Abstract
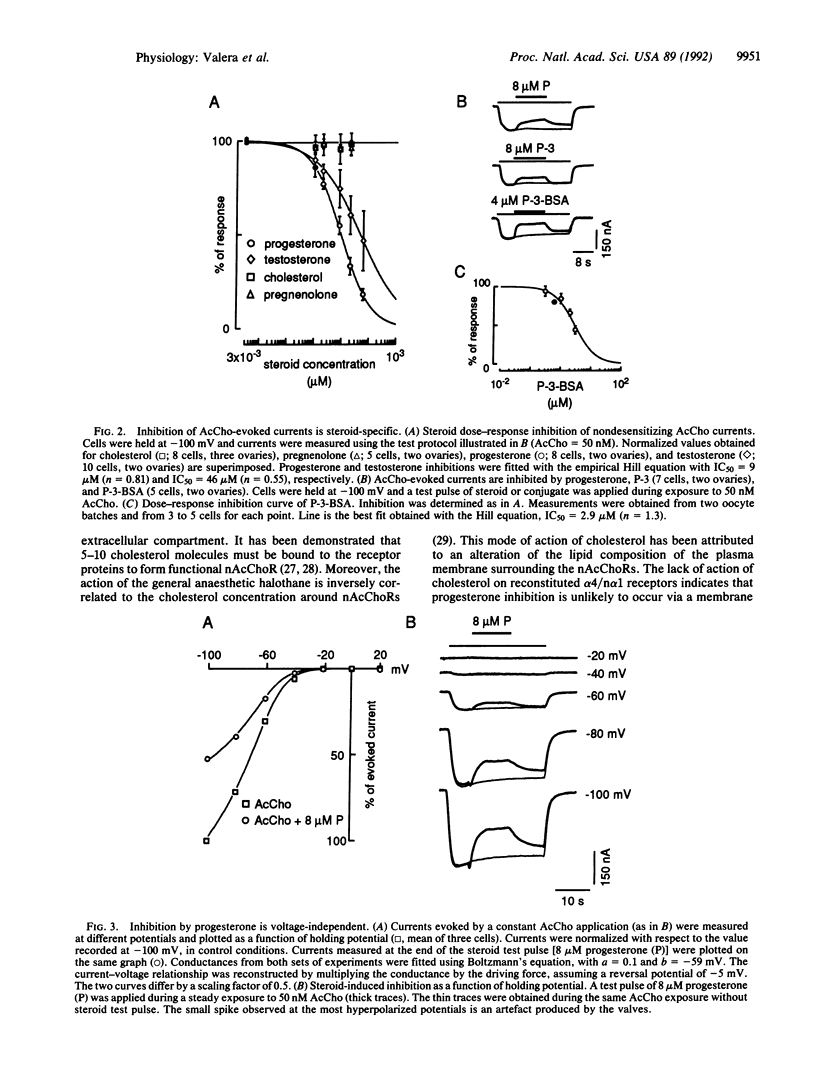
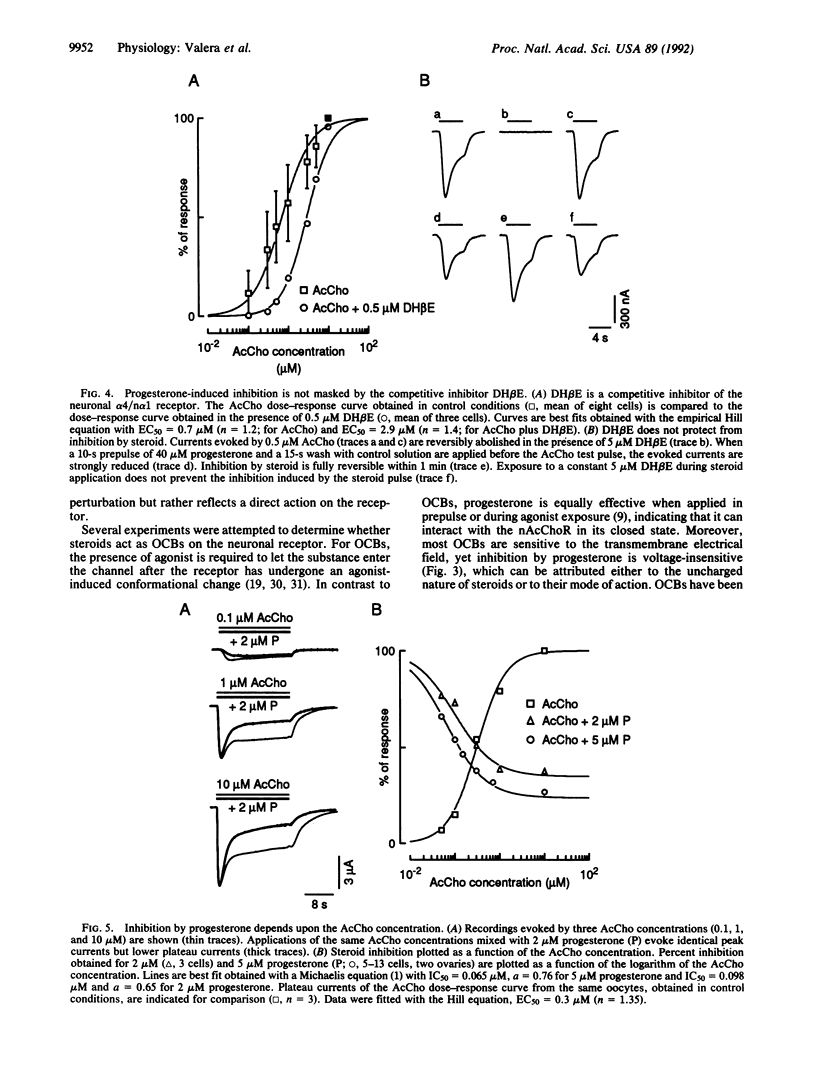
The major brain nicotinic acetylcholine receptor is assembled from two subunits termed alpha 4 and n alpha 1. When expressed in Xenopus oocytes, these subunits reconstitute a functional acetylcholine receptor that is inhibited by progesterone levels similar to those found in serum. In this report, we show that the steroid interacts with a site located on the extracellular part of the protein, thus confirming that inhibition by progesterone is not due to a nonspecific perturbation of the membrane bilayer or to the activation of second messengers. Because inhibition by progesterone does not require the presence of agonist, is voltage-independent, and does not alter receptor desensitization, we conclude that the steroid is not an open channel blocker. In addition, we show that progesterone is not a competitive inhibitor but may interact with the acetylcholine binding site and that its effect is independent of the ionic permeability of the receptor.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ascher P., Large W. A., Rang H. P. Studies on the mechanism of action of acetylcholine antagonists on rat parasympathetic ganglion cells. J Physiol. 1979 Oct;295:139–170. doi: 10.1113/jphysiol.1979.sp012958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baulieu E. E., Godeau F., Schorderet M., Schorderet-Slatkine S. Steroid-induced meiotic division in Xenopus laevis oocytes: surface and calcium. Nature. 1978 Oct 19;275(5681):593–598. doi: 10.1038/275593a0. [DOI] [PubMed] [Google Scholar]
- Baulieu E. E., Robel P. Neurosteroids: a new brain function? J Steroid Biochem Mol Biol. 1990 Nov 20;37(3):395–403. doi: 10.1016/0960-0760(90)90490-c. [DOI] [PubMed] [Google Scholar]
- Bertrand D., Bader C. R. DATAC: a multipurpose biological data analysis program based on a mathematical interpreter. Int J Biomed Comput. 1986 May;18(3-4):193–202. doi: 10.1016/0020-7101(86)90016-4. [DOI] [PubMed] [Google Scholar]
- Bertrand D., Ballivet M., Rungger D. Activation and blocking of neuronal nicotinic acetylcholine receptor reconstituted in Xenopus oocytes. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1993–1997. doi: 10.1073/pnas.87.5.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand D., Valera S., Bertrand S., Ballivet M., Rungger D. Steroids inhibit nicotinic acetylcholine receptors. Neuroreport. 1991 May;2(5):277–280. doi: 10.1097/00001756-199105000-00016. [DOI] [PubMed] [Google Scholar]
- Cachelin A. B., Colquhoun D. Desensitization of the acetylcholine receptor of frog end-plates measured in a Vaseline-gap voltage clamp. J Physiol. 1989 Aug;415:159–188. doi: 10.1113/jphysiol.1989.sp017717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changeux J. P., Pinset C., Ribera A. B. Effects of chlorpromazine and phencyclidine on mouse C2 acetylcholine receptor kinetics. J Physiol. 1986 Sep;378:497–513. doi: 10.1113/jphysiol.1986.sp016232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charnet P., Labarca C., Leonard R. J., Vogelaar N. J., Czyzyk L., Gouin A., Davidson N., Lester H. A. An open-channel blocker interacts with adjacent turns of alpha-helices in the nicotinic acetylcholine receptor. Neuron. 1990 Jan;4(1):87–95. doi: 10.1016/0896-6273(90)90445-l. [DOI] [PubMed] [Google Scholar]
- Cooper E., Couturier S., Ballivet M. Pentameric structure and subunit stoichiometry of a neuronal nicotinic acetylcholine receptor. Nature. 1991 Mar 21;350(6315):235–238. doi: 10.1038/350235a0. [DOI] [PubMed] [Google Scholar]
- Dodson B. A., Braswell L. M., Miller K. W. Barbiturates bind to an allosteric regulatory site on nicotinic acetylcholine receptor-rich membranes. Mol Pharmacol. 1987 Jul;32(1):119–126. [PubMed] [Google Scholar]
- Fesik S. W., Makriyannis A. Geometric requirements for membrane perturbation and anesthetic activity. Conformational analysis of alphaxalone and delta 16-alphaxalone and 2H NMR studies on their interactions with model membranes. Mol Pharmacol. 1985 Jun;27(6):624–629. [PubMed] [Google Scholar]
- Finidori-Lepicard J., Schorderet-Slatkine S., Hanoune J., Baulieu E. E. Progesterone inhibits membrane-bound adenylate cyclase in Xenopus laevis oocytes. Nature. 1981 Jul 16;292(5820):255–257. doi: 10.1038/292255a0. [DOI] [PubMed] [Google Scholar]
- Fong T. M., McNamee M. G. Correlation between acetylcholine receptor function and structural properties of membranes. Biochemistry. 1986 Feb 25;25(4):830–840. doi: 10.1021/bi00352a015. [DOI] [PubMed] [Google Scholar]
- Gee K. W., Bolger M. B., Brinton R. E., Coirini H., McEwen B. S. Steroid modulation of the chloride ionophore in rat brain: structure-activity requirements, regional dependence and mechanism of action. J Pharmacol Exp Ther. 1988 Aug;246(2):803–812. [PubMed] [Google Scholar]
- Harrison N. L., Majewska M. D., Harrington J. W., Barker J. L. Structure-activity relationships for steroid interaction with the gamma-aminobutyric acidA receptor complex. J Pharmacol Exp Ther. 1987 Apr;241(1):346–353. [PubMed] [Google Scholar]
- Ichikawa S., Sawada T., Nakamura Y., Morioka H. Ovarian secretion of pregnane compounds during the estrous cycle and pregnancy in rats. Endocrinology. 1974 Jun;94(6):1615–1620. doi: 10.1210/endo-94-6-1615. [DOI] [PubMed] [Google Scholar]
- Imoto K., Busch C., Sakmann B., Mishina M., Konno T., Nakai J., Bujo H., Mori Y., Fukuda K., Numa S. Rings of negatively charged amino acids determine the acetylcholine receptor channel conductance. Nature. 1988 Oct 13;335(6191):645–648. doi: 10.1038/335645a0. [DOI] [PubMed] [Google Scholar]
- Inoue M., Kuriyama H. Glucocorticoids inhibit acetylcholine-induced current in chromaffin cells. Am J Physiol. 1989 Nov;257(5 Pt 1):C906–C912. doi: 10.1152/ajpcell.1989.257.5.C906. [DOI] [PubMed] [Google Scholar]
- Jones O. T., McNamee M. G. Annular and nonannular binding sites for cholesterol associated with the nicotinic acetylcholine receptor. Biochemistry. 1988 Apr 5;27(7):2364–2374. doi: 10.1021/bi00407a018. [DOI] [PubMed] [Google Scholar]
- Lechleiter J., Wells M., Gruener R. Halothane-induced changes in acetylcholine receptor channel kinetics are attenuated by cholesterol. Biochim Biophys Acta. 1986 Apr 25;856(3):640–645. doi: 10.1016/0005-2736(86)90159-8. [DOI] [PubMed] [Google Scholar]
- Majewska M. D., Mienville J. M., Vicini S. Neurosteroid pregnenolone sulfate antagonizes electrophysiological responses to GABA in neurons. Neurosci Lett. 1988 Aug 1;90(3):279–284. doi: 10.1016/0304-3940(88)90202-9. [DOI] [PubMed] [Google Scholar]
- Maller J. L., Butcher F. R., Krebs E. G. Early effect of progesterone on levels of cyclic adenosine 3':5'-monophosphate in Xenopus oocytes. J Biol Chem. 1979 Feb 10;254(3):579–582. [PubMed] [Google Scholar]
- Ogden D. C., Siegelbaum S. A., Colquhoun D. Block of acetylcholine-activated ion channels by an uncharged local anaesthetic. Nature. 1981 Feb 12;289(5798):596–598. doi: 10.1038/289596a0. [DOI] [PubMed] [Google Scholar]
- Puia G., Santi M. R., Vicini S., Pritchett D. B., Purdy R. H., Paul S. M., Seeburg P. H., Costa E. Neurosteroids act on recombinant human GABAA receptors. Neuron. 1990 May;4(5):759–765. doi: 10.1016/0896-6273(90)90202-q. [DOI] [PubMed] [Google Scholar]
- Seeman P. The membrane actions of anesthetics and tranquilizers. Pharmacol Rev. 1972 Dec;24(4):583–655. [PubMed] [Google Scholar]
- Smith S. S. Progesterone administration attenuates excitatory amino acid responses of cerebellar Purkinje cells. Neuroscience. 1991;42(2):309–320. doi: 10.1016/0306-4522(91)90377-z. [DOI] [PubMed] [Google Scholar]
- Steinbach A. B. Alteration by xylocaine (lidocaine) and its derivatives of the time course of the end plate potential. J Gen Physiol. 1968 Jul;52(1):144–161. doi: 10.1085/jgp.52.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoshima C., Unwin N. Ion channel of acetylcholine receptor reconstructed from images of postsynaptic membranes. Nature. 1988 Nov 17;336(6196):247–250. doi: 10.1038/336247a0. [DOI] [PubMed] [Google Scholar]
- Unwin N. The structure of ion channels in membranes of excitable cells. Neuron. 1989 Dec;3(6):665–676. doi: 10.1016/0896-6273(89)90235-3. [DOI] [PubMed] [Google Scholar]
- Wasserman W. J., Pinto L. H., O'Connor C. M., Smith L. D. Progesterone induces a rapid increase in [Ca2+]in of Xenopus laevis oocytes. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1534–1536. doi: 10.1073/pnas.77.3.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F. S., Gibbs T. T., Farb D. H. Inverse modulation of gamma-aminobutyric acid- and glycine-induced currents by progesterone. Mol Pharmacol. 1990 May;37(5):597–602. [PubMed] [Google Scholar]