus, it is better to combine distinct and use individual combinations of genotyping markers in each geographic study area [23,67].
Previous studies conductes in the South and southeastern regions of Brazil analyzed the genetic variability of M. bovis isolates from 12 to 15-loci from MIRU-VNTR [19,40,64]. In the present study, the allelic diversity and, consequently, the discriminatory power of the 24 MIRU-VNTR loci in a convenience sample obtained in the Midwest Braizlian region, from 2010 to 2013, were investigated for the first time.
Spoligotyping showed a discriminatory index of 0.810 (Table 3), similar to previous studies [26,40,45], but higher than that described by Ramos et al. (2014) [19]. The 24-MIRU-VNTR typing, on the other hand, provided a discriminatory index of 0.980 and the combination of the methods presented a discrimination of 0.982 (Table 3), higher than those observed by Sola et al. (2003) [25], Parreiras et al. (2012) [40] and Ramos et al. (2014) [19]. Roring et al. (2002) [22] and Hilty et al. (2005) [23], when evaluating M. bovis isolates from Europe and Africa, showed that the MIRU-VNTR technique has greater ability to discriminate M. bovis isolates compared to spoligotyping. The slight difference in efficiency observed with or without adding spoligotyping to 24-MIRU-VNTR typing demonstrates that this technique by itself would be able to differentiate between M. bovis strains in the Brazilian Midwest. The main limitation of spoligotyping is that all genetic polymorphisms are restricted to a single genomic locus, the DR region, which limits resolution. While having the advantages of being considerably faster, spoligotyping alone still does not provide sufficient discrimination between M. bovis strains to be used as a sole typing method, and it is, thus, often combined with supplementary techniques [56,57,67].
MIRU-VNTR is considered the gold standard for MTC genotyping, since it is highly disriminatory and reproducible [68]. Its repeating units are located in loci scattered throughout the genome of MTC strains [31], with variable mutation rates for each locus [32,69]. The polymorphism of the strains is based on the variability of the number of copies of each repeating unit. The original MIRU-VNTR methodology included 12-loci was used in conjunction with spoligotyping for the first MTC genotyping. However, its discriminatory power was less than IS6110 RFLP [69,70]. Due to the low discriminatory power of the MIRU-VNTR 12-loci, current studies suggest the use of a set of 15-loci for molecular epidemiological studies and 24-loci for phylogenetic studies [32]. Currently, the method has a high yield due to multiplex-PCR application using primers labeled with different fluorophores. This amplified material is subjected to capillary electrophoresis in an automatic sequencer, to estimate the size of the PCR product [71,72]. The advantage of automated typing by MIRU-VNTR is the fact that method is highly reproducible, faster and less laborious than the original methodology, yielding more reliable results because of the computerized analysis of the generated fluorescent signals.
There is a consensus among different studies that, by associating the results of spoligotyping to those obtained by MIRU-VNTR, discrimination between strains is more effective, and, thus, the combination of methodology has been considered the best strategy for the molecular typing of M. bovis [21]. In addition, Sola et al. (2003) [25], Allix et al. (2006) [26] and Duarte et al. (2010) [27] demonstrated that the combination of these techniques has a good cost/benefit ratio due to speed, reproducibility and reliability of M. bovis genotyping.
Better discrimination between M. bovis strains by combining the spoligotyping and MIRU-VNTR results has also been described by Ramos et al. (2014) [19]. Figueiredo et al. (2011) [64] indicated considerable genetic variability between 12 isolates of M. bovis originated from a herd of 34 tuberculin-positive cows in the state of Rio de Janeiro. The authors grouped the isolates in two clusters and six orphan patterns. In another study [40], where 61 isolates from the five Brazilian macro regions (South, Southeast, Midwest, North and Northeast) were analyzed by spoligotyping and 12-loci MIRU-VNTR, the isolates were grouped in eight clusters containing 53 isolates and eight orphan patterns, confirming the genetic variability of M. bovis strains in the country.
Herein, five clusters with 13 isolates (35%) (Fig 2) were observed and interestingly, strains with orphan patterns were found predominantly in the state of Goiás (10/24), besides the clustered strains. In the state of Mato Grosso, clusters "C", "D" and "E" were found, along with 5 orphan patterns. Finally, in the state of Mato Grosso do Sul, strains were clustered in "A" and "B" and nine of them showed orphan patterns. Epidemiologically related isolates are derived from the clonal expansion of a single precursor and as a result, have common characteristics that differ from those that are unrelated epidemiologically [64].
The great genetic heterogeneity of M. bovis observed in the Brazilian Midwest can be explained by the animal movement that occurs between different regions and farms, thus causing the spread of numerous M. bovis strains in the herds of the region. Another important point to consider is that the Midwest region of the country is a dry border with other Latin American countries, such as Bolivia and Paraguay, over a wide range of territory, thus allowing contact between herds of both countries, resulting in the transfer of M. bovis strains to Brazil, which can be retained in the Midwest region, or possibly migrate to other, more remote, regions.
In the present study, the association of spoligotyping and 24-MIRU-VNTR for the molecular characterization of M. bovis isolates from the Brazilian Midwest was carried out for the first time and indicated that BTB in this geographical region is caused by M. bovis isolates with high genetic diversity, which may hinder in vivo diagnosis, control and eradication of the disease. The characterization of M. bovis circulating genotypes in the geographical region aids in tracking and sanitizing remaining outbreaks of disease, since BTB has a low prevalence in this region of Brazil.
Conclusions
Ten spoligotypes are present in the Brazilian Midwest region. The combination of spoligotyping with the 24-MIRU analysis rendered five clusters and 24 orphan patterns, confirming the high genotypic diversity among M. bovis strains circulating in the Midwest Brazil. The presence of different M. bovis genotypes in this region suggests movement of animals between regions or different sources of infection. Thus, it is possible to conclude that BTB in the Brazilian Midwest is caused by multiple M. bovis strains.
Acknowledgments
We thank the Brazilian funding agencies FAPEMAT/PRONEM (472914/2011), FAPERJ, FUNDECT (085/2015), CNPq/Universal (443235/2014-7), CAPES (AUX-PE PROCAD 2493/2008), CAPES/PNPD (23038.007901/2010-81), the Brazilian Agricultural Research Corporation EMBRAPA (project 02.13.16.002.00.00), and CNPq PhD grant (140492/2013-5) for financial support, and to National Agricultural Laboratory (LANAGRO/MAPA) and sequencing platform group (PDTIS-FIOCRUZ/RJ) for their assistance in research.
Data Availability
All relevant data are within the paper.
Funding Statement
The authors thank the Brazilian funding agencies for support: PRONEM/FAPEMAT (472914/2011) Dr. Eduardo Eustáquio de Souza Figueiredo; CNPq/Universal 14/2014 (443235/2014-7) Dr. Flábio Ribeiro de Araújo; EMBRAPA (02.13.16.002.00.00) Dr. Flábio Ribeiro de Araújo; FUNDECT (085/2015) Dr. Flábio Ribeiro de Araújo; CAPES/AUX-PE/PROCAD (2493/2008) Dra. Vânia Margaret Flosi Paschoalin; CAPES/PNPD (23038.007901/2010-81) Dra. Ana Carolina da Silva Carvalho; and CNPq PhD grant (140492/2013-5) Dr. Ricardo César Tavares Carvalho. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Medeiros LS, Marassi CD, Figueiredo EE, Lilenbaum W (2010) Potential application of new diagnostic methods for controlling bovine tuberculosis in Brazil. Braz J Microbiol 41: 531–541. 10.1590/S1517-83822010005000002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Araújo CP, Osorio AL, Jorge KS, Ramos CA, Filho AF, Vidal CE, et al. (2014). Detection of Mycobacterium bovis in bovine and bubaline tissues using nested-PCR for TbD1. PLoS One 9: e91023 10.1371/journal.pone.0091023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alzamora-Filho F, Vasconcellos SEG, Gomes HM, Cavalcante MP, Suffys PN, Costa JN (2014) Multiple strains of Mycobacterium bovis isolates identified by molecular typing of bovine animals slaughtered in slaughterhouse. Pesq Vet Bras 34:103–108. [Google Scholar]
- 4.Carvalho RCT, Furlanetto LV, Maruyama FH, Araújo CP, Barros SL, Ramos CA, et al. (2015) Evaluation of the efficiency of nested q-PCR in the detection of Mycobacterium tuberculosis complex directly from tuberculosis-suspected lesions in post-mortem macroscopic inspections of bovine carcasses slaughtered in the state of Mato Grosso, Brazil. Meat Sci 106:11–5. 10.1016/j.meatsci.2015.03.017 [DOI] [PubMed] [Google Scholar]
- 5.Acha PN, Szyfres B (2001) Zoonoses and Communicable Diseases Common to Man and Animals. Bacterioses and Mycoses. 3 ed: 395. [Google Scholar]
- 6.Grange JM (2001) Mycobacterium bovis infection in human beings. Tuberculosis (Edinb). 81:71–7. [DOI] [PubMed] [Google Scholar]
- 7.WHO—World Health Organization (1996). Guidelines for specification withing the Mycobacterium tuberculosis complex. WHO/EMC/ZOO/96.4. 2 ed. Available: http://whqlibdoc.who.int/hq/1996/who_emc_zoo_96.4.pdf. Accessed: 20 January 2016.
- 8.Brazil (2006) Programa Nacional de Controle e Erradicação da Brucelose e Tuberculose–PNCEBT. Manual Técnico, Ministério da Agricultura, Pecuária e Abastecimento, Brasília. Available: http://www.agricultura.gov.br/arq_editor/file/Aniamal/programa%20nacional%20sanidade%20brucelose/Manual%20do%20PNCEBT%20-%20Original.pdf. Accessed 10 January 2016.
- 9.Figueiredo EES, Conte Júnior CA, Furlanetto LV, Silva FGS, Duarte RS, Silva JT, et al. (2012) Molecular techniques for identification of species of the Mycobacterium tuberculosis complex: the use of multiplex PCR and an adapted HPLC method for identification of Mycobacterium bovis and Diagnosis of Bovine Tuberculosis. In: Cardona PJ, editor. Understanding Tuberculosis-Global Experiences and Innovative Approaches to the Diagnosis: InTech 411–32.
- 10.Murakami PS, Fuverki RBN, Nakatani SM, Filho IRB, Biondo AW (2009) Tuberculose Bovina: Saúde Animal e Saúde Pública. Arq Ciênc Vet Zool Unipar. 12:67–74. [Google Scholar]
- 11.Leite CQ, Anno IS, Leite SR, Roxo E, Morlock GP, Cooksey RC (2003) Isolation and identification of mycobacteria from livestock specimens and milk obtained in Brazil. Mem Inst Oswaldo Cruz. 98:319–23. [DOI] [PubMed] [Google Scholar]
- 12.OIE—World Organisation for Animal Health (2007) WAHID Interface. Available: http://www.oie.int/wahid-prod/public.php. Accessed 20 January 2016.
- 13.Carvalho RCT, Furlanetto LV, Duarte RS, Nakazato L, Lilenbaum W, Figueiredo EES, et al. (2015) Molecular Diagnostic Testing on Post Mortem Inspection and Rulings on Bovine Tuberculosis—An Experience Report in Brazil. In: Ribon W, editor. Tuberculosis—Expanding Knowledge: InTech 191–211.
- 14.Brazil (2015) Instituto Brasileiro de Geografia e Estatística. Produção Pecuária. Available: http://www.brasil.gov.br/economia-e-emprego/2015/10/rebanho-bovino-brasileiro-cresce-e-chega-a-212-3-milhoes-de-cabecas-de-gado. Accessed 10 January 2016.
- 15.Brazil (2014) Instituto Brasileiro de Geografia e Estatística. Produção Pecuária. Available: http://www.ibge.gov.br/home/estatistica/indicadores/agropecuaria/producaoagropecuaria/abate-leite-couro-ovos_201304_publ_completa.pdf, 2014. Accessed 10 January 2016.
- 16.Roxo E (2004) Situação Atual da Tuberculose Bovina no Brasil. Programa Nacional de Controle e Erradicação de Brucelose e Tuberculose Animal. Secretaria de Defesa Agropecuária. Docum. PNCE bovine tuberculosis, São Paulo.
- 17.Furlanetto LV, Figueiredo EES, Conte-Júnior CA, Silva FGS, Duarte RS, Silva JT, et al. (2012) Prevalence of bovine tuberculosis in herds and animals slaughtered in 2009 in the state of Mato Grosso, Brazil. Arq Bras Med Vet Zoot 64: 274–280. [Google Scholar]
- 18.OIE—World Organisation for Animal Health (2009) Bovine tuberculosis. Available: http://www.oie.int/fileadmin/Home/fr/Health_standards/tahm/2.04.07_BOVINE_TB.pdf). Accessed: 10 January 2016.
- 19.Ramos DF, Silva ABS, Fagundes MQ, Von-Groll A, Silva PE, Dellagostin OA (2014) Molecular typing of Mycobacterium bovis isolated in the south of Brazil. Braz J Microbiol 45: 657–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cazola DO, Jorge KSG, Zumárraga MJ, Souza-Filho AF, Araújo FR, Osório ALAR(2015) Identification and genotyping of Mycobacterium bovis from positive cattle in skin test for tuberculosis in the State of Mato Grosso do Sul, Brazil. Pesq Vet Bras 35: 141–147. [Google Scholar]
- 21.Mignard S, Pichat C, Carret G (2006) Mycobacterium bovis infection, Lyon, France. Emerg Infect Dis 12: 1431–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roring S, Scott AN, Brittain D, Walker I, Hewinson RG, Neill S, et al. (2002) Development of variable-number tandem repeat typing of Mycobacterium bovis: comparison of results with those obtained by using exact tandem repeats and spoligotyping. J Clin Microbiol 40: 2126–2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hilty M, Digimbaye C, Schelling E, Baggi F, Tanner M, Zinsstag J (2005) Evaluation of the discriminatory power of variable number of tandem repeat (VNTR) typing of Mycobacterium bovis strains. Vet Microbiol 109: 217–222. [DOI] [PubMed] [Google Scholar]
- 24.Vasconcellos SE, Acosta CC, Gomes LL, Conceição EC, Lima KV, de Araujo MI, et al. (2014) Strain classification of Mycobacterium tuberculosis isolates in Brazil based on genotypes obtained by spoligotyping, mycobacterial interspersed repetitive unit typing and the presence of large sequence and single nucleotide polymorphism. PLoS One 9: e107747 10.1371/journal.pone.0107747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sola C, Filliol IF, Legrand E, Lesjean S, Locht C, Supply P, et al. (2003). Genotyping of the Mycobacterium tuberculosis complex using MIRU-VNTR’s: association with VNTR and spoligotyping for molecular epidemiology and evolutionary genetics. Infect Genet Evol 3: 125–133. [DOI] [PubMed] [Google Scholar]
- 26.Allix C, Walravens K, Saegerman C, Godfroid J, Supply P, Fauville-Dufaux M (2006) Evaluation of the epidemiological relevance of variable-number tandem-repeat genotyping of Mycobacterium bovis and comparison of the method with IS6110 restriction fragment length polymorphism analysis and spoligotyping. J Clin Microbiol 44: 1951–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duarte EL, Domingos M, Amado A, Cunha MV, Botelho A (2010) MIRU-VNTR typing adds discriminatory value to groups of Mycobacterium bovis and Mycobacterium caprae strains defined by spoligotyping. Vet Microbiol 143: 299–306. 10.1016/j.vetmic.2009.11.027 [DOI] [PubMed] [Google Scholar]
- 28.Kamerbeek J, Schouls L, Kolk A, Van-Agterveld M, Van-Soolingen D, Kuijper S, et al. (1997) Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J Clin Microbiol 35: 907–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Le Flèche P, Fabre M, Denoeud F, Koeck JL, Vergnaud G (2002) High resolution, on-line identification of strains from the Mycobacterium tuberculosis complex based on tandem repeat typing. BMC Microbiol 2: 37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Supply P, Magdalena J, Himpens S, Locht C (1997) Identification of novel intergenic repetitive units in a mycobacterial two-component system operon. Mol Microbiol 26: 991–1003. [DOI] [PubMed] [Google Scholar]
- 31.Supply P, Mazars E, Lesjean S, Vincent V, Gicquel B, Locht C (2000) Variable human minisatellite-like regions in the Mycobacterium tuberculosis genome. Mol Microbiol 36: 762–71. [DOI] [PubMed] [Google Scholar]
- 32.Supply P, Allix C, Lesjean S, Cardoso-Oelemann M, Rüsch-Gerdes S, Willery E, et al. (2006) Proposal for standardization of optimized mycobacterial interspersed repetitive unit-variable-number tandem repeat typing of Mycobacterium tuberculosis. J Clin Microbiol 44: 4498–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mazars E, Lesjean S, Banuls AL, Gilbert M, Vincent V, Gicquel B, et al. (2001) High-resolution minisatellite-based typing as a portable approach to global analysis of Mycobacterium tuberculosis molecular epidemiology. Proc Natl Acad Sci U S A 98: 1901–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Allix C, Supply P, Fauville-Dufaux M (2004) Utility of fast mycobacterial interspersed repetitive unit-variable number tandem repeat genotyping in clinical mycobacteriological analysis. Clin Infect Dis 39: 783–9. [DOI] [PubMed] [Google Scholar]
- 35.Silva CF, Ueki MSY, Geiger PDC, Leão SC (2001) Hsp65 PCR-Restriction Enzyme Analysis (PRA) for identification of Mycobacteria in the clinical laboratory. Rev Inst Med Trop S Paulo 43: 25–28. [DOI] [PubMed] [Google Scholar]
- 36.Smith NH, Upton P (2012) Naming spoligotype patterns for the RD9-deleted lineage of the Mycobacterium tuberculosis complex. Infect Genet Evol 12(4):873–6. 10.1016/j.meegid.2011.08.002 [DOI] [PubMed] [Google Scholar]
- 37.De-Beer JL, Kremer K, Ködmön C, Supply P, Van Soolingen D (2012) First worldwide proficiency study on variable-number tandem-repeat typing of Mycobacterium tuberculosis complex strains. J Clin Microbiol 50: 662–9. 10.1128/JCM.00607-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hunter PR, Gaston MA (1988) Numerical index of the discriminatory ability of typing systems: an application of Simpson’s index of diversity. J Clin Microbiol 26: 2465–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rocha VCF, Figueiredo SC, Rosales CAR, Grisi-Filho JHH, Keid LB, Soares RM, et al. (2013) Molecular Discrimination of Mycobacterium bovis in São Paulo, Brazil. Vector Borne Zoonotic Dis 13: 17–21. 10.1089/vbz.2012.1035 [DOI] [PubMed] [Google Scholar]
- 40.Parreiras PM, Andrade GI, Nascimento TF, Oelemann MC, Gomes HM, Alencar AP, et al. (2012) Spoligotyping and variable number tandem repeat analysis of Mycobacterium bovis isolates from cattle in Brazil. Mem Inst Oswaldo Cruz 107: 64–73. [DOI] [PubMed] [Google Scholar]
- 41.Haddad N, Ostyn A, Karoui C, Masselot M, Thorel MF, Hughes SL, et al. (2001) Spoligotype diversity of Mycobacterium bovis strains isolated in France from 1979 to 2000. J Clin Microbiol 39: 3623–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hauer A, De Cruz K, Cochard T, Godreuil S, Karoui C, Henault S, et al. (2015) Genetic Evolution of Mycobacterium bovis Causing Tuberculosis in Livestock and Wildlife in France since 1978. PLoS One 10: e0117103 10.1371/journal.pone.0117103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boniotti MB, Goria M, Loda D, Garrone A, Benedetto A, Mondo A, et al. (2009) Molecular typing of Mycobacterium bovis strains isolated in Italy from 2000 to 2006 and evaluation of variable-number tandem repeats for geographically optimized genotyping. J Clin Microbiol 47: 636–644. 10.1128/JCM.01192-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Duarte EL, Domingos M, Amado A, Botelho A (2008) Spoligotype diversity of Mycobacterium bovis and Mycobacterium caprae animal isolates. Vet Microbiol 130: 415–421. 10.1016/j.vetmic.2008.02.012 [DOI] [PubMed] [Google Scholar]
- 45.Rodríguez S, Romero B, Bezos J, de Juan L, Alvarez J, Castellanos E, et al. (2010) High spoligotype diversity within a Mycobacterium bovis population: clues to understanding the demography of the pathogen in Europe. Vet Microbiol 141: 89–95. 10.1016/j.vetmic.2009.08.007 [DOI] [PubMed] [Google Scholar]
- 46.Sahraoui N, Muller B, Guetarni D, Boulahbal F, Yala D, Ouzrout R, et al. (2009) Molecular characterization of Mycobacterium bovis strains isolated from cattle slaughtered at two abattoirs in Algeria. BMC Vet Res 5: 4 10.1186/1746-6148-5-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Michel AL, Hlokwe TM, Coetzee ML, Mare L, Connoway L, Rutten VP, et al. (2008) High Mycobacterium bovis genetic diversity in a low prevalence setting. Vet. Microbiol 126: 151–159. [DOI] [PubMed] [Google Scholar]
- 48.Reyes JAG, Casanova LG, Torres CR, Gallegos SLS, Alarcón GJC, Mercado MP, et al. (2012) Population structure of Mycobacterium bovis isolates from cattle in Mexico. Prev Vet Med 106: 1–8. 10.1016/j.prevetmed.2012.05.008 [DOI] [PubMed] [Google Scholar]
- 49.Zumárraga MJ, Arriaga C, Barandiaran S, Cobos-Marín L, de Waard J, Estrada-Garcia I, et al. (2013) Understanding the relationship between Mycobacterium bovis spoligotypes from cattle in Latin American Countries. Res Vet Sci 94: 9–21. 10.1016/j.rvsc.2012.07.012 [DOI] [PubMed] [Google Scholar]
- 50.Rodriguez SC, Aranaz A, Juan L, Sáez-Llorente JL, Romero B, Bezos J, et al. (2011) Limitations of spoligotyping and variable-number tandem-repeat typing for molecular tracing of Mycobacterium bovis in a high-diversity setting. J Clin Microbiol 49: 3361–64. 10.1128/JCM.00301-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cobos-Marín L, Montes-Vargas J, Zumárraga M, Cataldi A, Romano MI, Estrada-Garcia I, et al. (2005) Spoligotype analysis of Mycobacterium bovis isolates from Northern México. Can J Microbiol 51:996–1000. [DOI] [PubMed] [Google Scholar]
- 52.Van Embden JD, Van GT, Kremer K, Jansen R, Van Der Zeijst BA, Schouls LM (2000) Genetic variation and evolutionary origin of the direct repeat locus of Mycobacterium tuberculosis complex bacteria. J Bacteriol 182: 2393–2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Skuce RA, McCorry TP, McCarroll JF, Roring SM, Scott AN, Brittain D, et al. (2002) Discrimination of Mycobacterium tuberculosis complex bacteria using novel VNTR-PCR targets. Microbiology 148: 519–28. [DOI] [PubMed] [Google Scholar]
- 54.Rodriguez CAR, Zumárraga MJ, Oliveira EMD, Cataldi AA, Romano MI, Otto HH, et al. (2004) Molecular characterization of Mycobacterium bovis isolates from the state of São Paulo, by the use of the spoligotyping Technique. Arq Inst Biol 71: 277–282. [Google Scholar]
- 55.Zumárraga M, Martín C, Samper S, Alito A, Latini O, Bigi F, et al. (1999) Usefulness of spoligotyping in molecular epidemiology of Mycobacterium bovis related infections in South America. J Clin Microbiol 37: 296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cousins D, Williams S, Liébana E, Aranaz A, Bunschoten A, Van Embden J, et al. (1998) Evaluation of four DNA typing techniques in epidemiological investigations of bovine tuberculosis. J Clin Microbiol 36: 168–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Costello E, O’Grady D, Flynn O, O'Brien R, Rogers M, et al. (1999) Study of restriction fragment length polymorphism analysis and spoligotyping for epidemiological investigation of Mycobacterium bovis infection. J Clin Microbiol 37: 3217–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gibson AL, Hewinson G, Goodchild T, Watt B, Story A, Inwald J, et al. (2004). Molecular epidemiology of disease due to Mycobacterium bovis in humans in the United Kingdom. J Clin Microbiol 42: 431–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hewinson RG, Vordermeier HM, Smith NH, Gordon SV (2006) Recent advances in our knowledge of Mycobacterium bovis: a feeling for the organism. Vet Microbiol 112: 127–39. [DOI] [PubMed] [Google Scholar]
- 60.Viana-Niero C, Rodriguez CA, Bigi F, Zanini MS, Ferreira-Neto JS, Cataldi A, et al. (2006) Identification of an IS6110 insertion site in plcD, the unique phospholipase C gene of Mycobacterium bovis. J Med Microbiol 5: 451–57. [DOI] [PubMed] [Google Scholar]
- 61.Serraino A, Marchetti G, Sanguinetti V, Rossi MC, Zanoni RG, Catozzi L, et al. (1999) Monitoring of transmission of tuberculosis between wild boars and cattle: genotypical analysis of strains by molecular epidemiology techniques. J Clin Microbiol 37: 2766–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zanella G, Durand B, Hars J, Moutou F, Garin-Bastuji B, Duvauchelle A, et al. (2008) Mycobacterium bovis in wildlife in France. J Wildl Dis 44: 99–108. [DOI] [PubMed] [Google Scholar]
- 63.Costa ACF, Silva NS, Rocha VCM, Rodrigues CAR, Estrela-Lima A, Moreira ELT, et al. (2010) Use of spoligotyping for genetic typing of Mycobacterium bovis isolates from slaughtered animals in the metropolitan area of Salvador, Bahia, Brazil. Arqs Inst Biológ. 77: 233–37. [Google Scholar]
- 64.Figueiredo EE, Ramos DF, Medeiros L, Silvestre FG, Lilenbaum W, Silva JT, et al. (2011) Multiple strains of Mycobacterium bovis revealed by molecular typing in a herd of cattle. Vet J 193: 296–98. 10.1016/j.tvjl.2011.11.006 [DOI] [PubMed] [Google Scholar]
- 65.Frothingham R, Meeker-O’Connell WA (1998) Genetic diversity in the Mycobacterium tuberculosis complex based on variable numbers of tandem DNA repeats. Microbiol 144: 1189–96. [DOI] [PubMed] [Google Scholar]
- 66.Campos SR, Navarro Y, Romero B, de-Juan L, Bezos J, Mateos A, et al. (2013) Splitting of a prevalent Mycobacterium bovis spoligotype by variable-number tandem-repeat typing reveals high heterogeneity in an evolving clonal group. J Clin Microbiol 51: 3658–65. 10.1128/JCM.01271-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Roring S, Scott AN, Hewinson RG, Neill SD, Skuce RA (2004) Evaluation of variable number tandem repeat (VNTR) loci in molecular of Mycobacterium bovis isolates from Ireland. Vet Microbiol 101: 65–73. [DOI] [PubMed] [Google Scholar]
- 68.Weniger T, Krawczyk J, Supply P, Niemann S, Harmsen D (2010) MIRU-VNTRplus: a web tool for polyphasic genotyping of Mycobacterium tuberculosis complex bacteria.Nucleic Acids Res 38: W326–W331. 10.1093/nar/gkq351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Iwamoto T, Yoshida S, Suzuki K, Tomita M, Fujiyama R, Tanaka N, et al. (2007) Hypervariable loci that enhance the discriminatory ability of newly proposed 15-loci and 24-loci variable-number tandem repeat typing method on Mycobacterium tuberculosis strains predominated by the Beijing family. FEMS Microbiol Lett 270: 67–74. [DOI] [PubMed] [Google Scholar]
- 70.Christianson S, Wolfe J, Orr P, Karlowsky J, Levett PN, Horsman GB, et al. (2010) Evaluation of 24 locus MIRU-VNTR genotyping of Mycobacterium tuberculosis isolates in Canada. Tuberculosis (Edinb) 90: 31–38. [DOI] [PubMed] [Google Scholar]
- 71.Supply P, Lesjean S, Savine E, Kremer K, van Soolingen D, Locht C (2001) Automated high-throughput genotyping for study of global epidemiology of Mycobacterium tuberculosis based on mycobacterial interspersed repetitive units. J Clin Microbiol 39: 3563–3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kato-Maeda M, Metcalfe JZ, Flores L (2011) Genotyping of Mycobacterium tuberculosis: application in epidemiologic studies. Future Microbiol 6: 203–216. 10.2217/fmb.10.165 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.