Abstract
To date no authentic embryonic stem cell (ESC) line or germline-competent-induced pluripotent stem cell (iPSC) line has been established for large animals. Despite this fact, there is an impression in the field that large animal ESCs or iPSCs are as good as mouse counterparts. Clarification of this issue is important for a healthy advancement of the stem cell field. Elucidation of the causes of this failure in obtaining high quality iPSCs/ESCs may offer essential clues for eventual establishment of authentic ESCs for large animals including humans. To this end, we first generated porcine iPSCs using nonintegrating replicating episomal plasmids. Although these porcine iPSCs met most pluripotency criteria, they could neither generate cloned piglets through nuclear transfer, nor contribute to later stage chimeras through morula injections or aggregations. We found that the reprogramming genes in iPSCs could not be removed even under negative selection, indicating they are required to maintain self-renewal. The persistent expression of these genes in porcine iPSCs in turn caused differentiation defects in vivo. Therefore, incomplete reprogramming manifested by a reliance on sustained expression of exogenous-reprogramming factors appears to be the main reason for the inability of porcine iPSCs to form iPSC-derived piglets.
Keywords: Pig, Induced pluripotent stem cells, Pluripotency, Nuclear transfer, Chimera, Transgene-free
Introduction
Recent advances in embryonic stem cell (ESC) research are opening up the way for development of gene therapy to treat patients with genetic diseases. Due to certain anatomical and physiological features shared with humans, the pig is considered an important animal model of human diseases with unique advantages in surgery and xenotransplantation studies [1–3]. Repeated attempts to isolate ESC-like cultures from porcine embryos have revealed that pig ESCs appear fundamentally different from counterparts in mouse [4, 5], rat [6, 7], rhesus monkey [8], and human [9]. Since the initial attempts [10–12], no porcine ESCs have been established that can maintain self-renewal and pluripotency in long-term culture akin to mouse or human ESCs [13]. As a replacement for ESCs, induced pluripotent stem cells (iPSCs) [14] generated from mouse differentiated cells can produce viable, fertile mice through nuclear transfer (NT) [15, 16] and tetraploid complementation (4N) [17, 18]. Several groups have reported derivation of porcine iPSCs [19–21]; however, to date no porcine iPSCs can be used to reliably produce viable, fertile offspring [22, 23]. Most importantly, whether porcine iPSCs have the ability to pass the crucial test of germ-line contribution needed to generate gene-modified piglets through chimera approaches remains to be demonstrated.
In the early stages, iPSCs had been mostly generated with viral vectors. This method has several problems including unpredictable mutations, uncontrolled silencing of exogenous factors, unregulated expression of residual transgenes [24], and strong immunogenicity [25]. Furthermore, residual expression of exogenous factors may interfere with the normal differentiation function of iPSCs [26] and affect their ability to form chimeras [24, 27, 28]. To solve these problems, scientists have made great efforts to generate transgene-free iPSCs. Conventional plasmids encoding reprogramming factors can generate both transgene-free mouse [29, 30] and human iPSCs [31]. However, the reprogramming procedure was laborious, and the reprogramming efficiency was much lower than that of the virus-based methods. To overcome these obstacles, Yu et al. initially established transgene-free human iPSCs with Epstein Barr virus-based self-replicating episomal vectors [32]. Optimized episomal vector systems have been reported by several groups including ours [25, 33, 34].
Here, we show that porcine iPSCs can be generated with nonintegrating episomal plasmids. Pluripotency was demonstrated by 2i/leukemia inhibitory factor (LIF) dependency, robust clonogenicity, reactivation of the X chromosome in female iPSCs, pluripotency gene expression, and teratoma formation. The iPSCs could be genetically modified throughout the porcine genome using piggyBac transposon-based vectors. However, we found that certain reprogramming genes could not be removed or silenced, and CpG sites in the endogenous OCT4 promoter region were highly methylated. These porcine iPSCs could develop into cloned embryos and chimeric blastocysts in vitro, and participated in the generation of inner cell mass (ICM) and trophectoderm (TE). However, nuclear transfer, early embryo injection, or embryo aggregation methods all failed to produce viable iPSC-derived piglets.
Materials and Methods
Cell Culture and Media
Porcine fetal fibroblasts (PFFs) were isolated from day 28 porcine embryos of pathogen-free laboratory mini-pigs. The PFFs were used within five passages to avoid replicative senescence. PFFs were maintained in serum-based EF medium (Dulbecco’s modified Eagle’s medium [DMEM] containing 10% fetal bovine serum [FBS], 1% nonessential amino acids [Invitrogen, CA, www.lifetechnologies.com], 1% penicillin-streptomycin [Gibco, CA, www.lifetechnologies.com]). The transfected cells were cultured on γ-ray-treated mouse embryonic fibroblasts (MEFs) in serum-based ESC medium (DMEM containing 10% FBS, 1× NEAA (Gibco), 1% penicillin-streptomycin (Gibco), 0.1 mM b-mercaptoethanol [Sigma Chemical Co., St. Louis, MO, www.sigmaaldrich.com], 106 unit/l mouse Lif [Gibco], supplemented with 600 mg/ml G418 [EMD Chemicals, Inc. San Diego, CA, www.emdchemicals.com]). Reprogramming and maintenance of porcine iPSCs were conducted in 2i/LIF medium (500 ml neurobasal medium [Gibco], 500 ml DMEM-F-12 medium [Gibco], 5 ml N2 supplement [Gibco], 10 ml B27 supplement [Gibco], 3 μM CHIR99021 [Selleck Chemicals, Houston, Texas, www.selleckchem.com], 1 μM PD0325901 [Selleck], 0.1 mM b-mercaptoethanol [Sigma], 1% penicillin-streptomycin [Invitrogen], and 106 unit/l mouse Lif [Gibco]). Colonies were counted 21 days after plating, and those colonies similar to mouse or rat ESCs were selected for further cultivation and evaluation.
Reprogramming of PFFs and Electrotransfection of iPSC Lines
Construction of the pMaster series of vectors was detailed in a previous report [19]. Four micrograms episomal plasmid DNA was electroplated into 106 PFFs with a Nucleofector 2b Device (Lonza, Cologne, Germany, www.lonza.com) with a 100-μl kit for primary fibroblasts using program A-024 or T-016. The transfected cells were replated onto 100-mm dishes covered with a MEF feeder layer. Cells were grown in a humidified 37°C/5% CO2 incubator. The culture medium was replaced the next day with mES medium for selection with G418 (600 mg/ml) for 5 days.
Immunofluorescence Analysis and Alkaline Phosphatase Staining
Pig iPSCs were grown on feeder cells in 12-well plates to 50%–60% confluence. Cells were fixed with 4% paraformaldehyde for 30 minutes, permeabilized with 0.3% Triton X-100 in phosphate-buffered saline for 10 minutes at 25°C, and blocked in 5% goat serum for 1 hour. Incubation with primary antibody was overnight at 4°C. The following primary antibodies were used: OCT4 (mouse IgG2b, 1:100, Santa Cruz, CA, www.scbt.com); NANOG (rabbit antibody, 1:400, Abcam, Cambridge, MA, www.abcam.com); SSEA-1 (mouse IgM, 1:100, DSHB, Iowa City, Iowa, dshb.biology.uiowa.edu); and SSEA-4, (mouse IgG3, 1:200, DSHB). The porcine iPSCs were incubated with the appropriate fluorescence labeled secondary antibodies (Life technologies, CA, www.lifetechnologies.com), and stained with 5 ng/ml dapi nucleic acid stain (DAPI) (Invitrogen). The alkaline phosphatase (AP) staining was performed using the AP substrate kit (Sigma).
PCR Analysis
Total DNA was extracted as described previously [35]. PCR was performed using GoTaq Green (Promega, Fitchburg, Wisconsin, www.promega.com) by denaturing DNA at 94°C for 5 minutes, followed by 30 cycles (94°C 30 seconds; 60°C 30 seconds; and 72°C 45 seconds) and a final 7-minute extension. The primer sequences used in PCR analysis are listed in Supporting Information Table S1.
Quantitative RT-PCR Analysis
Total RNA was isolated with Trizol reagent (Invitrogen). Synthesis of cDNA was performed by QuantiTect Reverse Transcription Kit (QIAGEN, Venlo, Netherlands, www.qiagen.com) according to the manufacturer’s procedure. RT-PCRs were performed using GoTaq Green (Promega) by denaturing cDNA at 95°C for 2 minutes, followed by 35 cycles (94°C 30 seconds; 60°C 30 seconds; and 72°C 20 seconds), and a final 3-minute extension. Quantitative PCR was performed using the Rotor-Gene SYBR Green PCR Kit (QIAGEN, Venlo, Netherlands, www.qiagen.com) on the ABI7900HT sequence detector (Applied Biosystems). The conditions for Q-PCR were as follows: 95°C, 5 minutes, followed by 35 amplification cycles (95°C, 10 seconds; 60°C, 10 seconds; 72°C, 10 seconds). Data were normalized to the expression level of GAPDH. The primer sequences used in PCR analysis are listed in Supporting Information Table S1.
Teratoma Formation
Approximately 1 million porcine iPSCs were injected into the axillary subcutaneous tissue of nude mice. After 37 days, the teratomas were excised, fixed in 4% paraformaldehyde, embedded in paraffin, sectioned, and stained with H&E.
In Vitro Maturation of Oocytes
Pig ovaries were collected from a local abattoir and transported to the laboratory within 3 hours in 0.9% NaCl at 35°C–38°C. Cumulus oocyte complexes (COCs) were aspirated from medium-sized (3–6 mm) follicles with a 12 gauge needle fixed to a vacuum-pumping system. The COCs were washed three times in tissue culture medium, and then 50 COCs per 500 μl were cultured in maturation medium covered with mineral oil in a four-well dish (Nunc, Roskilde, Denmark, www.thermoscientific.com), which was pre-equilibrated at 38.5°C, 5% CO2 in air for more than 4 hours. After 42–44 hours of culture, cumulous cells were removed by vigorous vortexing for 2 minutes in 0.1% hyaluronidase (Sigma, H3506).
Nuclear Transfer
The same procedure was used for PFFs or porcine iPSCs as donor cells. The fusion rate was calculated after reconstructed embryos were kept in T2 (HEPES-buffered Tissue Culture Medium-199 +2% FBS) for about 30 minutes. Fifty to eighty fused embryos were transferred into 500 μl of PZM-3 in a four-well dish covered with mineral oil and pre-equilibrated at 38.5°C, 5% CO2 in air for more than 4 hours. The embryos were then cultured at 38.5°C in 5% CO2, 5% O2, 90% N2 with maximum humidity. After the fusion/activation described above, reconstructed embryos were incubated in PZM-3 medium with 0.5 mM Scriptaid at 38.5°C in 5% CO2, 5% O2, and 90% N2 with maximum humidity for 16 hours.
Aggregation
At day 2 after nuclear transfer, the zona pellucidas of pig iPSC-embryos (red fluorescence) and PEF-embryos (green fluorescence) were removed. Only those with no damaged blastomeres were used. Aggregation was performed between two PEF-embryos and two pig iPSC-embryos in T2 supplemented with 0.4 mg/ml phytohemagglutinin. All aggregated embryos were further cultured by the well-of-the-well (WOW) system for 2–3 days until transfer into the recipient.
Morula Injection
Porcine embryos at the morula stage were prepared 4 days after nuclear transfer, and small clumps (15–30 cells) of iPSCs (red fluorescence) were slowly injected into each morula embryo. The injected embryos were then developed in PZM-3 medium to the blastocyst stage until transfer into the recipient.
Embryo Transfer
Aggerated blastocysts or injected blastocysts were surgically transferred into surrogate mothers. About 4 weeks later, the pregnancy status of the surrogates was diagnosed by ultrasonography. The piglets were delivered through natural birth.
Results
Derivation of Porcine iPSCs Using Episomal Plasmids
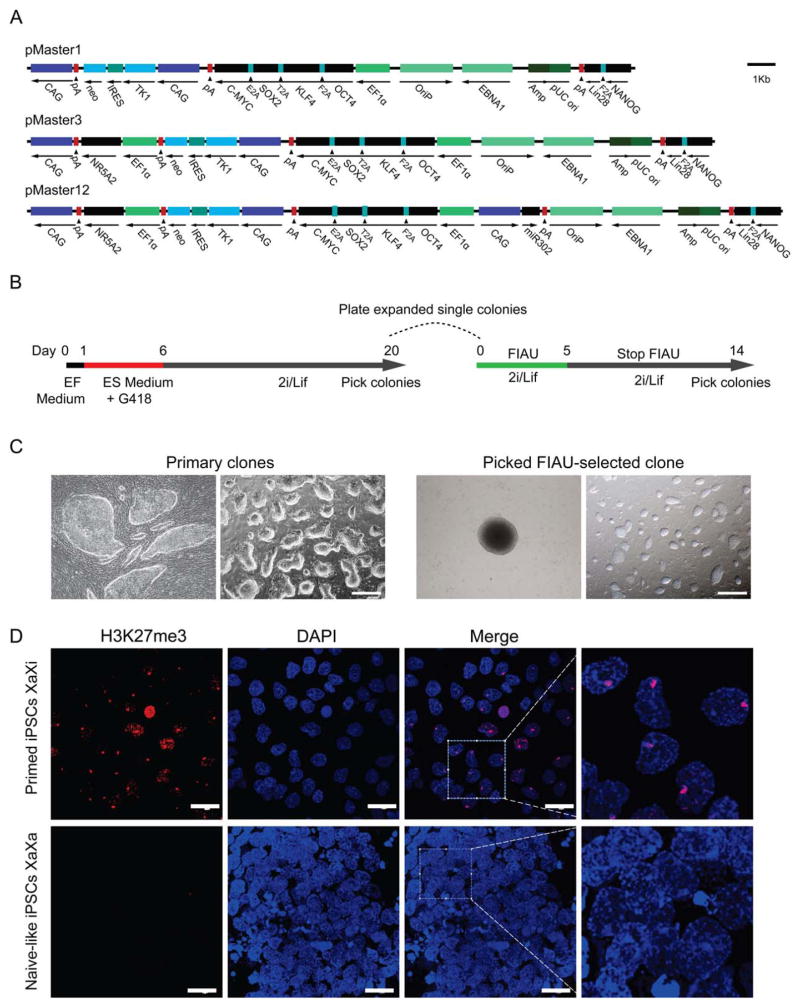
To generate transgene-free porcine iPSCs from female fetal fibroblasts, we used three episomal vectors generated previously [34], pMaster1, pMaster3, and pMaster12 (Fig. 1A), all of which encode human OCT4/POU5F1, SOX2, KLF4, C-MYC and NANOG, LIN28/LIN28A in two open reading frames linked by 2A peptides [30, 36]. In addition, pMaster3 also expresses the NR5A2 gene, and pMaster12 expresses NR5A2 and the miR302/367 cluster. Positive and negative selection cassettes, neo and HSVtk, were included in the episomal vectors to facilitate the selection process. These episomal plasmids were individually transfected into the PFFs by electroporation (Fig. 1B). At around day 6, iPSC colonies started to appear (Fig. 1C). By day 20, iPSCs showed tight and three-dimensional mouse ESC-like morphology (Fig. 1C). Colonies were then picked for further expansion and evaluation. The induction efficiency of the pMaster1 vector was very low (~0.001%), almost 2 orders of magnitude lower than that of the pMaster12 vector (Supporting Information Fig. S1A). These results suggest that NR5A2 and the miR302/367 cluster can significantly enhance the reprogramming efficiency of porcine iPSCs. Despite the obvious differences in reprogramming efficiency, iPSCs derived using different vectors exhibited remarkably consistent characteristics. They demonstrated morphology similar to that of mouse ESCs (Fig. 1C), and were AP positive (Supporting Information Fig. S1A). Immunofluorescence analysis showed that the iPSC lines uniformly expressed OCT4, NANOG, SSEA-1, and SSEA-4 (Supporting Information Fig. S1B). The iPSCs analyzed had normal karyotypes and a short cell cycle (doubling time of ~13 hours), and formed teratomas in severe combined immunodeficiency mice that contained derivatives of all three germ layers (Supporting Information Fig. S1C). Erasure of epigenetic silencing marks on the X chromosome was evidenced by loss of Xi-like H3K27me3 domains (Fig. 1D), indicating that iPSCs were epigenetically reprogrammed.
Figure 1.
Derivation of porcine iPSCs using episomal plasmids. (A): Schematic representation of the episomal reprogramming vectors. The pMaster1 vector contains six human reprogramming genes (OKSM+NANOG+LIN28); pMaster3 has seven genes (OKSM+NANOG+LIN28+NR5A2); pMaster12 has eight genes (OKSM+NANOG+LIN28+NR5A2+miR302/367 cluster). The neo and HSVtk resistance genes are separated by IRES sequences. (B): Outline of reprogramming mediated by pMaster vectors. EF medium, serum-based embryonic fibroblast medium; ES medium, serum/Lif-based ESC medium; 2i/Lif, serum-free ESC medium supplemented with PD0325901, CHIR90021 and Lif. (C): Bright-field image of porcine iPSCs derived with the pMaster12 vector. Primary iPSC clones obtained 6 days and 20 days after transfection; FIAU-resistant iPSC clone picked at day 14 showed an undifferentiated ESC-like morphology by expansion at serial passages. Scale bars =500 μm. (D): Immunostaining for H3K27me3 in primed and naïve-like porcine iPSCs. Histone H3K27 trimethylation spots were observed in primed porcine iPSCs (pM12-6-2, P6) cultured in serum/KOSR medium but not in naive-like iPSCs cultured in 2i/Lif medium. Scale bars =50 μm. Abbreviations: EF, embryonic fibroblast; ESC, embryonic stem cell; FIAU, Fialuridine; iPSCs, induced pluripotent stem cells.
Incomplete Removal of Transgenes from Porcine iPSCs
To obtain transgene-free porcine iPSCs, we used the HSVtk negative selection [34] on porcine iPSC clones induced from different episomal vectors, which had been confirmed to have the ability to form clones from single iPSC. According to previous studies, oriP/EBNA1-based episomal vectors replicate extrachromosomally once per cell cycle [37], and imperfect partitioning allows for the removal of episomal vectors from the primary iPSCs [25, 32, 33]. To facilitate selection of rare transgene-free iPSCs, we included built-in HSVtk negative selection cassette on our episomal vectors. We plated cells from a single primary iPS clone at low density in 10-cm dishes, followed by stringent negative fialuridine (FIAU) selection. During negative selection, more than 99% of the cells died, and only around 10 colonies survived the negative selection in each 10-cm dish. Due to low density plating and FIAU selection, these survived clones were single-cell colonies. We then picked and expanded these FIAU-resistant subclones for further analysis. Eleven iPSC clones were obtained from pMaster1 lines, while 32 and 56 clones were obtained from pMaster3 and pMaster12 lines, respectively.
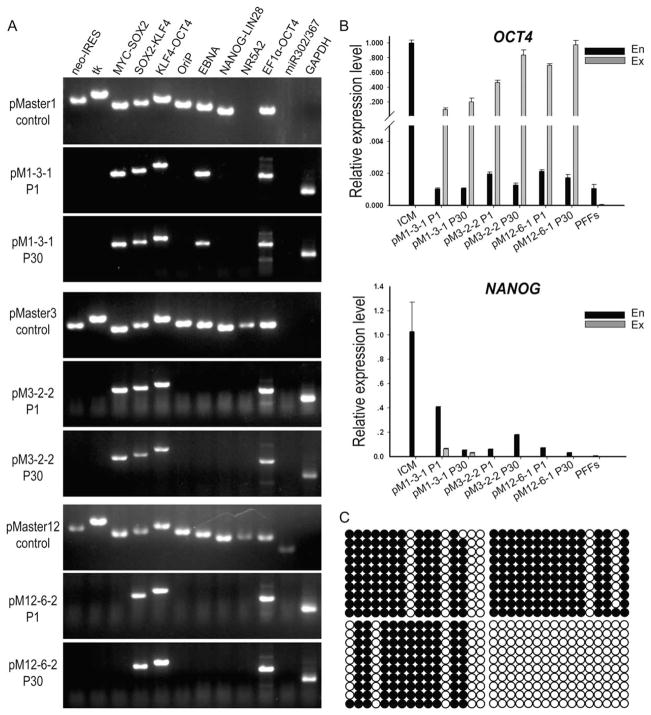
To examine whether episomal vector sequences persisted in FIAU-resistant porcine iPSCs, clones were expanded and screened by PCR using transgene-specific primer pairs (Supporting Information Table S2). Based on this rigorous PCR testing, 54.5% (54/99) of the candidate clones lacked an intact HSVtk/neo gene. Surprisingly, other gene fragments were found in these 54 iPSC clones. Although all clones derived with the pMaster12 vector (Supporting Information Fig. S2) were free of the episome backbone (OriP and EBNA1), some exogenous reprogramming genes were shown to remain intact. NANOG-LIN28, NR5A2, and the miR302/367 cluster were relatively more likely to be removed, but sequences encoding the four Yamanaka factors, C-MYC-SOX2 (90.7%, 49 out of 54), SOX2-KLF4 (88.9%, 48 out of 54), and KLF4-OCT4 (100%, 54 out of 54), were more likely to be retained. Furthermore, PCR of genomic DNA with primers specific for the exogenous human OCT4 gene confirmed retention of the OCT4 gene in all subclones (100%, 54 out of 54). Previous research suggested that transgene-free human iPSCs can be isolated through repeated passages, because OriP/EBNA1-based episomal vectors can gradually be lost from proliferating cells in the absence of selection [32]. We passaged the porcine iPSC clones pM1–3-1, pM3-2-2, and pM12-6-2, and tested for the presence of exogenous pluripotent factor genes until passage 30. However, extensive PCR analysis demonstrated that none of the porcine iPSC clones, maintained in continual cultures for more than 30 generations (about 120 days), had lost the entire episomal vector (Fig. 2A).
Figure 2.
The status of transgenes in porcine induced pluripotent stem cell (iPSC) lines derived by the episomal system. (A): PCR analysis indicates random integrations of episomal vectors. The sequences of transgenic DNA fragments were detected in clone pM1–3-1 (P1), pM1–3-1 (P30), pM3-2-2 (P1), pM3-2-2 (P30), pM12-6-2 (P1), and pM12-6-2 (P30) using 11 primer pairs covering the entire pMaster vector. (B): Quantitative PCR for exogenous and endogenous OCT4 and NANOG expression. Data are shown as relative expression to inner cell mass (ICM) from porcine in vitro fertilization blastocysts (7 days). Error bars indicate the SD generated from triplicates. (C): DNA methylation status of OCT4 in porcine iPSC pM12-6-2 (P30, upper right) and pM12-6-2 (P1, lower left), ICM from blastocysts (lower right), and PFFs (upper left) analyzed by sodium bisulfite sequencing. Open circles indicate unmethylated, and filled circles indicate methylated CpG dinucleotides, respectively. The methylation level (%) was based on the methylated CpGs/all examined CpGs. Abbreviation: PFF, porcine fetal fibroblast.
Regardless of origin, all porcine iPSCs derived using the three episomal vectors expressed the endogenous OCT4 gene at levels much lower than the integrated exogenous OCT4 transcript (Fig. 2B). CpG sites in the OCT4 promoter region were highly methylated in these iPSCs, almost on the same level with PFFs (Fig. 2C). This indicates that the endogenous OCT4 gene of iPSCs was not epigenetically reactivated. In contrast, there is robust activation of endogenous OCT4 expression in the ICM of pig blastocysts derived by in vitro fertilization (IVF). Compared with endogenous OCT4 in ICM, exogenous OCT4 in iPSCs showed a similar expression level. The integration of exogenous genes in the genome was confirmed by Southern blot analysis. These results suggest that the expression of exogenous transcription factors is necessary to maintain the morphology, stability, and pluripotency of the porcine iPSCs in 2i/Lif medium.
In contrast with OCT4, exogenous NANOG sequences were removed in some porcine iPSC lines, as demonstrated by DNA and RNA analysis. However, the endogenous NANOG gene remained in a partially activated state as determined by RNA level of endogenous NANOG in the ICM (Fig. 2B). These findings suggested that NANOG may not be indispensable to sustain porcine iPSC self-renewal in 2i/Lif medium.
Construction of Chimeric Embryos by iPSC Injection
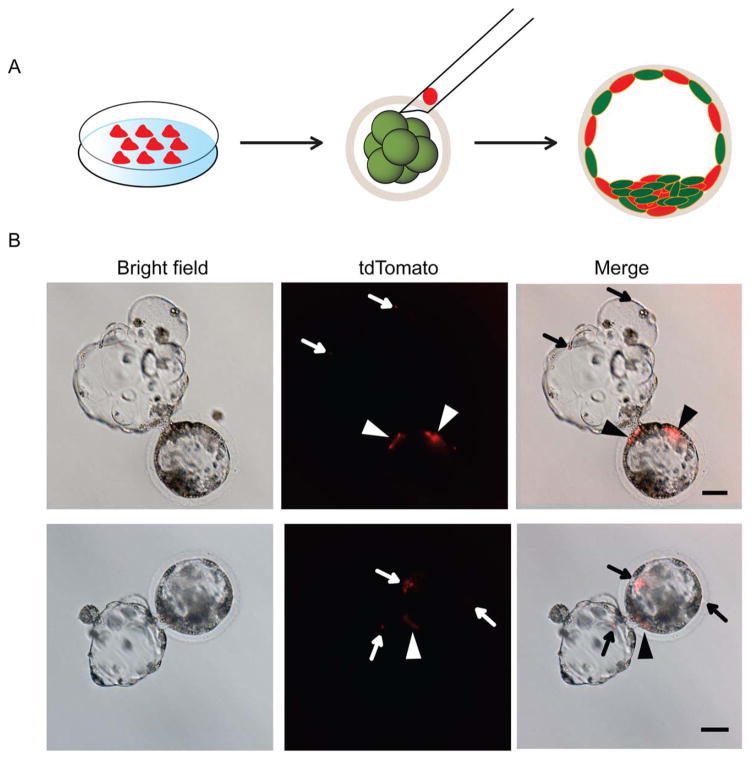
To directly observe the integration of iPSCs into preimplantation embryos, iPSCs labeled with red fluorescence (CellTracker Probes) were injected into porcine morulae derived by NT (Fig. 3A). Small clumps (15–30 cells) of porcine iPSCs were slowly injected into each morula embryo. Two days later, red fluorescent porcine iPSCs were detected in the ICM and in the TE of NT blastocysts (Fig. 3B). For chimera production, 483 chimeric embryos were transplanted into 7 surrogate uteruses. The pregnancy rate was about 28.6% (2/7), as detected by ultrasonography at 58 days after embryo transfer. The embryos in both of the pregnant recipients developed to term, and nine piglets were delivered after 114 days of gestation. However, PCR analysis on multiple tissues of each fetus to detect transgenes present in iPSCs failed to reveal any integration of iPSCs in the newborn piglets (Table 1).
Figure 3.
Morulae injection and development in vitro. (A): A schematic diagram showing production of chimeric blastocysts using red dye-tagged porcine naïve-like induced pluripotent stem cells (iPSCs) for injection into embryos. (B): Cells from the porcine iPSC line pM12-6-2 derived using an episomal vector were incorporated into the inner cell mass (arrow head) as well as into the trophectoderm (arrow) of hatching blastocysts 2 or 3 days after injection (upper). Scale bars =50 μm.
Table 1.
Injection of porcine induced pluripotent stem cells into morulae and subsequent development of embryos
| Donor cells (red dye-tagged) | Stage of host embryos | In vitro development of embryos
|
No. of recipients | In utero development of fetuses
|
|||
|---|---|---|---|---|---|---|---|
| No. of embryos injected | No. of chimeric blastocyst (%) | No. of transferred embryos | No. of pregnant recipients | No. of chimeric piglets | |||
| pM12-2-3, P5 | Morula | 320 | 263 (82.2) | – | – | – | – |
| pM12-6-2, P5 | Morula | 287 | 248 (86.4) | 483 | 7 | 2 | 0 |
| Blastomeres from NT embryos (3 days) | Morula | 53 | 47 (88.7) | – | – | – | – |
| ICM from NT embryos (7 days) | Morula | 26 | 24 (92.3) | – | – | – | – |
Abbreviations: ICM, inner cell mass; NT, nuclear transfer.
Production of Chimeras by Four-Cell Stage Embryo Aggregation
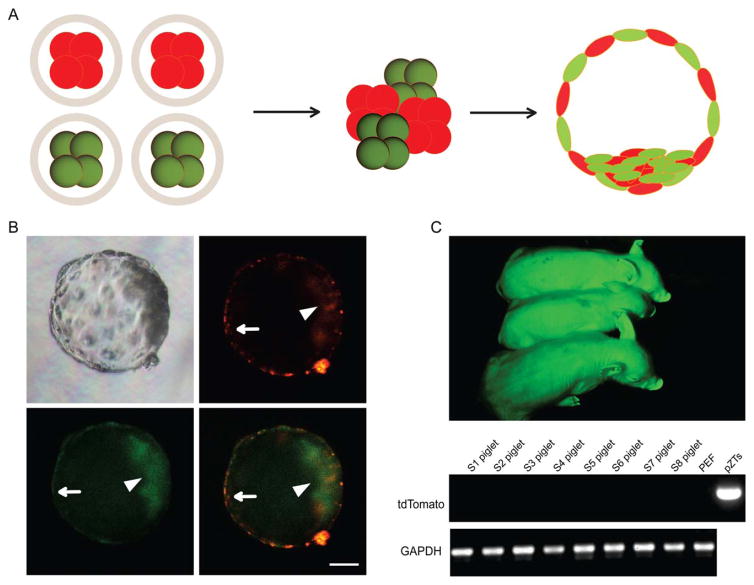
Another way to test the ability of porcine iPSCs to form chimeras is the aggregation method (Fig. 4A). To get a broader understanding and stringent validation of the chimera formation capability of the porcine iPSCs we derived, iPSCs were labeled with red fluorescence protein by piggyBac transposon vector pZT, while PFF donor cells were labeled with green fluorescence protein by piggyBac vector pZG (Supporting Information Fig. S2A). Aggregation was performed between two NT embryos derived from PFFs and two NT embryos derived from iPSCs. The aggregated embryos, cultured using the WOW system [38], were examined under a fluorescence microscope after 5 days (Supporting Information Fig. S2B). Both the ICM and the TE were composed of green and red cells, demonstrating the strong potential of iPSCs for chimera formation in vitro (Fig. 4B, Supporting Information Video S1). For chimera production, we generated 12,605 aggregates using PFF and iPSC morula stage embryos in the ratio of 2:2, and the aggregates were cultured to blastocyst stage. In summary, 1,608 aggregates (12.8%) developed to blastocysts after 5 days, of which 921 (7.3%) aggregates were chimeras based on their dual fluorescence (Table 2). The chimeric embryos were transplanted into nine synchronized recipients. The pregnancy rate was about 33.3% (3/9), as detected by ultrasonography at 62 days after embryo transfer and two recipients carried the pregnancy to term. After approximately 120 days of gestation, the surrogates delivered eight piglets naturally. None of these eight piglets showed red fluorescence, indicating no contribution of iPSC in these newborns. Further PCR analysis of viable piglets did not detect any pZT specific DNA fragments (Fig. 4C, Table 2).
Figure 4.
Construction of chimeric porcine embryos by embryo aggregation. (A): A schematic diagram showing production of chimeric blastocysts using GFP/tdTomato-expressing four-cell stage embryos. (B): Both induced pluripotent stem cell (iPSC)-derived and porcine fetal fibroblast-derived blastomeres were incorporated into the inner cell mass (arrow head) as well as the trophectoderm (arrow) of blastocysts 4 or 5 days after aggregation. Scale bar =50 μm. (C): One-week-old piglets derived from aggregated embryos. Only green fluorescence could be detected in newborns, indicating that iPSC-derived cells did not contribute to late stage chimera formation. PCR analysis using pZT-specific primers (for iPSCs) did not detect any contribution of pig iPSCs in eight fetuses (S1–S8).
Table 2.
Development of aggregated nuclear transfer embryos
| Donor cells (fluorescence protein) | Stage of host embryos | In vitro development of embryos
|
No. of recipients | In utero development of fetuses
|
|||
|---|---|---|---|---|---|---|---|
| No. of aggregated embryos | No. of blastocyst (%) | No. of chimeric blastocyst (%) | No. of pregnant recipients | No. of chimeric piglets | |||
| PFFs, P2 (GFP) | Four-cell embryo | 12,605 | 1,608 (12.8) | 921 (7.3) | 9 | 3 | 0 (0/8) |
| pM12-6-2, P5 (tdTomato) | Four-cell embryo | ||||||
Abbreviations: GFP, green fluorescent protein; PFFs, porcine fetal fibroblasts.
Construction of Cloned Embryos by Nuclear Transfer
To examine whether the porcine iPSCs could provide a better donor nucleus source for nuclear transfer (NT), due to their unlimited proliferative capacity and amenability to multiplex genetic manipulation compared to fibroblasts, we used six porcine iPSC lines generated by different episomal vectors as donor nuclei for reconstruction of NT embryos by conventional cloning (Supporting Information Fig. S3A). There was significant difference between porcine iPSC lines and PFFs with regard to fusion, cleavage, and blastocyst formation (Table 3). Of six iPSC lines, pM12-6-2 had the highest blastocyst rate with 15.1%, far lower than that of PFFs. In addition, DAPI staining demonstrated that there was also a notable difference in ratio of ICM and TE cells in reconstructed embryos between the two groups (Supporting Information Fig. S3B). To produce cloned pigs, 7,034 embryos from 6 porcine iPSC lines were introduced into 34 surrogates. Only four surrogates became pregnant as detected by ultrasonography on day 45 following embryo transfer. Three porcine iPSC lines could generate NT embryos that were able to implant, but none developed to term (Table 3). In contrast, a total of 5,307 cotransplanted cloned embryos from PFFs developed normally, with some carried to term (Table 3). This indicated that the failure of porcine iPSCs to produce cloned embryos was due to their inherent quality rather than our NT techniques.
Table 3.
Porcine induced pluripotent stem cell lines used for nuclear transfer experiments
| Donor cells | In vitro development of embryos
|
|
No. of recipients | In utero development of fetuses
|
|||
|---|---|---|---|---|---|---|---|
| Fusion rate (%) | Cleavage rate (%) | Blastocyst rate (%) | No. of transferred embryos | No. of pregnant recipients (45d) | No. of cloned piglets | ||
| pM1–3-1, P5 | 147/210 (70.0) | 112/147 (76.2) | 9/147 (6.1) | 976 | 5 | 1 | 0 |
| pM1–4-2, P5 | 154/230 (67.0) | 90/154 (58.4) | 9/154 (5.8) | 1,012 | 5 | 0 | 0 |
| pM3-2-2, P5 | 175/227 (77.1) | 129/175 (73.7) | 16/175 (9.1) | 1,104 | 5 | 0 | 0 |
| pM3–4-1, P5 | 143/219 (65.3) | 98/143 (68.5) | 8/14 (5.6) | 991 | 5 | 0 | 0 |
| pM12-2-3, P5 | 161/208 (77.4) | 122/161 (75.8) | 18/161 (11.2) | 1,446 | 7 | 1 | 0 |
| pM12-6-2, P5 | 166/205 (81.0) | 134/166 (80.7) | 25/166 (15.1) | 1,505 | 7 | 2 | 0 |
| PFFs, P3-P5 | 156/184 (84.8) | 137/156 (87.8) | 48/156 (30.8) | 5,307 | 20 | 14 | 69 |
Abbreviation: PFFs, porcine fetal fibroblasts.
Discussion
In this study, porcine iPSCs generated by episomal plasmids showed some characteristics associated with cells in a naïve state. They could contribute to the ICM and TE of chimeric blastocysts in vitro. However, we did not succeed in generating chimeric offspring with either the injection or aggregation methods. Attempts to generate cloned piglets from iPSCs through nuclear transfer also failed. In the field of human ESCs/iPSCs, the holy grail is to find the right culture conditions that can sustain human ESCs in a naive state, but the results are still inconclusive [39–42]. A major obstacle in human ESC/iPSC research is the inability to obtain in vivo data such as chimera formation and germline transmission. Therefore, the lessons learned from the behavior of porcine iPSCs in vivo may be of great significance for the induction and maintenance of human iPSC/ESC pluripotency.
First, we found that sustained expression of exogenous transcription factors was required to stabilize porcine iPSCs in cell culture. It is known that transgene-free iPSC lines in mice and humans can be established efficiently by oriP/EBNA1-based episomal plasmids [25, 32, 33]. Compared to other previously reported methods, our system has a built-in negative selection marker which helped us to obtain colonies from single cells. Despite extensive effort, no transgene-free pig iPSC subclones were ever obtained. We even tried subcloning of subclones without obtaining integration-free colonies for approximately 4 months. In comparison, with the same episomal vectors, we could readily obtain transgene-free mouse [34] and rat (unpublished data) iPSCs which were able to form chimeras. Such nonintegrated colonies were not detected for pig iPSCs suggesting that there was either the fragment of episomal plasmid DNA could be vital to porcine iPSCs and integrated into host genome, or integration-free colonies were unable to survive in the culture system in vitro.
We found that overexpression of NR5A2 and the miR302/ 367 cluster had a substantial effect on the efficiency and the timing of the reprogramming process. From detailed analysis of individual transgenes contained in the episomal vectors, it appeared that all reprogramming genes but OCT4 could be removed from porcine iPSCs. The reprogramming genes NANOG-LIN28, NR5A2, and the miR302/367 cluster were readily removed, while the four Yamanaka factor genes were almost always retained. We have shown recently in a separate study that, without ectopic expression of OCT4 by trimethoprim-inducible vectors, porcine iPSCs quickly differentiated [43]. Our results suggest that stable expression of the four exogenous Yamanaka factors, especially OCT4, is required for efficiently initiating porcine fibroblast reprogramming, sustaining an undifferentiated morphology in 2i/Lif medium and maintaining pluripotency of porcine iPSCs. Previous study in the mouse and rat also found that maintenance of Oct4 expression was crucial for successful establishment of rodent ESC lines [44]. Generation of transgenic reporter lines such as OCT4-GFP in the pig will provide essential tools for ESC derivation in large animals.
Second, sustained expression of exogenous transcription factors correlates with the absence of chimera formation potential of porcine iPSCs in vivo. To obtain adult chimaeras from mouse iPSC clones, transgenes must be strongly silenced [24, 27, 28]. Stochastic reactivation of c-Myc would lead to an elevated frequency of tumor formation in chimeric offspring [24]. Moreover, prolonged expression of transgenes during reprogramming might cause the resulting iPSCs to be unstable [45]. Continuous OCT4 expression could cause dysplasia by inhibiting cellular differentiation in a manner similar to that in embryonic cells [46]. Also, overexpression of KLF4, SOX2, or C-MYC was demonstrated to inhibit differentiation of pluripotent stem cells, and was found to drive iPSCs toward cell carcinoma [24, 47]. The constitutive expression of reprogramming genes in porcine iPSCs negatively impacts (or severely affects) their ability to form chimeras. All piglets produced by the aggregation method were derived entirely from the blastomere nuclei of PFFs, as was clearly confirmed by fluorescence microscopy and PCR.
Third, the inefficient chimera formation suggests that the porcine iPSCs have not attained an authentic pluripotent state. A key feature of stem cells in the pluripotent state is the ability to generate germline chimeric offspring [48, 49]. Only murine ESCs and iPSCs fulfill these criteria [24, 27, 50]. Efficient transgene silencing is essential for the derivation of naïve iPSC lines [24, 26, 51] and a prerequisite for normal cell differentiation [26], whereas incompletely reprogrammed iPSCs continue to express exogenous induction factors [24]. Some reports have claimed that porcine iPSCs that they have generated possessed characteristics similar to those of naive stem cells, maintained their self-renewal in 2i/Lif, and even produced chimeric fetuses [52–55]. However, no subsequent information regarding the production of adult chimeras has been reported. The failure of chimera formation and the reliance on exogenous factors to sustain self-renewal in 2i/Lif reveal that our porcine iPSCs do not constitute an authentic pluripotent state. This is likely to be a result of abolished differentiation due to constitutive expression of transgenes. Therefore, exogenous reprogramming factor silencing would probably be an essential characteristic of naive state porcine iPSCs.
Fourth, the failure to generate cloned pigs suggests that porcine iPSCs have an impaired ability to differentiate. Previous nuclear transfer experiments showed that viable and fertile pigs could be produced from different types of nuclear donors, including pronuclei containing karyoplasts [56], primordial germ cells [57], blastomere nuclei [58], and adult somatic cells [59]. However, whether porcine iPSCs are capable of generating offspring through nuclear transfer had not been tested. Ma et al. recently reported that genetic abnormalities may give rise to abnormal DNA methylation and transcriptome profiles in iPSCs compared to NT ESCs and IVF ESCs [60]. Similarly, porcine iPSCs that continue to express reprogramming genes would display abnormal pluripotency gene expression levels, telomere shortening [61], cell cycle disorder [62], and incomplete demethylation of pluripotency genes [63]. Any of these abnormalities could cause a failure to generate cloned piglets. In our experiments, although the porcine iPSCs retained normal karyotypes, we still failed (34 different iPSC lines, and >7,000 cloned embryos transferred) to produce cloned piglets by nuclear transfer (Table 3). In addition, bisulfite sequencing revealed that the promoter region of endogenous OCT4 was highly methylated in these porcine iPSCs. We conclude that the greatest barriers to cloned piglet formation from porcine iPSCs are the ectopic expression of exogenous transcription factors and hypermethylation of the endogenous OCT4 promoter region which affect the development and differentiation of iPSCs during implantation and later stages.
More importantly, as in vivo data are lacking for ESC/iPSC studies of humans, that is, no chimera/germline transmission data could be ethically and legally obtained, the true status of human ESCs/iPSCs are not determined. Therefore, large animal in vivo data on ESCs/iPSCs may offer some insight into the true stemness of human ESCs/iPSCs. In this manuscript, we present results of our extensive in vivo experiments with pig iPSCs. We found that incomplete reprogramming manifested by a reliance on sustained expression of exogenous-reprogramming factors appeared to be the main reason for the inability of porcine iPSCs to form iPSC-derived piglets. The abnormalities in pig iPSCs may include those in DNA methylation, histone modification, imprinting, and X chromosome inactivation. Elucidation of these abnormalities in the future should help us develop better reprogramming methods and culture conditions for eventual derivation of genuine pig iPSCs as well as ESCs. Similar to pigs, no germline-competent iPS or ESCs have been derived for cattle, goats, and sheep [64]. If barriers of deriving high quality iPS/ESCs can be solved in pigs, the solution may benefit other large animals including humans.
Conclusions
Episomal reprogramming plasmids have not been able to produce transgene-free pig iPSCs. Persistent expression of reprogramming genes in porcine iPSCs is required to maintain self-renewal with the current culture method. These partially transgene-free iPSCs cannot generate cloned piglets or contribute to late stage chimeric formation. The findings presented here contribute to our understanding of the obstacles in deriving genuine pluripotent stem cells for pigs, and other large animals as well.
Supplementary Material
Significance Statement.
Although pigs are becoming increasingly important animal models for human diseases and organ donors, no authentic pig ES cell line or germline-competent iPS cell line has been established. More importantly, as in vivo data are lacking for ESC/iPSC studies of humans, the true stemness status of human ES/iPS cells is not determined. Therefore, large animal in vivo data on ES/iPS cells may offer some insight of the true stemness of human ES/iPS cells, and provide clues on how genuine ES/iPS cells may be obtained for large animals including humans.
Acknowledgments
We thank Shuping Li, Chunlong Xu, Linlin Li, Qiuyan Li, Yan Li, and Huixia Song for help in cell culture and embryo manipulation; Qian Zhao and Jingxin Wang for everyday support of this project; and Anne Boulet for reading the manuscript. This work was supported by National Key Basic Research Program 2011CBA01002, the National High Technology Research and Development Program (2013AA102502), and Transgenic Research Grant 2013ZX08010-001.
Footnotes
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
Author Contributions
X.D.: conception and design, collection and/or assembly of data, data analysis and interpretation, and manuscript writing; T.F. and D.Y.: conception and design, collection and/or assembly of data, and data analysis and interpretation; Y.W.: conception and design; H.Z. and S.M.: collection and/or assembly of data; C.F., Y.H., H.O., X.H., and D.P.: provision of study material; N.L.: provision of study material, data analysis and interpretation, and financial support; S.W.: conception and design, financial support, data analysis and interpretation, manuscript writing, and final approval of manuscript; X.D., T.F., and D.Y. contributed equally to this article.
See www.StemCells.com for supporting information available online.
References
- 1.Prather RS, Hawley RJ, Carter DB, et al. Transgenic swine for biomedicine and agriculture. Theriogenology. 2003;59:115–123. doi: 10.1016/s0093-691x(02)01263-3. [DOI] [PubMed] [Google Scholar]
- 2.Brandl U, Michel S, Erhardt M, et al. Transgenic animals in experimental xenotransplantation models: Orthotopic heart transplantation in the pig-to-baboon model. Transplant Proc. 2007;39:577–578. doi: 10.1016/j.transproceed.2006.12.021. [DOI] [PubMed] [Google Scholar]
- 3.Piedrahita JA, Mir B. Cloning and transgenesis in mammals: Implications for xenotransplantation. Am J Transplant. 2004;4:43–50. doi: 10.1111/j.1600-6135.2004.0344.x. [DOI] [PubMed] [Google Scholar]
- 4.Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 5.Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci USA. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buehr M, Meek S, Blair K, et al. Capture of authentic embryonic stem cells from rat blastocysts. Cell. 2008;135:1287–1298. doi: 10.1016/j.cell.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 7.Liao J, Cui C, Chen S, et al. Generation of induced pluripotent stem cell lines from adult rat cells. Cell Stem Cell. 2008;4:11–15. doi: 10.1016/j.stem.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 8.Thomson JA, Kalishman J, Golos TG, et al. Isolation of a primate embryonic stem cell line. Proc Natl Acad Sci USA. 1995;92:7844–7848. doi: 10.1073/pnas.92.17.7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 10.Piedrahita JA, Anderson GB, Bondurant RH. On the isolation of embryonic stem-cells—Comparative behavior of murine, porcine and ovine embryos. Theriogenology. 1990;34:879–901. doi: 10.1016/0093-691x(90)90559-c. [DOI] [PubMed] [Google Scholar]
- 11.Notarianni E, Laurie S, Moor RM, et al. Maintenance and differentiation in culture of pluripotential embryonic-cell lines from pig blastocysts. J Reprod Fertil. 1990;41:51–56. [PubMed] [Google Scholar]
- 12.Strojek RM, Reed MA, Hoover JL, et al. A method for cultivating morphologically undifferentiated embryonic stem-cells from porcine blastocysts. Theriogenology. 1990;33:901–913. doi: 10.1016/0093-691x(90)90825-e. [DOI] [PubMed] [Google Scholar]
- 13.Telugu B, Ezashi T, Roberts RM. The promise of stem cell research in pigs and other ungulate species. Stem Cell Rev. 2010;6:31–41. doi: 10.1007/s12015-009-9101-1. [DOI] [PubMed] [Google Scholar]
- 14.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 15.Zhou S, Ding C, Zhao X, et al. Successful generation of cloned mice using nuclear transfer from induced pluripotent stem cells. Cell Res. 2010;20:850–853. doi: 10.1038/cr.2010.78. [DOI] [PubMed] [Google Scholar]
- 16.Kou Z, Kang L, Yuan Y, et al. Mice cloned from induced pluripotent stem cells (iPSCs) Biol Reprod. 2010;83:238–243. doi: 10.1095/biolreprod.110.084731. [DOI] [PubMed] [Google Scholar]
- 17.Zhao X-y, Li W, Lv Z, et al. iPS cells produce viable mice through tetraploid complementation. Nature. 2009;461:86–90. doi: 10.1038/nature08267. [DOI] [PubMed] [Google Scholar]
- 18.Boland MJ, Hazen JL, Nazor KL, et al. Adult mice generated from induced pluripotent stem cells. Nature. 2009;461:91–U94. doi: 10.1038/nature08310. [DOI] [PubMed] [Google Scholar]
- 19.Wu Z, Chen JJ, Ren JT, et al. Generation of pig induced pluripotent stem cells with a drug-inducible system. J Mol Cell Biol. 2009;1:46–54. doi: 10.1093/jmcb/mjp003. [DOI] [PubMed] [Google Scholar]
- 20.Esteban MA, Xu JY, Yang JY, et al. Generation of induced pluripotent stem cell lines from Tibetan miniature pig. J Biol Chem. 2009;284:17634–17640. doi: 10.1074/jbc.M109.008938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ezashi T, Telugu B, Alexenko AP, et al. Derivation of induced pluripotent stem cells from pig somatic cells. Proc Natl Acad Sci USA. 2009;106:10993–10998. doi: 10.1073/pnas.0905284106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.West FD, Terlouw SL, Kwon DJ, et al. Porcine induced pluripotent stem cells produce chimeric offspring. Stem Cells Dev. 2010;19:1211–1220. doi: 10.1089/scd.2009.0458. [DOI] [PubMed] [Google Scholar]
- 23.Fan NN, Chen JJ, Shang ZC, et al. Piglets cloned from induced pluripotent stem cells. Cell Res. 2013;23:162–166. doi: 10.1038/cr.2012.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- 25.Zhao TB, Zhang ZN, Rong ZL, et al. Immunogenicity of induced pluripotent stem cells. Nature. 2011;474:212–U251. doi: 10.1038/nature10135. [DOI] [PubMed] [Google Scholar]
- 26.Brambrink T, Foreman R, Welstead GG, et al. Sequential expression of pluripotency markers during direct reprogramming of mouse somatic cells. Cell Stem Cell. 2008;2:151–159. doi: 10.1016/j.stem.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maherali N, Sridharan R, Xie W, et al. Directly reprogrammed fibroblasts show global epigenetic remodeling and widespread tissue contribution. Cell Stem Cell. 2007;1:55–70. doi: 10.1016/j.stem.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 28.Wernig M, Meissner A, Foreman R, et al. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007;448:318–324. doi: 10.1038/nature05944. [DOI] [PubMed] [Google Scholar]
- 29.Gonzalez F, Monasterio MB, Tiscornia G, et al. Generation of mouse-induced pluripotent stem cells by transient expression of a single nonviral polycistronic vector. Proc Natl Acad Sci USA. 2009;106:8918–8922. doi: 10.1073/pnas.0901471106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okita K, Nakagawa M, Hong HJ, et al. Generation of mouse induced pluripotent stem cells without viral vectors. Science. 2008;322:949–953. doi: 10.1126/science.1164270. [DOI] [PubMed] [Google Scholar]
- 31.Si-Tayeb K, Noto FK, Sepac A, et al. Generation of human induced pluripotent stem cells by simple transient transfection of plasmid DNA encoding reprogramming factors. BMC Dev Biol. 2010;10:81. doi: 10.1186/1471-213X-10-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu JY, Hu KJ, Smuga-Otto K, et al. Human induced pluripotent stem cells free of vector and transgene sequences. Science. 2009;324:797–801. doi: 10.1126/science.1172482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okita K, Matsumura Y, Sato Y, et al. A more efficient method to generate integration-free human iPS cells. Nat Methods. 2011;8:409–412. doi: 10.1038/nmeth.1591. [DOI] [PubMed] [Google Scholar]
- 34.Wu S, Wu YY, Zhang X, et al. Efficient germ-line transmission obtained with transgene-free induced pluripotent stem cells. Proc Natl Acad Sci USA. 2014;111:10678–10683. doi: 10.1073/pnas.1409933111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu S, Ying G, Wu Q, et al. A protocol for constructing gene targeting vectors: Generating knockout mice for the cadherin family and beyond. Nat Protoc. 2008;3:1056–1076. doi: 10.1038/nprot.2008.70. [DOI] [PubMed] [Google Scholar]
- 36.Szymczak AL, Workman CJ, Wang Y, et al. Correction of multi-gene deficiency in vivo using a single ‘self-cleaving’ 2A peptide-based retroviral vector. Nat Biotechnol. 2004;22:589–594. doi: 10.1038/nbt957. [DOI] [PubMed] [Google Scholar]
- 37.Nanbo A, Sugden A, Sugden B. The coupling of synthesis and partitioning of EBV’s plasmid replicon is revealed in live cells. EMBO J. 2007;26:4252–4262. doi: 10.1038/sj.emboj.7601853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vajta G, Peura TT, Holm P, et al. New method for culture of zona-included or zona-free embryos: The Well of the Well (WOW) system. Mol Reprod Dev. 2000;55:256–264. doi: 10.1002/(SICI)1098-2795(200003)55:3<256::AID-MRD3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 39.Gafni O, Weinberger L, Mansour AA, et al. Derivation of novel human ground state naive pluripotent stem cells. Nature. 2013;504:282–286. doi: 10.1038/nature12745. [DOI] [PubMed] [Google Scholar]
- 40.Chan YS, Goke J, Ng JH, et al. Induction of a human pluripotent state with distinct regulatory circuitry that resembles preimplantation epiblast. Cell Stem Cell. 2013;13:663–675. doi: 10.1016/j.stem.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 41.Ware CB, Nelson AM, Mecham B, et al. Derivation of naive human embryonic stem cells. Proc Natl Acad Sci USA. 2014;111:4484–4489. doi: 10.1073/pnas.1319738111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Theunissen TW, Jaenisch R. Molecular control of induced pluripotency. Cell Stem Cell. 2014;14:720–734. doi: 10.1016/j.stem.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sui DD, Sun ZL, Xu CL, et al. Fine-tuning of iPSC derivation by an inducible reprogramming system at the protein level. Stem Cell Reports. 2014;2:721–733. doi: 10.1016/j.stemcr.2014.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Buehr M, Nichols J, Stenhouse F, et al. Rapid loss of Oct-4 and pluripotency in cultured rodent blastocysts and derivative cell lines. Biol Reprod. 2003;68:222–229. doi: 10.1095/biolreprod.102.006197. [DOI] [PubMed] [Google Scholar]
- 45.Okada M, Yoneda Y. The timing of retroviral silencing correlates with the quality of induced pluripotent stem cell lines. Biochim Biophys Acta. 2011;1810:226–235. doi: 10.1016/j.bbagen.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 46.Hochedlinger K, Yamada Y, Beard C, et al. Ectopic expression of Oct-4 blocks progenitor-cell differentiation and causes dysplasia in epithelial tissues. Cell. 2005;121:465–477. doi: 10.1016/j.cell.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 47.Nair V. Retrovirus-induced oncogenesis and safety of retroviral vectors. Curr Opin Mol Ther. 2008;10:431–438. [PubMed] [Google Scholar]
- 48.Bradley A, Evans M, Kaufman MH, et al. Formation of germ-line chimeras from embryo-derived teratocarcinoma cell-lines. Nature. 1984;309:255–256. doi: 10.1038/309255a0. [DOI] [PubMed] [Google Scholar]
- 49.Gardner RL. Contributions of blastocyst micromanipulation to the study of mammalian development. Bioessays. 1998;20:168–180. doi: 10.1002/(SICI)1521-1878(199802)20:2<168::AID-BIES9>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 50.Wernig M, Meissner A, Foreman R, et al. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007;448:318–U312. doi: 10.1038/nature05944. [DOI] [PubMed] [Google Scholar]
- 51.Stadtfeld M, Maherali N, Breault DT, et al. Defining molecular cornerstones during fibroblast to iPS cell reprogramming in mouse. Cell Stem Cell. 2008;2:230–240. doi: 10.1016/j.stem.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fujishiro S, Nakano K, Mizukami Y, et al. Generation of naive-like porcine-induced pluripotent stem cells capable of contributing to embryonic and fetal development. Stem Cells Dev. 2013;22:473–482. doi: 10.1089/scd.2012.0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Telugu BPVL, Ezashi T, Sinha S, et al. Leukemia inhibitory factor (LIF)-dependent, pluripotent stem cells established from inner cell mass of porcine embryos. J Biol Chem. 2011;286:28948–28953. doi: 10.1074/jbc.M111.229468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thomson AJ, Pierart H, Meek S, et al. Reprogramming pig fetal fibroblasts reveals a functional LIF signaling pathway. Cell Reprogram. 2012;14:112–122. doi: 10.1089/cell.2011.0078. [DOI] [PubMed] [Google Scholar]
- 55.Rodriguez A, Allegrucci C, Alberio R. Modulation of pluripotency in the porcine embryo and iPS cells. PLoS One. 2012;7:e49079. doi: 10.1371/journal.pone.0049079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Prather RS, Sims MM, First NL. Nuclear transplantation in early pig embryos. Biol Reprod. 1989;41:414–418. doi: 10.1095/biolreprod41.3.414. [DOI] [PubMed] [Google Scholar]
- 57.Liu L, Moor RM, Laurie S, et al. Nuclear remodelling and early development in cryopreserved, porcine primordial germ cells following nuclear transfer into in vitro-matured oocytes. Int J Dev Biol. 1995;39:639–644. [PubMed] [Google Scholar]
- 58.Polejaeva IA, Chen S-H, Vaught TD, et al. Cloned pigs produced by nuclear transfer from adult somatic cells. Nature. 2000;407:86–90. doi: 10.1038/35024082. [DOI] [PubMed] [Google Scholar]
- 59.Onishi A, Iwamoto M, Akita T, et al. Pig cloning by microinjection of fetal fibroblast nuclei. Science. 2000;289:1188–1190. doi: 10.1126/science.289.5482.1188. [DOI] [PubMed] [Google Scholar]
- 60.Ma H, Morey R, O’Neil RC, et al. Abnormalities in human pluripotent cells due to reprogramming mechanisms. Nature. 2014;511:177–183. doi: 10.1038/nature13551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ji GZ, Ruan WM, Liu K, et al. Telomere reprogramming and maintenance in porcine iPS cells. PLoS One. 2013;8:e74202. doi: 10.1371/journal.pone.0074202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yuan Y, Lee K, Park K-W, et al. Cell cycle synchronization of leukemia inhibitory factor (LIF)-dependent porcine-induced pluripotent stem cells and the generation of cloned embryos. Cell Cycle. 2014;13:1265–1276. doi: 10.4161/cc.28176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Arai Y, Ohgane J, Fujishiro S, et al. DNA methylation profiles provide a viable index for porcine pluripotent stem cells. Genesis. 2013;51:763–776. doi: 10.1002/dvg.22423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ezashi T, Telugu BP, Roberts RM. Induced pluripotent stem cells from pigs and other ungulate species: An alternative to embryonic stem cells? Reprod Domest Anim. 2012;47(suppl 4):92–97. doi: 10.1111/j.1439-0531.2012.02061.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.