Abstract
Purpose
The purpose of this study was to examine the effect of an oncologists’ exercise recommendation with and without exercise motivation package on the amount of exercise participation and quality of life (QOL) in breast and colon cancer survivors.
Methods
A total of 162 early stage breast and colorectal cancer survivors who completed primary and adjuvant treatments were recruited for this study. Participants were randomly assigned into one of three groups: 1) control (N=59), 2) Oncologists’ exercise recommendation (N=53), and 3) Oncologists’ exercise recommendation with exercise motivation package (N=50). At baseline and after 4 weeks, the level of exercise participation and QOL were assessed.
Results
A total of 130 (80.7%) participants completed the 4-week assessment. The result showed that participants who only received oncologists’ exercise recommendation did not increase their exercise participation level. But participants who received oncologist’s exercise recommendation with motivation package significantly increased the level of exercise participation [4.30±7.84 Metabolic Equivalent of Task (MET) hour per week, p<001] compared with that of the control group and significantly improved role functioning, pain and diarrhea.
Conclusion
Oncologists’ exercise recommendation may not be enough to increase exercise participation.. Exercise motivation package with oncologists’ exercise recommendation may be ideal to increase exercise participation to cancer survivor
Implications of cancer survivors
The providence of exercise motivation package in addition to oncologists’ exercise recommendation to increase the level of exercise among breast and colorectal cancer survivors should be considered.
Keywords: Physical activity recommendations, physical activity, quality of life, physical activity information package, oncologist, cancer
INTRODUCTION
Exercise and physical activity participation have been associated with reduced cancer-specific and all-cause mortality in breast and colorectal cancer survivors [1, 2]. Furthermore, PA participation is associated with better quality of life and psychological health of cancer survivors [1, 3–5]. Despite such evidence, more than two third of cancer patients are insufficiently active [6, 7]. Thus, promotion of health-enhancing PA in cancer survivor is one of the key components of cancer treatments.
Several exercise programs have been developed and tested for their safety and efficacy for cancer patients [8–15]. The goal of these programs is 1) to improve quality of life, 2) to alleviate the symptoms including fatigue, pain, and depression and 3) to improve fitness and surrogate markers which associated with patient survival. To encourage cancer patients to participate in PA and exercise, patient counseling is crucial factors [12, 16]. Oncologists, who are the most influential for cancer patients’ health-related decision-making [17], can significantly influence and increase a cancer patient’s participation in PA[18]. Several studies have indicated that healthcare sector (including the recommendations of oncologists, exercise advices, and exercise educations) has successfully motivated patients to increase their PA amounts especially when combined with telephone or community support [19, 20]. Jones et al. [18] found that an oncologist’s 30 second verbal exercise recommendation significantly increased the level of execise among cancer patients by 3.4 Metabolic Equivalent of Task (MET) per week. We have also recently found in a pilot study that the amount of exercise was significantly increased when stage 2–3 colorectal cancer survivors were given simple exercise advice, pedometer, and exercise diary [14]. Furthermore, Vallance et al. [11] also reported that providence of printed material on physical activity and pedometer is effective tool to increase the level of physical activity among breast cancer survivors. However, there is limited number of randomized controlled trials which examine whether oncologists’ exercise recommendation only or oncologists’ exercise recommendation which combined with motivation package such as exercise education, printed material, pedometer, and exercise diary would increase the level of exercise.
Several related studies have reported that the exercise interventions have improved quality of life for cancer survivors [21, 22]. In a recent meta-analysis, exercise interventions were shown to improve the quality of life for breast cancer survivors [22], and the authors recommended that exercise intervention should become targeted practice for improving patients’ quality of life. However, there are limited number studies that have examined the effect of increased exercise level on exercise interventions, consisting of oncologists’ exercise recommendations and exercise recommendations with exercise motivation package on the amount of exercise and quality of life for cancer survivors. There is also a need to determine which implementation strategies are most effective as exercise interventions for cancer survivors.
Therefore, the purposes of this study are to examine the effects of the 1) oncologists’ exercise recommendations and 2) oncologists’ exercise recommendations with exercise motivation package on the amount of self-reported exercise and the quality of life among stage I–III breast and colorectal cancer survivors.
METHODS
Study design
This study was a single-blind, three-armed, randomized controlled trial. Potential participants were screened for eligibility via a medical record review before their arrival at the clinic. Upon arrival at the clinic, oncologists asked patients if they were willing to participate in a study and participants who agreed to participate in the study were randomly assigned to one of three groups (control, oncologists’ exercise recommendations and, oncologists’ exercise recommendations with exercise motivation package). The research coordinator explained the study in detail and obtained a written consent. Each participant completed exercise and quality of life questionnaires at baseline and after four weeks. This research was conducted at Shinchon Severance Hospital Cancer Clinic, in Seoul, Korea. The study was approved by the Institutional Review Board of the Yonsei University College of Medicine.
Randomization
The simple randomization method was employed by drawing lot. The person who coordinated randomization process was not involved in any of the screening and outcome assessments.
Sample size
We calculated the required sample size from the previously published study using the breast cancer survivors during and after adjuvant therapy as the primary outcome on 450 subjects. A required n = 180 was obtained by applying Cohen’s formula for an expected Effect Size (ES) of 0.95 and an alpha of 0.05, powered at 0.95, using the G*Power program.
Participants and procedure of the study
The inclusion criteria was aged over 18 years old, completed primary and adjuvant treatments for colorectal and breast cancer (stage I, II, III) (within 36months after curative surgery), able to read and speak Korean. Participants who had any of the following characteristics or disorders were excluded from the study: participants who had a prior history of any cancer, medical, or current psychiatric illness (e.g. orthopedic problems); cardiovascular disease and/or diabetes; were engaged in ≥150 minutes moderate-vigorous exercise per week; or had any other condition that made them unsuitable for participation in this study.
Intervention
Oncologists’ exercise recommendation group only received oncologists’ exercise recommendation while oncologists’ exercise recommendation combined with exercise motivation package group received exercise recommendation as well as exercise motivation package. Both groups received the same oncologists’ recommendation for exercise. Participants in the control group received the conventional treatment consultation, but received no exercise recommendation. Control group and oncologists’ exercise recommendation group received exercise motivation packages after the completion of the study.
Oncologists’ exercise recommendation
The oncologists read the following recommendation to their patients:
“Studies showed that the participation of moderate PA more than 150 minute per week could reduce breast and colorectal cancer recurrence significantly. Therefore, it is highly recommended for breast and colorectal cancer survivors to participate at least 150 minute of moderate level PA and twice a week of strengthening exercise”.
Exercise motivation package: exercise motivation package included exercise DVDs, pedometer, exercise diary and 15 minute exercise education session.
Exercise DVD
There are two different DVDs (home-based low intensity and moderate intensity resistance training using their own body weight). The exercise program was consisted of three sets of seven different exercises (Supplementary Table).
Pedometer
Recommendation with exercise motivation package will provide a pedometer (DMC-03, Shinwoo electronic company, Seoul Korea) at the beginning of the study. Participants could record the number of the step walked per a day in exercise diary.
Exercise diary
The exercise diary include columns which help participants to keep track of resistance exercise (exercise DVD), aerobic exercise and steps walked per day.
Exercise education
One 15 minute exercise education session which covers how to use exercise motivation package. Research coordinators also explain the beneficial effect of exercise on cancer prognosis, types and methods of recommended exercise.
Main outcome measurement
The study’s primary outcome was the amount of exercise participation. The amount of exercise participation was assessed by the leisure score index (LSI) using the Godin Leisure-Time Exercise Questionnaire [23, 24]. The questionnaire contained several questions that assessed the average frequency of mild, moderate, or strenuous intensity exercise. The summary totals for each intensity time were calculated, along with the total exercise time within a week, and the MET per week. The weekly exercise intensity was categorized as follows: strenuous (9 MET), moderate (5 MET), and mild (3 MET).
Secondary outcome measurement
Factors relating to the patient’s quality of life and self-reported physical functioning were collected. These factors included global health status, physical functioning, role functioning, emotional functioning, cognitive functioning, social functioning, fatigue, nausea and vomiting, pain, dyspnea, insomnia, appetite loss, constipation, diarrhea, and financial difficulties. These factors were assessed with the European Organization for Research and Treatment of Cancer (EORTC) QLQ C-30 instrument, which has been widely used to assess quality of life of cancer survivors [25, 26].
Statistical analysis
Statistical analyses were performed using SPSS, Windows version 18.0. Baseline characteristics of the participants and the exercise amounts across three groups were compared using one-way ANOVA and χ2 test for categorical outcomes. To compare the amount of exercise change after four week, the delta value within group (post-pre) were calculated and their values were compared with the Wilcoxon test was used for non-normally distributed variables. The statistical significance level was set at p < 0.05. The number of participants was calculated using G*Power software based on the result of previous study [27].
RESULTS
Study population
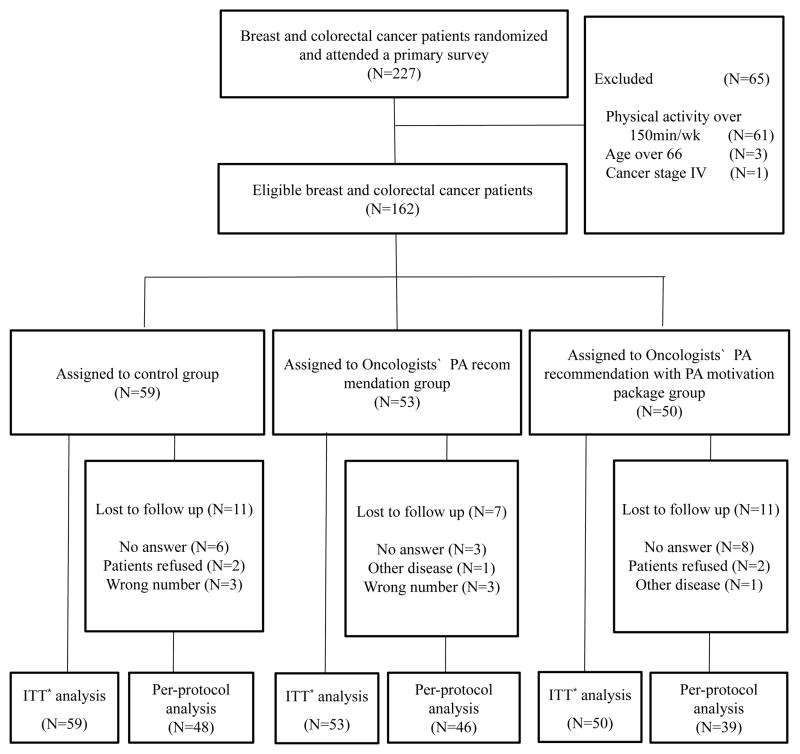
A total of 227 breast and colorectal cancer patients (168 breast cancer and 59 colorectal cancer patients) were screened for eligibility. Of these, 162 (71%) participants met the inclusion criteria also agreed to participate in the study and underwent randomization. Among 162 participants, 130 participants (80%) completed the study. The flow of the participants through the trial is described in Figure 1.
Figure 1.
Baseline characteristics of participants
The average age was 51.8 years, 88.3% of participants were women and 75.3 % of participants had breast cancer. Forty one percent of participants were diagnosed with stage I cancer and the average time since diagnosis was 23.12 months (Table 1). There was no statistical difference in the level of exercise and quality of life among three groups. The average weekly exercise participation including low intensity exercise was 244.19 minutes.
Table 1.
Demographic and medical characteristic
| Characteristic | Total (N=162) | Control (N=59) | Oncologists’ exercise recommendation (N=53) | Oncologists’ exercise recommendation with motivation package (N=50) | p-value | |
|---|---|---|---|---|---|---|
| Demographic | Male | 19(11.7) | 9(15.3) | 5(9.4) | 5(10.0) | .571 |
| Age (years) | 51.80±8.02 | 53.42±8.12 | 51.38±7.24 | 50.32±8.48 | .118 | |
| Married | 13.6(84) | 48(81.4) | 46(86.8) | 42(84.0) | .736 | |
| Family income over $50.000/year | 36(22.2) | 13(22.0) | 11(20.8) | 12(24.5) | .748 | |
| Completed university/college | 62(38.3) | 21(35.6) | 23(43.3) | 18(36.7) | .666 | |
| Employed full time | 64(39.5) | 24(42.1) | 21(42.0) | 19(38.8) | .928 | |
|
| ||||||
| Medical factor | Weight (kg) | 57.47±9.76 | 57.39±8.98 | 57.90±9.76 | 57.16±10.77 | .927 |
| BMI (kg/m2) | 22.72±3.32 | 22.76±3.47 | 22.91±3.17 | 22.47±3.34 | .796 | |
| Tumor Site | ||||||
| Breast | 122(75.3) | 41(69.5) | 42(79.2) | 39(78.6) | .425 | |
| Colorectal | 40(24.7) | 18(30.5) | 11(20.8) | 11(22.0) | ||
| Stage (N=148) | ||||||
| I | 66(40.7) | 22(40.0) | 25(49.0) | 19(45.2) | .877 | |
| II | 51(31.5) | 21(38.2) | 15(29.4) | 15(35.7) | ||
| III | 31(19.1) | 12(21.8) | 11(21.6) | 8(19.0) | ||
| Time since diagnosis (months) | 23.12±9.36 | 24.00±8.93 | 23.04±9.80 | 22.11±9.48 | .613 | |
| Time since surgery (months) | 20.35±8,78 | 21.53±8.18 | 20.20±9.41 | 19.11±8.78 | .401 | |
Data are presented as the mean ±SD and number (percent %)
Effect of oncologists’ exercise recommendations with and without exercise motivation packages on the amount of exercise
Participants who received oncologists’ exercise recommendations combined with exercise motivation package significantly increased moderate intensity exercise (51.57±94.14, 95% CI: 21.05~82.09 minute per week,), total exercise (60.99±148.84, 95% CI: 12.74~109.24 minute per week), and MET-hour per week (4.3±7.84, 95% CI: 1.75~6.84 MET-hour per week) when compared to the control group (Table 3). Participants who received oncologists’ exercise recommendations only did not increase their exercise level at 4 week of intervention. We further analyzed intention to treat analysis by including participants who dropped out of the study and observed the same statistical significance in the main outcome variable.
Table 3.
Mean change of level of exercise and EORTC QLQ-C30 between group
| Variable | Control (N=59) | Oncologists’ exercise recommendation (N=53) | Oncologists’ exercise recommendation with motivation package (N=50) | p-value | |
|---|---|---|---|---|---|
| Exercise (minute per week) | Strenuous intensity activity | −3.56(−8.61 to 1.49) | 4.53(−9.92 to 18.98) | .00(.00 to .00) | .422 |
| Moderate intensity activity | 10.34(−6.25 to 26.93) | 16.79(−4.54 to 38.12) | 40.22(15.88 to 64.57) | .000 | |
| Mild intensity activity | −47.35(−96.89 to 2.18) | −20.33(−65.68 to 25.02) | 7.35(−28.41 to 43.11) | .351 | |
| Total physical activity | −39.05(−8.28 to 11.18) | .99(−40.71 to 42.69) | 47.57(9.62 to 85.52) | .022 | |
| MET-hour/week | −1.81(−4.53 to .91) | 1.06(−1.65 to 3.78) | 4.14(1.70 to 6.58) | .004 | |
|
| |||||
| Quality of life | Global health status/QoL | −.56(−5.23 to 4.10) | 1.10(−3.34 to 5.54) | 1.67(−2.22 to 5.55) | .937 |
| Physical functioning | 6.55(3.35 to 9.75) | 6.16(2.90 to 9.42) | 6.00(1.93 to 10.07) | .870 | |
| Role functioning | .56(−4.47 to 5.60) | .31(−4.66 to 5.29) | 9.00(3.48 to 14.51) | .014 | |
| Emotional Functioning | 5.23(1.64 to 8.81) | 3.46(−1.66 to 8.58) | 4.33(.18 to 8.48) | .742 | |
| Cognitive functioning | 3.11(−.80 to 7.01) | 3.46(−1.50 to 8.42) | 5.10(1.55 to 8.65) | .635 | |
| Social functioning | 5.93(.07 to 11.79) | −.63(−7.78 to 6.52) | 1.33(−5.42 to 8.09) | .143 | |
| Fatigue | −10.73(−15.51 to −5.96) | −2.72(−7.17 to 1.72) | −7.78(−12.65 to −2.91) | .061 | |
| Nausea and vomiting | −1.69(−3.93 to .54) | .31(−2.15 to 2.78) | −1.67(−5.28 to 1.94) | .559 | |
| Pain | −2.26(−7.06 to 2.54) | −6.60(−12.67 to −.54) | −7.00(−10.59 to −3.41) | .087 | |
| Dyspnea | −3.95(−8.25 to .34) | −3.14(−8.32 to 2.03) | −1.33(−6.73 to 4.07) | .731 | |
| Insomnia | −.56(−6.49 to 5.36) | −10.06(−17.18 to −2.95) | −1.33(−17.57 to 14.90) | .109 | |
| Appetite loss | −4.52(−11.06 to 2.02) | −2.51(−7.23 to 2.20) | −9.33(−15.09 to −3.58) | .151 | |
| Constipation | −2.26(−5.82 to 1.30) | −.63(−8.16 to 6.91) | −3.33(−8.12 to 1.45) | .787 | |
| Diarrhea | −3.39(−6.49 to −.29) | −1.26(−4.84 to 2.33) | −9.33(−14.76 to −3.91) | .032 | |
| Financial difficulties | 2.82(−3.05 to 8.70) | −3.14(−9.19 to 2.90) | −2.67(−6.42 to 1.08) | .197 | |
Data are presented as the Delta(95%CI),
significantly different from control group,
significantly different from Oncologists’ exercise recommendation group (p<0.05)
Effect of oncologists’ exercise recommendations with and without exercise motivation packages on the QOL
There was no difference in the change of total QOL score among groups. However, we have found that participants who received oncologists’ exercise recommendations combined with exercise motivation package significantly increased role functioning (11.54±21.34, 95% CI:4.62~18.46) while reduced pain(−8.87±13.70, 95% CI: −13.42~−.4.53), and diarrhea(−11.96±20.92, 95%CI: −18.75~−5.18) compared to control and oncologists’ exercise recommendation only group in subdomain of QOL (Table 3). We further performed subgroup analysis based on the cancer type and observed increments in the amount of exercise in both breast and colorectal cancer.
DISCUSSION
The main purpose of the study was to investigate the effect of oncologists’ recommendations combined with exercise motivation packages on the amount of exercise and the quality of life in cancer survivors. The main finding of our study is that participants who received oncologists’ exercise recommendations combined with exercise motivation package significant increased the amount of exercise participation while exercise recommendation without motivation package failed to increase the level of exercise. This increase in amount of exercise was seen in the moderate and mild intensity levels of exercise as wells as the MET hours/week. Our finding suggests that oncologists’ exercise recommendation was not enough to elicit change in exercise level and it should accompany with pedometer, exercise education and exercise diary.
The lack of significant change in exercise when participants only received exercise recommendation from oncologists is noteworthy. Since ample solid evidence on beneficial effects of exercise and exercise for primary and secondary prevention of diseases is established, the role of healthcare sector to promote exercise participation has been evaluated. In 1990 the center for disease control funded the first initiative in the US to include physical activity counseling in primary and secondary prevent care for adults, called PACE project [28–31]. The focus of this project was to develop and test an approach for including routine assessment of patients’ current level of physical activity participation and provide a tailored counseling to increase physical activity level of patients[28]. However, early studies failed to find significant effect of physician’s counseling, brief and stand-alone intervention, on the level of physical activity in the context of a regular medical office visit [32–34]. Several studies have examined whether coupling physician’s physical activity recommendation with additional brief sessions with the health counselor or referral to a community resource can increase PA. Swinburn et al [35] combined brief physician’s physical activity counseling with post-visit outreach from exercise specialists and found 12 months increase in physical activity and activity-related outcomes such as self-rated health and vitality.
However, it is still noteworthy that physician’s physical activity counseling alone still elicit small but significant increase in the level of physical activity in cancer patients [11, 18]. Vallance et al. [11] and Jones et al. [18] reported that physical activity recommendation by oncologists without any other intervention still increased about 30 minute per week of physical activity. Although there has not been a study which examine whether the effect of physicians’ physical activity counseling would have different effect on the behavior change according to disease type including cancer, we can speculate that patients perceive cancer as more of life-threatening than other diseases and are more likely to follow physicians’ recommendation. Anyhow, we did not find significant increase in physical activity level after oncologists’ simple recommendation when it was not combined with PA motivation package in our study. This difference between our study and two other studies [11, 18] which found significant increase in physical activity level after oncologists’ recommendation is average time since diagnosis (less than 12 months vs. 23.76 months). Although there is some evidence that PA recommendation only still may elicit some change in the level of physical activity, cancer survivors may need more than just oncologists’ physical activity recommendations to elicit meaningful change in exercise behavior. Indeed, there are an ample number of studies which suggest that simple methods such as written exercise prescription, telephone advice and support, using a pedometer, or an internet program, can have a significant impact on engaging patients in being more active and staying that way [11, 14, 36].
In our study, we found that oncologists’ exercise recommendation accompanied with exercise motivation package increased the level of exercise by 80 minute per week compared with control group. This exercise motivation package included exercise DVDs, pedometer, exercise diary, and 15 minute exercise education. Since we did not measure independent impact of components of exercise motivation package, we cannot distinguish which part of exercise motivation package resulted in change in exercise level. Previous studies showed that PA recommendation only increased about 30 minute per week of physical activity [11, 18], however, additional increase in PA level was observed when PA recommendation was accompanied with printed material (70 min per week) as well as step pedometer (89 minute per week) in increasing the level of physical activity. Although it was not statistically significant, we have observed somewhat like dose dependent effect of physical activity recommendation only (17.5 minute/week of physical activity more than control) and physical activity recommendation combined with physical activity motivation package (80 minute/week of physical activity more than control). The increase of 80 minute of physical activity observed in the current study compared to that of control group is similar to the 87 minute per week of physical activity in combined printed material and step pedometer [11].
Relatively small increase in the level of PA significantly prevent cancer recurrence and improve all-cause mortality [1]. In breast cancer patients, patients who participated in 9 MET hour per week of PA had 50 percent reduction in the breast cancer-specific mortality and all-cause mortality [37]. In recent meta-analysis, we identified that even small amount of PA after cancer diagnosis could still produce significant improvement in colorectal-cancer specific mortality and all-cause mortality [38]. In the current study, we have found 80 minute per week of physical activity (estimated 6 MET hour per week) increase with exercise counseling and motivation package. Recently, American College of Sport Medicine (ACSM) launched a major initiative to “encourage physicians to counsel patients about exercise and prescribe exercise”. Major component of this initiative included followings: 1) more physicians to prescribe exercise and collect exercise data routinely and provide additional tools to physicians to promote exercise, 2) develop collaborative system between physicians and exercise specialists to promote PA, 3) encourage public to discuss about exercise with their MDs, 4) encourage policy developers to support the use of exercise as a way to prevent and treat disease.
The current study also showed that participants who received oncologists’ exercise recommendations significantly increased their role functioning, and had decreased pain and diarrhea, compared to the control group and the exercise recommendations group. A systemic review suggests that exercise interventions significantly improve the quality of life in cancer survivors [39, 40]. Similarly, Vallance et al. [11] reported that the printed materials combined with step pedometers helped to improve their quality of life and fatigue.
There are several limitations in this study that are important to consider. First, the current study started in fall and was completed in winter. Most patients participated in outdoor walking as exercise, but their frequency or duration of walking could have been affected by the seasonal change in weather conditions. Second, another limitation of the study relates to the change (dropouts) in the number of participants, during the follow-up between groups. The reason for these participant dropouts is unknown. Third, the reliance on self-report rather than objective measure of exercise behaviors may lead to imprecise measurements. Future research should strive to use objective measures in verifying exercise levels.
In conclusion, the current study was the first step in investigating the effect of oncologists’ exercise recommendations combined with exercise motivation packages of exercise on the amount of exercise and quality of life for cancer survivors. Although oncologists’ exercise recommendations are important strategy, it may not be enough to result in significant change in the level of exercise. Our study suggest that oncologists’ exercise recommendations should be combined with exercise information and tools which help cancer survivors to be motivated to participate in exercise. This study found that oncologists’ exercise recommendations and exercise motivation package significantly increase the level of exercise in the breast and colorectal cancer survivors.
Supplementary Material
Table 2.
Exercise and Quality of life characteristics of the participants
| Variables | Total (N=162) | Control (N=59) | Oncologists’ exercise recommendation (N=53) | Oncologists’ exercise recommendation with motivation package (N=50) | p-value | |
|---|---|---|---|---|---|---|
| Exercise (minutes/week) | Strenuous intensity exercise | 2.04(−.29 to 4.36) | 3.56(−1.49 to 8.61) | 2.26(−2.28 to 6.81) | .00(.00 to .00) | .429 |
| Moderate intensity exercise | 6.16(2.04 to 10.27) | .00(.00 to .00) | 8.63(−.12 to 17.39) | 10.80(1.09 to 20.51) | .058 | |
| Mild intensity exercise | 236.00(203.20 to 267.80) | 251.00(191.34 to 310.66) | 274.90(213.11 to 336.70) | 177.05(131.22 to 222.88) | .085 | |
| Total physical exercise | 244.19(211.59 to 276.79) | 254.56(194.94 to 314.18) | 285.80(225.48 to 346.12) | 187.85(141.09 to 234.61) | .078 | |
| MET-hour/week | 12.62(10.95 to 14.28) | 13.08(10.04 to 16.12) | 14.08(11.75 to 17.86) | 9.75(7.33 to 12.17) | .089 | |
|
| ||||||
| Quality of life | Global health status/QoL* | 66.41(63.43 to 69.38) | 64.69(59.46 to 69.92) | 66.98(61.61 to 72.35) | 67.83(62.72 to 72.94) | .712 |
| Physical functioning | 77.82(75.34 to 80.29) | 76.16(72.02 to 80.29) | 80.50(76.20 to 84.81) | 76.93(72.29 to 81.58) | .207 | |
| Role functioning | 82.41(78.79 to 86.02) | 83.05(77.38 to 88.72) | 85.85(79.45 to 92.24) | 78.00(70.94 to 85.06) | .075 | |
| Emotional Functioning | 77.47(74.60 to 80.33) | 76.98(72.22 to 81.74) | 78.30(73.34 to 83.26) | 77.17(71.63 to 82.70) | .958 | |
| Cognitive functioning | 79.22(76.42 to 82.01) | 80.79(76.61 to 84.97) | 78.30(72.78 to 83.82) | 78.33(73.16 to 83.51) | .853 | |
| Social functioning | 86.01(82.66 to 89.35) | 82.48(76.93 to 88.04) | 88.68(83.21 to 94.14) | 87.33(80.66 to 94.00) | .123 | |
| Fatigue | 33.61(30.35 to 36.87) | 36.72(30.42 to 43.02) | 28.30(23.26 to 33.38) | 35.55(30.18 to 40.93) | .098 | |
| Nausea and vomiting | 4.63(3.07 to 6.19) | 6.21(3.55 to 8.88) | 2.51(.06 to 4.96) | 5.00(1.94 to 8.06) | .031 | |
| Pain | 22.74(19.13 to 26.34) | 23.73(17.68 to 29.77) | 22.33(15.41 to 29.24) | 22.00(15.84 to 28.16) | .857 | |
| Dyspnea | 13.79(10.81 to16. 76) | 15.82(10.88 to 20.75) | 10.06(5.43 to 14.69) | 15.33(9.22 to 21.45) | .223 | |
| Insomnia | 24.28(19.85 to 28.71) | 22.60(15.49 to 29.71) | 25.16(16.72 to 33.59) | 25.33(17.31 to 33.35) | .874 | |
| Appetite loss | 12.76(9.34 to 16.17) | 13.56(7.08 to 20.03) | 10.06(4.48 to 15.64) | 14.67(8.87 to 20.46) | .335 | |
| Constipation | 16.46912.41 to 20.51) | 21.47(13.77 to 29.16) | 12.58(5.78 to 19.37) | 14.66(8.27 to 21.06) | .145 | |
| Diarrhea | 7.20(4.77 to 9.63) | 5.65(1.99 to 9.31) | 5.66(2.18 to 9.14) | 10.67(5.11 to 16.23) | .280 | |
| Financial difficulties | 17.90(13.66 to 22.14) | 17.51(11.19 to 23.85) | 16.98(9.42 to 24.54) | 19.33(10.54 to 28.12) | .909 | |
Data are presented as the mean±SD
Acknowledgments
The current study was supported by the national research foundation of Korea (NRF)(No.2011-0004892) and the National R & D program for Cancer Control, Ministry of Health and Welfare, Republic of Korea (No.1120230).
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Ballard-Barbash R, et al. Physical activity, biomarkers, and disease outcomes in cancer survivors: a systematic review. J Natl Cancer Inst. 2012;104(11):815–40. doi: 10.1093/jnci/djs207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Je Y, et al. Association between physical activity and mortality in colorectal cancer: a meta-analysis of prospective cohort studies. Int J Cancer. 2013;133(8):1905–13. doi: 10.1002/ijc.28208. [DOI] [PubMed] [Google Scholar]
- 3.Speck RM, et al. An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. J Cancer Surviv. 2010;4(2):87–100. doi: 10.1007/s11764-009-0110-5. [DOI] [PubMed] [Google Scholar]
- 4.Rogers LQ, et al. Physical activity and quality of life in head and neck cancer survivors. Support Care Cancer. 2006;14(10):1012–9. doi: 10.1007/s00520-006-0044-7. [DOI] [PubMed] [Google Scholar]
- 5.McNeely ML, et al. Effects of exercise on breast cancer patients and survivors: a systematic review and meta-analysis. CMAJ. 2006;175(1):34–41. doi: 10.1503/cmaj.051073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blanchard CM, Courneya KS, Stein K. Cancer survivors’ adherence to lifestyle behavior recommendations and associations with health-related quality of life: results from the American Cancer Society’s SCS-II. J Clin Oncol. 2008;26(13):2198–204. doi: 10.1200/JCO.2007.14.6217. [DOI] [PubMed] [Google Scholar]
- 7.Chung JY, et al. Patterns of physical activity participation across the cancer trajectory in colorectal cancer survivors. Support Care Cancer. 2013 doi: 10.1007/s00520-012-1703-5. [DOI] [PubMed] [Google Scholar]
- 8.Courneya KS, et al. Six-month follow-up of patient-rated outcomes in a randomized controlled trial of exercise training during breast cancer chemotherapy. Cancer Epidemiol Biomarkers Prev. 2007;16(12):2572–8. doi: 10.1158/1055-9965.EPI-07-0413. [DOI] [PubMed] [Google Scholar]
- 9.Schmitz KH, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42(7):1409–26. doi: 10.1249/MSS.0b013e3181e0c112. [DOI] [PubMed] [Google Scholar]
- 10.Courneya KS, et al. Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: a multicenter randomized controlled trial. J Clin Oncol. 2007;25(28):4396–404. doi: 10.1200/JCO.2006.08.2024. [DOI] [PubMed] [Google Scholar]
- 11.Vallance JK, et al. Randomized controlled trial of the effects of print materials and step pedometers on physical activity and quality of life in breast cancer survivors. J Clin Oncol. 2007;25(17):2352–9. doi: 10.1200/JCO.2006.07.9988. [DOI] [PubMed] [Google Scholar]
- 12.Rogers LQ, et al. Exercise stage of change, barriers, expectations, values and preferences among breast cancer patients during treatment: a pilot study. Eur J Cancer Care (Engl) 2007;16(1):55–66. doi: 10.1111/j.1365-2354.2006.00705.x. [DOI] [PubMed] [Google Scholar]
- 13.Karvinen KH, et al. Exercise programming and counseling preferences in bladder cancer survivors: a population-based study. J Cancer Surviv. 2007;1(1):27–34. doi: 10.1007/s11764-007-0010-5. [DOI] [PubMed] [Google Scholar]
- 14.Lee DH, et al. Effects of a 12-week home-based exercise program on the level of physical activity, insulin, and cytokines in colorectal cancer survivors: a pilot study. Support Care Cancer. 2013;21(9):2537–45. doi: 10.1007/s00520-013-1822-7. [DOI] [PubMed] [Google Scholar]
- 15.Ligibel JA, et al. Impact of a mixed strength and endurance exercise intervention on insulin levels in breast cancer survivors. J Clin Oncol. 2008;26(6):907–12. doi: 10.1200/JCO.2007.12.7357. [DOI] [PubMed] [Google Scholar]
- 16.Tulloch H, Fortier M, Hogg W. Physical activity counseling in primary care: who has and who should be counseling? Patient Educ Couns. 2006;64(1–3):6–20. doi: 10.1016/j.pec.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 17.Eggly S, et al. Oncologists’ recommendations of clinical trial participation to patients. Patient Educ Couns. 2008;70(1):143–8. doi: 10.1016/j.pec.2007.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones LW, et al. Effects of an oncologist’s recommendation to exercise on self-reported exercise behavior in newly diagnosed breast cancer survivors: a single-blind, randomized controlled trial. Ann Behav Med. 2004;28(2):105–13. doi: 10.1207/s15324796abm2802_5. [DOI] [PubMed] [Google Scholar]
- 19.Patrick K, Pratt M, Sallis RE. The healthcare sector’s role in the U.S. national physical activity plan. J Phys Act Health. 2009;6(Suppl 2):S211–9. [PubMed] [Google Scholar]
- 20.Weidinger KA, et al. How to make exercise counseling more effective: lessons from rural America. J Fam Pract. 2008;57(6):394–402. [PubMed] [Google Scholar]
- 21.Courneya KS, et al. A randomized trial of exercise and quality of life in colorectal cancer survivors. Eur J Cancer Care (Engl) 2003;12(4):347–57. doi: 10.1046/j.1365-2354.2003.00437.x. [DOI] [PubMed] [Google Scholar]
- 22.Bicego D, et al. Effects of Exercise on Quality of Life in Women Living with Breast Cancer: A Systematic Review. Breast Journal. 2009;15(1):45–51. doi: 10.1111/j.1524-4741.2008.00670.x. [DOI] [PubMed] [Google Scholar]
- 23.Godin G, Jobin J, Bouillon J. Assessment of leisure time exercise behavior by self-report: a concurrent validity study. Can J Public Health. 1986;77(5):359–62. [PubMed] [Google Scholar]
- 24.Godin G, Shephard RJ. A simple method to assess exercise behavior in the community. Can J Appl Sport Sci. 1985;10(3):141–6. [PubMed] [Google Scholar]
- 25.Aaronson N, et al. The European Organization for Research and Treatment for Cancer QLQ C-30: A quality of life instrument for use in international clinical trials in oncology. Journal of the National Cancer Institute. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 26.Osoba D, et al. Psychometric properties and responsiveness of the EORTC quality of Life Questionnaire (QLQ-C30) in patients with breast, ovarian and lung cancer. Qual Life Res. 1994;3(5):353–64. doi: 10.1007/BF00451727. [DOI] [PubMed] [Google Scholar]
- 27.Jones LW, et al. Oncologists’ opinions towards recommending exercise to patients with cancer: a Canadian national survey. Support Care Cancer. 2005;13(11):929–37. doi: 10.1007/s00520-005-0805-8. [DOI] [PubMed] [Google Scholar]
- 28.Horslen S, et al. PACE project: object-orientated modelling of paediatric practice. Med Inform (Lond) 1992;17(3):179–86. doi: 10.3109/14639239209096533. [DOI] [PubMed] [Google Scholar]
- 29.Figura SZ. Setting the pace. Long-term care project holds hope for future programs. Volunt Leader. 1997;38(3):11–2. [PubMed] [Google Scholar]
- 30.Eleazer GP, et al. Managed care for the frail elderly: the PACE Project. J S C Med Assoc. 1994;90(12):586–91. [PubMed] [Google Scholar]
- 31.Abdellah FG, Chow RK. The long-term care facility improvement campaign: the PACE Project. ARN J. 1976;1(7):3–4. [PubMed] [Google Scholar]
- 32.van Sluijs EM, et al. Effect of a tailored physical activity intervention delivered in general practice settings: results of a randomized controlled trial. Am J Public Health. 2005;95(10):1825–31. doi: 10.2105/AJPH.2004.044537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Norris SL, et al. Effectiveness of physician-based assessment and counseling for exercise in a staff model HMO. Prev Med. 2000;30(6):513–23. doi: 10.1006/pmed.2000.0673. [DOI] [PubMed] [Google Scholar]
- 34.Calfas KJ, et al. A controlled trial of physician counseling to promote the adoption of physical activity. Preventive Medicine. 1996;25(3):225–233. doi: 10.1006/pmed.1996.0050. [DOI] [PubMed] [Google Scholar]
- 35.Swinburn BA, et al. The green prescription study: a randomized controlled trial of written exercise advice provided by general practitioners. Am J Public Health. 1998;88(2):288–91. doi: 10.2105/ajph.88.2.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Orrow G, et al. Effectiveness of physical activity promotion based in primary care: systematic review and meta-analysis of randomised controlled trials. BMJ. 2012;344:e1389. doi: 10.1136/bmj.e1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holmes MD, et al. Physical activity and survival after breast cancer diagnosis. Jama-Journal of the American Medical Association. 2005;293(20):2479–2486. doi: 10.1001/jama.293.20.2479. [DOI] [PubMed] [Google Scholar]
- 38.Meyerhardt JA, et al. Physical activity and survival after colorectal cancer diagnosis. Journal of Clinical Oncology. 2006;24(22):3527–3534. doi: 10.1200/JCO.2006.06.0855. [DOI] [PubMed] [Google Scholar]
- 39.Ferrer RA, et al. Exercise interventions for cancer survivors: a meta-analysis of quality of life outcomes. Ann Behav Med. 2011;41(1):32–47. doi: 10.1007/s12160-010-9225-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Winters-Stone KM, Schwartz A, Nail LM. A review of exercise interventions to improve bone health in adult cancer survivors. J Cancer Surviv. 2010;4(3):187–201. doi: 10.1007/s11764-010-0122-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.