Abstract
Aedes aegypti is the primary vector of several medically relevant arboviruses including dengue virus (DENV) types 1–4. Ae. aegypti transmits DENV by inoculating virus-infected saliva into host skin during probing and feeding. Ae. aegypti saliva contains over one hundred unique proteins and these proteins have diverse functions, including facilitating blood feeding. Previously, we showed that Ae. aegypti salivary gland extracts (SGEs) enhanced dissemination of DENV to draining lymph nodes. In contrast, HPLC-fractionation revealed that some SGE components inhibited infection. Here, we show that D7 proteins are enriched in HPLC fractions that are inhibitory to DENV infection, and that recombinant D7 protein can inhibit DENV infection in vitro and in vivo. Further, binding assays indicate that D7 protein can directly interact with DENV virions and recombinant DENV envelope protein. These data reveal a novel role for D7 proteins, which inhibits arbovirus transmission to vertebrates through a direct interaction with virions.
Author Summary
Dengue virus (DENV) is transmitted to humans by Aedes aegypti during the blood feeding process. During blood feeding, DENV and saliva proteins are inoculated into human skin. D7 proteins are prevalent and immunogenic proteins present in Ae. aegypti saliva, and assist the blood feeding process by scavenging biogenic amines. Previous data suggests that antibodies against D7 protein from Culex spp. can increase West Nile virus infection. We hypothesized that D7 proteins may also have antiviral activity. Here, we show that recombinant Ae. aegypti D7 protein can inhibit DENV infection in vitro and in vivo, and that D7 can bind to DENV virions.
Introduction
Dengue virus (DENV) is a mosquito-borne arbovirus that is transmitted primarily by the species Aedes aegypti. The global burden of DENV has grown dramatically in the last few decades [1, 2]. We now expect approximately one hundred million clinically recognized cases of disease each year. Targeted therapeutics do not exist. Fortunately, conventional vaccines are in development and regulatory approval has been granted in a few countries. Development of a conventional DENV vaccine has been difficult due to the co-circulation of four serotypes [3, 4]. It is critical that conventional vaccines elicit robust antibody titers to avoid antibody-dependent enhancement, which occurs during a sub-neutralizing response. It is theoretically possible to target mosquito saliva or midgut proteins to block either transmission or acquisition of DENV [3, 5, 6]. This strategy would not be subject to antibody-dependent enhancement or viral genetic drift.
Ae. aegypti saliva contains over one hundred unique proteins that have been classified as D7 proteins, phosphatidylethanolamine binding proteins, odorant and juvenile hormone binding proteins, serpins and other protease inhibitors, a sialokinin vasodilator, nucleotidases, serine proteases, sugar digestion related proteins and other enzymes, lectins, angiopoietins, anti-microbial proteins and peptides, mucins and peritrophins, antigen 5 proteins, and many more proteins of unknown function [7–11]. Functional data is not available for the majority of these proteins, although it is expected that the saliva of all hematophagous arthropods have anti-coagulant, anti-platelet, and vasodilatory activities. It is also likely that saliva proteins serve to reduce host inflammation and prevent infection.
In addition to the normal physiological roles of hematophagous arthropod saliva, many vector-borne microorganisms have enhanced fitness in the presence of arthropod saliva. Arthropod saliva can enhance infectivity of West Nile virus, DENV, Rift Valley fever virus, and Powassan virus, among others [5, 12–18]. The exact mechanism of saliva-mediated infectivity enhancement is not known, although prior literature suggests that saliva proteins may locally modify the immune system in favor of arbovirus replication and/or stimulate dissemination by enhancing migration of target cells to draining lymph nodes [3]. Interestingly, individual saliva components can have inhibitory activities against arbovirus infection. For instance, the collagen-binding protein aegyptin decreased DENV infection in vivo [19]. Additionally, previous literature showed that vaccination of mice with a recombinant D7 protein from Culex spp. enhanced mortality in a West Nile virus mouse model, suggesting that D7 protein may be inhibitory in vivo [20]. Structural studies suggest that D7 proteins can simultaneously bind biogenic amines and cysteinyl leukotrienes, which is likely involved in preventing the host inflammatory response [21, 22]. Prevention of the host inflammatory response may reduce influx or activation of target cells.
Our previous work relied on high performance liquid chromatography (HPLC) to fractionate Ae. aegypti salivary gland extracts (SGEs) [5]. HPLC fractions were tested to see if they had virus enhancing or blocking activities in vitro. Here, we identified a number of fractions that inhibited DENV cell binding and then pooled these fractions for downstream tandem liquid chromatography tandem mass spectrometry (LC+MS/MS) analysis. D7 proteins were the most abundant proteins in the inhibitory fractions. We synthesized a recombinant D7 protein and found that it inhibited DENV infection in vitro and in vivo. Additionally, the D7 protein interacted directly with DENV virions and recombinant envelope protein. These data support a model whereby D7 proteins inhibit DENV infection through two independent mechanisms: (i) direct binding and neutralization of DENV virions, and (ii) inhibition of immune cell infiltration or activation, which reduces the number of permissive target cells. Characterization of virus-vector-host interactions at the transmission interface will further development of arthropod-based therapeutics and transmission-blocking vaccines.
Methods
Mosquito rearing and saliva material collection
Aedes aegypti were provided by staff at the Connecticut Agricultural Experiment Station. Mosquitoes were maintained in a sugar solution at 27°C and 80% humidity according to standard rearing procedures. Salivary glands and saliva were isolated as described previously [5]. Salivary gland extracts were prepared by placing 100 salivary glands in 100 μl sterile phosphate-buffered saline (PBS), freeze-thawing by placing on dry ice three times, and then removing insoluble debris by centrifugation at 5,000 × g for 10 min. Saliva was isolated using the immersion oil technique.
Cell culture and virus stocks
Mouse embryonic fibroblasts (MEFs) and a human monocyte-like (U937) cell line from the American Type Culture Collection were maintained in Dulbecco's modified Eagle medium (DMEM) containing 10% fetal bovine serum and antibiotics at 37°C with 5% CO2 (Gibco). C6/36 cells were maintained in DMEM containing 10% fetal bovine serum, tryptose phosphate, and antibiotics at 30°C. DENV2 was passaged in C6/36 cells. DENV2 New Guinea C strain was obtained from the Connecticut Agricultural Experiment Station and C6/36 cells were a kind gift from Erol Fikrig. Approximately 1 × 105 genome equivalents (GE) were used for in vitro infections of MEFs and U937 cells.
HPLC fractionation and LC+MS/MS
One hundred salivary glands were dissected from female Ae. aegypti and placed in 100 μl PBS. The sample was freeze-thawed three times at −80°C, and insoluble debris was pelleted by centrifugation at 5,000 × g for 10 min. The supernatant was reserved. SGE was either processed directly for LC+MS/MS analysis or fractionated by high-performance liquid chromatography (HPLC) on a nonporous reverse-phase column with a TFA buffer system into 80 100-μl fractions. Ten μl of each fraction was diluted into 90 μl PBS and used as SGE treatments for in vitro SGE-mediated cell binding assays as stated below. The remaining 90 μl from inhibitory fractions 31–49 were pooled and submitted for liquid chromatography tandem mass spectrometry (LC+MS/MS) analysis.
Proteins were digested with trypsin and analyzed using LC+MS/MS on a Thermo Scientific LTQ-Orbitrap XL mass spectrometer using Waters nanoACQUITY ultra-high-pressure liquid chromatographs (UPLC) for peptide separation. MS/MS spectra were searched in-house using the Mascot algorithm for uninterpreted MS/MS spectra after using the Mascot Distiller program to generate Mascot-compatible files. An A. aegypti database was used for searching. The Keck Biotechnology Resource at Yale University performed both HPLC and LC+MS/MS.
In vitro cell binding assay
MEFs were seeded at 25,000 cells/well in 48-well plates and grown to approximately 70% confluence overnight. Medium was aspirated, and cells were washed with PBS. Ten μl of HPLC fractions were diluted in a total volume of 100 μl PBS and inoculated into cells at room temperature for 10 min. Saliva material was removed, and then Approximately 1 × 105 GE of DENV2 was inoculated into cells in a total volume of 500 μl for 1 h at 37°C. Unbound virus was then removed, and fresh medium was added. Infections progressed for up to 18 h. Total RNA was extracted using RNeasy kits (Qiagen).
For analysis of relative DENV vRNA, total RNA was harvested using RNeasy kits (Qiagen). Amplification of both the viral target and reference gene target was performed using a duplex format in 0.2-ml, 96-well PCR plates (Bio-Rad) with a total reaction volume of 25 μl. Reverse transcription and quantitative PCR (RT-qPCR) were performed in the same closed tube with 250 ng of total RNA per reaction using the Quantitect RT-PCR kit (Qiagen).
All primers were used at a final concentration of 4 μM and were synthesized by the Keck Facility at Yale University. DENV2 vRNA was amplified using F 5’ CCACTGCCTCTGGAAAACTC 3’ and R 5’ GTACCAGCACCCATCCTCAC 3’ primers. Primers were developed using Gene Link Software (OligoAnalyzer 1.2 and OligoExplorer 1.2). All RT-qPCRs were performed using an iQ5 machine (Bio-Rad). Cycling conditions were 50°C for 30 min (reverse transcription) and 95°C for 15 min, followed by 42 cycles of 94°C for 15 s and 54.5°C for 1 min. Relative quantities of viral target cDNA were determined using REST software.
Cloning
Ae. aegypti total cellular RNA was converted to cDNA using random primers and the Superscript III First-Strand Synthesis System (ThermoFisher). Long form D7 protein (AAEL006424) was amplified using F 5’ GGAGGTACCGATGAAGCTGCCTCTATTACTCGCAATAGTTAC 3’ and R 5’ GGAGCGGCCGCAATTGTGGACACTGTTTACCGTCG 3’ primers and cloned into the pMT/BiP/V5-His A plasmid via BamHI and NotI restriction sites. pMT/BiP/D7/V5-His A and pCoHygro plasmids were transfected into S2 cells using the calcium phosphate method according to manufacturers’ instructions (ThermoFischer) and a stable cell line was generated through hygromycin selection. D7 synthesis and secretion was induced by treating cells with copper sulfate according to manufacturer’s instructions (ThermoFisher). D7 expression was confirmed by Coomassie Blue gel staining and Western blot using an anti-6 His antibody. Supernatants were also harvested from uninduced S2 cell supernatants and used as negative controls.
In vitro infectivity assay
D7 protein was purified using HisPur Cobalt Spin Columns (Thermo Scientific/Pierce, MA) according to manufacturer instructions. There were a total of 3 washes and 3 elutions. Samples were stored at -80°C until use. The U937 cell line (ATCC, VA) was used for the in vitro infection studies. The cells were grown at 37°C and 5% CO2 in DMEM supplemented with 10% fetal bovine serum (Gemini, CA), and 1% penicillin-streptomycin. D7 was used in 1/10, 1/100, and 1/1000 dilutions in complete media for final concentrations of 8, 0.8, and 0.08 ng/mL, respectively. Dilutions were added for pretreatment of cells in a total volume of 250 μL. Cells were then incubated for 1 hour at 37°C and then DENV was added to cells at a multiplicity of infection (MOI) of 1.0. For simultaneous treatments, ten-fold dilutions of D7 were mixed with DENV2 and these mixtures were pre-incubated for 1 hour at 37°C. D7-DENV2 mixtures were then inoculated onto cells. Unbound virions were removed by washing after 1 hour and then cells were incubated for 24 hours at 37°C. RNA was isolated from cells and qRT-PCR performed as described above. DENV2 vRNA was amplified using Forward: 5’ CAG ATC TCT GAT GAA TAA CCA ACG 3’ and Reverse: 5’ CAT TCC AAG TGA GAA TCT CTT TGT CA 3’ primers. Human B2M RNA was amplified using Forward: 5’ CTC CGT GGC CTT AGC TGT G 3’ and Reverse: 5’ TTT GGA GTA CGC TGG ATA GCC T 3’ primers.
Ethics statement
Animals were maintained and procedures were performed in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Research Council. Protocol 14051879 was approved by the University of Pittsburgh's IACUC committee. Approved euthanasia criteria were based on weight loss and morbidity.
In vivo infectivity assay
Mice deficient in receptors for type I and type II interferons, (IFNAGR-/-, AGB6) were bred under specific pathogen-free conditions. Groups of 4-5-week-old, age-matched, mixed-sex AGB6 mice were inoculated subcutaneously into both rear footpads with 20 μl containing either 107 genome equivalents (GE) of either DENV2 alone or DENV2 plus recombinant D7 protein. DENV2 alone samples contained purified S2 cell supernatant without D7 protein as a vehicle control. Forty-eight hours post-infection, mice were euthanized and left and right foot pads and left and right popliteal draining lymph nodes (DLN) were collected independently. Total RNA was extracted using an RNeasy kit (Qiagen). qRT-PCR was performed by adding equal amounts of RNA into each reaction and data were normalized to the actin reference gene. Mouse actin RNA was amplified using Forward: 5’ GGC TGT ATT CCC CTC CAT CG 3’ and Reverse: 5’ CCA GTT GGT AAC AAT GCC ATG T 3’ primers. Amplification of targets were performed using a duplex format in 0.2 ml, 96-well PCR plates (BIO-RAD) with a total reaction volume of 25 μl. Reverse transcription and quantitative PCR were performed in the same closed tube with 100 ng of total RNA per reaction using the Quantitect RT-PCR Kit (Qiagen).
Salivary gland extract-DENV virion binding assay
Seventy μg of anti-dengue virus type II antibody, clone 3H5-1 (Millipore) was covalently coupled to 25 μL amine-active resin according to the Pierce Co-Immunoprecipitation Kit manual (ThermoFisher Scientific). One hundred μg purified, formaldehyde-inactivated dengue virus type 2 virions (Microbix) was then mixed with 100 Ae. aegypti salivary gland equivalents and added to the antibody-containing resin with gentle end-over-end mixing for 2 hr at 4°C. Unbound proteins were washed away and bound proteins were eluted in a final volume of 50 μL. The experimental details are described in the Pierce Co-Immunoprecipitation Kit manual. Eluted proteins were identified by LC+MS/MS analysis as stated above.
Enzyme-linked immunosorbent assay
High binding 96-well plates were coated overnight at 4°C with a 1:1 dilution of D7 or matrix metalloproteinase protein (MMP; AAEL003012) in coating buffer (R&D systems) for a 0.01mg/mL final concentration. The next day, plates were rinsed twice with PBS and blocked with blocking buffer (1% BSA in 1XPBST) for 1 h at 37. Plates were incubated with 80μL of DENV 2 strain 186881 (2x105 p.f.u) or recombinant DENV Envelope protein (1μg/mL) (L2 Diagnostics, CT) overnight at 4°C. Plates were washed three times with buffer (1X PBST+ 0.01% Tween 20) and incubated with a 1:250 dilution of primary antibody for 1h at 37°C: mouse anti-DENV2 antibody (MAB10226, Millipore) to detect virus and mouse anti-E antibody (MA1-71251, Pierce) to detect envelope protein. After washing plates three times, wells were incubated with 100 μL of a 1:1000 dilution of anti-mouse IgG, HRP-linked antibody (Cell Signaling Technologies) and washed after 1h of incubation at 37°C. Reaction was visualized after incubating with 80 μL of TMB. After 3min the reaction was stopped with stop solution (2M Sulfuric acid) and plates were read at 450nm in a Synergy HT plate reader (Biotek Instruments Inc., VT).
Results
Identification of D7 proteins in inhibitory salivary gland extract high performance liquid chromatography fractions
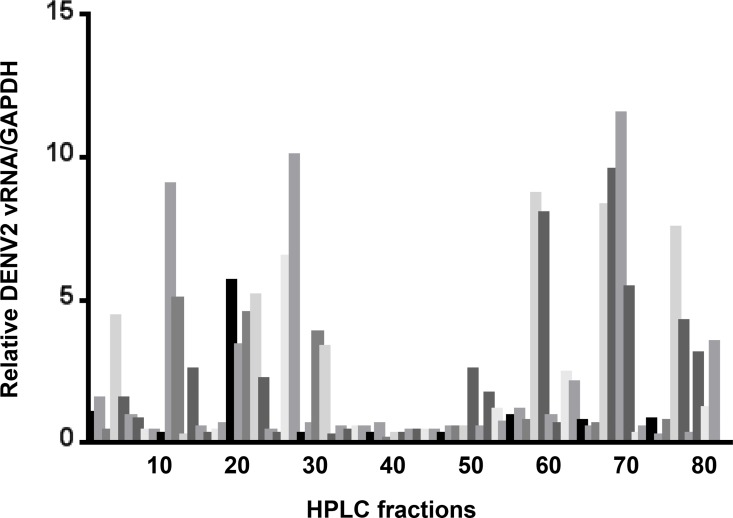
We previously showed that pre-treatment of mouse embryonic fibroblasts (MEFs) with salivary gland extract (SGE) increased DENV cell binding [5]. Reverse phase HPLC fractionation and LC+MS/MS analysis was then used to identify fractions of Ae. aegypti salivary gland extract (SGE) that increased DENV vRNA levels associated with mouse embryonic fibroblasts (MEFs)[5]. During this analysis, we also noted that a cluster of HPLC fractions (i.e., 31–49) inhibited DENV infection (Fig 1). These fractions were pooled and submitted for LC+MS/MS analysis and searched against the NCBI Ae. aegypti database to identify the most abundant proteins. Multiple proteins were identified with high score values, although long forms of the D7 protein family were the most prevalent (Table 1). A number of these proteins were not predicted as secreted proteins that would be present in saliva. To validate the proteins that are present in saliva and may play a role at the virus-vector-host interface, saliva was harvested from one hundred Ae. aegypti using the immersion oil technique [23]. Soluble proteins were extracted from immersion oil using phosphate buffered saline and the sample was submitted for LC+MS/MS analysis. We confirmed that 7 proteins in the inhibitory fraction are also present in saliva (Tables 1 and 2). Three of these proteins were either long or short form D7 proteins.
Fig 1. Impact of HPLC-fractionated Ae. aegypti salivary gland extract on DENV2 cell binding.
Individual HPLC fractions were used to pre-treat mouse embryonic fibroblasts prior to inoculation with DENV2. Total RNA was harvested and qRT-PCR was performed 18 hours post-infection. Relative levels of DENV2 were normalized to GAPDH. An untreated control was set to 1.0 and was placed on the far left of the graph (black bar).
Table 1. LC+MS/MS hits from pooled inhibitory HPLC SGE fractions.
| Identifier | Comments | Score | Present in saliva |
|---|---|---|---|
| AAEL006424 | D7 protein long | 2221 | Yes |
| AAEL006417 | D7 protein long | 2100 | Yes |
| AAEL010235 | 30 kDa salivary protein | 1852 | Yes |
| AAEL002761 | Tropomyosin | 1023 | No |
| AAEL011949 | Transferrin | 984 | No |
| AAEL006333 | Apyrase | 933 | Yes |
| AAEL006096 | Gelsolin | 898 | No |
| AAEL001005 | Calreticulin | 725 | No |
| AAEL010228 | 30 kDa allergen-like protein | 710 | Yes |
| AAEL006423 | D7 protein short | 707 | Yes |
| AAEL003566 | Galactose-specific C-type lectin | 688 | No |
| AAEL000533 | Antifreeze protein | 650 | No |
| AAEL012897 | Mitochondrial aconitase | 621 | No |
| AAEL006406 | Hypothetical protein | 603 | No |
| AAEL009852 | 14.5 kDa salivary protein | 586 | No |
| AAEL002539 | Calponin/transgelin | 579 | No |
| AAEL002417 | Troponin T isoform 1 | 564 | No |
| AAEL013528 | Peroxiredoxin | 467 | No |
| AAEL007394 | Hypothetical protein | 432 | No |
| AAEL010242 | 8.7 kDa salivary protein | 403 | No |
| AAEL003957 | Hypothetical protein | 387 | No |
| AAEL003600 | 34 kDa salivary protein | 382 | Yes |
| AAEL013279 | Cyclophilin | 344 | No |
| AAEL017096 | Translation elongation factor EF-1 alpha/Tu | 299 | No |
| AAEL013407 | Hypothetical protein | 293 | No |
| AAEL017096 | Lysozyme | 280 | No |
Table 2. LC+MS/MS hits from Aedes aegypti saliva.
| Identifier | Comments | Score |
|---|---|---|
| AAEL000732 | 62 kDa secreted protein | 1227 |
| AAEL003182 | Serine protease inhibitor (serpin) | 738 |
| AAEL005672 | Adenosine deaminase | 721 |
| AAEL006417 | D7 protein long | 652 |
| AAEL006347 | Apyrase precursor | 648 |
| AAEL003600 | 34 kDa salivary protein | 535 |
| AAEL003601 | 34 kDa salivary protein | 430 |
| AAEL002704 | Serine protease inhibitor (serpin) | 361 |
| AAEL006424 | D7 protein long | 344 |
| AAEL010235 | 30 kDa salivary gland protein | 336 |
| AAEL009081 | 56.5 kDa salivary protein | 329 |
| AAEL003053 | Allergen | 302 |
| AAEL006333 | Salivary apyrase | 286 |
| AAEL000748 | 62 kDa secreted protein | 270 |
| AAEL006485 | Inosine-uridine preferring nucleoside hydrolase | 210 |
| AAEL010228 | 30 kDa allergen-like protein | 199 |
| AAEL003100 | Salivary mucin | 181 |
| AAEL000793 | Venom allergen | 181 |
| AAEL005766 | Fructose-bisphosphate aldolase | 148 |
| AAEL011197 | Actin | 132 |
| AAEL003107 | Hypothetical | 123 |
| AAEL009992 | Hypothetical | 120 |
| AAEL006423 | D7 protein short | 107 |
| AAEL000490 | Histone H4 | 82 |
| AAEL009955 | Vitellogenin-like protein | 73 |
| AAEL006511 | Ubiquitin | 71 |
| AAEL009993 | SGS1 | 68 |
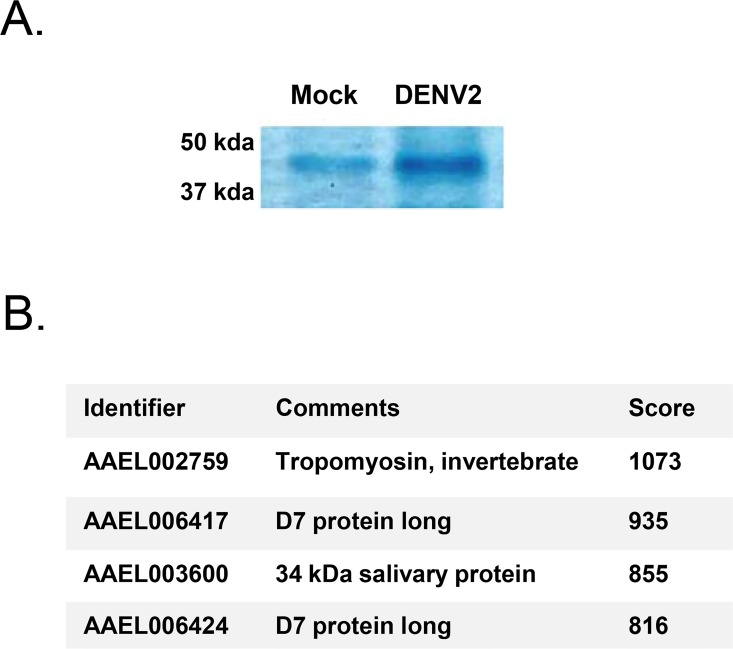
In a separate experiment, we sought to elucidate proteins that are upregulated in salivary glands during DENV2 infection. Ae. aegypti were either mock or infected with DENV2 via blood feeding using an artificial membrane. Twenty salivary glands were isolated from each group of mosquitoes 14 dpi and equal amounts of protein were loaded on to an SDS-PAGE gel for one-dimensional gel electrophoresis. Gels were stained with Coomassie Blue. Multiple bands were different between mock and DENV2 lanes, but one band appeared significantly darker in the DENV2-infected lane (Fig 2A). The band from the DENV2-infected lane was excised and submitted for mass spectrometry. Only four unique Ae. aegypti proteins were detected, which included tropomyosin, two long form D7 proteins, and a 34 kDa salivary protein (Fig 2B). We confirmed that both long and short form D7 proteins and the 34 kDa salivary protein are upregulated in Ae. aegypti salivary glands at the transcriptional level during DENV2 infection by assessing previously published RNA Seq data (Table 3) [11].
Fig 2. Identification of upregulated Ae. aegypti salivary gland proteins during DENV2 infection.
(A) Section of a Coomassie Blue stained gel containing approximately 1 μg salivary gland extracts from mock-infected and DENV2-infected Ae. aegypti. A single band was more intense during DENV2 infection. (B) Proteins identified within the DENV2-specific band as shown above by mass spectrometry.
Table 3. Gene accumulation levels in FPKM in mock and DENV2-infected Ae. aegypti salivary glands 14 days post-infection.
| Identifier | Comments | Mock | DENV2 |
|---|---|---|---|
| AAEL006417 | D7 protein long | 5,869 | 11,411 |
| AAEL006424 | D7 protein long | 13,440 | 28,913 |
| AAEL006423 | D7 protein short | 2,828 | 7,982 |
| AAEL006408 | D7 protein short | 1,960 | 2,336 |
| AAEL006406 | D7 protein short | 4,785 | 7,916 |
| AAEL007394 | D7 protein short | 1,529 | 6,718 |
| AAEL003600 | 34 kDa salivary protein | 6,287 | 14,361 |
| AAEL002759 | Tropomyosin, invertebrate | 36 | 11 |
Recombinant D7 protein inhibits dengue virus in vitro and in vivo
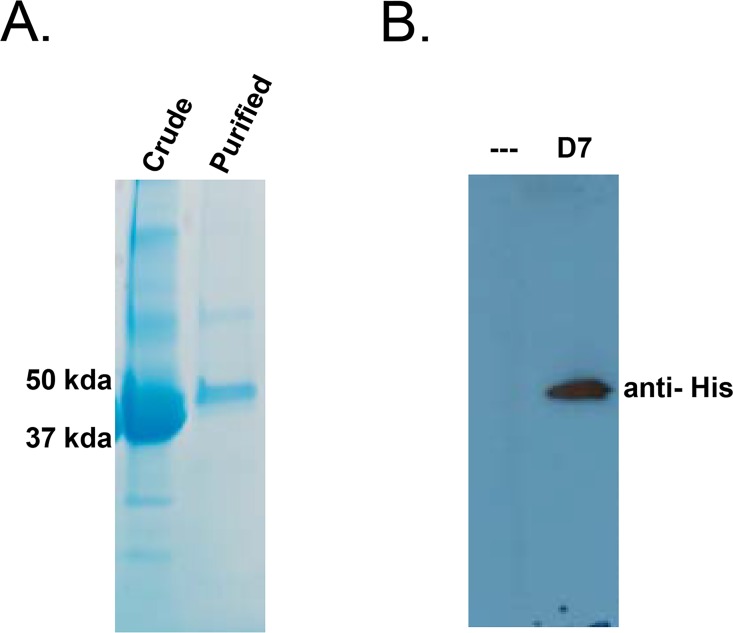
Ae. aegypti D7 proteins are multifunctional and have been shown to bind to cysteinyl leukotrienes (cysLT) and biogenic amines with high affinity. Based on the discovery of D7 proteins in inhibitory HPLC fractions, and of increased D7 protein expression in DENV2-infected salivary glands, we hypothesized that D7 proteins may bind to additional substrates with varying affinities, and that these interactions inhibit DENV cell binding and infectivity. To test this hypothesis, a recombinant D7 long form protein (AAEL006424) was produced in S2 cells and harvested from the supernatant at a concentration of 80 ng/mL (Fig 3A and 3B). We determined if D7 protein was toxic to cells by incubating ten-fold dilutions of D7 protein with monocyte-like U937 cells for 24 hours followed by analysis using Promega’s CellTox Green Cytotoxicity Assay. This assay measures membrane integrity that occurs as a result of cell death. Both positive and negative controls were included. None of the dilutions tested were toxic to U937 cells at the time point tested (Fig 4A).
Fig 3. Characterization of recombinant D7 protein.
(A) Coomassie Blue gel stain of crude S2 cell lysate containing recombinant D7 protein and D7 protein after purification using a HisPur Cobalt Spin Column. (B) Western blot of uninduced (—) and induced (D7) S2 cell supernatants using an anti-6 His antibody.
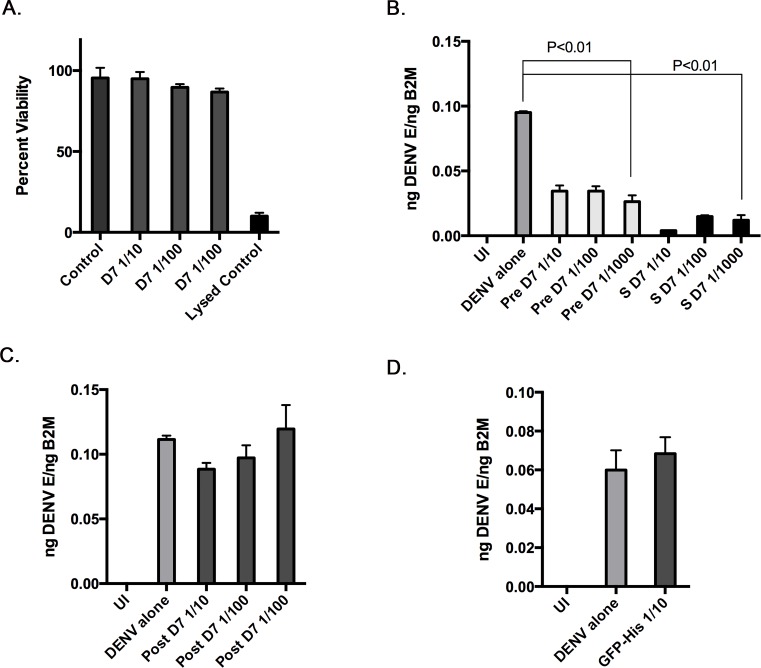
Fig 4. Recombinant D7 protein inhibits DENV2 infection in U937 cells.
(A) The Promega CellToxTM Green Cytotoxicity Assay was used to determine the toxicity of ten-fold dilutions of recombinant D7 protein on U937 cells 24 hours post-treatment. (B) U937 cells were either uninfected (UI), infected with DENV2 alone, pre-treated with 10-fold dilutions of recombinant D7 protein followed by DENV2 infection, or co-treated with 10-fold dilutions of recombinant D7 protein with concomitant DENV2 infection. Total RNA was harvested and qRT-PCR was performed 24 hours post-infection. Relative levels of DENV2 were normalized to B2M. (C) U937 cells were either uninfected (UI), infected with DENV2 alone, or infected followed by post-treatment with 10-fold dilutions of recombinant D7 protein. Post-treatment began 24 hpi and lasted 8 hours. Total RNA was harvested and qRT-PCR was performed 48 hours post-infection. Relative levels of DENV2 were normalized to B2M. (D) U937 cells were either uninfected (UI), infected with DENV2 alone, or pre-treated with recombinant Hig-tagged GFP, representing the highest concentration used for D7 protein followed by DENV2 infection. Total RNA was harvested and qRT-PCR was performed 24 hours post-infection. Relative levels of DENV2 were normalized to B2M. Data represent averages of at least 3 independent experiments. Student’s t tests were performed to assess statistical significance between groups.
We then tested if D7 protein had antiviral activity in vitro by either pre-incubating U937 cells with ten-fold dilutions of recombinant D7 protein for 1 hour at 37°C prior to inoculation with DENV2, or incubating ten-fold dilutions of D7 protein with DENV2 for 1 hour at 37°C prior to co-treatment with D7-DENV2 mixtures. The different treatment strategies were employed to determine if D7 needed to directly interact with virions for activity. Total RNA was extracted 24 hpi and the relative amount of DENV2 vRNA was normalized to a housekeeping gene. Both pre-treatment and co-treatment of recombinant D7 protein and DENV2 significantly reduced DENV2 vRNA levels in U937 cells and there wasn’t a statistical difference between these two treatment groups (Fig 4B). A post-treatment experiment was also performed to determine if D7 inhibited DENV at an early time point during infection. In this experiment, U937 cells were infected with DENV for 24 hours, followed by an 8 hour treatment with ten-fold dilutions of D7 protein. Total RNA was extracted 48 hpi and the relative amount of DENV2 vRNA was normalized to a housekeeping gene. This treatment strategy did not significantly inhibit DENV infection, suggesting that D7 inhibits DENV at an early time point (Fig 4C). We also tested if an irrelevant His-tagged protein could inhibit DENV infection. Recombinant His-tagged GFP was unable to inhibit DENV infection at the highest concentration tested for D7 (Fig 4D).
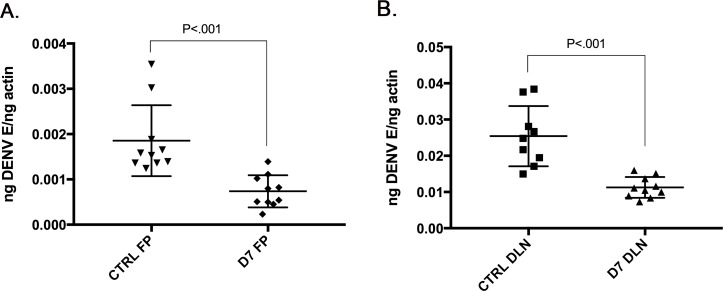
To determine if recombinant D7 can inhibit DENV2 infection in vivo, we challenged AGB6 mice with DENV2 with and without D7 protein by subcutaneous footpad inoculation. AGB6 mice were chosen because they are highly permissive to DENV2 infection and were a suitable model for early DENV2 replication and dissemination to draining lymph nodes. Briefly, 2 groups of 4-5-week-old, age-matched, sex-matched mice were inoculated into a single rear footpad with 20 μl containing 1 x 107 genome equivalents, DENV2 alone or in combination with approximately 1 ng recombinant D7 protein. In order to test the impact of D7 treatment on early replication and dissemination, RNA was harvested from footpads and draining lymph nodes 48 hpi and qRT-PCR was used to measure the relative levels of DENV2 normalized to an actin reference gene [5]. D7 protein significantly reduced DENV2 vRNA levels in both the footpads and draining lymph nodes 24 hpi (Fig 5A and 5B). DENV2 vRNA amplified at Ct values of 21–24 in footpad samples and 17–19 in draining lymph node samples.
Fig 5. Recombinant D7 protein inhibits DENV2 infection in AGB6 mice.
Mice deficient in receptors for type I and type II interferons, (IFNAGR-/-, AGB6) were inoculated subcutaneously into both rear footpads with 20 μl containing either 107 genome equivalents of either DENV2 alone or DENV2 plus recombinant D7 protein. Forty-eight hours post-infection, mice were euthanized and left and right foot pads and left and right popliteal draining lymph nodes (DLN) were collected independently. (A) Total RNA was harvested from footpad tissue and qRT-PCR was performed to assess relative levels of DENV2 vRNA. Relative DENV2 vRNA was normalized to actin gene expression. (B) Total RNA was harvested from DLNs and qRT-PCR was performed to assess relative levels of DENV2 vRNA. Relative DENV2 vRNA was normalized to actin gene expression. 5 mice were present in each group and the data represents a single experiment. Unpaired t tests were performed to assess statistical significance.
Recombinant D7 protein directly interacts with dengue virions and envelope protein
Our results suggest that D7 protein can inhibit DENV2 infection in vitro and in vivo, although it is unclear how D7 protein mediates its antiviral effect. Pre-treatment of U937 cells with recombinant D7 protein led to a significant decrease in DENV2 infection, suggesting that D7 protein may modulate the host cell. Although, co-treatment of D7 protein with DENV2 appeared to be more effective at preventing cell binding and/or infection of U937 cells. We hypothesized that D7 protein can interact with multiple substrates including proteins at the cell surface and viral proteins. To test this hypothesis, we performed binding assays to determine if D7 protein can bind to DENV virions and envelope protein.
First, anti-DENV2 antibody, clone 3H5-1 was covalently coupled to 25 μL amine-active resin in two separate columns. One hundred μg of purified, formaldehyde-inactivated dengue virus type 2 virions was then mixed with 100 Ae. aegypti salivary gland equivalents and added to the antibody-containing resin and incubated for 2 hr at 4°C. A negative control column containing anti-DENV2 antibody was also prepared with only 100 salivary gland equivalents. Unbound proteins were washed away and bound proteins were eluted. Eluted proteins were identified by LC+MS/MS analysis. Although Mascot scores were low, 8 proteins were identified that were unique and not in the negative control and three of these were present in saliva (Tables 2 and 4). This included the long form D7 protein (AAEL006424). To determine if the proteins we detected were simply correlated with their abundance, we performed LC+MS/MS analysis on Ae. aegypti salivary gland extracts (Table 5). Although actin and serine protease inhibitor (serpin) had Mascot scores in the top 3%, Mascot data suggested that the remaining proteins were less abundant, and that our co-immunoprecipitation data did not simply represent the most abundant proteins in salivary gland extracts.
Table 4. LC+MS/MS hits from SGE/DENV2 binding assay.
| Identifiera | Comments | Score | Present in saliva |
|---|---|---|---|
| AAEL001951 | Actin | 137 | No |
| AAEL003601 | 34 kDa salivary protein | 99 | Yes |
| AAEL005056 | Electron transfer flavoprotein beta subunit | 94 | No |
| AAEL003182 | Serine protease inhibitor (serpin) | 73 | Yes |
| AAEL012175 | F1 ATP synthase alpha subunit | 57 | No |
| AAEL006424 | D7 protein long | 53 | Yes |
| AAEL013144 | Translation initiation factor 3 subunit | 49 | No |
| AAEL010242 | 8.7 kDa secreted protein | 48 | No |
a Protein hits represent unique identifiers that were not present in the negative control.
Table 5. LC+MS/MS hits from Aedes aegypti salivary gland extract.
| Identifier | Comment | Score |
|---|---|---|
| AAEL009993 | SGS1 | 5240 |
| AAEL009992 | N/A | 4161 |
| AAEL002827 | ATP synthase beta subunit | 1343 |
| AAEL000732 | 62 kDa secreted protein, putative | 1238 |
| AAEL003182 | serine protease inhibitor (serpin) homologue—unlikely to be inhibitory | 1056 |
| AAEL011197 | actin | 1050 |
| AAEL005961 | actin | 954 |
| AAEL006347 | apyrase precursor | 887 |
| AAEL001928 | actin-1 | 880 |
| AAEL009955 | vitellogenin-like protein | 812 |
| AAEL000749 | angiopoietin-like protein splice variant | 798 |
| AAEL006333 | salivary apyrase, putative | 795 |
| AAEL008787 | V-type proton ATPase catalytic subunit A | 782 |
| AAEL000748 | 62 kDa secreted protein, putative | 778 |
| AAEL001951 | actin | 771 |
| AAEL005672 | adenosine deaminase | 737 |
| AAEL002851 | tubulin beta chain | 731 |
| AAEL012175 | ATP synthase alpha subunit mitochondrial | 728 |
| AAEL009081 | 56.5 kDa secreted protein, putative | 717 |
| AAEL009185 | arginine or creatine kinase | 701 |
| AAEL005733 | myosin heavy chain, nonmuscle or smooth muscle | 629 |
| AAEL013739 | electron transport oxidoreductase | 614 |
| AAEL006642 | tubulin alpha chain | 585 |
| AAEL003655 | N/A | 580 |
| AAEL005656 | myosin heavy chain, nonmuscle or smooth muscle | 577 |
| AAEL009691 | carboxylase:pyruvate/acetyl-coa/propionyl-coa | 576 |
| AAEL012576 | pyruvate kinase | 534 |
| AAEL010697 | 3-ketoacyl-coa thiolase, mitochondrial | 511 |
| AAEL004988 | phosphoglycerate kinase | 510 |
| AAEL003053 | allergen, putative | 510 |
| AAEL005766 | fructose-bisphosphate aldolase | 508 |
| AAEL002704 | serine protease inhibitor (serpin) homologue | 499 |
| AAEL003872 | translationally-controlled tumor protein homolog (TCTP) | 467 |
| AAEL010146 | 3-hydroxyacyl-coa dehyrogenase | 440 |
| AAEL011288 | elongation factor 1 gamma | 432 |
| AAEL007420 | serine protease inhibitor (serpin) homologue—unlikely to be inhibitory | 429 |
| AAEL003734 | aconitase, mitochondrial | 428 |
| AAEL006485 | inosine-uridine preferring nucleoside hydrolase | 417 |
| AAEL004338 | pyruvate dehydrogenase | 411 |
| AAEL002542 | triosephosphate isomerase | 411 |
| AAEL001593 | glycerol-3-phosphate dehydrogenase | 393 |
| AAEL000951 | elongation factor 1-beta2 | 375 |
| AAEL008166 | malate dehydrogenase | 372 |
| AAEL010821 | 60S acidic ribosomal protein P0 | 362 |
| AAEL013614 | clathrin heavy chain | 353 |
| AAEL005515 | heterogeneous nuclear ribonucleoprotein | 350 |
| AAEL010235 | 30 kDa salivary gland allergen Aed a 3 Precursor (Allergen Aed a 3) | 349 |
| AAEL006582 | calcium-transporting ATPase sarcoplasmic/endoplasmic reticulum type | 340 |
| AAEL006719 | alpha-amylase I precursor | 339 |
| AAEL012827 | endoplasmin | 330 |
| AAEL011584 | chaperonin-60kD, ch60 | 330 |
| AAEL011704 | heat shock protein | 328 |
| AAEL003100 | salivary mucin | 322 |
| AAEL005052 | tubulin beta chain | 312 |
| AAEL003107 | N/A | 311 |
| AAEL006417 | D7 protein, putative | 309 |
| AAEL006833 | succinyl-CoA synthetase small subunit, putative | 308 |
| AAEL003600 | 34 kDa salivary protein, putative | 306 |
| AAEL004297 | ATP-citrate synthase | 302 |
| AAEL006721 | 2-oxoglutarate dehydrogenase | 297 |
| AAEL014452 | acyl-coa dehydrogenase | 296 |
| AAEL004500 | eukaryotic translation elongation factor | 295 |
| AAEL008167 | aspartate ammonia lyase | 287 |
| AAEL010585 | spermatogenesis associated factor | 285 |
| AAEL000793 | venom allergen | 284 |
| AAEL001194 | fatty acid synthase | 284 |
| AAEL007555 | acyl-coa dehydrogenase | 280 |
| AAEL009651 | nascent polypeptide associated complex alpha subunit (nac alpha) | 270 |
| AAEL002764 | dihydrolipoamide succinyltransferase component of 2-oxoglutarate dehydrogenase | 269 |
| AAEL013613 | pyruvate dehydrogenase | 267 |
| AAEL002572 | myosin regulatory light chain 2 (mlc-2) | 266 |
| AAEL007065 | ADP-ribosylation factor, arf | 265 |
| AAEL006096 | gelsolin precursor | 263 |
| AAEL010464 | glutamate dehydrogenase | 262 |
| AAEL008192 | 40S ribosomal protein S3 | 239 |
| AAEL005931 | 6-phosphogluconate dehydrogenase | 237 |
| AAEL013904 | 3-hydroxyisobutyrate dehydrogenase | 234 |
| AAEL002841 | 3-hydroxyacyl-coa dehyrogenase | 233 |
| AAEL005798 | ATP synthase subunit beta vacuolar | 231 |
| AAEL006834 | glutamate semialdehyde dehydrogenase | 228 |
| AAEL007707 | malate dehydrogenase | 225 |
| AAEL013144 | eukaryotic translation initiation factor 3 subunit I (eIF3i) | 221 |
| AAEL000726 | fibrinogen and fibronectin | 221 |
| AAEL004294 | dihydrolipoamide acetyltransferase component of pyruvate dehydrogenase | 208 |
| AAEL003694 | N/A | 206 |
| AAEL002296 | trifunctional enzyme beta subunit (tp-beta) | 203 |
| AAEL006040 | coatomer | 201 |
| AAEL005084 | tubulin beta chain | 201 |
| AAEL004739 | acyl-coa dehydrogenase | 199 |
| AAEL007718 | eukaryotic translation initiation factor 3 subunit B (eIF3b) | 195 |
| AAEL013353 | profilin | 195 |
| AAEL003530 | acidic ribosomal protein P1, putative | 190 |
| AAEL000392 | maltase-like 1 | 189 |
| AAEL005422 | pyrroline-5-carboxylate dehydrogenase | 189 |
| AAEL001588 | glutamate carboxypeptidase | 184 |
| AAEL008619 | trypsin-like salivary secreted protein | 183 |
| AAEL003125 | acyl-coa dehydrogenase | 181 |
| AAEL008844 | calcium-binding protein, putative | 181 |
| AAEL002693 | venom allergen | 178 |
| AAEL013359 | DEAD box ATP-dependent RNA helicase | 176 |
| AAEL002309 | thioredoxin peroxidase | 174 |
| AAEL009029 | aldehyde dehydrogenase | 173 |
| AAEL006424 | 37 kDa salivary gland allergen Aed a 2 precursor (protein D7)(allergen Aed a 2) | 170 |
| AAEL003634 | hsp70-interacting protein, putative | 169 |
| AAEL008006 | 3-hydroxyacyl-coa dehyrogenase | 163 |
| AAEL007945 | eukaryotic translation initiation factor 3 subunit H (eIF3h) | 161 |
| AAEL009902 | eukaryotic translation initiation factor 3 subunit D (eIF3d) | 154 |
| AAEL001134 | probable methylmalonate-semialdehyde dehydrogenase [acylating], mitochondrial precursor | 151 |
| AAEL001668 | enolase | 147 |
| AAEL011746 | succinyl-coa synthetase beta chain | 147 |
| AAEL012359 | nucleoside-diphosphate kinase NBR-A, putative | 143 |
| AAEL003601 | 34 kDa secreted protein, putative | 140 |
| AAEL005069 | ras-related protein Rab-1A, putative | 139 |
| AAEL009313 | elongation factor -1 beta,delta | 138 |
| AAEL012746 | chaperonin | 138 |
| AAEL013675 | eukaryotic translation initiation factor | 136 |
| AAEL013625 | 40S ribosomal protein S5 | 135 |
| AAEL009747 | 40S ribosomal protein S18 | 134 |
| AAEL011756 | aldehyde dehydrogenase | 128 |
| AAEL001005 | calreticulin | 126 |
| AAEL000641 | protein disulfide isomerase | 126 |
| AAEL001218 | alanyl-tRNA synthetase | 126 |
| AAEL001128 | AMP dependent coa ligase | 126 |
| AAEL010143 | isocitrate dehydrogenase | 125 |
| AAEL006174 | proteasome subunit beta type | 123 |
| AAEL012207 | myosin light chain 1 | 122 |
| AAEL008848 | ATP synthase gamma subunit | 121 |
| AAEL000703 | glycogen phosphorylase | 121 |
| AAEL006634 | acetyl-coa acetyltransferase, mitochondrial (acetoacetyl-coa thiolase) | 121 |
| AAEL003993 | cyclohex-1-ene-1-carboxyl-CoA hydratase, putative | 119 |
| AAEL005567 | nucleosome assembly protein | 117 |
| AAEL006085 | methylenetetrahydrofolate dehydrogenase | 117 |
| AAEL008083 | 40S ribosomal protein SA | 114 |
| AAEL004378 | eukaryotic translation initiation factor 1A (eIF-1A) | 114 |
| AAEL013069 | receptor for activated protein kinase c (rack1) | 113 |
| AAEL002504 | ATP synthase delta chain, mitochondrial | 112 |
| AAEL004347 | eukaryotic translation initiation factor 3 subunit M (eIF3m) | 111 |
| AAEL009617 | eukaryotic translation initiation factor 3 subunit L (eIF3l) | 107 |
| AAEL012825 | bifunctional purine biosynthesis protein | 107 |
| AAEL006928 | dihydrolipoamide dehydrogenase | 106 |
| AAEL002833 | cathepsin l | 106 |
| AAEL002334 | eukaryotic translation initiation factor 3 subunit E (eIF3e) | 105 |
| AAEL009872 | alanine aminotransferase | 105 |
| AAEL011741 | glutathione transferase | 104 |
| AAEL007064 | Gram-negative Binding Protein (GNBP) or beta-1 3-glucan binding protein | 104 |
| AAEL005269 | ubiquinol-cytochrome c reductase complex core protein | 103 |
| AAEL007033 | pyrroline-5-carboxylate reductase | 103 |
| AAEL005407 | annexin x | 102 |
| AAEL001331 | mannose-1-phosphate guanyltransferase | 102 |
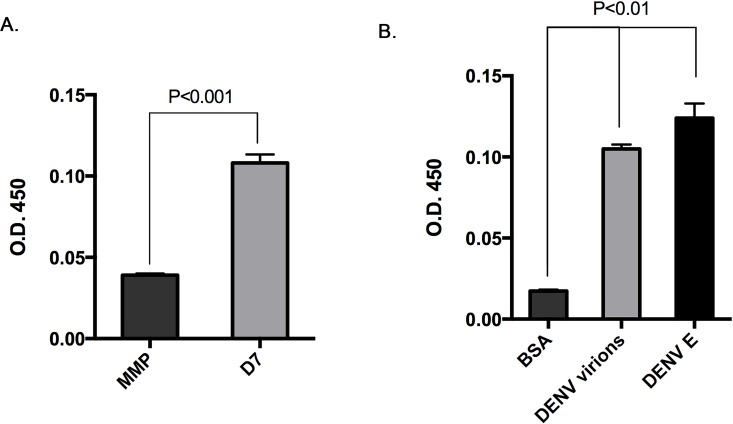
To confirm if D7 protein can directly interact with DENV2, we performed an enzyme-linked immunosorbent assay (ELISA) by binding a control MMP protein or recombinant D7 protein to a 96 well plate [24]. Plates were then incubated with DENV2 16881 virions. Unbound proteins were then removed by washing each well with buffer. The degree of association between immobilized proteins and DENV2 virions was assessed using an antibody that recognized DENV followed by a secondary antibody conjugated to horse radish peroxidase. DENV2 16881 virions bound to D7 protein more readily than MMP protein. (Fig 6A). Plates coated with D7 protein were then incubated with bovine serum albumin, DENV2 16881 virions, or recombinant DENV2 envelope protein. Unbound proteins were then removed by washing each well with buffer. The degree of association between D7 and the above proteins was assessed using an antibody recognizing DENV followed by a secondary antibody conjugated with horse radish peroxidase. We found that recombinant D7 interacted with both DENV2 16881 virions and recombinant DENV2 envelope protein (Fig 6B).
Fig 6. Recombinant D7 protein binds DENV2 virions and DENV2 envelope protein.
(A) An enzyme-linked immunosorbent assay was developed by coating plates with control MMP protein or recombinant D7 protein and then measuring binding of DENV2 virions. (B) An enzyme-linked immunosorbent assay was developed by coating plates with recombinant D7 protein and then measuring binding of bovine serum albumin (BSA), DENV2 virions, and recombinant DENV2 envelope protein. Interactions were detected using antibodies against DENV2 envelope protein and a secondary antibody conjugated to horse radish peroxidase. Absorbance was detected at OD 450 nm. Student’s t tests were performed to assess statistical significance between groups.
Discussion
Hematophagous arthropod saliva contains a complex mixture of proteins with anti-hemostatic, anti-inflammatory, and immunomodulatory properties. Hematophagous arthropod saliva can also enhance transmission of arboviruses, although the exact mechanism and saliva proteins involved in this process are not known [3]. Interestingly, individual saliva components can have inhibitory activities that prevent arbovirus infection [19]. Here, we identified D7 protein in biochemical fractions of salivary gland extracts that inhibited DENV2 cell binding and/or infection in mouse embryonic fibroblasts and then determined if this activity could be recapitulated with recombinant D7 protein. We found that recombinant D7 protein prevented DENV2 cell binding and/or infection in two permissive cell types and prevented infection and dissemination in a mouse model. Additionally, two separate binding assays suggest that D7 protein can physically interact with DENV virions and envelope protein. These data support the previous observation that D7 protein vaccination enhanced mortality in a West Nile virus mouse model and suggest that D7 protein inhibits virus transmission [20].
D7 proteins are some of the most abundant proteins expressed in the salivary glands of blood feeding Diptera. Long and short forms of D7 proteins exist in mosquitoes. Each of these proteins appear capable of binding either cysteinyl leukotrienes, and/or biogenic amines such as serotonin, histamine, and norepinephrine. These functions are predicted to antagonize the host’s inflammatory response, and ability to vasoconstrict, induce platelet-aggregation, and induce a sense of pain–each critical to efficiently obtaining a blood meal [21, 22].
It is theoretically possible that the functions of D7 proteins are counter to the needs of arboviruses during transmission to a vertebrate host. D7 proteins bind multiple substrates and it is possible that they bind other proteins with lower affinity, which may limit virus-host interactions. Additionally, limiting the host inflammatory response may reduce influx or activation of target cells, such as Langerhans cells, monocytes, or macrophage. Our data support that D7 protein mediates its antiviral effect through direct protein-protein interaction in vitro, although it is possible that modulation of the inflammatory response also occurs in vivo.
D7 proteins are some of the most abundant and immunogenic proteins present in mosquito saliva [25]. The presence of anti-D7 antibodies has been used as a marker of exposure to certain mosquito species [26–29]. Considering that individuals who are exposed to mosquitoes have high levels of anti-D7 antibodies, it is likely that these antibodies inhibit D7 protein function. In fact, the presence of anti-D7 antibodies has been linked to disease severity [29]. Although anti-D7 antibodies may prevent efficient blood feeding by a mosquito, it may also enhance disease transmission and disease severity. Characterizing the complex interplay of virus-vector-host interactions will lead to the development of better models of pathogenesis, strategies to limit disease transmission and promote the development of therapeutics, and transmission-blocking vaccines.
Acknowledgments
We thank Dr. John F. Anderson and the CAES (New Haven, CT) for mosquito rearing and experiment insights and L2 Diagnostics (New Haven, CT) for recombinant DENV2 envelope protein.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by NIH grants K22 AI103067-01 (Colpitts) and UO1 AI070343 (EF). EF is an Investigator of the Howard Hughes Medical Institute. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Shepard DS, Undurraga EA, Halasa YA, Stanaway JD. The global economic burden of dengue: a systematic analysis. Lancet Infect Dis. 2016. Epub 2016/04/20. doi: S1473-3099(16)00146-8 [pii] 10.1016/S1473-3099(16)00146-8 . [DOI] [PubMed] [Google Scholar]
- 2.Stanaway JD, Shepard DS, Undurraga EA, Halasa YA, Coffeng LE, Brady OJ, et al. The global burden of dengue: an analysis from the Global Burden of Disease Study 2013. Lancet Infect Dis. 2016. Epub 2016/02/15. doi: S1473-3099(16)00026-8 [pii] 10.1016/S1473-3099(16)00026-8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conway MJ, Colpitts TM, Fikrig E. Role of the Vector in Arbovirus Transmission. Annu Rev Virol. 2014;1(1):71–88. Epub 2014/11/03. 10.1146/annurev-virology-031413-085513 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chokephaibulkit K, Perng GC. Challenges for the formulation of a universal vaccine against dengue. Exp Biol Med (Maywood). 2013;238(5):566–78. Epub 2013/07/17. doi: 238/5/566 [pii] 10.1177/1535370212473703 . [DOI] [PubMed] [Google Scholar]
- 5.Conway MJ, Watson AM, Colpitts TM, Dragovic SM, Li Z, Wang P, et al. Mosquito saliva serine protease enhances dissemination of dengue virus into the mammalian host. J Virol. 2013. Epub 2013/10/18. doi: JVI.02235-13 [pii] 10.1128/JVI.02235-13 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Londono-Renteria B, Troupin A, Conway MJ, Vesely D, Ledizet M, Roundy CM, et al. Dengue Virus Infection of Aedes aegypti Requires a Putative Cysteine Rich Venom Protein. PLoS Pathog. 2015;11(10):e1005202 Epub 2015/10/23. 10.1371/journal.ppat.1005202 PPATHOGENS-D-15-01450 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ribeiro JM, Arca B, Lombardo F, Calvo E, Phan VM, Chandra PK, et al. An annotated catalogue of salivary gland transcripts in the adult female mosquito, Aedes aegypti. BMC Genomics. 2007;8:6. Epub 2007/01/06. doi: 1471-2164-8-6 [pii] 10.1186/1471-2164-8-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Champagne DE, Ribeiro JM. Sialokinin I and II: vasodilatory tachykinins from the yellow fever mosquito Aedes aegypti. Proc Natl Acad Sci U S A. 1994;91(1):138–42. Epub 1994/01/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chisenhall DM, Londono BL, Christofferson RC, McCracken MK, Mores CN. Effect of dengue-2 virus infection on protein expression in the salivary glands of Aedes aegypti mosquitoes. Am J Trop Med Hyg. 2014;90(3):431–7. Epub 2014/01/22. doi: ajtmh.13-0412 [pii] 10.4269/ajtmh.13-0412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chisenhall DM, Christofferson RC, McCracken MK, Johnson AM, Londono-Renteria B, Mores CN. Infection with dengue-2 virus alters proteins in naturally expectorated saliva of Aedes aegypti mosquitoes. Parasit Vectors. 2014;7:252. Epub 2014/06/03. doi: 1756-3305-7-252 [pii] 10.1186/1756-3305-7-252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonizzoni M, Dunn WA, Campbell CL, Olson KE, Marinotti O, James AA. Complex modulation of the Aedes aegypti transcriptome in response to dengue virus infection. PLoS One. 2012;7(11):e50512 Epub 2012/12/05. 10.1371/journal.pone.0050512 PONE-D-12-19714 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ader DB, Celluzzi C, Bisbing J, Gilmore L, Gunther V, Peachman KK, et al. Modulation of dengue virus infection of dendritic cells by Aedes aegypti saliva. Viral Immunol. 2004;17(2):252–65. Epub 2004/07/29. 10.1089/0882824041310496 . [DOI] [PubMed] [Google Scholar]
- 13.Le Coupanec A, Babin D, Fiette L, Jouvion G, Ave P, Misse D, et al. Aedes mosquito saliva modulates Rift Valley fever virus pathogenicity. PLoS Negl Trop Dis. 2013;7(6):e2237 Epub 2013/06/21. 10.1371/journal.pntd.0002237 PNTD-D-12-00684 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edwards JF, Higgs S, Beaty BJ. Mosquito feeding-induced enhancement of Cache Valley Virus (Bunyaviridae) infection in mice. J Med Entomol. 1998;35(3):261–5. Epub 1998/06/06. . [DOI] [PubMed] [Google Scholar]
- 15.Limesand KH, Higgs S, Pearson LD, Beaty BJ. Potentiation of vesicular stomatitis New Jersey virus infection in mice by mosquito saliva. Parasite Immunol. 2000;22(9):461–7. Epub 2000/09/06. doi: pim326 [pii]. . [DOI] [PubMed] [Google Scholar]
- 16.Schneider BS, Soong L, Girard YA, Campbell G, Mason P, Higgs S. Potentiation of West Nile encephalitis by mosquito feeding. Viral Immunol. 2006;19(1):74–82. Epub 2006/03/24. 10.1089/vim.2006.19.74 . [DOI] [PubMed] [Google Scholar]
- 17.Schneider BS, Soong L, Zeidner NS, Higgs S. Aedes aegypti salivary gland extracts modulate anti-viral and TH1/TH2 cytokine responses to sindbis virus infection. Viral Immunol. 2004;17(4):565–73. Epub 2005/01/27. 10.1089/vim.2004.17.565 . [DOI] [PubMed] [Google Scholar]
- 18.Hermance ME, Thangamani S. Tick Saliva Enhances Powassan Virus Transmission to the Host, Influencing Its Dissemination and the Course of Disease. J Virol. 2015;89(15):7852–60. Epub 2015/05/23. doi: JVI.01056-15 [pii] 10.1128/JVI.01056-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCracken MK, Christofferson RC, Grasperge BJ, Calvo E, Chisenhall DM, Mores CN. Aedes aegypti salivary protein "aegyptin" co-inoculation modulates dengue virus infection in the vertebrate host. Virology. 2014;468–470:133–9. Epub 2014/09/01. doi: S0042-6822(14)00327-4 [pii] 10.1016/j.virol.2014.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reagan KL, Machain-Williams C, Wang T, Blair CD. Immunization of mice with recombinant mosquito salivary protein D7 enhances mortality from subsequent West Nile virus infection via mosquito bite. PLoS Negl Trop Dis. 2012;6(12):e1935 Epub 2012/12/14. 10.1371/journal.pntd.0001935 PNTD-D-12-00770 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Calvo E, Mans BJ, Andersen JF, Ribeiro JM. Function and evolution of a mosquito salivary protein family. J Biol Chem. 2006;281(4):1935–42. Epub 2005/11/23. doi: M510359200 [pii] 10.1074/jbc.M510359200 . [DOI] [PubMed] [Google Scholar]
- 22.Calvo E, Mans BJ, Ribeiro JM, Andersen JF. Multifunctionality and mechanism of ligand binding in a mosquito antiinflammatory protein. Proc Natl Acad Sci U S A. 2009;106(10):3728–33. Epub 2009/02/24. doi: 0813190106 [pii] 10.1073/pnas.0813190106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anderson SL, Richards SL, Smartt CT. A simple method for determining arbovirus transmission in mosquitoes. J Am Mosq Control Assoc. 2010;26(1):108–11. Epub 2010/04/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Colpitts TM, Cox J, Vanlandingham DL, Feitosa FM, Cheng G, Kurscheid S, et al. Alterations in the Aedes aegypti transcriptome during infection with West Nile, dengue and yellow fever viruses. PLoS Pathog. 2011;7(9):e1002189 Epub 2011/09/13. 10.1371/journal.ppat.1002189 PPATHOGENS-D-11-00879 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.James AA, Blackmer K, Marinotti O, Ghosn CR, Racioppi JV. Isolation and characterization of the gene expressing the major salivary gland protein of the female mosquito, Aedes aegypti. Mol Biochem Parasitol. 1991;44(2):245–53. Epub 1991/02/01. . [DOI] [PubMed] [Google Scholar]
- 26.Brummer-Korvenkontio H, Lappalainen P, Reunala T, Palosuo T. Detection of mosquito saliva-specific IgE and IgG4 antibodies by immunoblotting. J Allergy Clin Immunol. 1994;93(3):551–5. Epub 1994/03/01. doi: S0091-6749(94)70066-4 [pii]. . [DOI] [PubMed] [Google Scholar]
- 27.Peng Z, Simons FE. Comparison of proteins, IgE, and IgG binding antigens, and skin reactivity in commercial and laboratory-made mosquito extracts. Ann Allergy Asthma Immunol. 1996;77(5):371–6. Epub 1996/11/01. doi: S1081-1206(10)63335-2 [pii] 10.1016/S1081-1206(10)63335-2 . [DOI] [PubMed] [Google Scholar]
- 28.Palosuo K, Brummer-Korvenkontio H, Mikkola J, Sahi T, Reunala T. Seasonal increase in human IgE and IgG4 antisaliva antibodies to Aedes mosquito bites. Int Arch Allergy Immunol. 1997;114(4):367–72. Epub 1997/12/31. . [DOI] [PubMed] [Google Scholar]
- 29.Machain-Williams C, Mammen MP Jr., Zeidner NS, Beaty BJ, Prenni JE, Nisalak A, et al. Association of human immune response to Aedes aegypti salivary proteins with dengue disease severity. Parasite Immunol. 2012;34(1):15–22. Epub 2011/10/15. 10.1111/j.1365-3024.2011.01339.x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.