Abstract
Previously we successfully produced a group of EGFP-expressing founder transgenic pigs by a newly developed efficient and simple pig transgenesis method based on cytoplasmic injection of piggyBac plasmids. In this study, we investigated the growth and reproduction performance, and characterized the transgene insertion, transmission and expression patterns in transgenic pigs generated by piggyBac transposition. Results showed that transgene has no injurious effect on the growth and reproduction of transgenic pigs. Multiple copies of monogenic EGFP transgene were inserted at noncoding sequences of host genome, and passed from founder transgenic pigs to their transgenic offspring in segregation or linkage manner. The EGFP transgene was ubiquitously expressed in transgenic pigs, and its expression intensity was associated with transgene copy number but not related to its promoter DNA methylation level. To the best of our knowledge, this is first study that fully described the growth and reproduction performance, transgene insertion, expression and transmission profiles in transgenic pigs produced by piggyBac system. It not only demonstrates that piggyBac transposition-mediated gene transfer is an effective and favourable approach for pig transgenesis, but also provides scientific information for understanding the transgene insertion, expression and transmission patterns in transgenic animals produced by piggyBac transposition.
Keywords: Transgenic pigs, piggyBac, transposition, copy number, methylation
Introduction
The horizontal transfer of genetic material into animals known as transgenesis has been around since 1980 [1]. However, the success rate of producing transgenic animals (TAs) was low. Therefore, improvements in animal transgenesis methods have been made since 1980, to facilitate more efficient and effective generation of TAs. The first new method developed to supplement the early transgenesis approach of pronuclear microinjection (PNI) [1] was intracytoplasmic sperm injection-mediated transgenesis (ICSI-Tr) [2] which was described in 1999. This method, like PNI, is a passive transgenesis technique and its main advantage over PNI is that it is possible to obtain TAs from individuals that have fertility issues. For example, if an animal has non-motile and therefore non-viable sperm, it is still possible to produce TAs if the sperm is complexed with linear transgene DNA and then microinjected into metaphase II (MII) arrested oocytes during ICSI-Tr. The ICSI-Tr procedure also allows the microinjection of very large transgenes into oocytes [3], which proves cumbersome when attempted with traditional PNI. ICSI-Tr, like PNI transgenesis, uses the oocyte DNA repair machinery to integrate the linear transgene into the genome of the host cells [4] and hence often results in concatemerised transgene insertions, just as PNI [5]. The efficiency of both ICSI-Tr and PNI was low, in the region of at best 5% (TAs/micromanipulated oocytes or embryos). In pigs the success rate of PNI was much reduced to around 1% [6]. For ICSI-Tr in pigs the efficiency also plummeted to 0.14% in some experiments [7]. The extremely low efficiency associated PNI and ISCI-Tr has prevented the application of these techniques in generation of transgenic pigs.
A lentivirual vectors-based efficient transgenic method, which was the first active transgenesis procedure, was introduced in 2002 [8]. This method was initially developed in rodents and then extended to farm animals [9,10]. While efficient in germline transgenesis, this method is not commonly used for production of transgenic founder animals, as a large number of treated embryos (~73%) die before implantation. Use of lentiviral vectors in transgenesis also cause other issues or concerns that are well documented elsewhere [11,12].
Currently mobile genetic elements known as transposons have emerged as an alternative active transgenesis technique [13,14], which was first implemented in rodents and now applied to pigs [15,16]. The transposases currently in use for these active transgenesis techniques are piggyBac (PB) and Sleeping Beauty (SB), which are Class II cut and paste transposase enzymes [14,17,18]. Both PB and SB transposase systems are highly efficient in producing transgenic pigs at a rate around 8% (TAs/micromanipulated embryos), which is much higher than the rates of PNI and ICSI-Tr. Furthermore, it has been shown that transgenic pigs produced by SB system exhibit normal fecundity, and germline transmission of the transgene [17,19].
Previously we have firstly employed PB transposition plasmids to successfully generate transgenic pigs via the cloning technique [20]. In a recent report [16] we also produced EGFP-expressing founder transgenic pigs at a high success rate of 6% (TAs/micromanipulated embryos), by the simple cytoplasmic injection (CPI) technique in combination with efficient gene transfer method mediated by a proprietary self-inactivating PB vector pmGENIE-3, which has been proven efficient for mouse transgenesis [21,22]. In the present study we investigated the growth and reproduction performance, and characterized the profiles of transgene integration, expression and transmission in transgenic pigs produced in our previous experiments by PB transposition [16].
Materials and methods
Ethics statement
This study was carried out in strict accordance with “The Instructive Notions with Respect to Caring for Laboratory Animals”, issued by the Ministry of Science and Technology of China. The animal experimental protocol was approved by the Institutional Animal Care and Use Committee of South China Agricultural University. All efforts were made to minimize animal suffering.
Animals
The wild-type sows (Yorkshire), F0 transgenic boars (Duroc) and their wild-type littermate boars (Duroc), and F1 transgenic pigs and their wild-type littermates used in the present study were raised in a same pig farm of Guangdong Wen’s Group located in Yunfu City, Guangdong Province, China.
Measurement of growth performance
Two growth traits, including average daily gain (ADG) and feed conversion efficiency (FCE), were measured during the growth period between 30–100kg of wild-type and transgenic pigs. The pigs were raised in a pen equipped with a Feed Intake Recording Equipment system (FIRE®; Osborne. Industries, Inc., KS, USA), which can pick up the ID signal transmitted from the electronic ear tag carried by the pigs when they come to feed. The system can automatically record the feed intake, body weight increment and days of measurement of each pig. At the end of the measurement, the ADG and FCE data for each pig were calculated by the system.
Measurement of reproduction performance
The farrowing rate, total born per litter and born alive per litter of sows mated by transgenic and wild-type boars, were measured. Farrowing rate was defined as number of farrowed sows/number of mated sows. Total born per litter was defined as number of live-born piglets and stillborn piglets/number of litters. Live-born per litter was defined as number of born alive piglets/number of litters.
Artificial insemination
Semen was collected from transgenic and wild-type boars at the age of 10 months, and diluted into multiple doses. Each dose contains 3 billion spermatozoa in 80 mL of semen extender. Sows showing sign of spontaneous estrus were artificially inseminated 3 times with 3 doses of semen, and with an interval of 12 h between each insemination.
Observation of EGFP expression in transgenic pigs and their organs
Transgenic pigs and their organs or tissues expressing EGFP were visualized by the Living Organism’s Fluorescent Protein Observation System, consisting of a blue light lamp with maximum excitation at 488 nm and goggles with light filters (Model: FBL, BLS Ltd., Hungary). Photographs of transgenic pigs, wild-type pigs, and their organs and tissues were taken under blue light or bright light by a camera equipped with and without light filters.
Analysis of EGFP expression in spermatids
F0 transgenic boars and wild-type boars were sacrificed at the age of 12 months. Their testis tissues were quickly removed, fixed in 4% paraformaldehyde, and embedded in paraffin. Embedded tissues were cut into sections and stained with hematoxylin and eosin. Stained sections were analyzed under fluorescence microscopy and photos were taken by a camera connected to the microscope.
Southern blot analysis
The Southern blot was performed as previously described [16]. Briefly: ear genomic DNA (10 μg) from each piglet was restriction digested with Hind III, purified by ethanol precipitation, and then separated by electrophoresis in a 0.80% agarose gel. The DNA was subsequently transferred to a nylon membrane (GE Life sciences, Shanghai, China) by the capillary transfer method. The membrane was prehybridized for 1 h and then hybridized overnight at 55°C with an EGFP gene probe labeled with DIG by using a PCR DIG Probe Synthesis Kit (Roche Applied Science, China). Hybridization and washing were performed with DIG-High Prime DNA Labeling and Detection Starter Kit II (Roche Applied Science, China). After hybridization, the membrane was incubated for 30 minutes in blocking solution and subsequently incubated for 30 minutes in anti-DIG-AP antibody solution. The membrane was next treated for 5–20 minutes following incubation with 1 ml of CSPD ready-to-use solution, and a Southern blot photograph was captured with an EC3 imaging system (UPA, CA, USA)
Inverse PCR analysis
One microgram of genomic DNA from TG7 boar was digested with Hind III. The digestion product was purified by a DNA purification column (Qiagen) and eluted with 100 μL of ddH2O. After adjustment with ddH2O and T4 ligase buffer to a finally required volume prior to ligase enzyme addition, T4 ligase was added with a final concentration of 10 U/μL to the 1000 μL final ligation mixture. The ligation reaction was allowed to proceed overnight by incubation at 16°C and ligated DNA was purified via a Qiagen DNA purification column, and 100 μL of ddH2O was used to elute the purified DNA from the column. A 2 μL elution aliquot was used as template for the PCR reaction, with primer sets: P13+P14 (for primer sequences see Li et al., 2014 [16]). The resulting PCR products were cloned by ligation into a TA vector (Life Technologies, Carlsbad, CA, U.S.A) and then sequenced. The obtained sequences were blasted against the sequence of the transgene vector pmGENIE-3-EGFP and the Sus scrofa (pig) genomic DNA sequence database (Build Sscrofa10.2) on NCBI BLAST website to find out the integration sites of the PB transposon.
Promoter DNA methylation analysis
The CAG promoter sequence of EGFP transgene was submitted to the website (http://cpgislands.usc.edu) for CpG islands search. Primer sequences for amplification of a selected CpG island were designed at website (http://www.urogene.org/methprimer). Genomic DNA was extracted from tissues by using E.Z.N.A. ® Tissue DNA Kit (Omega Bio-Tek). Extracted DNA was eluted into 200 μl elution buffer, and its concentration was measured by using NanoDrop 2000 (Thermo). Approximately 500ng purified genomic DNA was treated with sodium bisulfite to convert all unmethylated cytosine into uracil by using EZ DNA Methylation-Gold™ Kit (Zymo Research, Orange, CA) according to manufacturer’s recommendations. Bisulfite modified DNAs were amplified by PCR with primers (Forward: 5′-ATACATAACCCATATTACAATCC-3′; Reverse: 5′-TTAATAATTGATTAATAATTATTATTAGTT -3′. Nested PCRs were run by using HotStarTaq plus DNA polymerase (Qiagen) with 25~30 cycles for the first amplification reaction and 45 cycles for the second amplification reaction. The amplified PCR products were verified by electrophoresis on 3% agarose gels and then purified by using E.Z.N.A.® Gel Extraction Kit (Omega Bio-Tek). Purified PCR products were cloned into TA cloning vector pTZ57R/T (Fermentas). Positive colonies were confirmed by colony PCR and sent for sequencing. Sequences were analyzed by local BiQ Analyzer software and bead-diagram was plotted on the web site at http://biq-analyzer.bioinf.mpi-inf.mpg.de/tools/MethylationDiagrams/index.php.
Statistical analysis
All the data were analyzed using SPSS version 17 software (SPSS Inc., Chicago, IL, USA). T-test was used to compare difference in ADG, FCE and litter size between two groups, while chi-square analysis followed by Fisher’s exact test was used to determine difference in farrowing rate between the two groups.
Results
Comparison of growth and reproduction performance between transgenic and wild-type pigs
To investigate whether transgenic expression of EGFP gene integrated by PB transposition affects the growth performance of transgenic pigs, the average daily gain (ADG) and feed conversion efficiency (FCE) of four wild-type boars, and two EGFP-expressing transgenic littermate boars (TG7 and TG8 in reference [16]) generated by cytoplasmic injection of pmGENIE-3 constructs were measured. The results (Table 1) indicated that founder (F0) transgenic boars and their wild-type littermate boars have no significant (P>0.05) difference in ADG as well as FCE. Furthermore, the growth traits between transgenic and wide-type offspring of TG7 and TG8 also were compared. The results (Table 2) showed that F1 transgenic pigs and their wild-type littermates also exhibit similar performance in ADG and FCE. This suggests that the insertion and expression of the EGFP transgene did not affect the growth of transgenic pigs.
Table 1.
Comparison of growth performance between F0 transgenic boars produced by piggyBac based pmGENIE-3 transposition and their wild-type littermate boars.
| Growth performance | Transgenic boars (TG7 and TG8) | Wild-type boars (n=4, Mean±SEM) |
|---|---|---|
| ADG (kg) | 0.720 and 0.783 | 0.778±0.024 |
| FCE (kg/kg) | 3.159 and 2.468 | 2.702±0.213 |
The four wild-type Duroc boars that are used as controls were produced in our previous study (Li et al. 2014), they are littermates of TG7 and TG8, and should have similar genetic background with TG7 and TG8. Wild-type boars, TG7 and TG8 boars were raised under the same condition in the same pig farm. ADG=average daily gain (kg), FCE=feed conversion efficiency (kg of feed intake/kg of body weight increment). Two group’s values on the same row are not statistically (P>0.05) different from each other.
Table 2.
Comparison of growth performance between F1 transgenic pigs and their wild-type littermates.
| Growth performance | F1 Transgenic pigs (n=21) | F1 Wild-type littermates (n=9) |
|---|---|---|
| ADG (kg) (Mean±SEM) | 0.786±0.022 | 0.806±0.026 |
| FCE (kg/kg) (Mean±SEM) | 2.768±0.058 | 2.788±0.050 |
Transgenic and wild-type pigs were raised under the same condition in a same pig farm. ADG=average daily gain (kg), FCE=feed conversion efficiency (kg of feed intake/kg of body weight increment). Values on the same row are not statistically (P>0.05) different from each other.
To investigate whether the transgenic boars and wild-type littermate boars are different in fecundity, they were mated to wild-type sows by artificial insemination. The results (Table 3) indicated that the farrowing rate, total born per litter, and born alive per litter were not significantly different (P>0.05) between the two groups of sows mated by transgenic boars and wild-type boars. This suggests that integration and expression of the EGFP transgene did not change the reproduction performance of these genetically modified boars.
Table 3.
Comparison of reproduction performance between F0 transgenic boars produced by piggyBac based pmGENIE-3 transposition and their wild-type littermate boars.
| Reproduction performance | Sows mated by 2 transgenic boars (TG7 and TG8) | Sows mated by 4 wild-type boars |
|---|---|---|
| Farrowing rate | 100% (= 4/4) | 90.05% (= 19/21) |
| Total born per litter (Mean±SEM) | 10.50±1.32 | 10.926±0.864 |
| Born alive per litter (Mean±SEM) | 10.25±1.22 | 10.342±0.771 |
Transgenic boars and wild-type boars were mated to wild-type sows with similar genetic background by the same artificial insemination protocol and raised under the same condition in the same pig farm. Values on the same row are not significantly (P>0.05) different from each other.
Germline transmission of EGFP transgene in transgenic pigs produced by piggyBac transposition
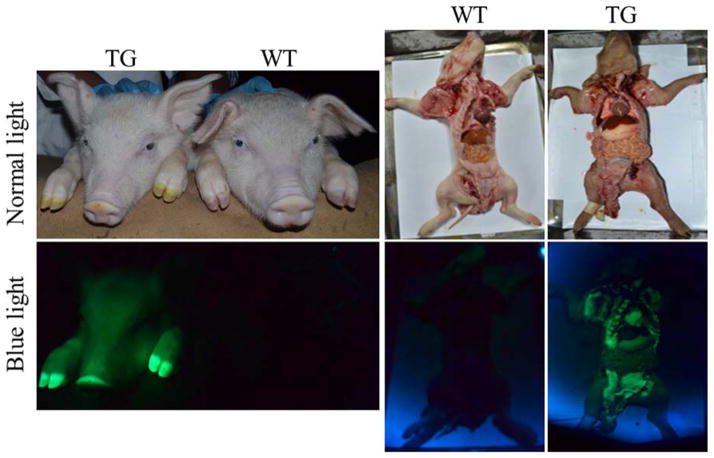
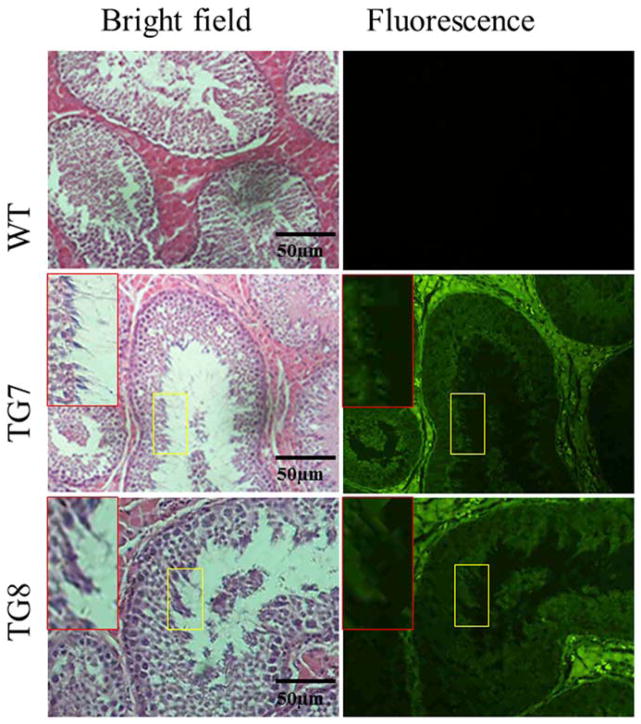
As shown in Table 4, four sows mated by transgenic founder boars delivered in total 42 piglets, of which 31(73.8%) were expressing EGFP that was observable on their bodies and their internal organs under blue light excitation (Fig. 1). This validates that the EGFP transgene was successfully transmitted from founder transgenic boars to their progeny. However, spermatozoa in semen collected from the two founder transgenic boars did not demonstrate EGFP expression under fluorescence excitation (data not shown), which might be due to the transcriptionally silent characteristic of mature sperm [23,24]. To test this point, testis sections of the two founder transgenic boars were used to analyze whether EGFP is expressed in the premature sperm cells, the spermatids, which usually are active in transcription and translation [23,24]. The results indicate that EGFP is expressed in transgenic founder boars’ spermatids (Fig. 2). This observation demonstrates that the EGFP transgene was inherited from transgenic founder boars to their offspring by germline transmission.
Table 4.
Germline transmission of the EGFP transgene in transgenic pigs produced by pmGENIE-3 transposition.
| ID. of F0 TG boars | ID. of WT sows | No. of born F1 piglets | Male/female | No. of EGFP+ piglets (%) | EGFP+ male/EGFP+ female |
|---|---|---|---|---|---|
| TG7 | A | 12 | 6/6 | 11 (91.7%) | 5/6 |
| B | 13 | 5/8 | 8 (61.5%) | 4/4 | |
| Subtotal | 25 | 11/14 | 19 (76.0%) | 9/10 | |
| TG8 | C | 7 | 3/4 | 6 (85.7%) | 3/3 |
| D | 10 | 6/4 | 6 (60.0%) | 3/3 | |
| Subtotal | 17 | 9/8 | 12 (70.6%) | 6/6 | |
| Total | 42 | 20/22 | 31 (73.8%) | 15/16 |
EGFP+ represents piglets which are expressing EGFP by epifluorescence observable under blue light excitation.
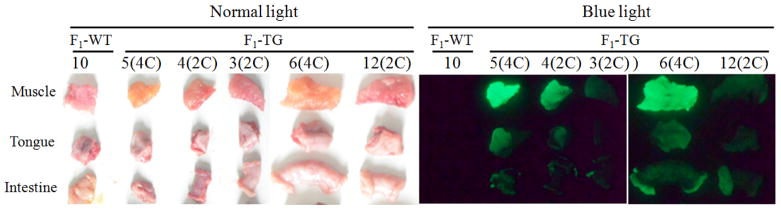
Figure 1.
EGFP transgene expression in F1 transgenic piglets and their internal organs and tissues.
Figure 2.
Analysis of EGFP transgene expression in spermatids of F0 transgenic boars. Red Box represent spermatids inside the yellow square frame zoomed out to a larger magnification.
Transmission of the monogenic piggyBac transposon from F0 to F1 transgenic pigs by segregation or linkage manner
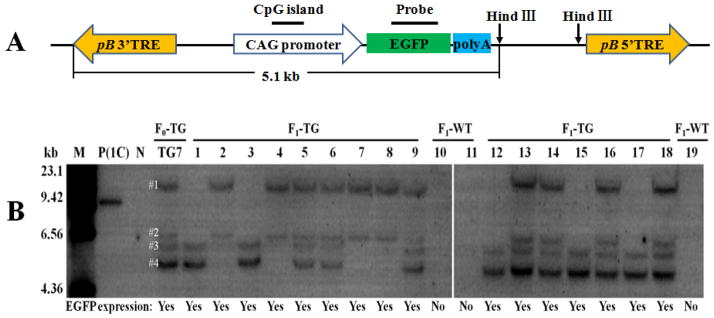
The Southern blot analysis result (Fig. 3) not only confirmed that the EGFP-expressing F1 piglets carry the transgene inherited from their fathers, but also indicated that the integrated transposons did not change their genomic location when they were transmitted from F0 transgenic pigs to F1 transgenic offspring, because the size of EGFP probe-reactive bands shown in F1 transgenic pigs is the same as that of corresponding bands shown in TG7. This suggests that no genomic insertion of an active PB transposase gene had occurred in transgenic founder TG7’s genome, and no endogenous PB-like transposase gene is present in the porcine genome, both of which will result in translocation of the transposon (transgene). Furthermore, Fig. 3 demonstrated that the four copies of monogenic PB transposons integrated in the genome of transgenic founder TG7 were transmitted to its transgenic progeny according to segregation and linkage rules. The #1 and #2 copies of transposons were always passed together to the transgenic progeny of founder TG7, as were the #3 and #4 copies of transposons (Fig. 3). Therefore, it is probable that #1 and #2 copies of transgenes were inserted in nearby loci of a same chromosome, while #3 and #4 copies of the transgene were inserted in another chromosome of transgenic founder TG7, and were linked together to be transmitted to next generation. To test this hypothesis, the transgene insertion sites in the genome of founder TG7 were detected by inverse PCR. As shown in Fig. 4, two copies of transgene were inserted on chromosome 3 while the other two copies of transgene were integrated on chromosome 7 of TG7 founder pig. This result confirms our hypothesis that two copies of transgenes were located on one chromosome while the other two copies of transgenes were linked on another chromosome in the genome of TG7 founder.
Figure 3.
Southern blot analysis of F0 transgenic boar TG7 and its F1 offspring’s genomic DNA. A. The location of probe and enzyme digestion sites for southern blot analysis in the transgene plasmid. pB 3′TRE, piggyBac transposon 3′ terminal repeat element. pB5′TREB, piggyBac transposon 5′ terminal repeat element. B. Southern blot analysis results. M=Marker; P(1C)=Positive control carrying 1 copy of EGFP gene, which was prepared by mixing 10μg of wild-type pig’s genomic DNA with 2.6 ×10−5 μg of a 9.0kb plasmid DNA fragment containing a EGFP gene; N=Negative control (Wild-type pig); TG=transgenic; WT=Wild-type; The F0 transgenic boar TG7 carries 4 copies (#1–#4) of EGFP transgene/piggyBac transposon.
Figure 4.
Identification of transposon (transgene) insertion sites in the genome of TG7 founder transgenic pig. A. Blast result and sequencing result of the first insertion site, which was at the non-coding intergenic sequences between nuclear receptor coactivator 1gene and collagen alpha-1 (I) chain-like gene on chromosome 3. B. Blast result and sequencing result of the second insertion site, which was at the non-coding intergenic sequences between two individual copies of beta-1,3-N-acetylglucosaminyltransferase lunatic fringe isoform X2 gene on chromosome 3. C. Blast result and sequencing result of the third insertion site, which was at the noncoding 8th intron of methymalonyl-CoA mutase gene on chromosome 7. D. Blast result and sequencing result of the fourth insertion site, which was at the non-coding intergenic sequences between signal sequence receptor alpha gene and cancer-associated gene 1 protein gene on chromosome 7. The PB 3′-TRE sites are flanked by the chromosomal sequences where the transposon inserted. The TTAA sequence on the border signifies transpositional integration of the PB transposon.
Luckily, the blast results indicated that founder TG7 carries 3 copies of transgene inserted at the noncoding intergenic sequences and 1 copy of transgene integrated at the noncoding 8th intron of the methymalonyl-CoA mutase (MCM) gene (Fig. 4), and founder TG8 also carries 3 copies of transgenes that were integrated at 3 noncoding intergenic regions by monogenic pattern (Figure 5). Studies have shown that functional mutation in the MCM gene will cause methylmalonic aciduria disorder (MMA) in human and mouse [25]. To investigate whether the transgene insertion at the 8th intron of MCM gene will cause MMA in transgenic pigs, we measured the urinary methylmalonic acid level, which is the diagnosis index for MMA, in 4 wild-type pigs and 4 transgenic pigs carrying a mutated MCM gene with EGFP insertion at its 8th intron. However, wild-type pigs and transgenic pigs showed no significant (P>0.05) difference in urinary methylmalonic acid level (data not shown). We also measured the MCM mRNA expression level in the kidney and liver tissues of 3 wild-type pigs and 3 transgenic pigs carrying the mutant MCM gene, but no significant (P>0.05) difference in MCM gene expression was found between transgenic pigs and their wild-type littermates (data not shown). These results suggest that transgene insertion at the noncoding 8th intron did not affect the expression and function of MCM gene in transgenic pigs.
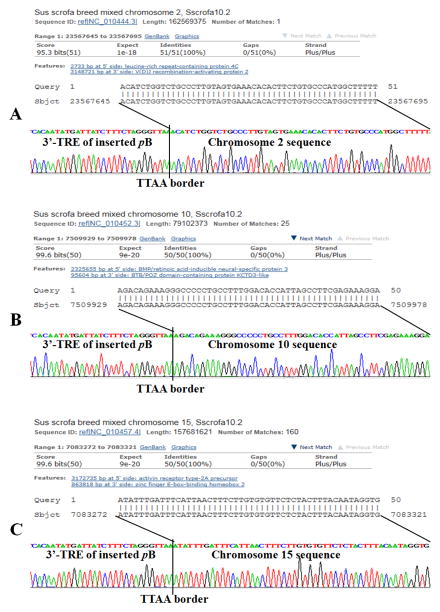
Figure 5.
Identification of transposon (transgene) insertion sites in the genome of TG8 founder transgenic pig. A. Blast result and sequencing result of the first insertion site, which was at the non-coding intergenic sequences between the leucine-rich repeat-containing protein 4C gene and the V(D)J recombination-activating protein 2 gene on chromosome 2. B. Blast result and sequencing result of the second insertion site, which was at the non-coding intergenic sequences between the BMP/retinoic acid-inducible neural-specific protein 3 gene and the BTB/POZ domain-containing protein KCTD3-like gene on chromosome 10. C. Blast result and sequencing result of the third insertion site, which was at the noncoding intergenic sequences between the activin receptor type-2A precursor gene and the zinc finger E-box-binding homeobox 2 gene on chromosome 15. The PB 3′-TRE sites are flanked by the chromosomal sequences where the transposon inserted. The TTAA sequence on the border signifies transpositional integration of the PB transposon.
Correlation between transgene expression level and transgene copy number or transgene’s promoter DNA methylation level
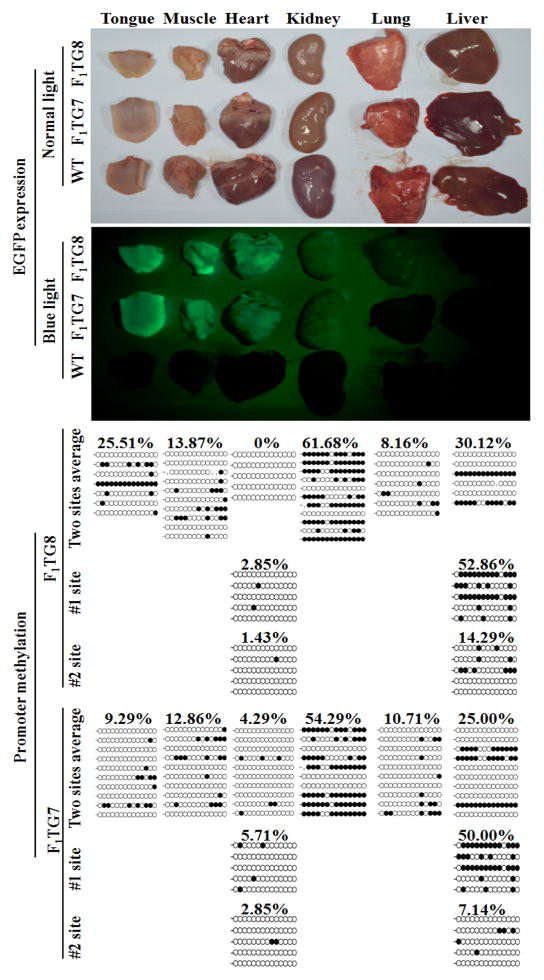
Although all F1 transgenic piglets expressed observable EGFP on their bodies and tissues, the intensity of the fluorescence chromophore seemed to have a positive correlation with transgene copy number. For example, F1 transgenic pigs number 5 and number 6 carrying four copies (4C) of transgene displayed stronger EGFP expression than number 4, number 3 and number 12 F1 transgenic pigs, all of which carry only two copies (2C) of transgene (Fig. 6). Although most of the F1 transgenic pig’s organs expressed EGFP, they expressed various level of EGFP (Fig. 7). To investigate whether the EGFP transgene expression level is related to its promoter DNA methylation degree, six different tissues (including tongue, muscle, heart, kidney, lung and liver) expressing different EGFP expression levels were obtained from two F1 transgenic pigs. Genomic DNA was extracted from these individual tissues for analysis of DNA methylation levels of the CAG promoter driving EGFP transgene expresion. The results (Fig. 7) demonstrated that the CAG promoter DNA methylation level is low in the lung tissue (8.16–10.71%) expressing very weak EGFP, while the CAG promoter is hypermethylated in the kidney tissue (54.29–61.68%) expressing moderate level of EGFP. This suggests that EGFP transgene expression level is not associated with the DNA methylation degree of its promoter.
Figure 6.
Association of expression intensity of EGFP transgene and its copy number in F1 transgenic pigs. The number 5 and number 6 F1 transgenic pigs carry 4 copies (4C) of EGFP transgene, while number 4, number 3 and number 12 F1 transgenic pigs carry 2 copies (2C) of EGFP transgene, as displayed in Figure 3.
Figure 7.
Association of expression intensity of EGFP transgene and its CAG promoter methylation in F1 transgenic pigs. #1 site and #2 site are the two integration sites on chromosome 3 (see Fig. 4A and 4B), which were analyzed by Southern blot and shown in Fig. 3.
The CAG promoter DNA methylation level in each organ showed in Figure 7 actually represents the average DNA methylation level of two copies of CAG promoter integrated at two different sites (#1 site and #2 site, see Fig. 3) in the genome of TG7 and TG8 of F1 generation. These two copies of CAG promoter might be different in DNA methylation level due to influence from integration locus. To test this hypothesis, we measured #1 site’s and #2 site’s CAG promoter methylation degree in two different tissues of F1-TG7 and F1-TG8 by using integration site-specific PCR primers. The results (Fig. 7) indicated that in the heart tissue the CAG promoter methylation levels at #1 site and #2 site are similar, yet in the liver tissue, #1 site’s CAG promoter methylation level is much higher than that at #2 site. This suggests that the DNA methylation degree of inserted CAG promoter could be affected by integration site.
Discussion
The genetic engineering of livestock is a costly and difficult task. It has been proven inefficient and expensive when attempted with passive transgenesis techniques in the past. The recent advances in genomic manipulation with transposase enzymes [15,16] and endonucleases such as clustered regularly interspaced short palindromic repeat (CRISPR)/Cas9 nuclease [26] promise better outcomes for the efficient production of transgenic livestock.
In our experiments we have demonstrated that pmGENIE-3 plasmids containing a 10.5 kb transposon can contribute to the generation of transgenic founder pigs (F0) by active transgenesis, at higher efficiencies than that reported with traditional transgenic methods [16]. The monogenic transgenes observed in the F0 founders were still active when hemizygous lines (F1) were created by breeding F0 transgenic male pigs to wild type females, with no observation of hopping, silencing or variegated expression of transgene. There was no reduction in growth and reproduction performance when transgenic pigs were compared with their wild type littermates. Our results demonstrate that PB transposase-catalyzed integration of foreign gene into the expression permissive loci of porcine genome is a viable approach for production of genetically modified pigs.
Through inverse PCR, we were able to identify the specific chromosomal locations of all inserted copies of transgene in founder TG7’s and TG8’s genome. The results demonstrated that all monogenic PB transposons were integrated at noncoding DNA sequences. This seems to imply that PB transposon prefers to insert at noncoding regions of mammalian genome. Yet this in fact may result from the “preselection” during production of founder transgenic pigs, since functional mutation caused by PB insertion at coding sequences may has injurious effects on transgenic pig development and usually only those transgenic founder pigs carrying foreign gene inserted at noncoding region will be born and survive.
Our data showed that the EGFP transgene expression level differs among different transgenic pigs and it is positively correlated with the copy number of transgene. This result is consistent with that reported by others [17,27]. However, a study [27] demonstrated that different tissues from the same transgenic pig express various level of EGFP, which has significant negative correlation with the transgene promoter methylation degree. Similar result was not found in the present study. Since other types of epigenetic modification such as histone methylation and acetylation also can affect gene expression, the variation of EGFP transgene expression level among different tissues from a same transgenic pig could be caused by histone modification rather than DNA methylation.
Conclusions
To the best of our knowledge, this is first study that fully characterized the growth and reproduction traits, transgene insertion, expression and transmission profiles in transgenic pigs produced by piggyBac transposition. The herein demonstrated normal growth and reproduction performance, harmless genomic insertion, stable transmission and expression of transgene in transgenic pigs generated with the PB transposase, together with the ability of the PB, Tol2 and SB transposases in integrating large size of bacterial artificial chromosome (BAC) constructs into TAs genomes [28–30], makes the transpositional “active transgenesis” an exciting method for production of TAs.
Acknowledgments
This work was supported by grants from Department of Science and Technology of Guangdong (grant numbers: 2011B060400029, 2014A030310500, 2014A030313464) and a National Institutes of Health Grant 2P20GM103457-06A1 to S.M.
References
- 1.Gordon JW, Scangos GA, Plotkin DJ, Barbosa JA, Ruddle FH. Genetic transformation of mouse embryos by microinjection of purified DNA. Proc Natl Acad Sci U S A. 1980;77:7380–7384. doi: 10.1073/pnas.77.12.7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perry AC, Wakayama T, Kishikawa H, Kasai T, Okabe M, Toyoda Y, Yanagimachi R. Mammalian transgenesis by intracytoplasmic sperm injection. Science. 1999;284:1180–1183. doi: 10.1126/science.284.5417.1180. [DOI] [PubMed] [Google Scholar]
- 3.Moreira PN, Pozueta J, Giraldo P, Gutierrez-Adan A, Montoliu L. Generation of yeast artificial chromosome transgenic mice by intracytoplasmic sperm injection. Methods Mol Biol. 2006;349:151–161. doi: 10.1385/1-59745-158-4:151. [DOI] [PubMed] [Google Scholar]
- 4.Perry AC. Hijacking oocyte DNA repair machinery in transgenesis? Mol Reprod Dev. 2000;56:319–324. doi: 10.1002/(SICI)1098-2795(200006)56:2+<319::AID-MRD24>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 5.Smith K. Theoretical mechanisms in targeted and random integration of transgene DNA. Reprod Nutr Dev. 2001;41:465–485. doi: 10.1051/rnd:2001102. [DOI] [PubMed] [Google Scholar]
- 6.Uchida M, Shimatsu Y, Onoe K, Matsuyama N, Niki R, Ikeda JE, Imai H. Production of transgenic miniature pigs by pronuclear microinjection. Transgenic Res. 2001;10:577–582. doi: 10.1023/a:1013059917280. [DOI] [PubMed] [Google Scholar]
- 7.Kurome M, Ueda H, Tomii R, Naruse K, Nagashima H. Production of transgenic-clone pigs by the combination of ICSI-mediated gene transfer with somatic cell nuclear transfer. Transgenic Res. 2006;15:229–240. doi: 10.1007/s11248-006-0004-5. [DOI] [PubMed] [Google Scholar]
- 8.Lois C, Hong EJ, Pease S, Brown EJ, Baltimore D. Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science. 2002;295:868–872. doi: 10.1126/science.1067081. [DOI] [PubMed] [Google Scholar]
- 9.Hofmann A, Kessler B, Ewerling S, Weppert M, Vogg B, Ludwig H, Stojkovic M, Boelhauve M, Brem G, Wolf E, Pfeifer A. Efficient transgenesis in farm animals by lentiviral vectors. EMBO Rep. 2003;4:1054–1060. doi: 10.1038/sj.embor.7400007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hofmann A, Zakhartchenko V, Weppert M, Sebald H, Wenigerkind H, Brem G, Wolf E, Pfeifer A. Generation of transgenic cattle by lentiviral gene transfer into oocytes. Biol Reprod. 2004;71:405–409. doi: 10.1095/biolreprod.104.028472. [DOI] [PubMed] [Google Scholar]
- 11.Page DC, Silber S, Brown LG. Men with infertility caused by AZFc deletion can produce sons by intracytoplasmic sperm injection, but are likely to transmit the deletion and infertility. Hum Reprod. 1999;14:1722–1726. doi: 10.1093/humrep/14.7.1722. [DOI] [PubMed] [Google Scholar]
- 12.Park F. Lentiviral vectors: are they the future of animal transgenesis? Physiol Genomics. 2007;31:159–173. doi: 10.1152/physiolgenomics.00069.2007. [DOI] [PubMed] [Google Scholar]
- 13.Katter K, Geurts AM, Hoffmann O, Mates L, Landa V, Hiripi L, Moreno C, Lazar J, Bashir S, Zidek V, Popova E, Jerchow B, Becker K, Devaraj A, Walter I, Grzybowksi M, Corbett M, Filho AR, Hodges MR, Bader M, Ivics Z, Jacob HJ, Pravenec M, Bosze Z, Rulicke T, Izsvak Z. Transposon-mediated transgenesis, transgenic rescue, and tissue-specific gene expression in rodents and rabbits. FASEB J. 2013;27:930–941. doi: 10.1096/fj.12-205526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marh J, Stoytcheva Z, Urschitz J, Sugawara A, Yamashiro H, Owens JB, Stoytchev I, Pelczar P, Yanagimachi R, Moisyadi S. Hyperactive self-inactivating piggyBac for transposase-enhanced pronuclear microinjection transgenesis. Proc Natl Acad Sci U S A. 2012;109:19184–19189. doi: 10.1073/pnas.1216473109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garrels W, Mates L, Holler S, Dalda A, Taylor U, Petersen B, Niemann H, Izsvak Z, Ivics Z, Kues WA. Germline transgenic pigs by Sleeping Beauty transposition in porcine zygotes and targeted integration in the pig genome. PLoS One. 2011;6:e23573. doi: 10.1371/journal.pone.0023573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Z, Zeng F, Meng F, Xu Z, Zhang X, Huang X, Tang F, Gao W, Shi J, He X, Liu D, Wang C, Urschitz J, Moisyadi S, Wu Z. Generation of transgenic pigs by cytoplasmic injection of piggyBac transposase-based pmGENIE-3 plasmids. Biol Reprod. 2014;90:93. doi: 10.1095/biolreprod.113.116905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garrels W, Holler S, Cleve N, Niemann H, Ivics Z, Kues WA. Assessment of fecundity and germ line transmission in two transgenic pig lines produced by sleeping beauty transposition. Genes (Basel) 2012;3:615–633. doi: 10.3390/genes3040615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Urschitz J, Moisyadi S. Transpositional transgenesis with piggyBac. Mob Genet Elements. 2013;3:e25167. doi: 10.4161/mge.25167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ivics Z, Garrels W, Mates L, Yau TY, Bashir S, Zidek V, Landa V, Geurts A, Pravenec M, Rulicke T, Kues WA, Izsvak Z. Germline transgenesis in pigs by cytoplasmic microinjection of Sleeping Beauty transposons. Nat Protoc. 2014;9:810–827. doi: 10.1038/nprot.2014.010. [DOI] [PubMed] [Google Scholar]
- 20.Wu Z, Xu Z, Zou X, Zeng F, Shi J, Liu D, Urschitz J, Moisyadi S, Li Z. Pig transgenesis by piggyBac transposition in combination with somatic cell nuclear transfer. Transgenic Res. 2013;22:1107–1118. doi: 10.1007/s11248-013-9729-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Urschitz J, Kawasumi M, Owens J, Morozumi K, Yamashiro H, Stoytchev I, Marh J, Dee JA, Kawamoto K, Coates CJ, Kaminski JM, Pelczar P, Yanagimachi R, Moisyadi S. Helper-independent piggyBac plasmids for gene delivery approaches: strategies for avoiding potential genotoxic effects. Proc Natl Acad Sci U S A. 2010;107:8117–8122. doi: 10.1073/pnas.1003674107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marh J, Stoytcheva Z, Urschitz J, Sugawara A, Yamashiro H, Owens JB, Stoytchev I, Pelczar P, Yanagimachi R, Moisyadi S. Hyperactive self-inactivating piggyBac for transposase-enhanced pronuclear microinjection transgenesis. Proc Natl Acad Sci U S A. 2012;109:19184–19189. doi: 10.1073/pnas.1216473109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steger K. Haploid spermatids exhibit translationally repressed mRNAs. Anat Embryol (Berl) 2001;203:323–334. doi: 10.1007/s004290100176. [DOI] [PubMed] [Google Scholar]
- 24.Ward WS, Zalensky AO. The unique, complex organization of the transcriptionally silent sperm chromatin. Crit Rev Eukaryot Gene Expr. 1996;6:139–147. doi: 10.1615/critreveukargeneexpr.v6.i2-3.30. [DOI] [PubMed] [Google Scholar]
- 25.Peters HL, Pitt JJ, Wood LR, Hamilton NJ, Sarsero JP, Buck NE. Mouse models for methylmalonic aciduria. PLoS One. 2012;7:e40609. doi: 10.1371/journal.pone.0040609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang D, Xu J, Zhu T, Fan J, Lai L, Zhang J, Chen YE. Effective gene targeting in rabbits using RNA-guided Cas9 nucleases. J Mol Cell Biol. 2014;6:97–99. doi: 10.1093/jmcb/mjt047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kong Q, Wu M, Huan Y, Zhang L, Liu H, Bou G, Luo Y, Mu Y, Liu Z. Transgene expression is associated with copy number and cytomegalovirus promoter methylation in transgenic pigs. PLoS One. 2009;4:e6679. doi: 10.1371/journal.pone.0006679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rostovskaya M, Fu J, Obst M, Baer I, Weidlich S, Wang H, Smith AJ, Anastassiadis K, Stewart AF. Transposon-mediated BAC transgenesis in human ES cells. Nucleic Acids Res. 2012;40:e150. doi: 10.1093/nar/gks643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suster ML, Abe G, Schouw A, Kawakami K. Transposon-mediated BAC transgenesis in zebrafish. Nat Protoc. 2011;6:1998–2021. doi: 10.1038/nprot.2011.416. [DOI] [PubMed] [Google Scholar]
- 30.Zhao L, Ng ET, Koopman P. A piggyBac transposon- and gateway-enhanced system for efficient BAC transgenesis. Dev Dyn. 2014;243:1086–1094. doi: 10.1002/dvdy.24153. [DOI] [PubMed] [Google Scholar]