Abstract
Background
Polymorphisms of IGF-1 and IGFBP-3 and environmental factors may work together to influence insulin-like growth factor (IGF) levels and thus breast cancer (BC) risk. However, very few studies have investigated high-order interactions among these variables.
Methods
A total of 277 newly diagnosed BC cases and 277 controls were recruited between October 2010 and July 2012. We collected each participant's demographic characteristics, dietary intake, and blood sample. IGF-1 rs1520220 and IGFBP-3 rs2854744 were then genotyped. A multi-analytic strategy combining unconditional logistic regression (ULR), generalized multifactor dimensionality reduction (GMDR), and classification and regression tree (CART) approaches was applied to systematically identify the interactions of the two single nucleotide polymorphisms (SNPs), body mass index (BMI), and daily intake of soy isoflavone (DISI) on BC susceptibility.
Results
In GMDR analyses, high-order interactions among BMI, DISI, and SNP rs2854744 were identified among overall and postmenopausal women. We also found significant dosage effects on BC risk with an increasing number of exposure factors, namely carrying the rs2854744 AA genotype, DISI <9.85 mg/day, and BMI ≥24 kg/m2 (P trend<0.05). Similarly, in CART analyses, compared with individuals having BMI<24kg/m2, DISI<9.85 mg/day, and the rs2854744 CC+CA genotype, BC risk increased significantly for those carrying the rs2854744 AA genotype, with BMI<24 kg/m2 and DISI<9.85 mg/day (OR = 1.95, 95%CI: 1.03–3.69), and also for those with BMI≥24kg/m2 and DISI<9.85 mg/day (OR = 2.13, 95%CI: 1.00–4.51). Similar interaction effects were observed among postmenopausal women.
Conclusions
This study suggests high-order interactions of the IGFBP-3 rs2854744 AA genotype, BMI≥24kg/m2, and DISI<9.85 mg/day on increased BC risk, particularly among postmenopausal women.
Introduction
Breast cancer (BC) is common among women worldwide, with about1.67 million new cases diagnosed in 2012, accounting for 25% of all cancers [1]. In China, the incidence of BC has steadily risen, from 126,227 in 2002 [2] to over 169,000 in 2008 [3], to 187,213 in 2012 [1]. Unless this trend slows, BC cases in China are expected to reach 2.5 million overall by 2021[4].
The insulin-like growth factor (IGF) system plays important roles in cellular proliferation, differentiation, and apoptosis [5–7]; therefore, the IGF system has long been known to be involved in BC carcinogenesis. The IGF system mainly consists of IGF-1/IGF-1 receptors, IGF-2/IGF-2 receptors, and IGF binding proteins (IGFBP-1 to 6). Within the IGF system, IGF-1 and IGFBP-3 are two key subunits involved in carcinogenesis.
Although the relationship between circulating IGFBP-3 concentration and BC remains inconsistent [8–11], high circulating IGF-1 levels have been observed in many in vivo and epidemiological studies to increase the risk and progression of BC [12–15]. Thus, determinants of circulating IGF-1 and IGFBP-3 levels, including genetic and environmental factors, may impact BC risk.
Among genetic factors, the G-C substitution in intron 3 of the IGF-1 gene (SNP rs1520220) may influence IGF-1 expression by altering the secondary structure of RNA or DNA [16, 17], and the A-C substitution at nucleotide-202 in the promoter region of the IGFBP-3 gene (SNP rs2854744) may reduce promoter activity, which would theoretically decrease circulating IGFBP-3 levels [18].
Apart from genetic polymorphisms, other factors may also play roles in serum IGF-1 or IGFBP-3 variation. Obesity is a known risk factor for BC [19]. Body mass index (BMI) has shown effects on circulating IGF-1 or IGFBP-3 concentrations. Fowke et al. observed IGFBP-3 levels tend to rise with BMI, regardless of race[20]. As for dietary factors, soy isoflavone is a type of phytoestrogen with similar molecular structure to endogenous estrogens. It is believed that high soy isoflavone intake contributes to the relatively low BC risk in Asian countries [21]. Evidence from in vitro studies indicates that genistein, a main component of soy isoflavones, may stimulate the IGF-1 signaling pathway in human breast cancer cells at pharmacological doses [22]. Given that genetic and environmental factors are associated with circulating IGF levels, the hypothesis that IGF-1 and IGFBP-3 polymorphisms, BMI, and dietary intake of soy isoflavone may work together in affecting BC risk warrants further investigation. Hakkak et al. found obese rats fed soy exhibited a significant decrease in IGFBP-3 levels [23]. Our previous study observed joint effects of carrying IGF-1rs1520220 and consuming soy isoflavone on women's circulating IGF-1 levels [24]. However, to the best of our knowledge, no previous study has examined potential interactions of IGF-1 and IGFBP-3 polymorphisms, BMI, and dietary soy isoflavone on BC risk. To test for these interactions, we conducted a case control study to (i) identify the gene-environment interactions of IGF-1 rs1520220, IGFBP-3 rs2854744, BMI, and soy isoflavone intake on BC risk; and (ii) estimate the effects of gene-environment interactions.
Materials and Methods
Ethics Statement
This study was approved by the institutional research ethics committee of Sichuan University, and written informed consent was obtained from each subject before completing the questionnaire survey and laboratory tests.
Population
From October 2010 to July 2012, 292 primary BC cases newly histopathologically diagnosed in the Second People’s Hospital of Sichuan Province (also known as Sichuan Cancer Hospital) were invited to participate in our study, among whom, 15 cases (5%) were excluded because their blood samples were not available. A total of 277 cases were enrolled, of which 7 were diagnosed with breast carcinoma in situ (DCIS), 246 with invasive ductal carcinoma, and 24 with other cancers (e.g., invasive lobular carcinoma, medullary carcinoma). According to pathologic reports and using the American Joint Committee on Cancer (AJCC) TNM System (0, I, II, III, IV, and unknown stage or not applicable), 20 cases were classified as stage 0, 87 as stage I, 91 as stage II, 40 as stage III, 2 as stage IV, and 37 as unknown. During the same period, 306 women undergoing routine physical examinations in Chengdu Women’s and Children’s Central Hospital were selected as potential controls. They were then given breast ultrasound to exclude malignant tumors; however, those with benign breast disease, such as lobular hyperplasia, were included. Each control was matched to one patient by age (±2 years), so that 277 healthy women (90.5% of the potential control group) were included in our study as controls. All participants were of Han ethnicity and had lived in Sichuan Province for more than 20 years. We further excluded those with occupational exposure, other malignant tumors, or psychiatric disorders.
Data collection
We used a structured questionnaire to collect all participants’ socio-demographic and reproductive characteristics, and a semi-quantitative dietary questionnaire to collect their long-term (≥5 years) dietary habits. Evaluation of the reliability and structural validity of the questionnaires and calculation of energy-adjusted dietary intake has been described in detail in our previous study [25]. In brief, we calculated the total daily intake of energy first, then used a residual method to adjust other dietary intake as energy-adjusted protein, fat, carbohydrate, dietary fiber, and daily intake of soy isoflavones (DISI). According to the Chinese Dietary Reference Intakes (DRIs) (formulated by the Chinese DRIs committee in 2000) for 18–50 year old women with moderate physical activity [26], we used the following dichotimization: 2300 kcal/day for total energy, 70 g/day for protein, and 77 g/day for fat. For those categories without recommended levels of dietary intake, the mean of dietary intakes (132.52 g/day for carbohydrate, 17.86 g/day for dietary fiber, and 9.85 mg/day for soy isoflavones) were selected as the cutoff values of high vs. low intake.
Genotype analyses
Five milliliters of whole blood was obtained from each participant via venipuncture into an anticoagulative tube and stored at -20°Cuntil DNA extraction. Genomic DNA was extracted from whole blood using a TIANamp Blood DNA Kit (TIANGEN, Beijing). DNA samples with purity between 1.8 and 2.0 qualified for genotyping. IGF-1 rs1520220 and IGFBP-3 rs2854744 were genotyped with TaqMan assays, which were performed with an ABI 7500 thermal cycler (Applied Biosystems, Foster City, CA). Primers and probes for IGF-1 rs1520220 were purchased as predesigned assays-on-demand from Applied Biosystems (ABI assay-on-demand C_2801118_10). For IGFBP-3 rs2854744, primers (forward: CACCTTGGTTCTTGTAGACGACAA; reverse: GGCGTGCAGCTCGAGACT) and probes (VIC-MGB-TCCTCGTGCGCACG and FAM-MGB-CTCGTGCTCACGCC) were used. All tests were performed in the molecular biology laboratory of West China School of Public Health, Chengdu, China. In addition, 5% of the total subjects were selected randomly for duplicate testing, and the determined genotypes of repeated tests were in complete concordance.
Statistical analyses
For each SNP, we checked the Hardy-Weinberg equilibrium (HWE) among all controls via a goodness-of-fit chi-square test. Differences in demographic characteristics, reproductive and dietary factors between cases and controls were compared with independent-sample T-tests (for continuous variables) or chi-square/Fisher's exact tests (for categorical variables). We applied multivariable unconditional logistic regression (ULR) to test the main effects of SNPs IGF-1 rs1520220 and IGFBP-3 rs2854744, and the joint effects of SNPs and BMI, and of SNPs and DISI on BC risk, by calculating odds ratios (ORs) and 95% confidence intervals (95% CIs). The Akaike Information Criterion (AIC) was used to determine the goodness of model fit.
According to the results of previous studies, IGF-1 levels were significantly lower for IGF-1 rs1520220 CC genotype carriers than for GC or GG carriers [27], and carrying the IGFBP-3 rs2854744 AA genotype was associated with higher circulating IGFBP-3 levels [28]. We therefore analyzed the effects of IGF-1 rs1520220 with CC vs. GC+GG and IGFBP-3 rs2854744 with AA vs. CC+CA. SPSS18.0 was used for statistical analyses.
GMDR analyses
We applied generalized multifactor dimensionality reduction (GMDR, version 0.9, obtained from http://www.ssg.uab.edu/gmdr/) to analyze possible high-order interactions among genetic and environmental factors, obtaining parameters such as balanced accuracy, sign test P value, and cross-validation (CV) consistency. The model with the maximum balanced accuracy, the maximum CV consistency score, and a P value of 0.05 or less was considered the best. The odds ratios (OR) with 95% confidence intervals (95% CIs) for the interaction effects of the variables in the best model were calculated using ULR analysis by classifying subjects into groups according to the number of exposure risk factors, and adjusting potential confounders as covariates [29].
CART analyses
CART analysis was performed with SPSS18.0. Via recursive partitioning, a CART is constructed by splitting a node into two child nodes step by step, beginning with the root node that contains the whole learning sample and ending with a decision tree. Before building a tree, a Gini criterion was used to choose the measurement for goodness of split that would yield the maximum homogeneity between two child nodes. Tree splitting was done until terminal nodes reached a pre-specified minimum size of 30 subjects. To avoid overfitting, a pruning procedure would be performed when a tree had grown to its full depth. Terminal nodes of the tree represent the subgroups with differential risk associations with BC, indicating the potential presence of interactions. Finally, the risk for these subgroups was evaluated using the ULR by treating the subgroup having the smallest percentage of cases as the reference and adjusting for potential confounder factors as covariates [30].
All the data analyses were also performed stratified by menopausal status.
Results
General demographic characteristics, related reproductive factors and dietary intake of study subjects
Among the participants, the 227 cases included 143 pre- and 134 postmenopausal women, while there were 187 pre- and 90 postmenopausal controls. Even though the menopausal status was not exactly equal between the two groups, the difference of mean (SD) ages between cases (49.29±11.04) and controls (47.77±9.04) was not significant (t = -1.76, P = 0.08).
Among all study participants (Table 1) as well in the pre- and postmenopausal subgroups, distributions of education, income, age at first pregnancy, parity, and breast feeding were significantly different between cases and controls (P<0.05). Cases tended to be less educated and have lower income, younger age at first pregnancy, more children and longer breast feeding than controls. BMI was also significantly different between cases and controls in the overall and postmenopausal groups. These factors were treated as priori chosen potential confounders and adjusted in ULR, GMDR and CART analyses. The ratios of women who ever used contraceptives and had a family history of BC were higher among postmenopausal women than premenopausal women.
Table 1. Demographic characteristics, reproductive factors and breast cancer among study participants.
| Factors | Cases (%) | Controls (%) | P |
|---|---|---|---|
| Education | |||
| Less than high school | 192 (69.3) | 62 (22.4) | <0.001 |
| High school or more | 85 (30.7) | 215 (77.6) | |
| Income (RMB per month) | |||
| <1500 | 203 (73.3) | 96 (34.7) | <0.001 |
| ≥1500 | 74 (26.7) | 181 (65.3) | |
| BMI (kg/m2) | |||
| <24 | 187 (67.5) | 222 (80.1) | 0.001 |
| ≥24 | 90 (32.5) | 55 (19.9) | |
| Smoking | |||
| No | 272 (98.2) | 271 (97.8) | 0.76 |
| Yes | 5 (1.8) | 6 (2.2) | |
| Alcohol consumption | |||
| No | 265 (95.7) | 265 (95.7) | 1.00 |
| Yes | 12 (4.3) | 12 (4.3) | |
| Menarche (years) | |||
| ≥13 | 232 (83.8) | 225 (81.2) | 0.43 |
| <13 | 45 (16.2) | 52 (18.8) | |
| Age at first pregnancy (years) | |||
| <25 | 189 (68.2) | 121 (43.7) | <0.001 |
| ≥25 | 88 (31.8) | 156 (56.3) | |
| Parity | |||
| 0 | 3 (1.1) | 6 (2.2) | |
| 1–2 | 224 (80.9) | 265 (95.7) | <0.001 |
| ≥3 | 50 (18.1) | 6 (2.2) | |
| No. of abortions | |||
| 0 | 57 (20.6) | 57 (20.6) | |
| 1–2 | 148 (53.4) | 164 (59.2) | 0.24 |
| ≥3 | 72 (26.0) | 56 (20.2) | |
| Breast feeding (months) | |||
| ≥3 | 237 (85.6) | 190 (68.6) | <0.001 |
| <3 | 40 (14.4) | 87 (31.4) | |
| Contraceptive use | |||
| Never | 222 (80.1) | 227 (81.9) | 0.59 |
| Ever | 55 (19.9) | 50 (18.1) | |
| History of benign breast disease | |||
| No | 229 (82.7) | 217 (78.3) | 0.20 |
| Yes | 48 (17.3) | 60 (21.7) | |
| Family history of breast cancer | |||
| No | 266 (96.0) | 269 (97.1) | 0.48 |
| Yes | 11 (4.0) | 8 (2.9) | |
Table 2 shows the dietary intake of our subjects. Among study participants, energy-adjusted protein, fat, dietary fiber, and DISI were significantly different between cases and controls (P<0.05). However, the results stratified by menopausal status had some differences. There were significant differences in energy-adjusted protein, fat, carbohydrate, and dietary fiber intake between premenopausal cases and controls, while for postmenopausal subjects, the only significant differences were in DISI consumption.
Table 2. Dietary intake and breast cancer by menopausal status.
| Dietary intake | Total | Premenopausal | Postmenopausal | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases (%) | Controls (%) | χ2 (P) | Cases (%) | Controls (%) | χ2 (P) | Cases (%) | Controls (%) | χ2 (P) | |||
| Total energy intake (kcal/day) | |||||||||||
| <2300 | 246 (88.8) | 241 (87.0) | 124 (86.7) | 160 (85.6) | 122 (91.0) | 81 (90.0) | |||||
| ≥2300 | 31 (11.2) | 36 (13.0) | 0.42 (0.52) | 19 (13.3) | 27 (14.4) | 0.09 (0.77) | 12 (9.0) | 9 (10.0) | 0.07 (0.79) | ||
| Protein intake (g/day)a | |||||||||||
| <70 | 239 (86.3) | 203 (73.3) | 125 (87.4) | 134 (71.7) | 114 (85.1) | 69 (76.7) | |||||
| ≥70 | 38 (13.7) | 74 (26.7) | 14.50 (<0.001) | 18 (12.6) | 53 (28.3) | 11.91 (0.001) | 20 (14.9) | 21 (23.3) | 2.55 (0.11) | ||
| Fat intake(g/day) a | |||||||||||
| <77 | 51 (18.4) | 99 (35.7) | 23 (16.1) | 76 (40.6) | 28 (20.9) | 23 (25.6) | |||||
| ≥77 | 226 (81.6) | 178 (64.3) | 21.06 (<0.001) | 120 (83.9) | 111(59.4) | 23.27 (<0.001) | 106 (79.1) | 67 (74.4) | 0.67 (0.42) | ||
| Carbohydrate intake(g/day) a | |||||||||||
| <132.52 | 152(54.9) | 137(49.5) | 83(58.0) | 80(42.8) | 69(51.5) | 57(63.3) | |||||
| ≥132.52 | 125(45.1) | 140(50.5) | 1.63(0.20) | 60(42.0) | 107(57.2) | 7.55(0.006) | 65(48.5) | 33(36.7) | 3.07(0.08) | ||
| Dietary fiber intake(g/day) a | |||||||||||
| <17.86 | 165(59.6) | 133(48.0) | 86(60.1) | 80(42.8) | 79(59.0) | 53(58.9) | |||||
| ≥17.86 | 112(40.4) | 144(52.0) | 7.44(0.01) | 57(39.9) | 107(57.2) | 9.77(0.002) | 55(41.0) | 37(41.1) | 0.001(0.99) | ||
| DISI (mg/day) a | |||||||||||
| ≥9.85 | 112(40.4) | 135(48.7) | 59(41.3) | 84(44.9) | 53(39.6) | 51(56.7) | |||||
| <9.85 | 165(59.6) | 142(51.3) | 3.87 (0.049) | 84(58.7) | 103(55.1) | 0.44(0.51) | 81(60.4) | 39(43.3) | 6.34(0.01) | ||
a: The dietary key nutrient intake values, including protein, fat, carbohydrates, dietary fiber, and soy isoflavones, were adjusted for energy by the residual method
IGF-1 rs1520220 and IGFBP-3 rs28 54744 genotypes and BC
Among all controls, the frequencies of the C allele for IGF-1 rs1520220 and the C allele for IGFBP-3 rs2854744 were 55.8% and 25.0%, respectively. Genotypes of IGF-1 rs1520220 and IGFBP-3 rs2854744 accorded with HWE (IGF-1 rs1520220: χ2 = 3.63, P = 0.06; IGFBP-3 rs2854744: χ2 = 0.56, P = 0.45). The main effects of IGF-1 rs1520220 and IGFBP-3 rs2854744 on BC are shown in the S1 Table. Results from multivariable ULR analysis showed that after adjustment for confounders, IGF-1 rs1520220 and IGFBP-3 rs2854744 were not associated with BC risk.
Joint effects of IGF-1 rs1520220, IGFBP-3 rs2854744 and BMI or DISI
Joint effects of IGF-1 rs1520220, IGFBP-3 rs2854744 and BMI are shown in the S2 Table. Significant joint effects were mainly found among postmenopausal women. We observed that when using the IGF-1 GG+GC genotype and BMI<24 kg/m2 as the reference group, carrying the IGF-1 GG+GC genotype with BMI ≥24 kg/m2 increased BC risk. Compared with women with the IGFBP-3 CC+CA genotype and BMI<24 kg/m2, other groups (IGFBP-3 AA& BMI<24 kg/m2; IGFBP-3 CC+CA& BMI ≥24 kg/m2; IGFBP-3 AA& BMI≥24 kg/m2) had a significantly higher risk for BC. Also, we found carrying the IGFBP-3 AA genotype worked jointly with low soy intake (DISI <9.85 mg/day) to increase BC risk among postmenopausal women (S3 Table).
GMDR analyses
To further explore gene-environment interactions, we performed a GMDR analysis (S4 Table). A three-factor interaction model of BMI, DISI, and IGFBP-3 rs2854744 was identified as the best model among overall and postmenopausal women, with the maximum balanced accuracy for the training set (58.48% for overall and 66.56% for postmenopausal women), balanced accuracy for the calibration set (58.09% for overall and 64.51% for postmenopausal women), the maximum CV consistency of 10/10, and a sign test P value of 0.01 and 0.001 for overall and postmenopausal women, respectively. The results indicated potential interactions among BMI, DISI, and IGFBP-3 rs2854744. The interaction effects were then estimated using ULR analyses (Table 3). Subjects were classified into four subgroups by the number of exposure factors, defined as the IGFBP-3 rs2854744 AA genotype, DISI<9.85 mg/day, and BMI ≥24 kg/m2. A significant dose-response relationship was observed for BC risk among overall and postmenopausal women (P trend is 0.015 for overall and <0.001 for postmenopausal women). Compared with subjects carrying no exposure factors, those with two risk factors had higher BC risk (for overall women: OR = 1.73, 95%CI: 0.98–3.06; for postmenopausal women: OR = 4.96, 95%CI: 1.94–12.66), and the risk increased further for those carrying three factors (for overall women: OR = 2.74, 95%CI: 1.25–6.03; for postmenopausal women: OR = 5.76, 95%CI: 1.62–20.45).
Table 3. Cumulative effects of IGFBP-3 rs2854744, DISI, and BMI on breast cancer risk.
| NO. a | Total | Premenopausal | Postmenopausal | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Cases (%) | Controls (%) | OR (95%CI)b | Cases (%) | Controls (%) | OR (95%CI)c | Cases (%) | Controls (%) | OR (95%CI)d | |
| 0 | 28(10.1) | 36(13.0) | 1.00 | 18(12.6) | 20(10.7) | 1.00 | 10(7.5) | 16(17.8) | 1.00 |
| 1 | 101(36.5) | 140(50.5) | 0.93(0.53–1.62) | 57(39.9) | 91(48.7) | 0.70(0.34–1.43) | 44(32.8) | 49(54.4) | 1.44(0.59–3.49) |
| 2 | 116(41.9) | 86(31.0) | 1.73(0.98–3.06) | 54(37.8) | 66(35.3) | 0.91(0.44–1.89) | 62(46.3) | 20(22.2) | 4.96(1.94–12.66) |
| 3 | 32(11.6) | 15(5.4) | 2.74(1.25–6.03) | 14(9.8) | 10(5.3) | 1.56(0.56–4.36) | 18(13.4) | 5(5.6) | 5.76(1.62–20.45) |
| P trend | 0.015 | 0.489 | <0.001 | ||||||
a: The number of exposure factors, defined as theIGFBP-3 rs2854744 AA genotype, DISI <9.85 mg/day, and BMI ≥24 kg/m2
b: adjusted for education, income, age at first pregnancy, parity, breast feeding, and energy-adjusted protein, fat and dietary fiber intake
c: adjusted for education, income, age at first pregnancy, parity, breast feeding, and energy-adjusted protein, fat, carbohydrate and dietary fiber intake
d: adjusted for education, income, age at first pregnancy, parity, breast feeding, and contraceptive use
CART analyses
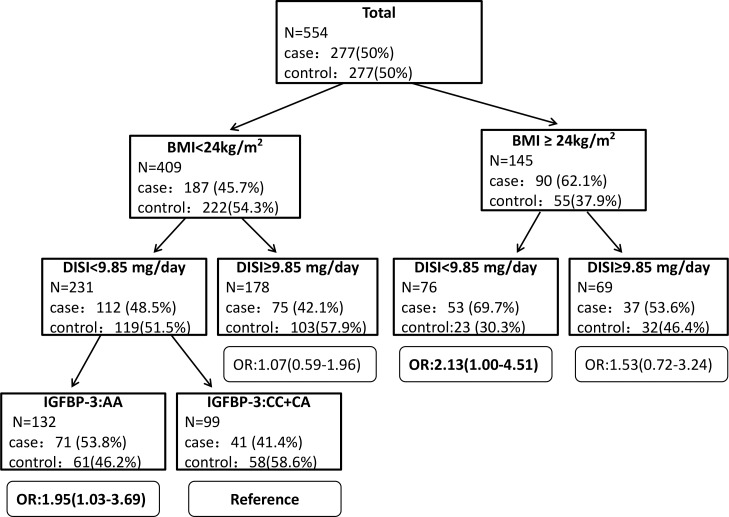
To further validate the gene-environment interactions defined by GMDR, we performed classification and regression tree (CART) analyses. Fig 1 depicts the resulting tree structure generated for study participants. There was an initial split on BMI, confirming that BMI was the most important risk factor for BC among the factors considered. With the smallest percentage of cases (41.4%), the subgroup with BMI<24kg/m2, DISI<9.85 mg/day and the IGFBP-3 rs2854744 CC+CA genotype was treated as a reference. Compared with the reference subgroup, BC risk was significantly higher for those with the IGFBP-3 rs2854744 AA genotype, BMI<24 kg/m2, and DISI<9.85 mg/day (OR = 1.95, 95%CI: 1.03–3.69), and for those with BMI≥24 kg/m2, DISI<9.85 mg/day and either IGFBP-3 rs2854744 genotype (OR = 2.13, 95%CI: 1.00–4.51). However, we did not observe significant gene-environment interactions among the variables considered in premenopausal women (data not shown).
Fig 1. CART analysis of IGF-1 and IGFBP-3 genetic polymorphisms and environmental factors among study participants.
After CART analysis, the risk for subgroups identified in different terminal nodes was evaluated using the ULR by treating the subgroup having the smallest percentage of cases as the reference and adjusting for potential confounder factors, including education, income, age at first pregnancy, parity, breast feeding, and energy-adjusted protein, fat, and dietary fiber intake. The results of ULR, ORs, and their 95% confidence intervals for each subgroup are shown by the side of each terminal node of the tree.
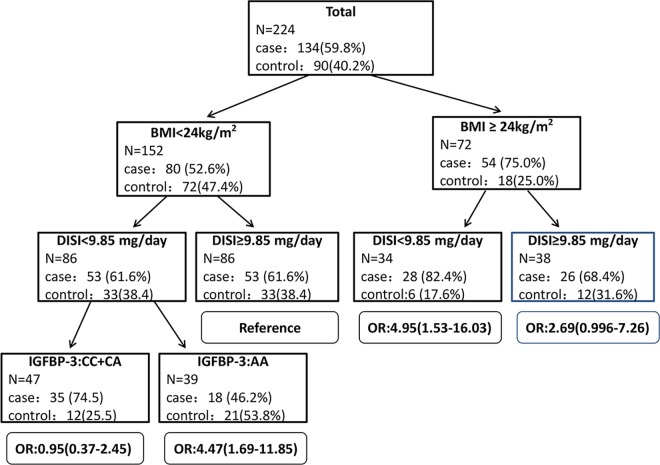
The results for postmenopausal women are summarized in Fig 2. Here the tree also split initially on BMI, and the reference group was that with BMI<24kg/m2 and DISI≥9.85 and either IGFBP-3 rs2854744 genotype. Compared with the reference, the BC risk for those with BMI≥24kg/m2 was higher (OR = 2.69, 95%CI: 0.996–7.26), and the risk was still higher in the subgroups with both BMI≥24 kg/m2 and DISI<9.85 mg/day (OR = 4.95, 95%CI: 1.53–16.03), and both DISI<9.85 mg/day and the IGFBP-3 rs2854744 AA genotype (OR = 4.47, 95%CI: 1.69–11.85).
Fig 2. CART analysis of IGF-1 and IGFBP-3 genetic polymorphisms and environmental factors among postmenopausal women.
This figure summarizes the results of CART analysis for postmenopausal women. The risk for subgroups was also evaluated using ULR with adjustment for education, income, age at first pregnancy, parity, breast feeding, and estrogen use. The results of ULR, ORs, and their 95% confidence intervals for each subgroup are shown by the side of each terminal node of the tree.
Discussion
In this study, we applied a multiple-pronged strategy combining ULR, GMDR, and CART analyses to systematically examine the association between BC risk and a series of risk factors. These included SNPs of IGF-1 rs1520220 and IGFBP-3 rs2854744, BMI, and soy isoflavone intake. Results from the GMDR and CART analyses consistently revealed a high-order interaction of the IGFBP-3 rs2854744 genotype, BMI, and DISI on BC risk. Having the IGFBP-3 rs2854744 AA genotype, BMI≥24 kg/m2, and DISI<9.85 mg/day may synergistically increase women's BC risk, particularly among postmenopausal women.
IGF-1 rs1520220, IGFBP-3 rs2854744, and BC
Given the association between high levels of circulating IGF-1 and increased risk and progression of BC, it is believed that genetic polymorphisms associated with serum IGF-1 variation can also affect BC risk. Numerous epidemiologic studies have examined the relationship between genes encoding IGF-1and BC risk (reviewed in [28]), but with respect to SNP IGF-1 rs1520220, the results are inconsistent. For example, Al-Zahrani et al. reported that women carrying the C allele of IGF-1 rs1520220 had a 1.41-fold higher BC risk [31], while Qian et al. observed that this SNP predicted circulating IGF levels but not breast cancer risk among Chinese women [27]. As in Qian et al.’s study, we did not detect a significant relationship between SNP IGF-1 rs1520220 and BC risk. The differing results can be explained by heredity. A SNP database at the National Center of Biotechnology Information reveals that the IGF-1 rs1520220 C allele frequency is 45.8–63.3% among Asians (55.8% in our study), much less than that among Europeans (75.0–100%) [32].
Although the A allele of IGFBP-3 rs2854744 is positively associated with circulating IGFBP-3 levels, with a distinct dose-response relationship [33–35], there remains conflicting evidence about the association between IGFBP-3 rs2854744 and BC risk. A study in Europe with 8,760 subjects reported that women carrying the A allele of IGFBP-3 had an 87% lower risk [31]. A case-control study from Shanghai of 2,503 women showed a 1.6-fold higher risk conferred by the IGFBP-3 C allele [36]. However, several studies have found no association between this SNP and breast cancer risk [27, 28, 33, 37], including the present study. In vitro studies found that IGFBP-3 gene expression varied by approximately 50% between A- and C- containing alleles, whereas circulating levels varied according to genotype to a lesser extent (7.7%) [33]. Since this SNP has only weak effects on circulating IGFBP-3 level, it is not easy to detect a significant relationship between the IGFBP-3 rs2854744 genotype alone and BC risk.
High-order interactions among IGFBP-3 genetic polymorphisms, body mass index, and soy isoflavone intake on BC risk
Although we did not observe effects of IGF-1 rs1520220 and IGFBP-3 rs2854744 alone on BC, we did find joint effects of IGF-1 rs1520220 and BMI, IGFBP-3 rs2854744 and BMI, and IGFBP-3 rs2854744 and DISI using multivariable ULR. To further explore possible high-order gene-environment interactions, we performed GMDR and CART analyses and consistently obtained the most interesting findings in this study, suggesting there were high-order gene-environment interactions of BMI, DISI, and IGFBP-3 rs2854744 on BC risk among overall and postmenopausal women. Furthermore, ULR analyses indicated that BC risk was associated with three risk factors in a dose dependent manner: the IGFBP-3 rs2854744 AA genotype, DISI <9.85 mg/day, and BMI ≥24 kg/m2. The ORs for interaction effects among these factors ranged from 1.73 to 2.74 for overall and from 2.69 to 5.76 for postmenopausal women. These results are biologically plausible since the IGFBP-3 rs2854744 AA genotype, DISI<9.85 mg/day, and BMI ≥24 kg/m2 may work together to increase circulating IGFBP-3 levels, which has been observed to be positively associated with the risk of BC among Chinese women [10]. Some researchers have observed that IGFBP-3 levels tend to rise with BMI [20, 38, 39]. Deal et al. demonstrated a synergetic effect of BMI>27 kg/m2 and carrying the IGFBP-3 rs2854744-A allele on increasing IGFBP-3 levels [33].The protective effect of dietary soy intake against breast cancer has been demonstrated by a number of studies in Asia (reviewed in [40]). Hakkaket al. found obese rats fed with soy exhibited a significant decrease in IGFBP-3 levels [23]. Population studies suggested there was trend toward decreased IGFBP-3 concentrations in women with increasing isoflavone consumption [41, 42]. We therefore suggest that increased IGFBP-3 level maybe a key mediator of the association between BC risk, the IGFBP-3 rs2854744 AA genotype, DISI<9.85 mg/day, and BMI ≥24 kg/m2 in our study population.
It is notable that the interactions were limited to postmenopausal women. Postmenopausal women are more susceptible to the effects of soy isoflavone [43], an exogenous phytoestrogen, because of their sharply decreased hormone levels. Moreover, postmenopausal women have a higher average BMI than that of premenopausal women [44]. Therefore, interactions of soy isoflavone intake, BMI, and gene polymorphisms may be more easily detected among postmenopausal women. However, the exact underlying mechanisms for the differences in interaction effects between pre- and postmenopausal women remain to be elucidated.
Strengths
There are two main strengths of our study. First, to the best of our current knowledge, this is the first study exploring the complex gene-environment interactions of IGF-1 rs1520220 and IGFBP-3 rs2854744 polymorphisms, BMI, and soy isoflavone intake on BC susceptibility. Moreover, we used complex statistical analyses, including GMDR and CART, to explore high-order gene-environment interactions. Compared with logistic regression, GMDR and CART are high powered for identifying high-order interactions [45, 46].These methods have been applied widely to explore high-order gene-gene and gene-environment interactions on cancer risk [29, 47, 48]. However, GMDR and CART are non-parametric data mining approaches, with a disadvantage in estimating ORs and 95% CIs for interaction effects. We thus used ULR to calculate interaction effects of variables defined in the best model of GMDR and those defined by CART. We obtained similar estimations of interaction effects based on GMDR (Table 1) and CART (Fig 1 and Fig 2). Thus, we believe our results are robust.
Limitations
This study has several limitations. First, the cases and controls were not closely matched in general demographic characteristics, related reproductive factors and dietary intake (Table 1 and Table 2). In particular, the results showing that cases had a younger age at first pregnancy, more children, and longer breast feeding time than controls were contrary to established knowledge [49].This may be because cases were from both urban (78%) and rural areas (22%) while controls were only from urban areas, which may have introduced selection bias. However, we adjusted for potential confounders in our analyses to reduce the bias, making it highly likely that the high-order gene-environment interaction results in our study are valid.
Second, our subjects were exclusively Chinese women, so the results may not be generalizable to women from other countries with different ethnicities, dietary habits, and lifestyles. However, our study results add a new clue on the effects of gene-environment interactions on BC susceptibility.
Third, the sample size in this study was limited, which may lead to unstable results. Since the estimates of interaction based on different methods were similar, we believe our results are robust, and not likely to be influenced by the relatively small sample size.
Conclusions
In conclusion, our study explored complex gene-environment interactions among genetic polymorphisms of the IGF system, BMI, and soy isoflavone intake on BC susceptibility. The results showed that having the IGFBP-3 rs2854744 AA genotype, BMI≥24 kg/m2, and DISI<9.85 mg/day may synergistically increase women’s BC risk, particularly among postmenopausal women. Our results have public health implications, suggesting that losing weight and increasing soy isoflavone intake may reduce BC risk for women with a susceptible IGFBP-3 rs2854744 genotype.
Supporting Information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We thank Dr. Hui Li at the Second People’s Hospital of Sichuan Province and Dr. Jing Zhang at Chengdu Women’s and Children’s Central Hospital for assisting us in the successful completion of this project.
Data Availability
All relevant data are within the paper and Supporting Information files.
Funding Statement
Interpretation of data and manuscript writing was supported by: (1) Medical Scientific Research Foundation of Guangdong Province (funding number: A2015443). The first author QW received this funding. The URLs of funder's website is http://www.gdwst.gov.cn/. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript; (2) National Natural Science Foundation of China (funding number: 81602819). The first author QW received this funding. The URLs of funder's website is http://www.nsfc.gov.cn/. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript; (3) National Natural Science Foundation of China (funding number: 81302500). The corresponding author JL received this funding. The URLs of funder's website is http://www.nsfc.gov.cn/. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript; (4) Medical Scientific Research Foundation of Sichuan Province (funding number: 120520). The co-first author LL received this funding. The URLs of funder's website is http://www.scwst.gov.cn/. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Ferlay J, Soerjomataram I, Ervik M. GLOBOCAN 2012 v1.0. Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. Lyon, France: International Agency for Research on Cancer. Available at http://globocan.iarc.fr. Last accessed December 12, 2013. 2012.
- 2.Ferlay J, Bray F, Pisani P, Parkin D M. GLOBOCAN 2002 V2.0. Cancer Incidence, Mortality and Prevalence Worldwide: IARC CancerBase No. 5. Lyon, France: International Agency for Research on Cancer. Available at http://www-dep.iarc.fr/. Last accessed April 13, 2007. 2002.
- 3.Ferlay J, Shin HR, Bray F, Forman D, Mathers C. GLOBOCAN 2008 v1.2, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10. Lyon, France: International Agency for Research on Cancer. Available at http://globocan.iarc.fr. Last accessed December 11, 2012. 2008.
- 4.Linos E, Spanos D, Rosner BA, Linos K, Hesketh T, Qu JD, et al. Effects of reproductive and demographic changes on breast cancer incidence in China: a modeling analysis. J Natl Cancer Inst. 2008; 100(19): 1352–1360. 10.1093/jnci/djn305 10.1093/jnci/djn305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pollak MN, Schernhammer ES, Hankinson SE. Insulin-like growth factors and neoplasia. Nat Rev Cancer. 2004; 4(7): 505–518. 10.1038/nrc1387 [DOI] [PubMed] [Google Scholar]
- 6.Sarkissyan S, Sarkissyan M, Wu Y, Cardenas J, Koeffler HP, Vadgama JV. IGF-1 regulates Cyr61 induced breast cancer cell proliferation and invasion. PLoS One. 2014; 9(7): e103534 10.1371/journal.pone.0103534 eCollection 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qiu J, Yang R, Rao Y, Du Y, Kalembo FW. Risk factors for breast cancer and expression of insulin-like growth factor-2 (IGF-2) in women with breast cancer in Wuhan City, China. PLoS One. 2012; 7(5): e36497 10.1371/journal.pone.0036497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Renehan AG, Zwahlen M, Minder C, O'Dwyer ST, Shalet SM. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet. 2004; 363(9418): 1346–1353. 10.1016/S0140-6736(04)16044-3 [DOI] [PubMed] [Google Scholar]
- 9.Kaaks R, Lundin E, Manjer J, Biessy C. Prospective study of IGF-1, IGF-binding proteins, and breast cancer risk, in Northern and Southern Sweden. Cancer Causes Control. 2002; 13(4): 307–316. 10.1023/A:1015270324325 [DOI] [PubMed] [Google Scholar]
- 10.Yu H, Jin F, Shu XO, Li BD, Dai Q. Insulin-like growth factors and breast cancer risk in Chinese women. Cancer Epidemiol Biomarkers Prev. 2002; 11(8): 705–712. [PubMed] [Google Scholar]
- 11.Krajcik R A, Borofsky N D, Massardo S, Orentreich N. Insulin-like growth factor I (IGF-1), IGF binding proteins, and breast cancer. Cancer Epidemiol Biomarkers Prev. 2002; 11(12): 1566–1573. [PubMed] [Google Scholar]
- 12.Renehan A G, Harvie M, Howell A. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and breast cancer risk: eight years on. Endocr Relat Cancer. 2006; 13(2): 273–278. 10.1677/erc.1.01219 [DOI] [PubMed] [Google Scholar]
- 13.Rinaldi S, Peeters PH, Berrino F, Dossus L, Biessy C. IGF-1, IGFBP-3 and breast cancer risk in women: the European Prospective Investigation into Cancer And Nutrition (EPIC). Endocrine Rel Cancer. 2006; 13(2): 593–605. 10.1677/erc.1.01150 [DOI] [PubMed] [Google Scholar]
- 14.Lagiou P, Hsieh CC, Lipworth L, Samoli E, Okulicz W. Insulin-like growth factor levels in cord blood, birth weight and breast cancer risk. Br J Cancer. 2009; 100(11): 1794–1798. 10.1038/sj.bjc.6605074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu Y, Cui K, Miyoshi K, Hennighausen L, Green JE. Reduced circulating insulin-like growth factor I levels delay the onset of chemically and genetically induced mammary tumors. Cancer Res. 2003; 63(15): 4384–4388. [PubMed] [Google Scholar]
- 16.Lu L, Katsaros D, Mayne ST, Risch HA, Benedetto C, Canuto EM. Functional study of risk loci of stem cell-associated gene lin-28B and associations with disease survival outcomes in epithelial ovarian cancer. Carcinogenesis. 2012; 33(11): 2119–2125. 10.1093/carcin/bgs243 [DOI] [PubMed] [Google Scholar]
- 17.Lu L, Risch E, Deng Q, Biglia N, Picardo E, Katsaros D. An insulin-like growth factor-II intronic variant affects local DNA conformation and ovarian cancer survival. Carcinogenesis. 2013; 34(9): 2024–2030. 10.1093/carcin/bgt168 [DOI] [PubMed] [Google Scholar]
- 18.Canzian F, McKay JD, Cleveland RJ, Dossus L, Biessy C, Rinaldi S. Polymorphisms of genes coding for insulin-like growth factor 1 and its major binding proteins, circulating levels of IGF-I and IGFBP-3 and breast cancer risk: results from the EPIC study. Br J Cancer. 2006; 94(2): 299–307. 10.1038/sj.bjc.6602936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang J, Yang DL, Chen ZZ, Gou BF. Associations of body mass index with cancer incidence among populations, genders, and menopausal status: A systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2016; 42: 1–8. 10.1016/j.canep.2016.02.010 [DOI] [PubMed] [Google Scholar]
- 20.Fowke JH, Matthews CE, Yu H, Cai Q, Cohen S, Buchowski MS, et al. Racial differences in the association between body mass index and serum IGF1, IGF2, and IGFBP3. Endocr Relat Cancer. 2010; 17(1): 51–60. 10.1677/ERC-09-0023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dong JY,Qin L. Soy isoflavones consumption and risk of breast cancer incidence or recurrence: a meta analysis of prospective studies. Breast Cancer Res Treat. 2011; 125(2): 315–323. 10.1007/s10549-010-1270-8 [DOI] [PubMed] [Google Scholar]
- 22.Chen WF,Wong MS. Genistein enhances insulin-like growth factor signaling pathway in human breast cancer (MCF-7) cells. J Clin Endocrinol Metab. 2004; 89(5): 2351–2359. 10.1210/jc.2003-032065 [DOI] [PubMed] [Google Scholar]
- 23.Hakkak R, Shaaf S, Jo C H, Macleod S, Korourian S. Effects of high-isoflavone soy diet vs. casein protein diet and obesity on DMBA-induced mammary tumor development. Oncol Lett. 2011; 2(1): 29–36. 10.3892/ol.2010.202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Q, Liu L, Li H, McCullough LE, Qi YN, Li JY. Genetic and dietary determinants of insulin-like growth factor (IGF)-1 and IGF binding protein (BP)-3 levels among Chinese women. PloS one. 2014; 9(10): e108934 10.1371/journal.pone.0108934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Q, Li H, Tao P, Wang YP, Yuan P. Soy isoflavones, CYP1A1, CYP1B1, and COMT polymorphisms, and breast cancer: a case-control study in southwestern China. DNA Cell Biol. 2011; 30(8): 585–595. 10.1089/dna.2010.1195 [DOI] [PubMed] [Google Scholar]
- 26.Chinese Nutrition Society, Chinese Dietary Reference Intakes Website.Available: http://www.cnsoc.org.cn. Accessed 2014 Jan 1.
- 27.Qian B, Zheng H, Yu H, Chen K. Genotypes and phenotypes of IGF-I and IGFBP-3 in breast tumors among Chinese women. Breast Cancer Res Treat. 2011; 130(1): 217–226. 10.1007/s10549-011-1552-9 [DOI] [PubMed] [Google Scholar]
- 28.Fletcher O, Gibson L, Johnson N, Altmann DR, Holly JM, Ashworth A. Polymorphisms and circulating levels in the insulin-like growth factor system and risk of breast cancer: a systematic review. Cancer Epidemiol Biomarkers Prev 2005; 14(1): 2–19. [PubMed] [Google Scholar]
- 29.Hu X, Yuan P, Yan J, Feng F, Li X, Liu W, et al. Gene Polymorphisms of ADIPOQ +45T>G, UCP2 -866G>A, and FABP2 Ala54Thr on the Risk of Colorectal Cancer: A Matched Case-Control Study. PLoS One. 2013; 8(6): e67275 10.1371/journal.pone.0067275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu L, Wu C, Wang Y, Zhong R. Association of candidate genetic variations with gastric cardia adenocarcinoma in Chinese population: a multiple interaction analysis. Carcinogenesis. 2011; 32(3): 336–342. 10.1093/carcin/bgq264 [DOI] [PubMed] [Google Scholar]
- 31.Al-Zahrani A, Sandhu MS, Luben RN, Thompson D, Baynes C, Pooley KA. IGF1 and IGFBP3 tagging polymorphisms are associated with circulating levels of IGF1, IGFBP3 and risk of breast cancer. Hum Mol Genet. 2006; 15(1): 1–10. 10.1093/hmg/ddi398 [DOI] [PubMed] [Google Scholar]
- 32.NCBI, Database of single nucleotide polymorphisms (SNPs). Available at http://www.ncbi.nlm.nih.gov/. Last accessed December 12, 2015. 2015.
- 33.Deal C, Ma J, Wilkin F, Aquette J, Rozen F. Novel promoter polymorphism in insulin-like growth factor-binding protein-3: correlation with serum levels and interaction with known regulators. J Clin Endocrinol Metab. 2001; 86(3): 1274–1280. 10.1210/jc.86.3.1274 [DOI] [PubMed] [Google Scholar]
- 34.Jernstrom H, Chu W, Vesprini D, Tao Y, Majeed N, Deal C. Genetic factors related to racial variation in plasma levels of insulin-like growth factor-1: implications for premenopausal breast cancer risk. Mol Genet Metab. 2001; 72(2): 144–154. 10.1006/mgme.2000.3130 [DOI] [PubMed] [Google Scholar]
- 35.Schernhammer ES, Hankinson SE, Hunter DJ, Blouin MJ, Pollak MN. Polymorphic variation at the -202 locus in IGFBP3: Influence on serum levels of insulin-like growth factors, interaction with plasma retinol and vitamin D and breast cancer risk. Int J Cancer. 2003; 197(1): 60–64. 10.1002/ijc.11358 [DOI] [PubMed] [Google Scholar]
- 36.Ren Z, Cai Q, Shu XO, Cai H, Li C, Yu H. Genetic polymorphisms in the IGFBP3 gene: association with breast cancer risk and blood IGFBP-3 protein levels among Chinese women. Cancer Epidemiol Biomarkers Prev. 2004; 13(8): 1290–1295. [PubMed] [Google Scholar]
- 37.Patel AV, Cheng I, Canzian F, Marchand L, Thun MJ, Berg CD. IGF-1, IGFBP-1, and IGFBP-3 polymorphisms predict circulating IGF levels but not breast cancer risk: findings from the Breast and Prostate Cancer Cohort Consortium (BPC3). PloS one. 2008; 3(7): e2578 10.1371/journal.pone.0002578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gram IT, Norat T, Rinaldi S, Dossus L, Lukanova A, Téhard B, et al. Body mass index, waist circumference and waist-hip ratio and serum levels of IGF-I and IGFBP-3 in European women. Int J Obes (Lond). 2006; 30(11): 1623–1631. [DOI] [PubMed] [Google Scholar]
- 39.Crowe FL, Key TJ, Allen NE, Appleby PN, Overvad K, Grønbæk H, et al. A cross-sectional analysis of the associations between adult height, BMI and serum concentrations of IGF-I and IGFBP-1–2 and -3 in the European Prospective Investigation into Cancer and Nutrition (EPIC). Ann Hum Biol. 2011; 38(2): 194–202. 10.3109/03014460.2010.507221 [DOI] [PubMed] [Google Scholar]
- 40.Liu XO, Huang YB, Gao Y, Chen C, Yan Y, Dai HJ. Association between dietary factors and breast cancer risk among Chinese females: systematic review and meta-analysis. Asian Pac J Cancer Prev. 2014; 15(3): 1291–1298. 10.7314/APJCP.2014.15.3.1291 [DOI] [PubMed] [Google Scholar]
- 41.Wangen KE, Duncan AM, Merz-Demlow BE, Xu X, Marcus R. Effects of soy isoflavones on markers of bone turnover in premenopausal and postmenopausal women. J Clin Endocrinol Metab. 2000; 85(9): 3043–3048. 10.1210/jc.85.9.3043 [DOI] [PubMed] [Google Scholar]
- 42.Gann PH, Kazer R, Chatterton R, Gapstur S, Thedford K, Helenowski I, et al. Sequential, randomized trial of a low-fat, high-fiber diet and soy supplementation: effects on circulating IGF-I and its binding proteins in premenopausal women. Int J Cancer. 2005; 116(2): 297–303. [DOI] [PubMed] [Google Scholar]
- 43.Wang Q, Wang YP, Li JY, Yuan P, Yang F, Li H. Polymorphic catechol-O-methyltransferase gene, soy isoflavone intake and breast cancer in postmenopausal women: a case control study. Chin J Cancer. 2010; 29(7): 683–688. [DOI] [PubMed] [Google Scholar]
- 44.Biglia N, Peano E, Sgandurra P, Moggio G, Pecchio S, Maggiorotto F, et al. Body mass index (BMI) and breast cancer: impact on tumor histopathologic features, cancer subtypes and recurrence rate in pre and postmenopausal women. Gynecol Endocrinol. 2013; 29(3): 263–267. 10.3109/09513590.2012.736559 [DOI] [PubMed] [Google Scholar]
- 45.Hahn LW, Ritchie MD, Moore JH. Multifactor dimensionality reduction software for detecting gene-gene and gene-environment interactions. Bioinformatics. 2003; 19(3): 376–382. 10.1093/bioinformatics/btf869 [DOI] [PubMed] [Google Scholar]
- 46.García-Magariños M, López-de-Ullibarri I, Cao R, Salas A. Evaluating the ability of tree-based methods and logistic regression for the detection of SNP-SNP interaction. Ann Hum Genet 2009; 74(Pt 3): 360–369. 10.1111/j.1469-1809.2009.00511.x [DOI] [PubMed] [Google Scholar]
- 47.Zhai R, Chen F, Liu G, Su L, Kulke MH. Interactions among genetic variants in apoptosis pathway genes, reflux symptoms, body mass index, and smoking indicate two distinct etiologic patterns of esophageal adenocarcinoma. J Clin Oncol. 2010; 28(14): 2445–2451. 10.1200/JCO.2009.26.2790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Andrew AS, Karagas MR, Nelson H H, Guarrera S, Polidoro S, Gamberini S. DNA repair polymorphisms modify bladder cancer risk: a multi-factor analytic strategy. Hum Hered. 2008; 65(2): 105–118. 10.1159/000108942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tao P, Hu YY, Huang Y, Li JY. Risk factors of breast cancer in Asian women: a meta-analysis. Zhonghua Liu Xing Bing Xue Za Zhi. 2011; 32(2): 164–169. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and Supporting Information files.