Abstract
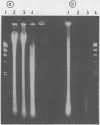
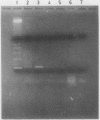
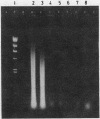
Several DNA extraction techniques were quantitatively and qualitatively compared using both fresh and paraffin wax embedded tissue and their suitability investigated for providing DNA and RNA for the polymerase chain reaction (PCR). A one hour incubation with proteinase K was the most efficient DNA extraction procedure for fresh tissue. For paraffin wax embedded tissue a five day incubation with proteinase K was required to produce good yields of DNA. Incubation with sodium dodecyl sulphate produced very poor yields, while boiling produced 20% as much DNA as long enzyme digestion. DNA extracted by these methods was suitable for the PCR amplification of a single copy gene. Proteinase K digestion also produced considerable amounts of RNA which has previously been shown to be suitable for PCR analysis. A delay before fixation had no effect on the amount of DNA obtained while fixation in Carnoy's reagent results in a much better preservation of DNA than formalin fixation, allowing greater yields to be extracted.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burmer G. C., Rabinovitch P. S., Loeb L. A. Analysis of c-Ki-ras mutations in human colon carcinoma by cell sorting, polymerase chain reaction, and DNA sequencing. Cancer Res. 1989 Apr 15;49(8):2141–2146. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Dixit R., Harrison M. W., Dixit S. N. Isolation and partial characterization of a novel basement membrane collagen. Biochem Biophys Res Commun. 1985 Jul 16;130(1):1–8. doi: 10.1016/0006-291x(85)90373-0. [DOI] [PubMed] [Google Scholar]
- Dubeau L., Chandler L. A., Gralow J. R., Nichols P. W., Jones P. A. Southern blot analysis of DNA extracted from formalin-fixed pathology specimens. Cancer Res. 1986 Jun;46(6):2964–2969. [PubMed] [Google Scholar]
- Kogan S. C., Doherty M., Gitschier J. An improved method for prenatal diagnosis of genetic diseases by analysis of amplified DNA sequences. Application to hemophilia A. N Engl J Med. 1987 Oct 15;317(16):985–990. doi: 10.1056/NEJM198710153171603. [DOI] [PubMed] [Google Scholar]
- Lench N., Stanier P., Williamson R. Simple non-invasive method to obtain DNA for gene analysis. Lancet. 1988 Jun 18;1(8599):1356–1358. doi: 10.1016/s0140-6736(88)92178-2. [DOI] [PubMed] [Google Scholar]
- Lynas C., Cook S. D., Laycock K. A., Bradfield J. W., Maitland N. J. Detection of latent virus mRNA in tissues using the polymerase chain reaction. J Pathol. 1989 Apr;157(4):285–289. doi: 10.1002/path.1711570404. [DOI] [PubMed] [Google Scholar]
- Multicentre randomised clinical trial of chorion villus sampling and amniocentesis. First report. Canadian Collaborative CVS-Amniocentesis Clinical Trial Group. Lancet. 1989 Jan 7;1(8628):1–6. [PubMed] [Google Scholar]