Abstract
Objectives: To evaluate the accuracy of transvaginal sonography (TVS) in the diagnosis of deep infiltrating endometriosis (DIE) of the posterior compartment (rectovaginal septum, uterosacral ligaments, rectosigmoid colon, vagina) when undertaken by physicians of varying experience and to investigate if size of the nodule is relevant in influencing diagnostic accuracy. Methods: 381 patients who were operated on between January 2007 and December 2010 for suspected pelvic endometriosis were prospectively recruited. Clinical, surgical and histopathologic data were collected and a preoperative TVS was carried out. Comparison was made between the diagnostic accuracy of TVS performed by two groups of physicians of different expertise. Results: One hundred and thirty‐six patients underwent removal of deep endometriotic lesions of the posterior compartment. Sensitivity, specificity, positive and negative predictive value and the overall diagnostic accuracy of the expert operators were 77%, 95%, 90%, 87% and 88% respectively. Corresponding values for first‐level operators were: 45%, 98%, 92%, 79% and 81%. In patients with positive findings at TVS, the mean diameter of the endometriotic nodule was of 4.7 ± 3.4 cm while, in cases with negative findings, the average diameter was 2.6 ± 1.1 cm (P < 0.05). Conclusions: TVS is accurate in detecting the presence of DIE. Accuracy is dependent on the experience of the physician and the size of the nodule.
Keywords: deep infiltrating, endometriosis, rectovaginal septum, transvaginal ultrasound
Introduction
Deep infiltrating posterior endometriosis (DIE) is defined as the presence of endometrial glands and stroma penetrating the retroperitoneal space to a depth of at least 5 mm. 1 , 2 Such a condition is accompanied by fibrosis of the retroperitoneum and proliferation of smooth muscle cells 3 , 4 which cause distortion of the pelvic anatomy. Clinically, DIE is responsible for sterility and painful symptoms, such as dysmenorrhea, dyspareunia, dyschezia and chronic pelvic pain which limit the quality of life of young patients. Several different anatomic structures can be affected by DIE, mainly the anterior wall of the rectosigmoid colon, uterosacral ligaments, rectouterine space, vagina and the rectovaginal septum. The aim of surgical treatment is the reduction of pain and the restoration of fertility, achieved by removal of the entire endometriotic lesion and attainment of a normal anatomical relationship between the pelvic organs. 5 Exact preoperative knowledge of the presence and extent of DIE would help in planning proper treatment (medical versus surgical), in counselling women about the risks and complications of surgery, and in deciding whether to send the patient to a skilled laparoscopist working in a recognised centre for the treatment of endometriosis. Transvaginal sonography (TVS) is the method of choice for investigating the female pelvic anatomy, and it has been demonstrated to be useful in the diagnosis of DIE. 6 – 10 Published reports on the diagnostic accuracy of TVS in the diagnosis of DIE are based on a restricted number of expert physicians performing the ultrasound scans. Moreover, the relationship between diagnostic accuracy and lesion size has never been evaluated.
We conducted this prospective study with two aims: to test the accuracy of TVS in relation to the size of DIE in a population of patients with symptoms suggesting the presence of endometriosis, and to determine whether training and experience are factors influencing the preoperative detection of DIE.
Methods
We prospectively collected data at the Department of Gynecology and Obstetrics of the University of Bologna from January 2007 to December 2010 from those patients who had been examined by TVS due to symptoms suggesting the presence of endometriosis. Of over 506 women examined, 381 patients (mean age 33.6 ± 5.9 years) underwent laparoscopic surgery during the study period and constituted the patient population. Three hundred and forty (89%) were nulliparous, 61 (16%) were on cyclic oral contraceptives, 38 (10%) were on continuous oral contraceptives and 53 (14%) were being treated with GnRHa. Preoperative ultrasound scans were performed during the week before surgery by physicians of either Group A (residents in training with 12–24 months of experience in gynecological ultrasound) or Group B (gynecologists specialised in gynecologic ultrasound, with more than ten years of experience in diagnosis and treatment of endometriosis). The patients were randomly allocated to one group of examiners or another, depending on the day of the week in which the ultrasound scan was scheduled, irrespective of the day of the cycle.
All sonographic examinations were carried out in a systematic and predetermined manner, with an examination time of about 10–15 minutes, using the same sonographic equipment (Esaote My Lab Twice, Genova, Italy and Samsung Medison Accuvix V20 Prestige, Milan, Italy) equipped with a wide‐band 5.0–9.0 MHz vaginal transducer. Detailed sonographic reports were recorded, and representative digital images and videoclips of every patient were saved and stored on a hard disk for subsequent review and analysis.
The indications for TVS (Table 1) were one or more of the following: history of infertility (36%), suspicion of an ovarian cyst (18%), follow‐up after previous surgery for endometriosis (9%), dysmenorrhea (50%), deep dyspareunia (28%), dyschezia (20%), chronic pelvic pain (43%) or a palpable nodule at bimanual gynecologic examination (29%). Transvaginal scans of the pelvic organs were performed first on longitudinal and transverse sections to evaluate the anatomy of the uterus and the ovaries. Then, the posterior pelvic compartment (i.e., consisting of the rectosigmoid colon, pouch of Douglas, uterosacral ligaments, rectovaginal septum, vagine) was investigated by positioning; the transducer in the posterior vaginal fornix; and tilting the scan towards the rectum, looking for the fresence of hypoechoic irregular‐shaped nodules with a hyperechoic rim, located behind the uterine cervix (Figure 1) or lateral to it (Figure 2). 6 – 10 The rectovaginal septum was considered to ba involved when a hypoechoic nodule was seen axtending downwards from the anterior wall of the rectum and accupying the space between the rectum and the vaginal wall. (Figure 3). DIE on the sigmoid colon appeared as hypoechoic nodules on the antimesenteric portion of the descending colon, located above the level of the uterine fundus, far from the tip of the probe.
Table 1.
Indications for TVS (506 patients).
| Indications for TVS | No. | (%) |
|---|---|---|
| Evaluation of an ovarian cyst | 91 | 18 |
| Follow up for endometriosis | 46 | 9 |
| Dysmenorrhea | 253 | 50 |
| Dyspareunia | 142 | 28 |
| Dyschezia | 101 | 20 |
| Chronic pelvic pain | 218 | 43 |
| Palpable posterior nodule | 147 | 29 |
| History of infertility | 182 | 36 |
Figure 1.
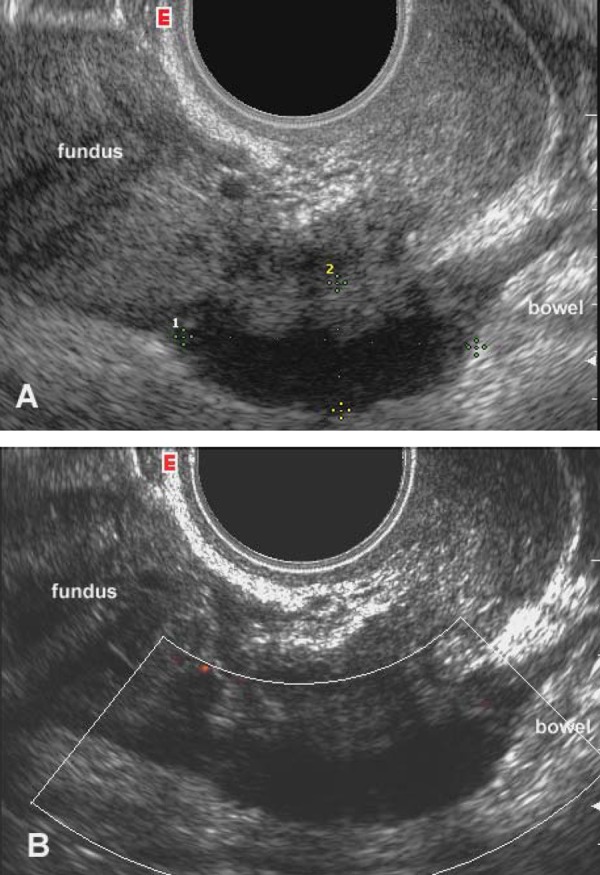
Transvaginal sagittal scan of the posterior compartment of the pelvis, including the pouch of Douglas. A) Greyscale image: a solid hypoechoic nodule with blurred margins and a hyperechoic rim (calipers) suggestive of the presence of DIE is seen at the level of the anterior wall of the rectum. B) Power Doppler reveals the absence of blood vessels inside the implant.
Figure 2.
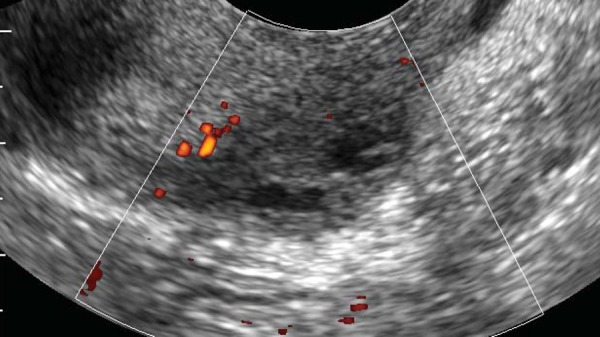
Transvaginal transverse sonogram at the level of the uterine cervix, showing the presence of a hypoechoic nodule with irregular outer margins and scarce vascularisation located in the median third of the left utero‐sacral ligament.
Figure 3.
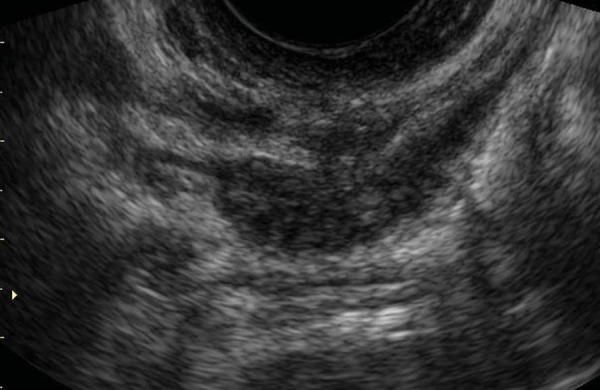
Transvaginal sagittal scan of the posterior compartment of the pelvis, showing the presence of an endometriotic nodule extending caudally toward the rectovaginal septum. DIE appears as a solid hypoechoic nodule with spiculated margins and a hyperechoic rim, close to the tip of the vaginal probe.
DIE was diagnosed when at least one of the above mentioned structures was involved. 9 When required, a transabdominal scan was performed, based on the sonographers' judgment. The investigators were blind to the results of the bimanual pelvic examination but could ask the patients about their symptoms. In fact, it is well known that there is a strong correlation between pain symptoms and the anatomic location of deep infiltrating endometriosis 11 ; therefore, an exhaustive clinical examination should always be carried out in order to search for the eventual presence of DIE responsible for a given symptom.
During the study period, 381 patients underwent operative laparoscopy performed by one skilled gynecologic surgeon specialised in endometriosis (RS) who was aware of pre‐operative ultrasound findings prior to performing the surgery. In each case, the laparoscopy consisted of a diagnostic phase during which the stage of pelvic endometriosis was scored according to the revised American Fertility Society (rAFS) classification. 12 Whenever the presence of DIE was confirmed, the removal of all visible lesions was carried out, with varying techniques according to the site involved, lesion size and depth of invasion. The essential points of the operation were: lysis of adhesions, drainage and stripping of endometriomas and suspension of the ovaries to the round ligaments. Then, dissection of the two pararectal spaces was performed with exposure of the ureters; mobilisation of the obliterated cul‐de‐sac was obtained with en bloc resection of the DIE, by a transverse incision across the uterine cervix above the point of adherence of the bowel which was carried out laterally across the base of each uterosacral ligament. The dissection was continued caudally up to the rectovaginal septum to expose the normal healthy rectal wall below the nodule. In this way, all the DIE comes to lie on the anterior wall of the bowel as one mass containing the uterosacral ligaments, the rectovaginal septum, the vaginal wall (if found to be involved), the obliterated pouch of Douglas and the infiltrated anterior rectal wall. At this point, skinning of the rectal wall was performed, if judged feasible, or intestinal resection was carried out in the cases in which stenosis of the lumen and full thickness infiltration of the rectal wall was seen. All removed specimens were sent for histopathological analysis. Confirmation of DIE was based on the presence of endometrial glands and stroma together with fibrosis and smooth muscle cell hyperplasia and hypertrophy. 13
The sensitivity, specificity, positive and negative predictive values and accuracy of TVS were calculated in relation to the different groups of physicians performing the scans and to the size of the removed DIE lesion as measured by the pathologist. Cohen's kappa statistic was used for the evaluation of agreement between each diagnostic system and the histological outcome. According to Landis and Koch, 14 the degree of agreement was considered to be poor when k < 0.2; fair 0.2 < k ≤ 0.4; moderate 0.4 < k ≤ 0.6; good 0.6 < k ≤ 0.8 and very good 0.8 < k ≤ 1. The level of significance was set at P < 0.05.
Results
Of the 381 patients, surgery and subsequently histopathology confirmed the presence of DIE in 136 (36%) cases. The findings at laparoscopy are summarised in Table 2. Associated pelvic pathologies were: one or more endometriotic ovarian cysts in 150 (39%) cases, ovarian cysts other than endometriotic in 23 (6%), pelvic adhesions in 182 (48%), superficial peritoneal endometriosis in 137 (36%), bladder endometriosis in 50 (13%), myomas in 59 (15%), sactosalpinges or tubal blockage at dye test in 76 (20%) and upper intestinal tract endometriosis in 10 (3%).
Table 2.
Findings at laparoscopy (381 patients, mean age 33.6 ± 5.9 years).
| No. | (%) | ||
|---|---|---|---|
| DIE | Associated pelvic pathologies | 136 | 36 |
| Pelvic adhesions | 95 | 70 | |
| Endometriotic cyst(s) | 79 | 58 | |
| Superficial peritoneal endometriosis | 60 | 44 | |
| Sactosalpinges or tubal blockage at dye test | 37 | 27 | |
| Bladder endometriosis | 32 | 24 | |
| Non‐endometriotic ovarian cyst(s) | 18 | 13 | |
| Myoma(s) | 18 | 13 | |
| Upper intestinal tract endometriosis | 10 | 7 | |
| Normal posterior compartment | Associated pelvic pathologies | 245 | 64 |
| Pelvic adhesions | 87 | 36 | |
| Superficial peritoneal endometriosis | 77 | 31 | |
| Endometriotic ovarian cyst(s) | 71 | 29 | |
| Normal pelvic anatomy | 53 | 22 | |
| Myoma(s) | 41 | 17 | |
| Sactosalpinges or tubal blockage at dye test | 39 | 16 | |
| Bladder endometriosis | 18 | 7 | |
| Non‐endometriotic ovarian cyst(s) | 5 | 2 | |
| Overall | 381 | 100 |
The group of residents in training evaluated 163 patients, of whom 53 (33%) turned out to have DIE at surgery while the group physicians expert in TVS evaluated 218 patients, of whom 83 (38%) were affected by DIE. Overall, 88 patients were correctly diagnosed as having DIE while 236 patients were true negatives.
The sensitivity, specificity, positive and negative predictive values and overall accuracy for TVS with the corresponding features for expert and first‐level operators are given in Table 3. Diagnostic differences of first‐ and second level physicians are statistically significant with P < 0.05. In 48 women (29 in Group A, 19 in Group B), TVS failed to diagnose DIE (in 20 cases, the DIE consisted of small nodules located laterally on one uterosacral ligament, not infiltrating the rectal wall while in 18 women the DIE consisted of a bulky nodule behind the uterus; 10 patients had a nodule located in the upper pelvis, far from the tip of the vaginal probe). There were nine false positive cases (two in Group A and seven in Group B). In patients with true positive findings at TVS, the mean diameter of the endometriotic nodule as measured at histopathology was 4.7 ± 3.4 cm while, in cases with negative findings, the mean diameter was 2.6 ± 1.1 cm (P < 0.05). No statistical difference was found in the mean diameter of DIE between the group of patients evaluated by the expert physicians (4.1 ± 3.1 cm) as compared to the group of patients evaluated by the less experienced physicians (3.7 ± 2.9 cm) (P > 0.05).
Table 3.
Sensitivity, specificity, positive and negative predictive values of TVS in diagnosing DIE.
| Sensitivity(%) | Specificity(%) | PPV(%) | NPV(%) | Accuracy(%) | |
|---|---|---|---|---|---|
| First‐level TVS (Group A) | 24/53 (45.3) | 108/110 (98.2) | 24/26 (92.3) | 108/137 (78.8) | 132/163 (80.9) |
| Expert TVS (Group B) | 64/83 (77.1) | 128/135 (94.8) | 64/71 (90.1) | 128/147 (87.1) | 192/218 (88.1) |
| Overall | 88/136 (64.7) | 236/245 (96.3) | 88/97 (90.7) | 236/284 (83.1) | 324/381 (85.0) |
Discussion
The present study confirms the value of TVS in the preoperative evaluation of patients with suspected endometriosis. Overall diagnostic accuracy is comparable to that previously reported in other patient populations. 7 – 10 We demonstrated that, when TVS is performed by adequately trained personnel, the sensitivity, specificity, positive and negative predictive values are significantly higher than when TVS is performed by physicians with less experience in the field. It is well known that, in the preoperative work‐up of a patient suspected of having endometriosis, it is crucial to accurately predict the risks and difficulties of the operation, to stress the need for a skilled laparoscopic surgeon and sometimes even the need for a general surgeon to be on call. Moreover, the duration of the operation, the length of the hospital stay and surgery‐related complications are useful data when counselling a patient likely to undergo surgery for endometriosis. This information is based on a thorough sonographic evaluation of the female pelvis, focusing not only on the ovaries but, most of all, on the search for DIE. In fact, the removal of DIE requires good technical skills, a long operating time and carries risks and heavy complications. 15 – 17 TVS is an expeditious and cost‐effective diagnostic method widely available in every gynecologic and radiological department. We demonstrated, in a large patient population, that the accuracy of TVS in diagnosing DIE is strongly operator‐dependent. We believe that proper training in the ultrasound lab is crucial in order to be aware of the different sonographic appearances of DIE and of the technique of searching for it. This will help in diagnosing a disease which is responsible for severe symptoms which limit the quality of life and which must be known preoperatively in order to counsel the patient so as to enable the surgeon to perform difficult, risky and time‐consuming surgery. In the present study, we intended to group together deep infiltrating endometriotic nodules from somewhat different sites (rectosigmoid colon, pouch of Douglas, uterosacral ligaments, rectovaginal septum, vagina). In fact, in our experience, at TVS and often even at surgery it is difficult to determine on which anatomical structure the DIE originated. Basically, this is true for two reasons: first, with time, DIE tends to grow and to involve several adjacent structures, forming a bulky nodule composed of endometrial glands and stroma, smooth muscle cells, fibroblasts, collagen and retroperitoneal fatty tissue. Such nodules are located centrally behind the uterus, at slightly different levels (isthmus or cervix, rarely below the pure rectovaginal septum). Second, the chronic inflammation induced by the ectopic endometrium causes distortion of the pelvic anatomy, mainly because of the fibrotic retraction towards the pouch of Douglas which produces a radial scar involving the uterosacral ligaments, anterior rectal wall and posterior vaginal fornix all fused together.
Histological and TVS findings have not previously been compared in terms of the size of the endometriotic lesions and the accuracy of the preoperative diagnosis. In our series, a strong relationship was found between mean lesion size and findings at TVS. In particular, the group of less experienced operators at TVS showed a lower sensitivity due to the high number of nodules missed. In fact, in patients with false negative findings, the average diameter of the DIE as measured by the pathologist was significantly less than in cases with true positive findings at TVS (2.6 ± 1.1 cm v 4.7 ± 3.4 cm, P < 0.05). It seems reasonable to hypothesize that the bigger the lesion, the easier its detection at TVS. Small nodules might be difficult to visualize because they tend to be superficial, without creating a bulky hypoechoic mass behind the cervix or slightly lateral to it. However, smaller nodules of less than two centimetres are easier to remove with less risk of compromising the integrity of the bowel wall or the ureters. In fact, in the group of patients with negative findings at TVS, no intestinal resection was necessary in order to remove the lesion. We agree with Bazot and colleagues 9 that a retroflexed uterus, subserous posterior wall leiomyomas and endometriotic ovarian cysts lying on the uterosacral ligaments hinder the diagnosis of DIE. Moreover, it is difficult to detect deep infiltrating nodules located purely on the sigmoid, not in the true pelvis and far from the tip of the probe at TVS.
In conclusion, TVS should be performed by adequately trained physicians because accuracy of this technique is dependent on the operator's knowledge and skills. Moreover, it should be known that small, superficial endometriotic nodules are hardly visible at TVS, but such lesions rarely represent a big challenge at surgery.
References
- 1. Koninckx PR, Meuleman C, Demeyere S, Lessafre E, Cornillie FJ. Suggestive evidence that pelvic endometriosis is a progressive disease, whereas deeply infiltrating endometriosis is associated with pelvic pain. Fertil Steril 1991; 55: 759–65. [DOI] [PubMed] [Google Scholar]
- 2. Cornillie FJ, Oosterlynck D, Lauwereyns JM, Koninckx PR. Deeply infiltrating endometriosis: histological and clinical significance. Fertil Steril 1990; 53: 978–83. [DOI] [PubMed] [Google Scholar]
- 3. Anaf V, Simon P, Fay TI, Noel J. Smooth muscles are frequent components of endometriotic lesions. Hum Reprod 2000; 15: 767–71. [DOI] [PubMed] [Google Scholar]
- 4. Clement MD. Diseases of the peritoneum. In Blaunstein's Pathology of the Female Genital Tract (5th Edn), Kurman RJ. (ed.). Springer‐Verlag: New York NY; 2002. pp 729–89. [Google Scholar]
- 5. Chapron C, Jacob S, Dubuisson JB, Vieira M, Liaras E, Fauconnier A. Laparoscopically assisted vaginal management of deep endometriosis infiltrating the rectovaginal septum. Acta Obstet Gynecol Scand 2001; 80: 349–54. [PubMed] [Google Scholar]
- 6. Koga K, Osuga Y, Yano T, Momoeda M, Yoshino O, Hirota Y, et al. Characteristic images of deeply infiltrating rectosigmoid endometriosis on transvaginal and transrectal ultrasonography. Hum Reprod 2003; 18: 1328–33. [DOI] [PubMed] [Google Scholar]
- 7. Exacoustos C, Zupi E, Carusotti C, Rinaldo D, Marconi D, Lanzi G, et al. Staging of pelvic endometriosis: role of sonographic appearance in determining extension of disease and modulating surgical approach. J Am Assoc Gynecol Laparosc 2003; 10: 378–82. [DOI] [PubMed] [Google Scholar]
- 8. Bazot M, Detchev R, Cortez A, Amouyal P, Uzan S, Darai E. Transvaginal sonography and rectal endoscopic sonography for the assessment of pelvic endometriosis: a preliminary comparison. Hum Reprod 2003; 8: 1686–92. [DOI] [PubMed] [Google Scholar]
- 9. Bazot M, Thomassin I, Hourani R, Cortez A, Darai E. Diagnostic accuracy of transvaginal sonography for deep pelvic endometriosis. Ultrasound Obstet Gynecol 2004; 24: 180–85. [DOI] [PubMed] [Google Scholar]
- 10. Bazot M, Malzy P, Cortez A, Roseau G, Amouyal P, Daraï E. Accuracy of transvaginal sonography and rectal endoscopic sonography in the diagnosis of deep infiltrating endometriosis. Ultrasound Obstet Gynecol 2007; 30: 994–1001. [DOI] [PubMed] [Google Scholar]
- 11. Fauconnier A, Chapron C, Dubuisson JB, Vieira M, Dousset B, Bréart G. Relation between pain symptoms and the anatomic location of deep infiltrating endometriosis. Fertil Steril 2002; 78: 719–26. [DOI] [PubMed] [Google Scholar]
- 12. American Fertility Society . Revised American Fertility Society Classification of Endometriosis. Fertil Steril 1985; 43: 351–52. [DOI] [PubMed] [Google Scholar]
- 13. Adamson GD, Nelson HP. Surgical treatment of endometriosis. Obstet Gynecol Clin North Am 1997; 24: 375–409. [DOI] [PubMed] [Google Scholar]
- 14. Landis JR, Koch G. The measurement of observer agreement for categorical data. Biometrics 1977; 33: 159–74. [PubMed] [Google Scholar]
- 15. Richardson R, Sutton C. Complications of first entry: a prospective lparoscopy audit. Gynaecol Endosc 1999; 8: 327–34. [Google Scholar]
- 16. Redwine DB. Surgical management of endometriosis. Martin Dunitz. New York: Taylor & Francis; 2004. [Google Scholar]
- 17. Landi S, Ceccaroni M, Perutelli A, Allodi C, Barbieri F, Fiaccavento A, et al. Laparoscopic nerve‐sparing complete excision of deep endometriosis: is it feasible? Hum Reprod 2006; 21: 774–81. [DOI] [PubMed] [Google Scholar]
- 18. Valentin L. High‐quality gynaecological ultrasound can be highly beneficial, but poor‐quality gynaecological ultrasound can do harm. Ultrasound Obstet Gynecol 1999; 13: 1–7. [DOI] [PubMed] [Google Scholar]


