Abstract
It is suspected that microbial infections take part in the pathogenesis of diabetes mellitus type 1 (T1DM). Glucose-induced insulin secretion is accompanied by the release of free arachidonic acid (AA) mainly by cytosolic- and calcium independent phospholipases A2 (cPLA2 and iPLA2). Insulinoma cell line (INS-1E) was infected with E. coli isolated from the blood culture of a patient with sepsis. Invasion assay, Scanning Electron Microscopy and Transmission Electron Microscopy demonstrated the capacity of E. coli to enter cells, which was reduced by PLA2 inhibitors. Glucose-induced insulin secretion was significantly increased after acute infection (8h) but significantly decreased after chronic infection (72h). PLA2 activities, cPLA2, iPLA2, phospho-cPLA2, and COX-2 expressions were increased after acute and, even more, after chronic E. coli infection. The silencing of the two isoforms of PLA2s, with specific cPLA2- or iPLA2-siRNAs, reduced insulin secretion after acute infection and determined a rise in insulin release after chronic infection. Prostaglandins E2 (PGE2) production was significantly elevated in INS-1E after long-term E. coli infection and the restored insulin secretion in presence of L798106, a specific EP3 antagonist, and NS-398, a COX-2 inhibitor, and the reduction of insulin secretion in presence of sulprostone, a specific EP3 agonist, revealed their involvement in the effects triggered by bacterial infection. The results obtained demonstrated that cPLA2 and iPLA2 play a key role in insulin secretion process after E. coli infection. The high concentration of AA released is transformed into PGE2, which could be responsible for the reduced insulin secretion.
Introduction
Research in recent years has turned its attention to the bacterial infections that develop in patients with diabetes [1, 2]. But could it be that a generalized bacterial infection is able to reduce the secretion of insulin by pancreatic cells and consequently have a causal role in diabetes? Microbes, viruses in particular, have been the focal point of diabetes research for several decades but proving a causal role between infection and the onset of diabetes mellitus type 1 (T1DM) is, however, extremely difficult. One of the reasons is the long period between exposure and the clinical onset of the disease. Another problem is that affected individuals often experience multiple infections over the years before the onset of T1DM, as do non-diabetics in the population [3].
Several mechanisms have been proposed for explaining how bacteria are able to damage pancreatic cells. Streptomyces strains may act by producing a toxin that would affect the pancreatic ß cells causing their lysis [4]. In other cases the bacterial infection would result in the activation of lymphocytes and an increase in the concentration of cytokines in close proximity of the pancreatic cells [5, 6]. It has been demonstrated that endotoxins, released during bacterial infection, induced apoptosis in insulin secreting (INS-1) cells [7], caused acute insulin resistance, followed by long-lasting tissue-specific dysfunctions of lipid and glucose metabolism [8] and could deteriorate insulin secretion in a rodent model of metabolic syndrome [9].
In addition, hyperglycemia, associated with hypoinsulinemia, may be the normal pathophysiological response in children with meningococcal sepsis [10] suffering from frequent and significant hyperglycemic episodes associated with low insulin levels in the plasma during the acute phase of the disease [11]. The results of a study of obese and non-obese dogs show that Staphylococcus intermedius infection is able to reduce insulin sensitivity in mongrel dogs [12]. Salmonella typhimurium has been identified as a causative agent of acute pancreatitis [13]; Salmonella persistent infection is characterized by a loss of pancreatic acinar cells and accumulation of inflammatory cells, being able to colonize the pancreas in vivo, and to invade cultured pancreatic acinar cells in vitro [14]. Moreover, acute pancreatitis is a recognized complication of Hemolytic Uremic Syndrome in the setting of E. coli infection [15]. There may be a percentage of patients with E. coli colitis with undiagnosed pancreatitis [16]. It has been demonstrated, in a cat model, that bacterial infection is able to trigger acute pancreatitis [17]. In rabbit, acute pancreatitis can be induced by infected bile, which causes an interstitial-edematous trait with occasional acinar necrosis, its severity depending on the bacterial species, including E. coli [18].
E. coli normally colonizes the gastrointestinal tract in infants a few hours after birth. These commensal strains of E. coli rarely cause disease except in immuno-compromised patients [19] or where the normal gastrointestinal barriers have been altered as in the case of peritonitis [20]. However, there are several E. coli strains which acquire specific virulent characteristics, becoming capable of adapting to new niches. These attributes of virulence are often encoded on genetic elements that make some E. coli strains capable of causing diseases in healthy individuals [21].
Most of the pathogenic E. coli strains remain extracellular, but enteroinvasive E. coli (EIEC) is a true intracellular pathogen that is capable of invading and replicating within epithelial cells and macrophages [22].
The early phase of EIEC pathogenesis comprises epithelial cell penetration, followed by lysis of the endocytic vacuole, intracellular multiplication, directional movement through the cytoplasm and extension into adjacent epithelial cells [23]. Movement within the cytoplasm is mediated by nucleation of cellular actin into a ‘tail’ that extends from one pole of the bacterium [24]. Through this pathogenic mechanism, E. coli could infect different organs including the pancreas, leading to a reduction of insulin secretion. On the other hand, it is right to report that in vitro studies have shown that the presence of bacteria can reduce or even increase insulin secretion in cultures of pancreatic tissue, depending on the type of infecting microorganism. The infection by Pseudomonas causes reduction of insulin secretion while Enterobacter and Staphylococcus determined an increase in insulin secretion [25]. These conflicting results require further studies that may elucidate the molecular mechanism that induces the onset of diabetes in patients with bacterial infection.
Arachidonic acid (AA) is released from membrane phospholipids by the action of the different isoforms of phospholipases A2 (PLA2) and converted into prostaglandin (PGs) or leukotrienes (LTs) by the action of cyclooxygenases (COX-1 and COX-2) and 5-lipoxygenase, respectively. Cytosolic PLA2 (cPLA2), Ca2+-independent PLA2 (iPLA2), and Ca2+-dependent secretory PLA2 (sPLA2) differ from each other in terms of substrate specificity, Ca2+-requirement, modification of lipids, translocation to cell membranes, and the release of AA [26].
The cPLA2, present in many cell types, including pancreatic ß cells (cPLA2ß), requires phosphorylation at Ser-505 and binding with Ca2+ for its activity [27]. cPLA2ß stimulates insulin exocytosis by accelerating granule mobilization and “overfilling” of the readily releasable pool so that more granules are available for release once intracellular Ca2+ concentration rises to exocytotic levels. Activation of cPLA2ß during the ß-cell stimulus/secretion coupling would cause translocation of the enzyme to the secretory granules and accumulation of AA and lysophospholipids in the membrane, leading to changes in membrane structure or fluidity [27]. It has also been demonstrated that cPLA2ß plays a role in the maintenance of insulin stores, but it is not required for the initiation of insulin secretion from ß-cells [28]. Moreover, overexpression of cPLA2 results in severe impairment of the calcium and secretory responses of ß-cells to glucose through upregulation of mithocondrial uncoupling protein-2 [29]. In pancreatic ß cells, the enzyme iPLA2ß does not require Ca2+ for the catalytic activity and it is inhibited by the suicide substrate bromoenol lactone (BEL) [30]. It has been shown that iPLA2ß is involved in apoptosis of ß-cells of the pancreas during diabetes and its inhibition is able to reduce apoptosis, thus preventing cell dysfunction associated with diabetes [31]. Type IB sPLA2 is contained in insulin secretory granules of pancreatic islet ß-cells, it is co-secreted with insulin from glucose-stimulated islets [32] and it is expressed in human islets of transplanted pancreas after the recurrence of type 1 diabetes mellitus with insulitis [33].
The AA, produced by PLA2 activities, has a significant regulatory action on insulin secretion in pancreatic ß cells [34].
Our previous studies showed the significant role of cPLA2, iPLA2 and PKCα/ERK/MAPK signalling pathways during E. coli infection of microvascular endothelial cells [35, 36]. Moreover, we demonstrated that S. aureus chronic infection of INS-1 cells causes a decrease in insulin release and a significant increase of cPLA2, iPLA2 activity/expression and COX-2 protein expression [37].
The objective of this study was to investigate the role of the PLA2s in the E. coli infection of ß cells and the molecular mechanisms which could lead to T1DM pathogenesis.
To carry out this study, we used E. coli isolated from the blood of a female patient dying from severe sepsis with underlying acute pyelonephritis and subsequent multiple-organ failure. To gain insight in the correlation between bacterial infection-response of the pancreas in terms of insulin secretion, we performed E. coli-infection experiments by using the insulin-producing INS-1E rat cell line, which is widely used as a pancreatic ß-cell model, retaining glucose-stimulated insulin secretion and a high degree of differentiation [38]. Here we demonstrated that E. coli is able to enter INS-1E cells in a time-dependent manner. Chronic infection causes a significant decrease in insulin release and a significant PLA2s and COX-2 protein activation. Furthermore, we provide data suggesting that prostaglandin E2 (PGE2) production plays a key role in the reduction of insulin secretion after long-term infection and that insulin secretion by E. coli-infected ß-cells could be restored by using specific siRNAs against cPLA2 and iPLA2 isoforms.
Material and Methods
All reagents and antibodies were purchased from Sigma or E. Merck unless otherwise indicated. Phospholipase A2 inhibitors, arachidonoyl trifluoromethyl ketone (AACOCF3), and bromoenol lactone (BEL) were from Calbiochem. Phospholipase A2 (cytosolic and calcium independent), sPLA2 specific activity assay kits, rabbit polyclonal against iPLA2 antibody and Sulprostone were from Cayman Chemical (Ann Arbor, MI). cPLA2 (mouse monoclonal), phospho-cPLA2 (rabbit polyclonal), anti-α-actin (mouse monoclonal), anti-COX-1 and anti-COX-2 (mouse monoclonal) were purchased from Santa Cruz Biotechnology.
Bacterial strains and culture conditions
E. coli strain belongs of a collection of a hospital laboratory of Clinical Microbiology and has been isolated from blood culture of a patient with sepsis. All patient data have followed the required anonymity procedure, being the patient identified with alphanumeric code. As the strain is of collection, the Ethics Committee did not have to be approached.
E. coli was grown in tryptic soy broth (TSB) at 37°C for 14h. The culture was centrifuged at 4300xg for 10 min, and the supernatant discarded. The bacterial pellet was washed with PBS and serially diluted to the desired concentration. The density of bacteria was measured by enumerating the number of CFU on LB agar plates (Difco).
Cell cultures
Rat insulinoma β-cell line (INS-1E) was kindly provided by Dr. C. B. Wollheim, (Médical Universitaire, Genève, Switzerland). Cells were cultured in RPMI-1640 medium containing 5 mM glucose, supplemented with 10% heat-inactivated fetal bovine serum, 100 U/mL penicillin, 100 U/mL streptomycin, 1 mM sodium pyruvate and 50 μm β-mercaptoethanol in 5% CO2 atmosphere at 37°C [39].
Invasion assay
Cell monolayers (grown in 6-well tissue culture plate at a density of 8 x 105 cells/well) were infected with E. coli (107 CFU/well) for 2h, 4h, 6h and 8h in a serum free medium. At the end of the incubation times, gentamicin at 100 μg/ml (Sigma-Aldrich) was added and left for 1 h to kill extracellular bacteria. Cells were then washed three times with PBS, lysed with 1 ml of 0.1% Triton X-100 in PBS, and plated onto LB agar plates with the appropriate antibiotics. Invasion frequencies were calculated as the number of bacteria surviving the incubation with gentamicin divided by the total number of bacteria present in the absence of the antibiotic. The experiments were performed three times in triplicate on separate days, and the data was expressed as percentage of invasion.
Cell viability
In order to determine the viability of INS-1E cells after short-term and long-term E. coli treatment, cells were trypsinized, cell suspensions were mixed with a 0.4% (w/v) trypan blue solution, and the number of live cells was determined using a haemocytometer. Cells failing to exclude the dye were considered non-viable. Each infection was performed in triplicate and counted four times each.
Electron microscopy
For Scanning Electron Microscopy (SEM) preparations, INS-1E cells, grown on sterile circular cover glasses, inserted into 6-well chamber slide (8 x 105 cells/well), and infected for 8h with E. coli (107 CFU/well) were fixed with 1.5% glutaraldehyde in 0.12 M phosphate buffer (pH 7.5) overnight at 4°C. Cells were then postfixed in 1% OsO4 for 1 h at 4°C. Following washing with distilled water, the cells were dehydrated in graded ethanol, critical point dried, and sputtered with a 5-nm gold layer using an EmscopeSM300 (Emscope Laboratories, Ashford, United Kingdom). They were then observed using a Hitachi S-4000 (Hitachi High-Technologies America, Inc., Schaumburg, IL) field emission scanning electron microscope. For transmission electron microscopy (TEM), after being dehydrated in a graded series of acetone, cells were embedded in Durcupan ACM (Fluka Chemika-Biochemika, Buchs, Switzerland). Ultrathin sections were cut perpendicularly from the membrane using a Reichert Ultracut E microtome and double stained with uranyl acetate and lead citrate. Observations were carried out using a Hitachi H-7000 transmission electron microscope (Hitachi High-Technologies Europe GmbH, Krefeld, Germany).
Insulin secretion assay
Glucose-induced insulin secretion was evaluated as previously described [39]. INS-1E cells (8 x 105 cells/well) were seeded in 6-well plates and incubated for 8h in a serum free medium containing E. coli (107 CFU/well). At the end of the incubation period, the medium containing bacteria was removed and gentamicin at 100 μg/ml (Sigma-Aldrich) was added and left for 1h to kill extracellular bacteria. Cells were then washed three times with PBS and cultures were randomly divided into two groups to mimic an acute and a chronic infection. The first group (short-term infection) was stopped at this point (after incubation with E. coli for 8h) miming an acute infection. The second group (long-term infection), after 8h of infection with E. coli, was further incubated for another 72h in a bacteria-free medium containing 5 mM glucose in order to allow bacterial proliferation inside the cells and to mimic a chronic infection. Cells from the two groups were then incubated for 1h at 37°C in Krebs-Ringer-HEPES buffer (KRHB) [39] containing 2.7 mM glucose (starvation). Thereafter, cells were washed with KRHB and incubated for 2h in the same buffer containing different concentrations of glucose (5.5 mM, 11.1 mM, 16.6 mM and 22.2 mM). Aliquots of supernatant were taken for the measurement of insulin secretion, while total protein content was determined by using BCA protein assay (Pierce). Non-infected cells (control cells) were incubated in a bacteria-free medium for the same incubation time as infected cells, for 8h (control cells of short-term E. coli infection) and for 8h plus 72h (control cells of long-term E. coli infection). In the experiments in presence of inhibitors, INS-1E cells were pre-incubated for 60 min in culture medium supplemented or not with 5 μM NS-398, COX-2 specific inhibitor, or 20 μM L-798106, specific EP3 antagonist, or 10 nM sulprostone, specific EP3 agonist.
The cells were then re-fed with fresh culture medium containing the inhibitors in presence or in absence of E. coli for 8h (short-term infection) or for 8h and subsequently further incubated for 72h (long-term infection).
Insulin levels in the culture media were measured by ELISA kit (Millipore). Data were expressed as percentage of maximal secretion showed in INS-1E cells, which for glucose stimulation was obtained at 16.6 mM.
Phospholipases A2 activity assay
INS-1E cells were pre-incubated for 1h in RPMI 1640 medium containing 5 mM glucose, supplemented or not with either 50 μM AACOCF3 (Arachidonoyl trifluoromethyl ketone, both PLA2s activity blocker) or 2.5 μM BEL (Bromoenol lactone, specific iPLA2 inhibitor) or 5 mM EDTA (cPLA2 inhibitor). The cells were then re-fed with fresh culture medium containing the inhibitors, in the presence or in the absence of E. coli (107 CFU/well) for 8h. At the end of the incubation period, cells were divided into two groups and processed as described in order to mime an acute and a chronic infection. Cultures from short-term infection (cells incubated for 8h with E. coli in the presence or absence of inhibitors) and from long-term infection (cells incubated for 8h with E. coli in the presence or absence of inhibitors and subsequently further incubated for 72h in the presence or absence of inhibitors) were lysed as previously described [40], and lysates were used for cPLA2 and sPLA2 activity assays, following the manufacturer’s instructions. Results were expressed as a percentage compared to the control non-infected cells.
Immunoblotting
For immunoblotting, INS-1E cells from short and long-term E. coli infection were collected by trypsinization. Controls were performed with non-infected cells. The lysates of INS-1E cells were prepared for Western blotting as previously described [41, 42]. The protein content of the cell lysate was quantified by BCA assay. Membranes were incubated with primary antibodies (1:500 dilution) against total cPLA2, iPLA2, phospho-cPLA2, COX-1, COX-2 and α-actin, and then incubated with secondary antibodies for 1h at room temperature.
Transfection of siRNAs
The cPLA2 and iPLA2 knock-down in INS-1E cells was carried out by using rat ON-TARGET plus SMART pool siRNA duplex (Dharmacon, Chicago, IL), transfected by Lipofectamine RNAiMax (Life Technologies, CA, USA). Two sets of oligonucleotides were used: the first direct against cPLA2 (Gene Bank NM_133551) and the second one direct against iPLA2 (Gene Bank NM_001005560). siRNA used were provided as SMART pool designed against shared and conserved regions in order to ensure efficient and specific target silencing for cPLA2 α, β and γ, as well as iPLA2, as indicated by the provider. A siRNA non targeting was used as negative control, according to the manufacturer’s instruction. Western blot analysis confirmed the reduction of the protein target level. After transfection with iPLA2-siRNA or cPLA2-siRNA, the cells were infected for 8h with E. coli (107 CFU/well). At the end of the incubation period, the cells were divided in two groups, as described (long- and short-term infection), and insulin release was determined.
Determination of PGE2 production
To determine PGE2 production, INS-1E were pre-incubated for 60 min in culture medium supplemented or not with either 50 mM AACOCF3 or 2.5 mM BEL. The cells were then re-fed with fresh culture medium containing the inhibitors in presence or in absence of E. coli for 8h (short-term infection) or for 8h and subsequently further incubated for 72h (long-term infection). Supernatants were collected and aliquots were employed for PGE2 determination, by kit from Cayman Chemicals, Ann Arbor, MI, USA. For PGE2, the detection range was 7.8–1000 pg ml-1.
Statistical analysis
Data is reported as mean ± standard deviation (SD). Statistical significance between two groups was analyzed by Student’s t-test. One-way analysis of variance (ANOVA), followed by Tukey’s post-hoc test, was used to compare the means for the multiple groups. GraphPad Prism was used to generate bar graphs. The p value <0.05 was considered statistically significant.
Results
Capability of E. coli to enter INS-1E
To evaluate the capability of E. coli to enter INS-1E cells, invasion assays were performed (Fig 1A). The percentage of invasion at 4h, 6h and 8h increased 1.6-, 2.2 and 2.9 fold respectively in comparison with invasion after 2h. The number of invasive bacteria recovered after 10h was very similar to that of 8h, indicating that the greatest number of bacteria was able to enter cells after 8h of incubation. For this reason, 8h incubation time was chosen for all the infection experiments. As shown in Fig 2B, the infection with E. coli for 8h of INS-1E in presence of PLA2 activity dual blocker AACOCF3 or iPLA2 inhibitor BEL caused a significant inhibition of invasion by about 50% and 40%, respectively, compared to invasion in absence of inhibitors. Trypan blue exclusion test demonstrated that short-term and long-term infection with E. coli (see Materials and Methods) did not affected cell viability (panel C).
Fig 1. Invasion assay, in absence or in presence of PLA2 inhibitors, and viability after incubation of INS-1E cells with E. coli.
Panel A: invasion of INS-1E cells with E. coli for 2h, 4h, 6h, 8h and 10h. Values are expressed as a percentage of invasion ± SD by three independent experiments performed in triplicate. Statistically significant differences, by one-way ANOVA and the Tukey post-test are indicated (*p<0.05 vs 2h invasion). Panel B: effect of PLA2 inhibitors (50 μM AACOCF3 or 2.5 μM BEL) on 8h E. coli invasion of INS-1E cells. Values are expressed as a percentage of invasion ± SD by three independent experiments performed in triplicate. Statistically significant differences, by one-way ANOVA and the Tukey post-test are indicated (*p<0.05 vs 8h invasion without inhibitors). Panel C: number of live non-infected cells and after short-term and long-term infection (see Materials and Methods). Values, in percentage compared to control cells incubated in absence of bacteria (mean ± SD) are from three independent experiments (n = 3).
Fig 2. Scanning Electron Microscopy (SEM) and Transmission Electron Microscopy (TEM) of INS-1E cells infected with E. coli for 8h.
(A) The surface of the cells shows microvilli variable in size and in shape and some bacteria on cell surface (white arrow) are visible (magnification, x 2000). (B) Numerous bacteria are present between the cells, in contact with pseudopod-like structures on the surface of the cells and some bacteria are engulfed in the cytoplasm of the cells (arrows). Bar 500 nm. (C) The bacteria appear to be in close contact with the cell membranes which encircle the microorganism (arrows). Some bacteria are engulfed intracellularly inside membrane-bound vacuoles (arrow). Bar 500 nm.
Fig 2A shows Scanning Electron Microscopy (SEM) images of INS-1E cells after 8h infection with E. coli. Some bacteria on the surface of the cells are visible (white arrow). Fig 2B and 2C show Transmission Electron Microscopy (TEM) images of INS-1E cells after 8h infection with bacteria. Pseudopod-like structures indicate that invasion requires cytoskeletal rearrangements. Some bacteria are present in the cytoplasm of the cells within vacuoles (black arrows).
Insulin secretion after acute and chronic E. coli infection
Insulin release was determined in INS-1E cells after short-term (8h, acute) infection (Fig 3A) and after long-term (72h, chronic) infection (Fig 3B). In non-infected cells (control), the insulin release in presence of 16.6 mM glucose (maximal secretion) was 0.94 ± 0.05 ng/μg protein/h. The insulin release after short-term infection significantly increased 1.5 fold in presence of 16.6 mM glucose concentration in comparison to non-infected cells (panel A). After long-term infection, insulin release in presence of 16.6 mM glucose concentration significantly decreased 5.5 fold in comparison to non-infected cells at the same glucose concentration (panel B).
Fig 3. Insulin release in INS-1E cells infected for short-term (8h, panel A) or long-term (8h plus 72h, B) with E. coli.
Control cells were incubated in a medium without bacteria for 8h (in short-term E. coli infection experiments) or for 8h plus 72h (in long-term infection experiments). Values are expressed as ng/μg protein (mean ± SD measured by three independent experiments performed in triplicate). Statistically significant differences, by one-way ANOVA and the Tukey post-test (p< 0.05) are indicated: (*) infected vs non-infected cells at the same glucose concentrations; (**) non-infected at different glucose concentrations vs non-infected cells at 2.7 mM glucose concentrations; (§) infected at different glucose concentrations vs infected cells at 2.7 mM glucose concentration.
PLA2 activities
Fig 4 shows PLA2 activities in INS-1E cells non-infected or infected (short- and long-term infection) with E. coli in absence or in presence of PLA2 inhibitors. The use of EDTA, and BEL in control and infected cells allowed us to discriminate between cPLA2 and iPLA2 activity. The enzyme activity insensitive to BEL represents Ca2+-dependent PLA2 contribution, whereas the enzyme insensitive to EDTA represents Ca2+-independent PLA2. None of these components, used at the specified concentration, affected cell viability, as verified by trypan blue exclusion test (data not shown). Results, pmol of ATPC hydrolyzed per minute and per milligram protein, were expressed in percentage compared to control cells.
Fig 4. Phospholipase A2 activities in INS-1E cells.
cPLA2 and iPLA2 activities in non-infected cells and after short-term infection (panel A) or after long-term infection (panel B) in absence or in presence of 50 μM AACOCF3 or 2.5 μM BEL or 5 mM EDTA (see Materials and Methods). Panel C: sPLA2 activity in non-infected and in infected cells (short-term and long-term infection) with E. coli. Values, in percentage compared to control cells incubated in absence of bacteria (mean ± SD) are from three independent experiments (n = 3). Statistically significant differences, by one-way ANOVA and the Tukey post-test (p< 0.05) are indicated: (*) non-infected cells with inhibitors vs non-infected w/o inhibitor cells; (§) infected cells with inhibitors vs infected cells w/o inhibitors; (**) infected vs non-infected in absence or in presence of the same inhibitor.
Total PLA2 specific activity was 20.1 ± 1.8 pmol/min/mg protein in non infected cells, 32.2 ± 2.3 pmol/min/mg protein after short-term E. coli infection and 42.3 ± 3.5 pmol/min/mg protein after long-term E. coli infection in absence of inhibitors. PLA2 activity of non-infected INS-1E cells (white bars) decreased almost 2.0 and 1.6 fold in presence of EDTA and BEL, respectively, compared to control cells in absence of inhibitors. As expected, the dual (cPLA2 and iPLA2) phospholipase blocker AACOCF3 almost totally reduced the specific activity to a very low level (panel A). PLA2 activity of infected INS-1E cells (black bars) was significantly activated (almost 1.4 fold) compared to non-infected cells. The incubation of INS-1E cells with E. coli in presence of EDTA caused a significant decrease of PLA2 activity, 2.1 fold in comparison with infected cells; BEL decreased PLA2 activity 1.2 fold, highlighting that, following E. coli acute infection, cPLA2 activity is mainly responsible for the AA production. Moreover, AACOCF3 almost completely reduced PLA2 activity of infected INS-1E cells.
After long-term infection, PLA2 activity of INS-1E cells (black bars) was significantly activated (almost 2.1 fold) compared to non-infected cells. The incubation of INS-1E cells with E. coli in presence of EDTA caused a significant decrease of PLA2 activity, 1.5 fold, in comparison to appropriate control; BEL decreased PLA2 activity, 2.5 fold, highlighting that, following long-term E. coli infection, iPLA2 activity is mainly responsible for AA production. AACOCF3 almost completely reduced PLA2 activity of long-term infected INS-1E cells. Moreover, sPLA2 activity was assayed in INS-1E cells after bacterial infection (panel B). No differences in sPLA2 activity were found after short- or long-term infection, indicating that sPLA2 is not involved in the response of the cells after E. coli infection.
Bacterial PLA2 was also assayed, but its contribution in the bacterial concentration used in our experiments was undetectable.
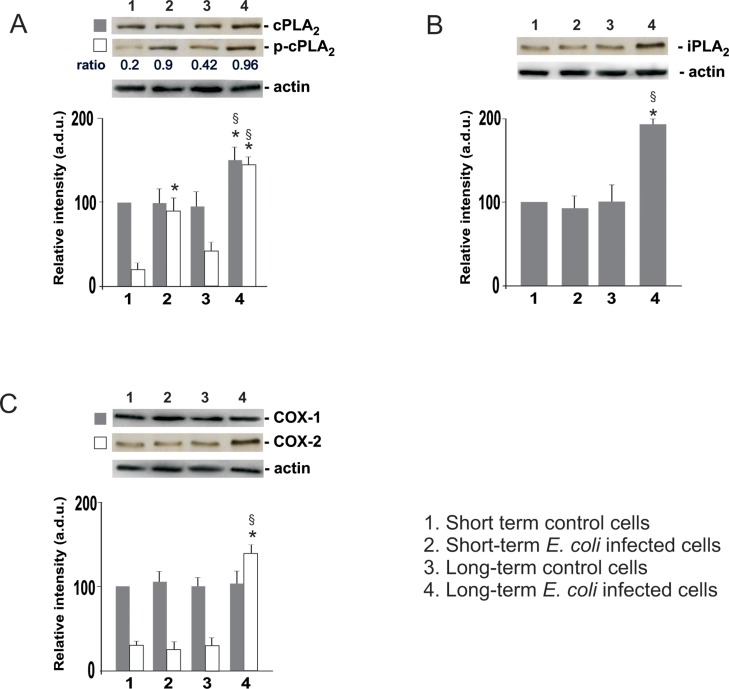
cPLA2, p-cPLA2, iPLA2, COX-1 and COX-2 expressions after E. coli infection
Western blot analyses in Fig 5, panel A, show the total cPLA2 and its phosphorylated levels, in INS-1E cells non-infected or infected (acute and chronic infection) with E. coli.
Fig 5.
Western blot analysis of cPLA2 and p-cPLA2 (A), iPLA2 (B), and COX-1/2 (C) in INS-1E cells after short and long-term infection with E. coli. The values, expressed as arbitrary densitometric units (a.d.u.) were obtained by reading the blots using the ImageJ program and are the mean ± SD from three independent experiments (n = 3) performed in triplicate. Control cells: non infected cells. Statistically significant differences, determined by one-way ANOVA and the Tukey post test, are indicated (p< 0.05). (*) infected vs non-infected cells at the same incubation period; (§) long-term infected vs short-term infected cells (line 4 vs line 2).
After short-term infection, no changes in the protein levels of total cPLA2 in infected INS-1E in comparison to non-infected cells were observed whereas cPLA2 expression increased about 1.5 fold in E. coli treated cells after long-term infection in comparison to non-infected cells in the same incubation period and to short-term infected cells. The phosphorylated form of cPLA2 increased in the cells after short-term infection 4.5 fold (0.9 vs 0.2 ratio p-cPLA2/cPLA2) and after long-term infection 3.1 fold (0.96 vs 0.42 ratio p-cPLA2/cPLA2), in comparison to the respective non-infected cells in the same incubation period. Moreover, long-term infection increased p-cPLA2 expression 1.6 fold in comparison to short-term infected cells (line 4 vs line 2).
Calcium-independent PLA2 expression (panel B) did not change after short-term infection, whereas iPLA2 expression increased 1.8 fold in E. coli treated INS-1E cells after long-term infection in comparison to control cells in the same incubation period (line 4 vs line 3) and to short-term infected cells (line 4 vs line 2).
Furthermore, COX-2 expression (panel C) significantly increased in the cells after long-term infection 4.6 fold in comparison to the respective control non-infected cells in the same incubation period, and 5.1 fold in comparison to short-term infected cells. No changes in COX-1 expression were observed.
Transfection of cPLA2- and iPLA2-siRNA
In order to shed light on the role played by cPLA2 and iPLA2 in insulin secretion after short-term or long-term infection, their respective mRNA were silenced using specific siRNA. Western blot of INS-1E lysates from three separate preparations of cells after transfection revealed the specificity of siRNAs used (panel A): both PLA2 protein basal expressions were strongly attenuated in transfected/non infected cells (Fig 6A, lanes 3 and 4, respectively), compared to non-transfected cells (control INS-1E, lane 1) or transfected with non targeting siRNA (lane 2). Insulin release was determined in transfected INS-1E cells without E. coli infection, after short-term infection and after long-term infection and the comparison among the three conditions at the most significant glucose concentration of 16.6 mM, is reported in Fig 6, panel B. In non-infected cells, the insulin release at 16.6 mM glucose concentration significantly decreased 3.3 and 1.5 fold in cPLA2- and iPLA2-siRNA transfected cells, respectively, in comparison to non-transfected INS-1E cells. After short-term infection, insulin release at 16.6 mM glucose concentration in non-transfected/infected cells significantly increased 1.6 fold in comparison to non-transfected/non-infected cells at the same glucose concentration. Moreover, insulin release at 16.6 mM glucose concentration significantly decreased 1.8 and 1.2 fold in cPLA2- and iPLA2-siRNA transfected cells, respectively, in comparison to non-transfected INS-1E cells. After long-term infection, insulin release at 16.6 mM in non-transfected/infected cells decreased 2.8 fold in comparison to non-transfected/non-infected cells at the same glucose concentration (value equal to 100). In these conditions, insulin release by cPLA2- and iPLA2-siRNA transfected INS-1E cells at 16.6 mM glucose concentrations increased 1.7 and 2.5 fold respectively, in comparison to non-transfected/infected cells.
Fig 6. Insulin release in INS-1E cells transfected with PLA2-siRNAs.
(A): cell lysates were immunoblotted to confirm the reduction of PLA2 protein levels. (B): insulin secretion of PLA2-siRNA transfected cells, infected or non infected with E. coli. Data is expressed as percentage of maximal secretion shown in non-infected/non-transfected INS-1E cells (mean ± SD measured by three independent experiments performed in triplicate), which for glucose stimulation is obtained at 16.6 mM glucose concentration. Statistically significant differences, by one-way ANOVA and the Tukey post-test (p< 0.05) are indicated: (*) cPLA2- and iPLA2-siRNA transfected/non infected cells vs non-transfected/non-infected cells. (**) non-transfected/infected cells vs non-transfected/non-infected cells (equal to 100%) at 16.6 mM glucose concentration w/o transfection. (§) cPLA2- and iPLA2-siRNA transfected/infected cells vs non-transfected/infected cells.
These results demonstrate the involvement of cPLA2 and iPLA2 activities in insulin release after E. coli infection and in particular the different responses of the transfected cells to acute and chronic infection. In acute infection, the silencing of iPLA2 and, even more so, cPLA2 reduces insulin secretion, confirming the involvement of these enzymes. However, in chronic infection, insulin release decreases in comparison to non-infected cells and the silencing of the cPLA2 and, even more so, iPLA2 determines a rise in the values of insulin release. The results demonstrate that iPLA2 is the main factor responsible for the decrease of insulin secretion after chronic infection: its activation leads to the release of the AA metabolism products into the cells, such as prostaglandins, whose effect could be manifested by the reduction of insulin release.
PGE2 production after E. coli infection
PGE2 production was measured in non-infected or short- and long-term infected INS-1E cells. As shown in Table 1, a 1.1-fold increase after E. coli short-term infection was observed in comparison to the respective non-infected cells. E. coli incubation in presence of 50 μM AACOCF3 or 2.5 μM BEL decreased PGE2 production 1.4 and 1.3 fold, respectively. After long-term infection, PGE2 production increased 3.2 fold in comparison to the respective control and the presence of AACOCF3 or BEL reduced PGE2 levels 3.5 and 2.8 fold, respectively. The results in presence of BEL demonstrated the cPLA2 contribution in PGE2 release. In long-term infection experiments, most of PGE2 produced could be a result of iPLA2 activity.
Table 1. PGE2 production in INS-1E cells stimulated and non-stimulated by E. coli.
| Control cells PGE2 secretion (pg/ml) | Cells + E. coli PGE2 secretion (pg/ml) | |
|---|---|---|
| Short-term infection | ||
| INS-1E | 115 ± 13.8 | 130 ± 12.1* |
| INS-1E + AACOCF3 | 98 ± 8.3a | 91 ± 7.9a |
| INS-1E + BEL | 104 ± 9.9 | 95 ± 8.9a |
| Long-term infection | ||
| INS-1E | 122 ± 10.6b | 388 ± 22.3b* |
| INS-1E + AACOCF3 | 97 ± 8.5a | 110 ± 10.1a |
| INS-1E + BEL | 108 ± 9.5a | 138 ± 12.6a |
a. The statistically significant differences in PGE2 production of cultures incubated with PLA2 inhibitors in comparison with the respective in absence of inhibitors.
b. The statistically significant differences, between long-time infected and non-infected cultures versus the respective short-time infected cultures.
INS-1E cells (8 x 105 cells/well) were pre-incubated for 60 min in culture medium supplemented or not with either 50 mM AACOCF3 or 2.5 mM BEL. The cells were then re-fed with fresh culture medium containing the inhibitors in presence or in absence of E. coli (107 CFU/well) for 8h (short-term infection) or for 8h and subsequently further incubated for 72h (long-term infection).
Cell culture supernatants were assayed for PGE2 production. Values (means ± SEM) are from three independent experiments (n = 3). ANOVA and the Tukey post-test were used to compare PGE2 production in the different experimental conditions (P < 0.05). Stimulated cells versus control cultures (not stimulated by bacteria), are indicated by asterisk (*).
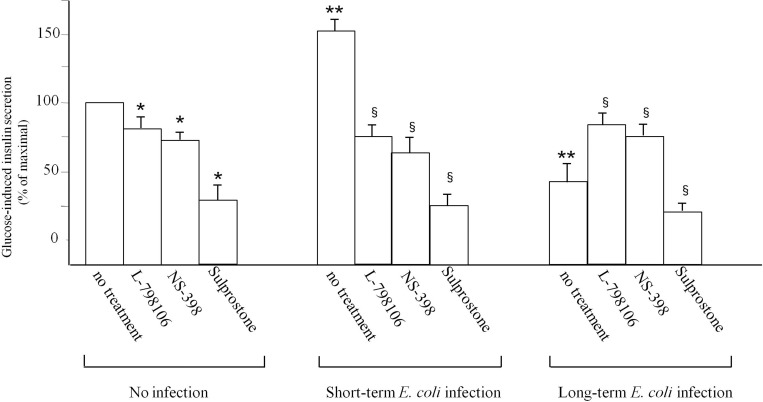
PGE2 imbalance causes dysfunction of insulin secretion
The increased production of PGE2 raised the possibility of an autocrine-paracrine loop in INS-1E cells after E. coli infection, causing insulin secretion reduction. For this reason, we hypothesized that it would be possible to modulate insulin secretion by acting on the EP3 receptor. Insulin secretion in non-infected INS-1E cells either after short-term infection or after long-term infection in absence or presence of L-798106, a specific EP3 antagonist, or NS-398, COX-2 inhibitor, or sulprostone, a specific EP3 agonist, was determined. The comparison among the three conditions at the most significant glucose concentration of 16.6 mM, is reported in Fig 7. In non-infected cells the insulin release significantly decreased by 20%, 26% and 73% in presence of L-798106, NS-398 and sulprostone, respectively, in comparison to control INS-1E cells in absence of inhibitors. After short-term infection, insulin release in infected cells significantly increased 1.6 fold in comparison to non-infected cells at the same glucose concentration. Moreover, insulin release significantly decreased by 51%, 58% and 84% in presence of L-798106, NS-398 and sulprostone, respectively, in comparison to short-term infected cells in absence of inhibitors. After long-term infection, insulin release in infected cells significantly decreased by 60% in comparison to non-infected cells. The presence of sulprostone further reduced insulin secretion by 80% in comparison to infected cells in absence of the inhibitor. However, the presence of NS-398 or L-798106 led to a recovery of insulin secretion by 35% and 50%, respectively. The results demonstrate that PGE2 production is responsible for insulin release dysfunction after E. coli infection.
Fig 7. Insulin release in non-infected INS-1E cells, or infected with E. coli for short-term or long-term.
INS-1E were pre-incubated for 60 min in culture medium supplemented or not with 5 μM NS-398, COX-2 specific inhibitor, or 20 μM L-798106, specific EP3 antagonist, or 10 nM sulprostone, specific EP3 agonist. Data is expressed as a percentage of maximal secretion shown in INS-1E cells (mean ± SD measured by three independent experiments performed in triplicate), which for glucose stimulation is obtained at 16.6 mM. Statistically significant differences, by one-way ANOVA and the Tukey post-test (p< 0.05) are indicated: (*) Treated non-infected cells vs non infected cells with no treatment. (**) Infected cells vs non-infected cells (equal to 100%) at 16.6 mM glucose concentration. (§) Treated infected cells vs infected cells with no treatment.
Discussion
The onset of T1DM in most cases is a gradual process, with a long period of subclinical disease that precedes the onset of clinical disease. For this reason, the identification of the factors responsible for the initiation of the disease process, years before the diagnosis of diabetes, is a goal of great importance in the study of the pathogenesis of T1DM.
Apart from cigarette smoking and alcohol consumption, the remaining cases of chronic pancreatitis are considered idiopathic in nature [43].
Experimental studies on the pathogenesis of pancreatitis have shown that the translocation of bacteria in the mesenteric lymph nodes, peritoneal fluid and blood, and, therefore, in the pancreas, plays a significant role and that the main mechanisms that govern this process are probably related to the proliferation of enteric flora after intestinal dysfunction, damage to the intestinal permeability, and impairment of host immunity [1]. From human cases of pancreatic abscesses, the organisms reported are Escherichia coli, Klebsiella species, Proteus species, Pseudomonas species, Enterobacter species, Candida species, Staphylococcus aureus, Enterococcus fecalis, Citrobacter species, Bacteroides species, Haemophilus influenzae and Mycobacterium tuberculosis [44]. A certain relationship between diabetes mellitus and E. coli infection was evidenced, the increased incidence of insulin-dependent diabetes mellitus in E. coli-infected pediatric patients having been demonstrated [45]. The infection of a host by E. coli is facilitated by virulence factors, which are coded by virulence-associated genes. E. coli can express a wide variety of virulence factors, involved in colonization, adhesion and invasion. These factors are adhesins, invasins and toxins which lead to different infection mechanisms [46]. However, EIEC strains are capable of invading and replicating within the cells [28] and therefore of infecting different organs including the pancreas. Despite intensive research, a final conclusion concerning the causal role of microbes in the pathogenesis of T1DM has not been found.
In this study, we used two experimental models, one miming an acute infection (short-term infection) and the other miming a chronic infection (long-term infection) by E. coli of INS-1E cells, able to secrete insulin in response to elevated glucose concentrations. The glucose concentration-dependence curve for these cells is similar to that of rat islets and, for this reason, INS-1E cells represent a stable and valuable ß-cell model [47].
It has been demonstrated that bacterial infection may reduce or increase the secretion of insulin based on the type of micro-organisms which penetrate the pancreatic tissue [48]. In the experiments here reported, E. coli was already able to adhere and invade INS-1E cells after 2h of incubation, and, once inside the cell, it could survive within vacuoles. The fate of E. coli in pancreatic cells is largely unexplored and, at present, its role in affecting PLA2 activities and insulin secretion is unknown.
The results obtained in this study show that, after short-term E. coli infection, PLA2 activities were increased and pancreatic cells continue to secrete insulin that results in even higher amounts in the E. coli-infected cells compared to uninfected ones. When the infection is carried out long-term, insulin secretion is significantly reduced and at the same time extremely high PLA2 activities and expressions were observed. We speculated that E. coli long-term infection determines excessive PLA2 activation leading to a significant imbalance in AA concentration. In isolated human islets it has been demonstrated that AA itself, rather than one or more of its metabolites, is responsible for an enhanced secretory response [49]. However, the fact that high concentrations of AA could cause a dangerous imbalance cannot be neglected because this polyunsaturated fatty acid up-regulates COX-2 enzymes which release a high amount of prostaglandins. Our results demonstrated that COX-2 expression is higher after long-term infection in comparison to protein expression in non-infected cells. Instead, short-term infection did not allow new synthesis of COX-2 enzymes. In addition, PGE2 production was significantly higher in INS-1E cells after long-term E. coli infection and the release was significantly reduced in presence of BEL. The residual low production of PGE2s in the presence of BEL testifies the significant contribution of iPLA2 in PGE2 production. Therefore one might speculate that cPLA2 plays a leading role in INS-1E under physiological conditions, contributing to the release of insulin. However, after chronic infection, iPLA2 was significantly activated, releasing greater amounts of AA and, then, determining COX-2 activation, resulting in increased PGE2 production. Consequently, iPLA2 could be the isoform primarily responsible for the PGE2 imbalance in INS-1E after E. coli long-term infection. This is confirmed by the results of enzymatic activity shown in Fig 4.
In diabetic islet, it has been demonstrated that increased PGE2 production, coupled with increased prostaglandin receptor 3, mediates a negative autocrine-paracrine signalling pathway which antagonizes GLP-1 receptor signalling, via a negative effect on cAMP production, contributing to the ß cell dysfunction [50].
In our study, cPLA2 and iPLA2 differently respond to ßacterial infection after 8h or 72h. After short-term infection, cPLA2 activity is higher than iPLA2, indicating that cPLA2 is the main factor responsible for AA release after acute infection. Differently, after long-term infection, iPLA2 was higher than cPLA2. These results highlight that the two isoforms play a different role after acute and chronic infection.
The role of cPLA2 in the ß-cells is controversial. It seems that cPLA2 is not required for the initiation of insulin secretion from ß-cells, but for the maintenance of ß-cell insulin stores [28]; on the other hand, it has been demonstrated that cPLA2ß plays an important role in controlling the rate of exocytosis in ß-cells and requires the combined actions of AA and lysophosphatidylcholine [28]. Its overexpression results in severe impairment of insulin secretion through uncoupling of mitochondrial metabolism [29]. Concerning iPLA2, it has been demonstrated that it participates in glucose-stimulated insulin secretion in pancreatic ß-cells [24, 25] and a dual role for this enzyme has been proposed, being able to amplify insulin secretion [51] or to contribute to apoptosis [31, 52] when overexpressed.
Numerous studies examining the role of PLA2s and AA in insulin release have focused on short-term signalling [53]. However, long term exposure to high levels of fatty acids has been shown to be detrimental to ß-cell function [34, 54] in contrast to the stimulatory short-term effects of exogenous AA and other fatty acids on the ß-cells [53]. It has been demonstrated that acute exposure of pancreatic ß-cells to saturated non-esterified fatty acids, including AA, increases glucose-induced insulin release, whereas chronic exposure results in desensitization and suppression of secretion, followed by induction of apoptosis [55]. We demonstrated that cPLA2, releasing AA, is activated by phosphorylation after short- and, even more, after long-term infection. Moreover, after 72h of bacterial incubation, new synthesis of cPLA2 and iPLA2 proteins indicates a specific response of the cells to counteract bacterial infection.
In view of our findings we propose a model of inflammatory response of INS-1E cells to E. coli infection in which cPLA2 and iPLA2, acting in concert, are involved. Their contribution is highlighted by the fact that their silencing by cPLA2- and iPLA2-siRNA transfection significantly reduces the glucose-stimulated insulin secretion in uninfected or short-term infected cells in comparison to non-transfected cells. When, after long-term E. coli infection, INS-1E no longer respond to high concentrations of glucose, the siRNA-iPLA2 transfection allows a response to glucose with consequent insulin secretion from INS-1E cells to be restored, even if to a lesser extent compared to that of non-infected cells. In particular, the insulin secretion is higher in siRNA-iPLA2 than in siRNA-cPLA2 transfected cells, highlighting that iPLA2 plays a main role in insulin secretion after chronic infection of INS-1E cells. Thus, iPLA2 could represent a therapeutic target to moderate the AA concentration imbalance, presumed responsible for the insulin secretion reduction in damaged ß cells.
Moreover, insulin release, increased after short-term infection, was significantly reduced in presence of L-798106, PGE2 receptor antagonist, NS-398, COX-2 inhibitor, and sulprostone, an EP3 agonist, demonstrating that PGE2 are responsible for the INS-1E dysfunction. Instead, insulin secretion, significantly reduced after long-term infection, is restored in presence of NS-398 and L-798106, further confirming the PGE2 role after E. coli infection. We hypothesized that an increase in insulin synthesis after short-term infection could be the initial response to infection of cells with the activation of phospholipases and COX-2, releasing PGE2, which, in balanced amounts inside the cell, would have a stimulatory effect on insulin secretion. In fact, it has been demonstrated that the phospholipases participate in amplifying glucose-induced insulin secretion [27, 56].
Conversely, when the infection continues for a longer time, the bacterial growth within the cells would cause excessive phospholipase and COX-2 activation, leading to an imbalance in AA and PGE2 concentration which may be responsible for the damage to INS-1E and consequently for the reduction of insulin secretion. In fact, in these conditions, blocking PGE2 synthesis or blocking the binding to their receptor, reduces their dangerous effect on INS-1E and restores insulin secretion.
So the main “culprits” as responsible for the reduction of insulin secretion would not be primarily AA and PGE2, which are able to induce, at physiological concentrations, an increase of the above synthesis, but the excessive concentration of AA and PGE2 within the cells. An imbalance in their concentration would be the mechanism by which E. coli would lead to the dysfunction of INS-1E. In this regard, it has been demonstrated that sulprostone and PGE2, are capable of eliciting a dose-dependent decrease of insulin secretion in glucose-stimulated INS-1 cells [50].
The E. coli infection is the result of the cumulative effect of numerous molecules acting on different target proteins of intracellular signalling pathways, by changing the functions of the host cell. In particular, type III secretion system (T3SS) and secreted proteins EspA, EspB and EspD, form a traslocom that is essential for protein secretion and for the translocation of multiple effectors into the host cells. These molecules have biochemical functions that make E. coli able to exert an accurate control of the host cell [57, 58] and are able to trigger cross-talk between bacterial and host cells [59]. It has been shown that molecules produced by E. coli are able to act on specific signalling pathways that can keep the infected cells alive and consequently may allow the bacteria to multiply within them and to colonize other tissues [60]. In particular, NleE, NleB, NleC, NleD and NleH are targeting specific proteins within inflammatory signalling, which presumably allows the bacteria to establish infection and avoid immediate elimination by the host innate immune response [61]. It has also been demonstrated that Shiga toxin-producing E. coli induce the activation of intracellular second messenger molecules, including inositol triphosphate and intracellular calcium, in infected eukaryotic cells in tissue culture [62–64]. Moreover, bacteria are able to cause post-translational modifications in the host cells through a variety of bacterial effectors present on their surface or secreted. These effectors can interact with intracellular proteins, by implementing different post-translational modifications. In particular, it has been shown that E. coli is capable of determining the deamidation of Rho GTPases through the production of CNF-1 (Cytotoxic Necrotizing Factor-1) [65]. Previous studies suggested that the ability of E. coli to raise intracellular calcium levels and generate diacylglycerol (DAG) led to the proposal that EPEC activates calcium- dependent protein kinases, including protein kinase C (PKC), in host epithelia [66]. It has been also reported that activation of PKC results in up-regulation of iPLA2ß expression that leads to activation of RhoA/Rho kinase/CPI-17 signalling [67, 68]. In this way, E. coli could regulate iPLA2. It has also been demonstrated that the three mitogen- activated protein kinases (MAPK), ERK1/2, p38, and JNK were phosphorylated in E. coli-infected human colonic cell lines T84 [67] and the tyrosine phosphorylation of host cell proteins [65].
As the infection strategy used by E. coli is post-translational modification, which targets central signalling pathways in the host cell, such as the NF-kB and MAP kinase pathways, it is likely that PLA2 is, in turn, activated by MAPK and by increasing intracellular calcium concentration.
We realize the fact that this study was only conducted on INS-1E cells and that further studies are needed to demonstrate the presence of this mechanism also in other models using several types of pancreatic cells. For the time being, the results provide a key to understanding the mechanisms by which E. coli could damage pancreatic cells.
In conclusion, PLA2s, and mainly iPLA2, play a key role in the response to the chronic E. coli infection of INS-1E cells, by producing AA, the COX-2 substrate for PGE2 synthesis. Further studies on the ability of the bacteria to modulate insulin secretion are needed to understand the mechanism through which they could cause diabetes, in order to develop strategies for the prevention of insulin imbalance and for the implementation of new therapeutic approaches.
Acknowledgments
This study was supported by the National Grant PON01- 00110.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by National Grant PON01- 00110. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Arrellano-Valdez F, Urrutia-Osorio M, Arroyo C, Soto-Vega E. A comprehensive review of urologic complications in patients with diabetes. Springerplus. 2014;3:549–56. 10.1186/2193-1801-3-549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crouzet J, Lavigne JP, Richard JL, Sotto A. Diabetic foot infection: a critical review of recent randomized clinical trials on antibiotic therapy. Int J Infect Dis. 2011;15:601–10. [DOI] [PubMed] [Google Scholar]
- 3.Von Herrath MG. Obstacles to identifying viruses that cause autoimmune disease. J of Neuroimmunology 2000;107:154–60. [DOI] [PubMed] [Google Scholar]
- 4.Myers MA, Mackay IR, Rowley MJ, Zimmet PZ. Dietary microbial toxins and type 1 diabetes – a new meaning for seed and soil. Diabetologia. 2001;44(9):1199–2000. [DOI] [PubMed] [Google Scholar]
- 5.Roep BO. The role of T-cells in the pathogenesis of type 1 diabetes: from cause to cure. Diabetologia. 2003;46(3):305–21. [DOI] [PubMed] [Google Scholar]
- 6.Lammi N, Karvonen M, Tuomilehto J. Do microbes have a causal role in type 1 diabetes? Med Sci Monit. 2005;11(3):RA63–69. [PubMed] [Google Scholar]
- 7.DU SC, Ge QM, Lin N, Dong Y, Su Q. ROS-mediated lipopolysaccharide-induced apoptosis in INS-1 cells by modulation of Bcl-2 and Bax. Cell Mol Biol (Noisy-le-grand). 2012;58:1654–9. [PubMed] [Google Scholar]
- 8.Osto M, Zini E, Franchini M, Wolfrum C, Guscetti F, Hafner M, et al. Subacute endotoxemia induces adipose inflammation and changes in lipid and lipoprotein metabolism in cats. Endocrinology. 2011;152:804–15. 10.1210/en.2010-0999 [DOI] [PubMed] [Google Scholar]
- 9.Hsieh PS. Inflammatory change of fatty liver induced by intraportal low-dose lipopolysaccharide infusion deteriorates pancreatic insulin secretion in fructose-induced insulin-resistant rats. Liver Int. 2008;28:1167–75. 10.1111/j.1478-3231.2008.01714.x [DOI] [PubMed] [Google Scholar]
- 10.Van Waardenburg DA, Jansen TC, Vos GD, Buurman WA. Hyperglycemia in children with meningococcal sepsis and septic shock: the relation between plasma levels of insulin and inflammatory mediators. J Clin Endocrinol Metab 2006;91(10):3916–21. [DOI] [PubMed] [Google Scholar]
- 11.Faustino EV, Apkon M. Persistent hyperglycemia in critically ill children. J Pediatr. 2005;146:30–34. [DOI] [PubMed] [Google Scholar]
- 12.Slavov E1, Georgiev IP, Dzhelebov P, Kanelov I, Andonova M, Mircheva G, et al. High-fat feeding and Staphylococcus intermedius infection impair beta cell function and insulin sensitivity in mongrel dogs. Vet Res Commun. 2010;34(3):205–15. 10.1007/s11259-010-9345-x [DOI] [PubMed] [Google Scholar]
- 13.Pezzilli R, Morselli-Labate AM, Barakat B, Romboli E, Ceciliato R, Piscitelli L, et al. Pancreatic involvement in Salmonella infection. JOP. 2003;4:200–6. [PubMed] [Google Scholar]
- 14.Del Giorno KE, Tam JW, Hall JC, Thotakura G, Crawford HC, van der Velden AW. Persistent salmonellosis causes pancreatitis in a murine model of infection. PLOS ONE. 2014;9:e92807 10.1371/journal.pone.0092807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gianantonio CA, Vitacco M, Mendilaharzu F, Gallo GE, Sojo ET. The hemolytic-uremic syndrome. Nephron. 1973;11:174–192. [DOI] [PubMed] [Google Scholar]
- 16.Sass DA, Chopra KB, Regueiro MD. Pancreatitis and E. coli O157:H7 colitis without hemolytic uremic syndrome. Dig Dis Sci. 2003;48:415–6. [DOI] [PubMed] [Google Scholar]
- 17.Arendt T. Bile-induced acute pancreatitis in cats. Roles of bile, bacteria, and pancreatic duct pressure. Dig Dis Sci. 1993;38:39–44. [DOI] [PubMed] [Google Scholar]
- 18.Arendt T, Nizze H, Stüber E, Mönig H, Kloehn S, Fölsch UR. Infected bile-induced acute pancreatitis in rabbits. The role of bacteria. Int J Pancreatol. 1998;24(2):111–6. [DOI] [PubMed] [Google Scholar]
- 19.Safi M, Achour W, Baaboura R, El Fatmi R, Ben Othmen T, Ben Hassen A. Distribution of virulence associated traits among urine Escherichia coli isolates from patients in onco-hematology. J Infect Chemother. 2016;22(4):221–4. 10.1016/j.jiac.2015.12.017 [DOI] [PubMed] [Google Scholar]
- 20.Taşer N, Doğan Z, Bahşi R, Sentürk S, Simşek M, Kekilli M. Extended-spectrum β-lactamase Escherichia coli in a community-acquired spontaneous bacterial peritonitis. Eur J Gastroenterol Hepatol. 2014;26(8):937–8. [DOI] [PubMed] [Google Scholar]
- 21.Sanchez-Villamil J, Navarro-Garcia F. Role of virulence factors on host inflammatory response induced by diarrheagenic Escherichia coli pathotypes. Future Microbiol. 2015;10(6):1009–33. 10.2217/fmb.15.17 [DOI] [PubMed] [Google Scholar]
- 22.Smith EJ, Thompson AP, O'Driscoll A, Clarke DJ. Pathogenesis of adherent-invasive Escherichia coli. Future Microbiol. 2013;8(10):1289–300. 10.2217/fmb.13.94 [DOI] [PubMed] [Google Scholar]
- 23.Sansonetti P. Host–pathogen interactions: the seduction of molecular cross talk. Gut. 2002;50, Suppl. 3 S2–S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaper JB1, Nataro JP, Mobley HL. Pathogenic Escherichia coli Nat Rev Microbiol. 2004;2(2):123–40. [DOI] [PubMed] [Google Scholar]
- 25.Nikolić DM. Effects of bacterial infection on insulin secretory capacity of human adult pancreatic islets. Br J Biomed Sci. 2011;68:181–4. [DOI] [PubMed] [Google Scholar]
- 26.Alberghina M. Phospholipase A(2): new lessons from endothelial cells. Microvasc Res. 2010;80:280–5. 10.1016/j.mvr.2010.03.013 [DOI] [PubMed] [Google Scholar]
- 27.Juhl K, Høy M, Olsen HL, Bokvist K, Efanov AM, Hoffmann EK. cPLA2alpha-evoked formation of arachidonic acid and lysophospholipids is required for exocytosis in mouse pancreatic beta-cells. Am J Physiol Endocrinol Metab. 2003;285:73–81. [DOI] [PubMed] [Google Scholar]
- 28.Persaud SJ, Roderigo-Milne HM, Squires PE, Sugden D, Wheeler-Jones CP, Marsh PJ, et al. A key role for beta-cell cytosolic phospholipase A(2) in the maintenance of insulin stores but not in the initiation of insulin secretion. Diabetes. 2002;51:98–104. [DOI] [PubMed] [Google Scholar]
- 29.Milne HM, Burns CJ, Squires PE, Evans ND, Pickup J, Jones PM, et al. Uncoupling of nutrient metabolism from insulin secretion by overexpression of cytosolic phospholipase A(2). Diabetes. 2005;54:116–24. [DOI] [PubMed] [Google Scholar]
- 30.Song H, Wohltmann M, Tan M, Bao S, Ladenson JH, Turk J. Group VIA PLA2 (iPLA2β) is activated upstream of p38 mitogen-activated protein kinase (MAPK) in pancreatic islet β-cell signalling. J Biol Chem. 2012;287:5528–41. 10.1074/jbc.M111.285114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ali T, Kokotos G, Magrioti V, Bone RN, Mobley JA, Hancock W. Characterization of FKGK18 as inhibitor of group VIA Ca2+-independent phospholipase A2 (iPLA2β): candidate drug for preventing beta-cell apoptosis and diabetes. PLoS One. 2013;8:e71748 10.1371/journal.pone.0071748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramanadham S, Ma Z, Arita H, Zhang S, Turk J. Type IB secretory phospholipase A2 is contained in insulin secretory granules of pancreatic islet beta-cells and is co-secreted with insulin from glucose-stimulated islets. Biochim Biophys Acta. 1998;1390:301–12. [DOI] [PubMed] [Google Scholar]
- 33.Ishida-Oku M, Iwase M, Sonoki K, Sasaki N, Imoto H, Uchizono Y. Expression of secretory phospholipase A2 in insulitis of human transplanted pancreas and its insulinotropic effect on isolated rat islets. Islets. 2010;2:274–7. [DOI] [PubMed] [Google Scholar]
- 34.Keane DC, Takahashi HK, Dhayal S, Morgan NG, Curi R, Newsholme P. Arachidonic acid actions on functional integrity and attenuation of the negative effects of palmitic acid in a clonal pancreatic β-cell line. Clin Sci (Lond). 2011;120:195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salmeri M, Motta C, Mastrojeni S, Amodeo A, Anfuso CD, Giurdanella G, et al. Involvement of PKCα-MAPK/ERK-phospholipase A(2) pathway in the Escherichia coli invasion of brain microvascular endothelial cells. Neurosci Lett. 2012;511:33–7. 10.1016/j.neulet.2012.01.031 [DOI] [PubMed] [Google Scholar]
- 36.Salmeri M, Motta C, Anfuso CD, Amodeo A, Scalia M, Toscano MA, et al. VEGF receptor-1 involvement in pericyte loss induced by Escherichia coli in an in vitro model of blood brain barrier. Cell Microbiol. 2013;15:1367–84. 10.1111/cmi.12121 [DOI] [PubMed] [Google Scholar]
- 37.Caporarello N, Salmeri M, Scalia M, Motta C, Parrino C, Frittitta L, et al. Role of cytosolic and calcium independent phospholipases A2 in insulin secretion impairment of INS-1E cells infected by S. aureus. FEBS Letters. 2015; 589:3969–3976. 10.1016/j.febslet.2015.11.035 [DOI] [PubMed] [Google Scholar]
- 38.D'Hertog W, Maris M, Thorrez L, Waelkens E, Overbergh L, Mathieu C. Two-dimensional gel proteome reference map of INS-1E cells. Proteomics. 2011;11:1365–9. 10.1002/pmic.201000006 [DOI] [PubMed] [Google Scholar]
- 39.Patané G, Caporarello N, Marchetti P, Parrino C, Sudano D, Marselli L, et al. Adiponectin increases glucose-induced insulin secretion through the activation of lipid oxidation. Acta Diabetol. 2013;50:851–7. 10.1007/s00592-013-0458-x [DOI] [PubMed] [Google Scholar]
- 40.Anfuso CD, Lupo G, Romeo L, Giurdanella G, Motta C, Pascale A, et al. Endothelial cell-pericyte cocultures induce PLA2 protein expression through activation of PKC alpha and the MAPK/ERK cascade. J Lipid Res. 2007;48:782–793. [DOI] [PubMed] [Google Scholar]
- 41.Giurdanella G, Motta C, Muriana S, Arena V, Anfuso CD, Lupo G, et al. Cytosolic and calcium-independent phospholipase A2 mediate glioma-enhanced proangiogenic activity of brain endothelial cells. Microvasc Res. 2011;81:1–17. 10.1016/j.mvr.2010.11.005 [DOI] [PubMed] [Google Scholar]
- 42.Anfuso CD, Motta C, Giurdanella G, Arena V, Alberghina M, Lupo G. Endothelial PKCa-MAPK/ERK-phospholipase A2 pathway activation as a response of glioma in a triple culture model. A new role for pericytes? Biochimie. 2014;99:77–87. 10.1016/j.biochi.2013.11.013 [DOI] [PubMed] [Google Scholar]
- 43.Culetto A, Bournet B, Haennig A, Alric L, Peron JM, Buscail L. Prospective evaluation of the aetiological profile of acute pancreatitis in young adult patients. Dig Liver Dis. 2015;47:584–9. 10.1016/j.dld.2015.03.009 [DOI] [PubMed] [Google Scholar]
- 44.Baker S. Diagnosis& management of acute pancreatitis. Crit Care Resusc 2004;6:17–27. [PubMed] [Google Scholar]
- 45.Suri RS, Mahon JL, Clark WF, Moist LM, Salvadori M, Garg AX. Relationship between Escherichia coli O157:H7 and diabetes mellitus. Kidney Int Suppl. 2009;112:S44–6. 10.1038/ki.2008.619 [DOI] [PubMed] [Google Scholar]
- 46.Frömmel U1, Lehmann W, Rödiger S, Böhm A, Nitschke J, Weinreich J, et al. Adhesion of human and animal Escherichia coli strains in association with their virulence-associated genes and phylogenetic origins. App Envir Microbiol. 2013;79:5814–5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Skelin M, Rupnik M, Cencič A. Pancreatic beta cell lines and their applications in diabetes mellitus research. ALTEX. 2010;27:105–113. [DOI] [PubMed] [Google Scholar]
- 48.Nikolic DM. Effects of bacterial infection on insulin secretory capacity of human adult pancreatic islets. Br J Biomed Sci. 2011;68:181–4. [DOI] [PubMed] [Google Scholar]
- 49.Persaud SJ, Muller D, Belin VD, Kitsou-Mylona I, Asare-Anane H, Papadimitriou A, et al. The role of arachidonic acid and its metabolites in insulin secretion from human islets of Langerhans. Diabetes. 2007;56:197–203. [DOI] [PubMed] [Google Scholar]
- 50.Kimple ME, Keller MP, Rabaglia MR, Pasker RL, Neuman JC, Truchan NA, et al. Prostaglandin E2 Receptor, EP3, is induced in diabetic islets and negatively regulates glucose- and hormone-stimulated insulin secretion. Diabetes. 2013;62:1904–1912. 10.2337/db12-0769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bao S, Jacobson DA, Wohltmann M, Bohrer A, Jin W, Philipson LH, et al. Glucose homeostasis, insulin secretion, and islet phospholipids in mice that overexpress iPLA2beta in pancreatic beta-cells and in iPLA2beta-null mice. Am J Physiol Endocrinol Metab. 2008;294:217–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bone RN, Gai Y, Magrioti V, Kokotou MG, Ali T, Lei X, et al. Inhibition of Ca2+-independent phospholipase A2β (iPLA2β) ameliorates islet infiltration and incidence of diabetes in NOD mice. Diabetes. 2015;64:541–54. 10.2337/db14-0097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nolan CJ, Madiraju MS, Delghingaro-Augusto V, Peyot ML, Prentki M. Fatty acid signaling in the beta-cell and insulin secretion. Diabetes. 2006;55:S16–23. [DOI] [PubMed] [Google Scholar]
- 54.Iizuka K, Nakajima H, Namba M, Miyagawa Ji, Miyazaki J, Hanafusa T, et al. Metabolic consequence of long-term exposure of pancreatic beta cells to free fatty acid with special reference to glucose insensitivity. Biochim Biophys Acta. 2002;1586:23–31. [DOI] [PubMed] [Google Scholar]
- 55.Cao Y, Pearman AT, Zimmerman GA, McIntyre TM, Prescott SM. Intracellular unesterified arachidonic acid signals apoptosis. Proc Natl Acad Sci U S A. 2000;97:11280–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shunzhong B, Jacobson D, Wohltmann M, Bohrer A, Jin W, Philipson LH, et al. Glucose Homeostasis, Insulin Secretion, and Islet Phospholipids in Mice that Overexpress iPLA2β in Pancreatic β-Cells and in iPLA2β-Null Mice. Am J Physiol Endocrinol Metab. 2008; 294(2): E217–E229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Buttner D, Bonas U. Port of entry the type III secretion translocon. Trends Microbiol. 2002;10:186–192. [DOI] [PubMed] [Google Scholar]
- 58.Wong AR, Pearson JS, Bright MD, Munera D, Robinson KS, Lee SF, et al. Enteropathogenic and enterohaemorrhagic Escherichia coli: even more subversive elements. Mol Microbiol. 2011;80(6):1420–38. 10.1111/j.1365-2958.2011.07661.x [DOI] [PubMed] [Google Scholar]
- 59.Hughes DT, Sperandio, V. Inter-kingdom signalling: communication between bacteria and their hosts. Nat Rev Microbiol. 2008;6:111–120. 10.1038/nrmicro1836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pearson JS, Giogha C, Ong SY, Kennedy CL, Kelly M, Robinson KS, et al. A type III effector antagonizes death receptor signalling during bacterial gut infection. Nature. 2013;501(7466):247–51. 10.1038/nature12524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sharma R, Tesfay S, Tomson FL, Kanteti RP, Viswanathan VK, Hecht G. Balance of bacterial pro- and anti-inflammatory mediators dictates net effect of enteropathogenic Escherichia coli on intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2006;290: G685–G694. [DOI] [PubMed] [Google Scholar]
- 62.Ismaili A, McWhirter E, Handelsman MY, Brunton JL, Sherman PM. Divergent signal transduction responses to infection with attaching and effacing Escherichia coli. Infect Immun. 1998;66(4):1688–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brown MD, Bry L, Li Z, Sacks DB. Actin pedestal formation by enteropathogenic Escherichia coli is regulated by IQGAP1, calcium, and calmodulin. J BiolChem. 2008; 283: 35212–35222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thumbikat P, Berry RE, Zhou G, Billips BK, Yaggie RE, Zaichuk T, et al. Bacteria-induced uroplakin signaling mediates bladder response to infection. PLoS Pathog. 2009; 5, e1000415 10.1371/journal.ppat.1000415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Doye A, Mettouchi A, Bossis G, Clément R, Buisson-Touati C, Flatau G, et al. CNF1 exploits the ubiquitin-proteasome machinery to restrict Rho GTPase activation for bacterial host cell invasion. Cell. 2002; 111(4):553–64. [DOI] [PubMed] [Google Scholar]
- 66.Shen-Tu G, Kim H, Liu M, Johnson-Henry KC, Sherman PM. Protein kinase C mediates enterohemorrhagic Escherichia coli O157:H7-induced attaching and effacing lesions. Infect Immun. 2014;82(4):1648–56. 10.1128/IAI.00534-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xie Z, Gong MC, Su W, Xie D, Turk J, Guo Z. Role of calcium-independent phospholipase A2beta in high glucose-induced activation of RhoA, Rho kinase, and CPI-17 in cultured vascular smooth muscle cells and vascular smooth muscle hypercontractility in diabetic animals. J Biol Chem. 2010; 285(12):8628–38. 10.1074/jbc.M109.057711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dahan S, Busuttil V, Imbert V, Peyron JF, Rampal P, Czerucka D. Enterohemorrhagic Escherichia coli infection induces interleukin-8 production via activation of mitogen-activated protein kinases and the transcription factors NF-kappaB and AP-1 in T84 cells. Infect Immun. 2002;70(5):2304–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.