Abstract
The umbilical cord is a helical and tubular blood conduit connecting the foetus to the placenta. The umbilical cord achieves its final form by the 12th week of gestation and normally contains two arteries and a single vein, all embedded in Wharton's jelly. The structure of the umbilical cord receives only a cursory glance during many obstetric ultrasound examinations: with imaging limited to documenting the number of vessels within the cord and the insertion sites at the foetus and placenta. Extensive research into blood flow characteristics of the umbilical cord arteries has been undertaken and is now widely applied in contemporary ultrasound practice. In contrast, investigation of umbilical vein blood flow is only instigated in instances of foetal compromise when the spectral waveform of the ductus venosus and pulsations in the vein are scrutinised. The current level of ultrasound imaging of the umbilical vein demonstrates a lack of appreciation and knowledge about a structure that is crucial to sustaining foetal life.
The goal of this review is to increase awareness of the importance of the umbilical cord. In addition, this review will provide an information platform for undertaking and critically analysing research into the umbilical cord by providing a summary of cord embryology, structure, foetal venous circulation and mechanisms of blood flow within the umbilical cord vein.
Keywords: umbilical cord, umbilical cord vein, venous blood flow
Introduction
The umbilical cord provides the pathway for unhindered blood transport from the placenta to the foetus and vice versa. Aristotle (384–322 BC) originally identified the umbilical cord as the connection between the mother and unborn child. 1
This article is the consequence of a systemic review of established texts, peer reviewed articles and credible websites which was undertaken to develop a knowledge base about the development, structure and blood movement within the foetal venous system in preparation for undertaking research into quantifiable aspects of the umbilical cord vein.
The following pages review the development, structure, foetal venous circulation and mechanisms of blood flow within the umbilical cord vein with the aim of fostering an increased appreciation of a structure that is often neglected during routine obstetric ultrasound examinations and to provide an information platform for research into the foetal venous network.
Umbilical cord embryology
The rudimentary umbilical cord is formed during the 4th to 8th weeks of gestation (calculated from the first day of the last menstrual cycle) by the expanding amnion enveloping tissue from the body stalk, the omphalomesenteric duct and the umbilical coelom. 2 Blood flow is established within the umbilical cord by the end of the 5th week of gestation. 3
Coursing through the body stalk are two umbilical arteries, two umbilical veins and the allantois. Initially, the left and right umbilical arteries are caudal continuations of the primitive dorsal aortae, but after several revisions finally arise from the internal iliac arteries. 4 After birth, the proximal portions of the intra‐abdominal umbilical arteries become the internal iliac and superior vesical arteries, while the distal portions are obliterated and form the medial umbilical ligaments. 5 The umbilical veins arise from a convergence of venules that drain the extra‐embryonic allantois. 4 Obliteration of the right umbilical vein by the end of the 6th week of gestation 6 results in a single persisting left umbilical vein. Obliteration of the intra‐abdominal umbilical vein at birth produces a hepatic remnant termed the ligamentum teres. 5 The allantois arises as a diverticulum of the yolk sac extending from the early foetal bladder into the body stalk and contributes to umbilical vessel development. Regression of the allantois occurs between the 6th and 8th weeks of gestation 7 and the remnant is located between the two arteries within the cord. The intra‐abdominal remnant of the allantois involutes to a thick tube termed the urachus or the median umbilical ligament. 8
In addition to the body stalk, the expanding amnion envelopes the umbilical coelom and the omphalomesenteric duct. The umbilical coelom connects the extra‐embryonic coelom with the intra‐embryonic coelom and regresses by the 12th week of gestation. The omphalomesenteric or vitelline duct connects the primitive intestines with the yolk sac and usually regresses between the 7th and 10th weeks of gestation, leaving a solid, intra‐abdominal cord from the ileum to the umbilicus. 9
Umbilical cord structure
The fully developed umbilical cord normally contains two umbilical arteries, one umbilical vein, the remnant of the allantois all embedded in Wharton's jelly and surrounded by a single layer of amnion 10 , 11 as shown in Figure 1, 12 and Figure 2a.
Figure 1.
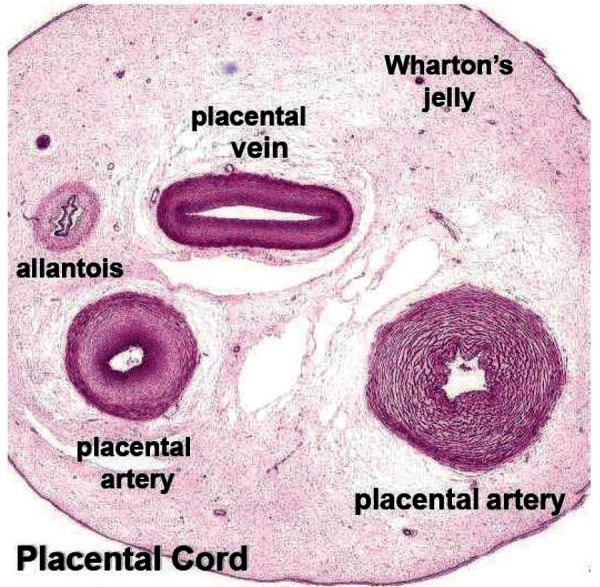
A cross‐sectional image of a postpartum umbilical cord showing the major structures (Hill M. UNSW Embryology. Placenta Histology. [Internet] 2010 [updated 2010 26th May; cited 2011 7th March]; Available from: http://embryology.med.unsw.edu.au/notes/placen‐ta5.htm).
Figure 2.
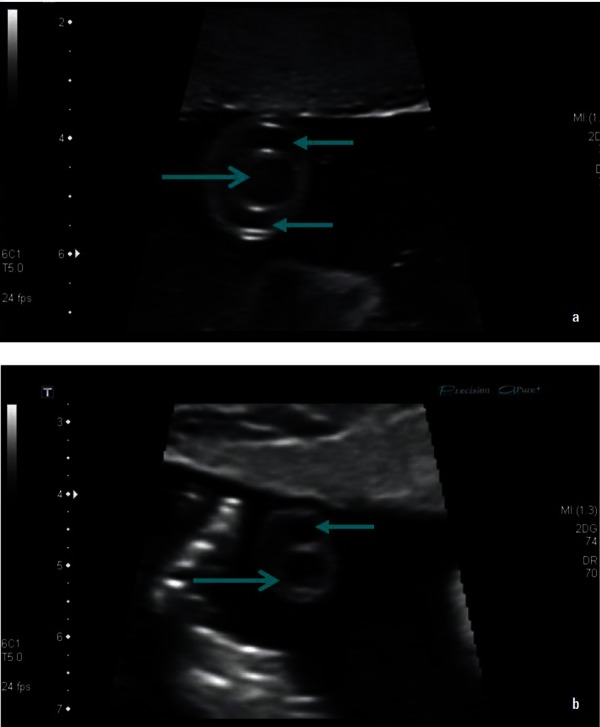
B mode images of a transverse section of the umbilical cord with (a) showing a normal three‐vessel cord and (b) showing a single umbilical artery (←) and vein (→).
At term the umbilical cord has a average length of 50–60 cm. 13 Normal cord length can range from 30 cm to 100 cm, with less than 30 cm considered short. 14 Excessively long cords may be associated with prolapse, looping of the cord around the foetal neck, 15 entanglement, distress and foetal demise. 14 On the other hand very short cords may be associated with delayed foetal descent, premature placental separation, 15 growth restriction, congenital abnormalities, foetal distress and demise. 14
In a study involving 368 healthy pregnant women the average diameter of the umbilical cord was shown to range from 3.19 ± 0.40 mm at 10 weeks gestation, to a peak of 16.72 ± 2.57 mm at 33–35 weeks and then decline to 14.42 ± 1.50 mm at 42 weeks gestation. 13 The decline in cord diameter towards term is attributed to a reduction in the water content of Wharton's jelly 16 which is a mucous connective tissue surrounding the umbilical vessels. 10
The diameter of the umbilical cord vein increases from 4.1 mm at 20 weeks to 8.3 mm at 38 weeks gestation. 17 Togni, et al. 18 demonstrated an increase in the cross‐sectional area of the umbilical cord vein from 28 mm 2 at 24 weeks gestation to a maximum of approximately 58mm 2 between 34–38 weeks, followed by a slight decline from the 39th week. Another variation in the umbilical cord vein is a decrease in the diameter of vessel by approximately 1 mm between the placental and foetal ends. 19
The area of the umbilical cord vein is approximately 30% larger than the combined areas of the arteries and as such the velocity in the vein is approximately half the velocity in either artery, 20 with the velocity in the umbilical cord vein ranging from 10–22 cms/s. 21
The umbilical cord arteries and vein are unlike their counterparts in the remainder of the foetal body as the umbilical cord vein transports oxygenated blood to the foetal heart 22 while the arteries return oxygen‐depleted blood to the placenta. The walls of the umbilical cord artery lack an internal and external elastic lamina and the adventitia found in other arteries is replaced by mucous connective tissue. The umbilical cord vein has a thickened muscularis layer with intermingling circular, longitudinal and oblique smooth muscle fibres as well as an internal elastic lamina. 10
In some cases, one of the umbilical cord arteries may undergo atresia, aplasia or agenesis resulting in a single umbilical cord artery (Figure 2b), with the left umbilical artery being absent more frequently. 23 A single umbilical artery may be associated with aneuploid foetuses, or with intrauterine growth restriction and renal anomalies in euploid foetuses. 23
Located within 3 cm of the cord insertion into the placenta surface 14 there is a 1.5–2 cm long shunt between the umbilical cord arteries, termed the Hyrtl anastomosis. 24 The functions of the Hyrtl anastomosis are to equalise pressure between the umbilical arteries before they enter the placenta and to act as a safety valve in case of placental compression or blockage of an umbilical artery. 25
The two umbilical arteries commonly form a cylindrical helix around the umbilical vein (Figure 3a). The normal umbilical cord has one coil per 5 cm of cord length. 23 The umbilical cord may develop up to 40 spirals and there may be straight portions or reversal of spiral direction in different segments. 26 In most cases the umbilical arteries twist over the vein, however, in 4.2% of cases the vein may twist around straight or hypocoiled arteries 23 (Figure 3b). The helices or frequently termed “spirals” of the umbilical cord are dextral in approximately 90% of cases and sinistral in the remaining. 20 Spiralling is attributed to the helical muscle layers in the umbilical artery walls, 6 however, foetal rotational movements, asymmetry in the sizes and growth rate of the umbilical arteries and asymmetrical contractions of the uterus 26 have also been proposed as causes of umbilical cord helices. In the clinical setting uncoiled or hypocoiled umbilical cords have been associated with suboptimal pregnancy outcomes including increased incidence of interventional deliveries, higher cord pH and heart rate disturbances. 27 However, mechanical testing has proven that uncoiled cords suffer no more ill effects of external compression, twisting and pressure than normal cords and that mechanical features of coiling are not a contributing factor to perinatal morbidity. 28 Hyper coiled umbilical cords have been associated with preterm labour, preterm birth and growth restriction. 29
Figure 3.
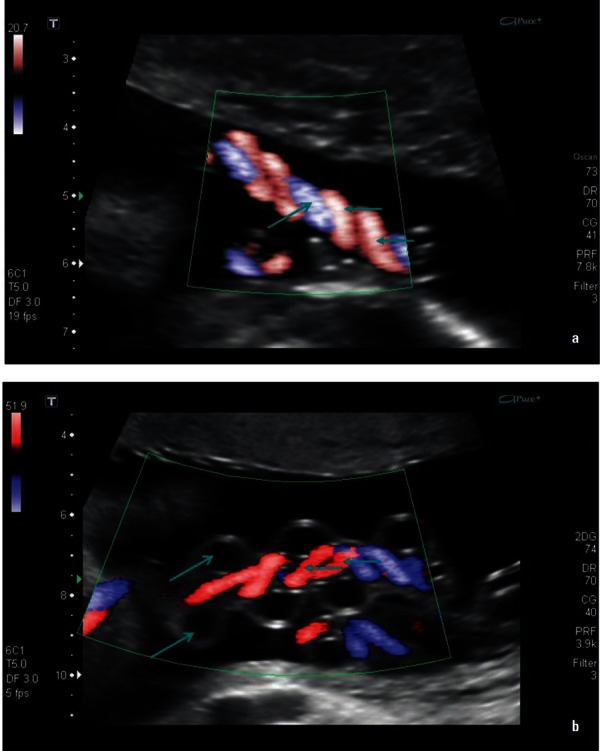
Longitudinal images of the umbilical cord with (a) showing paired arteries (←) spiralling around the umbilical vein (→) and (b) showing the vein looping around central coiled arteries.
Foetal venous circulation
The foetal venous system develops from three embryological paired veins; the vitelline veins from the yolk sac, the umbilical veins from the chorion and the cardinal veins from the embryo. 22 , 30 Venous system development results in the obliteration of the right umbilical vein and the development of the ductus venosus between the left umbilical vein and the inferior vena cava (IVC). 22
The persisting left umbilical vein travels from the placenta to enter the foetal abdomen at the umbilicus and courses into the liver. Within the liver, oxygenated blood passes through the ductus venosus to enter the left hepatic vein near its confluence with the IVC. In an uncomplicated pregnancy, 30% of blood is diverted through the ductus venosus at 20 weeks gestation with a reduction to 20% from 30 weeks gestation until term. 31 The remaining oxygenated blood from the umbilical vein enters the left and right portal veins of the liver, and drains into the IVC via the right hepatic vein.
The blood from the ductus venosus enters the IVC along with blood returning from the hepatic veins, lower extremities and abdominal wall, and flows into the right atrium. In the right atrium, there are two pathways as shown in Figure 4. 21
Figure 4.
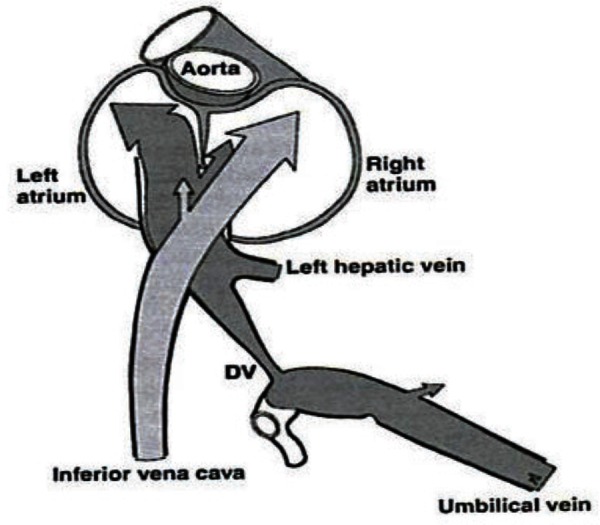
Pathways for blood flow from the IVC and the ductus venosus (Kiserud T. Physiology of fetal circulation. Semin Fet Neonat Med 2005;10 (6): 493–503).
-
1
Blood from the IVC combines with blood from the superior vena cava (SVC) and passes through the tricuspid valve into the right ventricle. From the right ventricle, blood enters the pulmonary trunk and the ductus arteriosus to flow into the descending aorta for distribution to the organs of the abdomen, pelvis and lower extremities, and returns to the placenta via the paired umbilical arteries.
-
2
The blood diverted through the ductus venosus enters the left atrium via the foramen ovale. This bypass of the right ventricle occurs because of the higher velocity of the ductus venosus blood, valve placement within the IVC 32 and to streaming of well‐oxygenated blood towards the medial aspect of the IVC. 14 The blood in the left atrium joins blood from the pulmonary veins to enter the ascending aorta for distribution to the heart, head and upper extremities, and eventual return to the heart via the SVC.
Mechanisms of blood flow in the umbilical cord vein
The placenta provides a large volume of blood awaiting transportation to the foetus. “The quantity of the blood flowing from the fetus to the placenta very nearly equals that flowing from the placenta to the fetus” 20 and as such the foetus can be considered a closed system.
Movement of oxygenated blood from the placenta to the foetus occurs by the following methods:
-
1The umbilical cord vein pressure increases from 4.5 mmHg at 18 weeks gestation to 6 mmHg at term 21 and the blood pressure distending the umbilical vein is higher than that in the fetal IVC. 20 This gradient is due to at least two mechanisms:
-
1.1Normal foetal heart contractions producing a pressure gradient between the atria and ventricles, which in turn diminishes the preload in the venous circulation and allows the blood in the umbilical vein to move towards the heart 30
- 1.2
-
1.1
-
2
Passive pressure changes in the umbilical cord vein due to longitudinal distortion of the arteries with each foetal heart beat. The pressure peaks in the umbilical cord artery and vein are out of phase by 180° which results in the addition of the effect of numerous, small pressure changes along the length of the cord and the subsequent movement of blood through the umbilical cord vein. 20
Conclusion
The umbilical cord normally contains two umbilical arteries, a single umbilical vein, an obliterated allantois duct, all surrounded by Wharton's jelly and contained within an outer layer of amnion. The structure of the umbilical cord can vary in the number of umbilical arteries, the length and diameter of the cord, and the direction and number of spirals of the cord. The umbilical cord vein is the remnant of embryological venous development that results in the obliteration of the right umbilical cord vein and the establishment of two pathways through the liver and heart for oxygenated blood travelling from the placenta to the foetus via the persisting left umbilical cord vein. Pressure gradients caused by foetal heart contractions, foetal breathing and distortions of the umbilical cord arteries transports oxygenated blood from the placenta to the foetus through the umbilical cord vein.
An understanding of the embryology, anatomy and physiology of the umbilical cord, especially the vein, may lead to more comprehensive ultrasound imaging, heighten appreciation of the importance of this structure and encourage further research into this critical blood conduit.
Acknowledgements
I am indebted to my colleagues and supervisors for their constructive review of this article. I am grateful to the NSW Department of Health and Charles Sturt University for their financial assistance during my higher research degree.
References
- 1. Gill RW, Kossoff G, Warren PS, Garrett WJ. Umbilical venous flow in normal and complicated pregnancy. Ultrasound Med Biol 1984; 10 (3): 349–63. [DOI] [PubMed] [Google Scholar]
- 2. Schöni‐Affolter F, Dubuis‐Grieder C, Strauch E. The umbilical cord. [Internet] 2007 [updated 15th October 2007; cited 2012 10th March]; Available from: http://www.embryology.ch/anglais/fplacenta/cordon01.html.
- 3. Cochard LR. Netter's Atlas of Human Embryology. 1st ed. New Jersey: Icon Learning Systems; 2002. [Google Scholar]
- 4. Standring S, ed. Gray's Anatomy: The Anatomical Basis of Clinical Practice. 40 ed. Edinburgh: Churchill Livingstone Elsevier; 2008. [Google Scholar]
- 5. Blackburn ST. Maternal, Fetal, & Neonatal Physiology: A Clinical Perspective. 3rd ed. St. Louis: Sanders Elsevier; 2007. [Google Scholar]
- 6. Callen PW. Ultrasonography in Obstetrics and Gynecology. 4th ed. Philadelphia: WB Saunders Company; 2000. [Google Scholar]
- 7. Larsen WJ. Human Embryology. 3rd ed. Philadelphia: Churchill Livingstone; 2001. [Google Scholar]
- 8. Moore KL, Persaud TV. The Developing Human: Clinically Orientated Embrylogy. 8th ed. Philadelphia: Saunders Elsevier; 2008. [Google Scholar]
- 9. Khati N, Enquist E, Javitt M. Imaging of the umbilicus and periumbilical region. Radiographics 1998; 18 (2): 413–31. [DOI] [PubMed] [Google Scholar]
- 10. Bergman RA, Afifi AK, Heidger PM. Altas of microscopic anatomy – a functional approach. [Internet] 2012 [updated 2012 24th January; cited 2012 2nd March]; Available from: http://www.anatomyatlases.org/MicroscopicAnatomy/Section13/Plate13261.shtml.
- 11. Ferguson VL, Dodson RB. Bioengineering aspects of the umbilical cord. Eur J Obstet Gynecol Reprod Biol 2009; 144 (Supplement 1): S108–13. [DOI] [PubMed] [Google Scholar]
- 12. Hill M. UNSW Embryology. Placenta Histology. [Internet] 2010 [updated 2010 26th May; cited 2011. 7th March]; Available from: http://embryology.med.unsw.edu.au/notes/placenta5.htm.
- 13. Di Naro E, Ghezzi F, Raio L, Franchi M, D'Addario V. Umbilical cord morphology and pregnancy outcome. Eur J Obstet Gynecol Reprod Biol 2001; 96 (2): 150–57. [DOI] [PubMed] [Google Scholar]
- 14. Cunningham FG, Leveno KJ, Bloom SL, Hauth JC, Rouse DJ, Spong CY. Williams Obstetrics. 23rd ed. New York: McGraw Hill Medical; 2010. [Google Scholar]
- 15. Hanretty K. Obstetrics Illustrated. Edinburgh: Churchill Livingstone Elsevier; 2010. [Google Scholar]
- 16. Weissman A, Jakobi P, Bronshtein M, Goldstein I. Sonographic measurements of the umbilical cord and vessels during normal pregnancies. J Ultrasound Med 1994; 13: 11–14. [DOI] [PubMed] [Google Scholar]
- 17. Barbera A, Galan HL, Ferrazzi E, Rigano S, Józwik M, Battaglia FC, et al. Relationship of umbilical vein blood flow to growth parameters in the human fetus. Am J Obstet Gynecol 1999; 181: 174–79. [DOI] [PubMed] [Google Scholar]
- 18. Togni FA, Araujo Júnior E, Vasques FA, Moron AF, Torloni MR, Nardozza LM. The cross‐sectional area of umbilical cord components in normal pregnancy. Int J Gynaecol Obstet 2007; 96: 156–61. [DOI] [PubMed] [Google Scholar]
- 19. Li WC, Ruan XZ, Zhang HM, Zeng YJ. Biomechanical properties of different segments of human umbilical cord vein and its value for clinical application. J Biomed Mater Res B Appl Biomater 2006; 76B: 93–7. [DOI] [PubMed] [Google Scholar]
- 20. Reynolds SR. Mechanisms of placentofetal blood flow. Obstet Gynecol 1978; 51: 245–49. [PubMed] [Google Scholar]
- 21. Kiserud T. Physiology of fetal circulation. Semin Fetal Neonatal Med 2005; 10: 493–503. [DOI] [PubMed] [Google Scholar]
- 22. Hofstaetter C, Plath H, Hansmann M. Prenatal diagnosis of abnormalities of the fetal venous system. Ultrasound Obstet Gynecol 2000; 15: 231–41. [DOI] [PubMed] [Google Scholar]
- 23. Predanic M. Sonographic assessment of the umbilical cord. Ultrasound Rev Obstet Gynecol 2005; 5: 105–10. [Google Scholar]
- 24. Raio L, Ghezzi F, Di Naro E, Franchi M, Bruhwiler H. Prenatal assessment of the Hyrtl anastomosis and evaluation of its function. Hum Reprod 1999; 14: 1890–93. [DOI] [PubMed] [Google Scholar]
- 25. Gordon Z, Eytan O, Jaffa AJ, Elad D. Hemodynamic analysis of Hyrtl anastomosis in human placenta. Am J Physiol Regul Integr Comp Physiol 2007; 292: R977–82. [DOI] [PubMed] [Google Scholar]
- 26. Edmonds HW. The spiral twist of the normal umbilical cord in twins and singletons. Am J Obstet Gynecol 1959; 67: 102–20. [DOI] [PubMed] [Google Scholar]
- 27. Di Naro E, Ghezzi F, Raio L, Franchi M, D'Addario V, Lanzillotti G, et al. Umbilical vein blood flow in fetuses with normal and lean umbilical cord. Ultrasound Obstet Gynecol 2001; 17: 224–28. [DOI] [PubMed] [Google Scholar]
- 28. Dado GM, Dobrin PB, Mrkvicka RS. Venous flow through coiled and noncoiled umbilical cords. Effects of external compression, twisting and longitudinal stretching. J Reprod Med 1997; 42: 576–80. [PubMed] [Google Scholar]
- 29. Kumar S. Handbook of Fetal Medicine, [monograph online]: Cambridge University Press; 2010. [cited 2012 12th March]; Available from: http://CSUAU.eblib.com/patron/FullRecord.aspx?p=542863. [Google Scholar]
- 30. Fasouliotis SJ, Achiron R, Kivilevitch Z, Yagel S. The human fetal venous system: normal embryologic, anatomic, and physiologic characteristics and developmental abnormalities. J Ultrasound Med 2002; 21(10): 1145–58. [DOI] [PubMed] [Google Scholar]
- 31. Kiserud T, Acharya G. The fetal circulation. Prenat Diagn 2004; 24: 1049–59. [DOI] [PubMed] [Google Scholar]
- 32. Williams PL, ed. Gray's Anatomy. 38th ed. New York: Churchill Livingstone; 1995. [Google Scholar]
- 33. Vasques FA, Moron AF, Murta CG, Carvalho FH, Barbosa MM, Marcolino LA. The assessment of fetal well‐being by venous Doppler velocimetry. Ultrasound Rev Obstet Gynecol 2004; 4: 121–25. [Google Scholar]


