Abstract
The Asian citrus psyllid Diaphorina citri Kuwayama (Hemiptera: Liviidae) is an economically important pest of citrus because it serves as a vector of the causal pathogens of huanglongbing (HLB) also known as citrus greening disease. The increased use of insecticides for control of D. citri negatively impacts several natural enemies including some effective ladybeetle species which are not available commercially. The two-spotted ladybeetle, Adalia bipunctata (Coleoptera: Coccinellidae) is found in some crop and forest ecosystems of Asia, Europe and North America and available commercially. It is known to attack aphids and mealybugs but there are no published records of feeding on psyllids. We evaluated suitability and preference of A. bipunctata for nymphs of D. citri compared to corn leaf aphid Rhopalosiphum maidis (Hemiptera: Aphididae) a global pest of cereal crops and prey for many predaceous insects. We also compared development and reproduction of A. bipunctata on these two species with frozen eggs of the Mediterranean flour moth Ephestia kuehniella (Lepidoptera: Pyralidae) at 25°C. Initially, more D. citri than R. maidis nymphs were consumed in the no-choice tests although final consumption by larva and adult of A. bipunctata did not differ in the choice and no-choice tests. Larval development was prolonged by one day on D. citri compared to R. maidis nymphs but did not differ between either of these diets and E. kuehniella. Larval survival to adult averaged 93–100% and was not impacted by diet. Adult life span did not differ between diets although those on D. citri and R. maidis nymphs weighed less and produced fewer but more fertile eggs than on E. kuehniella eggs. Significant reduction of D. citri nymphs averaging 54% was observed in colonies caged with adult A. bipunctata on field planted citrus. R° (net reproductive rate) was least for beetles fed R. maidis, but otherwise there were no significant differences in demographic parameters. Successful feeding, development and reproductive performance of A. bipunctata suggest its usefulness as biological control agent of D. citri as well as aphid species exemplified by R. maidis.
Introduction
The Asian citrus psyllid [ACP] Diaphorina citri Kuwayama [Hemiptera: Liviidae] serves as a vector of the causal pathogens of huanglongbing [HLB] also known as citrus greening disease. Thus ACP is an economically important pest of citrus in HLB affected regions [1]. Diaphorina citri was first described in Taiwan [2], later found in Punjab Pakistan and the rest of the region [3]. Diaphorina citri was reported from Brazil in 1940 [4] and from Florida in 1998 [5]. Huanglongbing in Florida was detected in 2005 [6] one year after it was identified in Brazil in 2004 [7,8].
Biological control plays an important role in citrus pest management in Florida [9,10,11]. Ladybeetles, syrphid flies, lacewings and spiders are common predators of D. citri. Ladybeetles are considered major contributor to natural mortality of D. citri eggs and nymphs. Michaud and Olsen [12] found that Olla v-nigrum (Mulsant), Harmonia axyridis (Pallas), Curinus coeruleus (Mulsant), and Exochomus childreni (Mulsant) developed and reproduced on a diet of D. citri nymphs whereas Cycloneda sanguinea (L.) did not reproduce. Michaud [10] reported H. axyridis, O. v-nigrum, C. sanguinea and E. childreni as key predators of D. citri nymphs in central Florida. Qureshi and Stansly [11] attributed most of the 90–100% observed mortality of D. citri nymphs in southwest Florida citrus to ladybeetles O. v-nigrum, C. coeruleus, H. axyridis and C. sanguinea.
The use of insecticides has increased tremendously in Florida citrus with some growers using up to 12 sprays to control ACP and reduce incidence and intensity of HLB [13]. Such intense use of insecticides may accelerate selection for pest resistance and negatively impact naturally occurring biological control by ladybeetles, other predators and parasitoids thus reducing control of D. citri and promoting secondary pest outbreaks [14–17]. The populations of ladybeetles shown to be effective against D. citri have notably declined over recent years [Qureshi, unpublished data], presumably due to the increased use of insecticides. It is therefore important to evaluate the performance of commercially available natural enemies against D. citri and other pests in order to augment biological control. Commercially available predators that have been tested against D. citri include the convergent ladybeetle Hippodamia convergens Guérin-Méneville (Coleoptera: Coccinellidae), which developed and reproduced on the diets of D. citri, brown citrus aphid Toxoptera citricida Kirkaldy and green citrus aphid or spirea aphid Aphis spiraecola (Homoptera: Aphididae) [18]. Similarly a predatory mite Amblyseius swirskii Athias-Henriot (Acari: Phytoseiidae) was effective in reducing D. citri through feeding on its eggs and neonates [19].
The two-spotted ladybeetle Adalia bipunctata (L.) is a commercially available species used for aphid control in many countries [20–24]. It shares six percent of overall world market for aphid preferring predators and in Western Europe, is largely used for aphid control in the urban landscape (R. Timmer, Koppert BV, Netherlands). Adalia bipunctata was reported during a survey of predatory beetles in field crops and forests in Faisalabad, Pakistan [25]. Later, it was also reported among twelve species of predatory Coccinellids found in Chitral district of Pakistan [26]. However, its role against D. citri was never investigated. The objectives of our investigations were to evaluate the preference and suitability of larvae and adults of A. bipunctata for nymphs of D. citri compared to a common and available aphid, Rhopalosiphum maidis Fitch (Hemiptera: Aphididae) as well as survival, development and reproduction on these two species and frozen eggs of the Mediterranean flour moth Ephestia kuehniella (Lepidoptera: Pyralidae). We included R. maidis because it is one of the global pests of cereal crops, is commonly used in banker plants, and important to survival of several predators which forage multiple crops including A. bipunctata. Ephestia kuehniella eggs are commonly used in the laboratory to rear predators and support the development and reproduction of several ladybeetle species [12,18].
Materials and Methods
Study location, insects and experimental conditions
Experiments were conducted at the Southwest Florida Research and Education Center (SWFREC) of the University of Florida-IFAS, Immokalee, FL, USA (Latitude: 26.484 N, Longitude: 81.435 W). No permit or specific permission was required. These studies did not involve endangered or protected species. Colonies of D. citri and R. maidis were established at the SWFREC, Immokalee, FL. Diaphorina citri was reared on orange jasmine Murraya paniculata (L.), a close relative of citrus and one of the preferred hosts of D. citri. Rhopalosiphum maidis was reared on Sorghum bicolor (L.) Moench, a grass species cultivated for grain to feed animals and humans, and for ethanol production. Both colonies were maintained in a climate controlled glasshouse set at 28°C. Adalia bipunctata larvae were originally obtained from BIOBEST (BIOBEST-NV, USA Inc. Detroit MI 48201–2311) and reared through adulthood on frozen eggs of E. kuehniella (Koppert Biological Systems, Romulus MI 48174). Individual larvae were reared in experimental arenas (petri dishes 9 cm diameter by 1.5 cm high) and provided with water on a small cube of moist sponge. Upon emergence, adults were placed in 3-litre ventilated plastic jars (15 adults per jar) to initiate a colony which was maintained on eggs of E. kuehniella. Shoots of M. paniculata were provided as substrate for oviposition. The colony was maintained in an incubator (Percival, Model I36LLC8, Percival Scientific Inc. Perry, Iowa, USA) set to a photoperiod of 16:8 (L:D) at 25°C. Eggs and emerging larvae were collected on daily basis and kept under above conditions. Same conditions were used for the experiments.
Choice and no-choice tests
First instar larvae or 4–5 day old adults of A. bipunctata were tested in choice and no-choice tests. Fifteen replicates for larva or 14 for adults were used for the three treatments; i) 20 nymphs of D. citri alone (no-choice test) ii) 20 nymphs of R. maidis alone (no-choice test) and iii) 10 nymphs each of D. citri and R. maidis together (choice test). Second and third instar nymphs of D. citri and R. maidis were provided to an individual larva or adult in experimental arena. The petri dish was covered with perforated parafilm to prevent escape of nymphs and to provide ventilation. Larvae and adults were starved for 24 hr prior to exposure to prey to motivate foraging [27]. Prey consumption was calculated at 3, 6 and 12 hrs after exposure by deducting the number of live nymphs from the total provided.
Development and reproduction
Development of 48 h old larvae of A. bipunctata was evaluated through adult emergence followed by reproduction on three diets. Larvae were obtained from multiple egg batches of A. bipunctata stock colony and transferred individually using a soft camel hair brush to experimental arenas designated at random for one of the three diets; i) nymphs of D. citri ii) nymphs of R. maidis and iii) frozen eggs of E. kuehniella. Diaphorina citri nymphs on untreated citrus shoots, aphids on untreated sorghum leaves and E. kuehniella eggs on clean citrus leaves were provided to larvae on a daily basis through pupation. Water was provided on a small cube of sponge. Dates of larval death, pupation and adult emergence were recorded every 24-hrs. Freshly emerged adults were weighed on an A-200D balance (Denver Instrument, 5 Orville Dr. Bohemia, NY).
To determine effects of diet on larval instars of A. bipunctata, egg batches obtained from female A. bipunctata fed separately on diets of D. citri, R. maidis and E. kuehniella were kept in the incubator under conditions described above for the colony. Upon emergence, 1st instar larvae of A. bipunctata were transferred to snap cap cups (Crystalware Clear Plastic Cups 9 oz) using camel hair brush and observed through pupation. There were 14 replicates for each diet. Rearing cup caps were perforated with needle for ventilation. Diets were changed every 24 hr and exuviae collected.
One week old adults of A. bipunctata developed on nymphs of D. citri or R. maidis and eggs of E. kuehniella were released in 5 liter plastic ventilated jars marked for respective diets and observed for mating. Ten mating pairs from each diet were transferred individually to experimental arenas and provided with the same diet on which they were reared. Psyllid nymphs on untreated M. paniculata shoots, aphids on untreated sorghum shoots and E. kuehniella eggs on a clean citrus leaf were provided daily ad libitum. A small 2 inch piece of folded paper towel and 3–4 clean leaves of M. paniculata were added as additional substrates for oviposition. Water was provided on a small cube of sponge. Eggs were counted and removed daily, along with the material on which they were laid and incubated in a separate Petri dish under the same conditions as above. Petri dishes with beetles were replaced every other day or earlier if eggs were laid on the dish. Newly hatched larvae were counted and removed every day with a soft camel’s-hair brush to avoid cannibalism of sibling or remaining eggs. Dates of beetle death were recorded.
Field test of the A. bipunctata predation on D. citri
The field experiment was conducted in a citrus orchard at UF SWFREC, Immokalee, Florida. Twenty, 4 year-old 1.2–1.5 m tall ‘Hamlin’ orange trees were trimmed to encourage production of young shoots needed by D. citri to reproduce and develop. Twenty new shoots infested with D. citri nymphs were selected and examined to remove eggs and mature instars leaving a uniform cohort of 2nd and 3rd instars averaging 30 ± 9 per shoot. Selected shoots with nymphs were covered with sleeve cages to protect against additional oviposition, parasitism or predation. One week old adults of A. bipunctata from the laboratory colony fed on the eggs of E. kuehniella were released in 10 randomly selected sleeve cages at one adult per cage, leaving 10 caged shoots as a control without beetles. Cages were examined from 2–4 days to count psyllid adults and nymphs and transfer beetles to new shoots caged with counted numbers of 2nd and 3rd instar nymphs. Experiment was continued until all beetles died.
Statistical analysis
A generalized linear model with Poisson errors (SAS PROC GENMOD) was used at P = 0.05 to evaluate treatment effects on number of D. citri or R. maidis nymphs consumed by larvae or adult A. bipunctata in choice and no-choice tests [28]. Data on larval survival until pupation or adult eclosion were analyzed by using the SAS GLIMMIXMACRO model with a logit link function to transform data [28]. Development times, adult weight, longevity, fecundity, and fertility were tested for diet effect using the Mixed procedure analysis and Tukey’s test for pairwise comparison of treatment means [t-tests] at a probability level of 0.05 [28]. Survival and fecundity data on all three diets given a sex ratio of 1:1 were used to estimate and analyze life table parameters of A. bipunctata according to the method proposed by Maia et al. [28, 29]. Percent reduction of psyllids in nymphal colonies with beetles was corrected using reduction in control colonies and Abbott’s formula [30] and analyzed using SAS Mixed model procedure. The relationship between female age and fecundity or colony size and consumption rate was evaluated with Pearson correlation statistics using the SAS Corr procedure [28].
Results
Choice and no-choice tests
Larvae or adult A. bipunctata did not show preference between nymphs of D. citri and R. maidis in choice tests (P > 0.05, Table 1). Although significantly more D. citri nymphs than R. maidis nymphs were consumed by larvae at 6 h after initiation of no-choice test (χ2 = 6.1, df = 1, P = 0.014, Table 1) no differences were seen at 12 h (P > 0.05). Adult consumption of D. citri nymphs was significantly more than R. maidis nymphs in the no-choice tests at 3 h (χ2 = 22.6, df = 1, P < 0.0001, Table 1) and 6 h (χ2 = 7.2, df = 1, P = 0.007, Table 1) but not at 12 h (P > 0.05). A total of 18–20 nymphs of one or both species were consumed within 12 h by a single larva or adult of A. bipunctata in choice and no-choice test.
Table 1. Mean number (±SEM) of nymphs of D. citri or R. maidis consumed by larvae and adults of A. bipunctata in two-way choice and no-choice tests.
| Choice test | No-choice test | |||
|---|---|---|---|---|
| Observation time | D. citri | R. maidis | D. citri | R. maidis |
| Larva of A. bipunctata | ||||
| 3 hours | 3.1 ± 0.6 a | 4.1 ± 0.6 a | 4.4 ± 0.9 a | 3.1 ± 1.0 a |
| 6 hours | 8.8 ± 0.3 a | 9.1 ± 0.4 a | 12.1 ± 1.5 a | 9.1 ± 1.3 b |
| 12 hours | 9.3 ± 0.2 a | 9.8 ± 0.1 a | 19.4 ± 0.8 a | 18.6 ± 0.2 a |
| Adult of A. bipunctata | ||||
| 3 hours | 5.1 ± 0.6 a | 4.1 ± 0.5 a | 9.9 ± 0.8 a | 5.0 ± 0.7 b |
| 6 hours | 8.6 ± 0.4 a | 7.3 ± 0.5 a | 15.3 ± 0.9 a | 11.6 ± 0.9 b |
| 12 hours | 9.7 ± 0.2 a | 8.9 ± 0.5 a | 19.6 ± 0.2 a | 17.6 ± 0.7 a |
Newly enclosed first instar larvae were used that were not exposed to any diet prior to the test. Adults from laboratory colony reared on eggs of E. kuehniella were starved for 24 h before the test. Means within a column followed by the same letter are not significantly different for the larva or adult (P > 0.05).
Development and reproduction
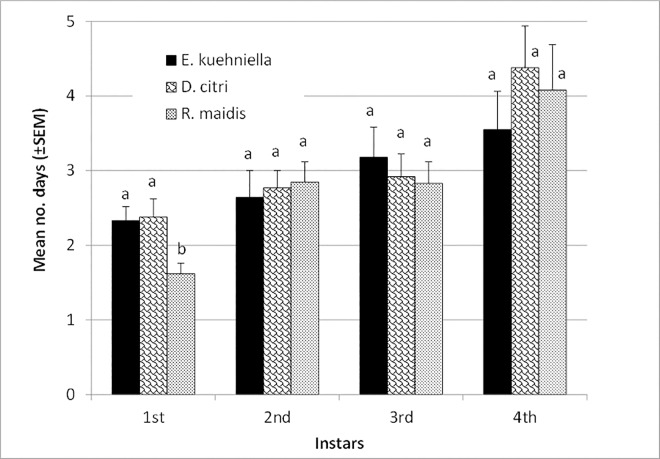
No effect of diet on the egg incubation time was observed (P > 0.05, Table 2). Larval survival to pupation averaged 93–100% and adult emergence from pupae was 100% on all diets, without significant impact of diet on larval or pupal survival (P > 0.05). A significant effect of diet on larval development was only observed for the first of the 4 larval instars (F = 4.95; df = 2, 35; P = 0.0128, Fig 1). First instar larvae developed faster on R. maidis nymphs compared to D. citri nymphs (P = 0.0205) or E. kuehniella eggs (P = 0.0346), with no significant difference between these later two diets (P = 0.9814). These differences diminished in later instars (P > 0.05). Overall a significant effect of diet on the larval development was detected (F = 3.84; df = 2, 78; P = 0.0257, Table 2), which was slightly prolonged (P = 0.0271) on the nymphs of D. citri (11.8 ± 0.4 days) compared to R. maidis (10.7 ± 0.3 days). Development on E. kuehniella eggs (10.9 ± 0.3 days) and either of the two nymphal diets did not differ (P > 0.05, Table 2). Pupation time averaged 4.5–4.7 days with no significant diet effect (P > 0.05, Table 2). However, a significant effect of diet on the adult weight was seen (F = 4.88; df = 2, 40; P = 0.0127, Table 2), with heavier adults developing on E. kuehniella eggs compared to R. maidis nymphs (P = 0.0095) and those fed on D. citri nymphs being intermediate.
Table 2. Mean (±SEM) development times of immature stages and fresh weight of adult A. bipunctata reared on diets of E. kuehniella eggs, D. citri nymphs and R. maidis nymphs.
| Diet | Egg (days) | Larva (days) | Pupa (days) | Adult weight (g) |
|---|---|---|---|---|
| E. kuehniella | 3.6 ± 0.1 a | 10.9 ± 0.3 ab | 4.5 ± 0.2 a | 0.009 ± 0.0004 a |
| D. citri | 3.5 ± 0.1 a | 11.8 ± 0.4 a | 4.7 ± 0.4 a | 0.008 ± 0.0003 ab |
| R. maidis | 3.6 ± 0.1 a | 10.7 ± 0.3 b | 4.6 ± 0.4 a | 0.007 ± 0.0004 b |
Eggs and larvae were obtained from the adults developed on respective diets. Larval survival to pupation averaged 93–100% and 100% pupae resulted in adults on all three diets (P > 0.05). Means within a column followed by the same letter are not significantly different (P > 0.05)
Fig 1. Mean (±SEM) development times of the larval instars of A. bipunctata on diets of E. kuehniella eggs, D. citri nymphs and R. maidis nymphs.
Means with the same letter are not significantly different for respective instar (P>0.05)
Longevity of males and females was not impacted by diet (P > 0.05, Table 3). No diet-by-week interaction on fecundity was detected (F = 0.45; df = 10, 150; P = 0.9216), although significant effects of diet (F = 4.19; df = 2, 150; P = 0.0170) and date (F = 9.77; df = 5, 150; P < 0.0001) were seen.
Table 3. Mean (±SEM) adult longevity, life time fecundity and fertility of A. bipunctata on diets of E. kuehniella eggs, D. citri nymphs, and R. maidis nymphs.
| Diet | Male longevity (days) | Female longevity (days) | Fecundity (no. of eggs/ female) | Fertility (% eggs hatched) | Fertility (no. of larvae/female) |
|---|---|---|---|---|---|
| E. kuehniella | 34 ± 1.8 a | 39 ± 3.2 a | 300 ± 32.7 a | 49 ± 3.5 b | 103 ± 27.4 a |
| D. citri | 32 ± 2.0 a | 34 ± 1.9 a | 222 ± 28.7 ab | 66 ± 3.1 a | 106 ± 25.1 a |
| R. maidis | 30 ± 1.5 a | 32 ± 2.1 a | 186 ± 20.6 b | 68 ± 3.6 a | 86 ± 21.9 a |
Means within columns sharing the same letter are not significantly different (P>0.05)
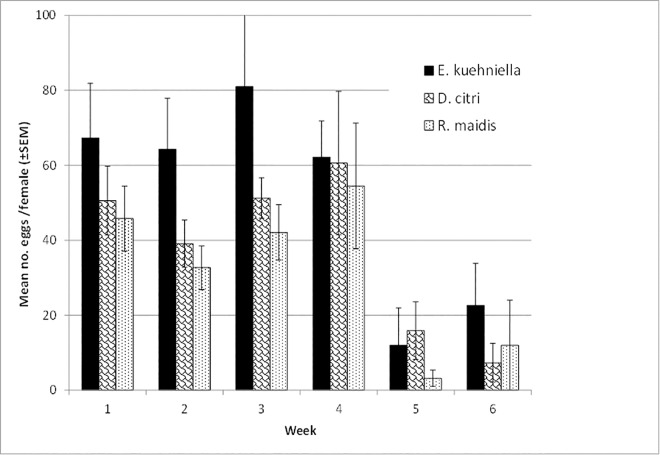
In total, 441 oviposition events were observed from all females of which 44% occurred on leaves, followed by 29% on paper and 27% on petri dishes. Females fed on R. maidis nymphs produced significantly fewer eggs than those fed on E. kuehniella eggs (P = 0.0153), with no difference between diets of R. maidis and D. citri (P = 0.6943) or D. citri and E. kuehniella (P = 0.1163). A significant negative relationship between increasing female age and weekly fecundity rate was detected (r = -0.66, P = 0.003, Fig 2). Fertility of eggs was affected by diet (F = 9.2; df = 2, 275; P = 0.0001, Table 3) with only 49% hatching from females fed on E. kuehniella eggs compared to 66% and 68%, respectively, from the females fed on the nymphs of D. citri and R. maidis (P = 0.0008 and 0.0001, respectively) which did not differ (P = 0.6287) (Table 3). However, the number of live larvae per female was not significantly different among diets (F = 0.19; df = 2, 27; P = 0.8285, Table 3).
Fig 2. Mean (± SEM) weekly fecundity of A. bipunctata on diets of E. kuehniella eggs, D. citri nymphs, and R. maidis nymphs.
Weekly means were not significantly different among diets (P >0.05).
Life table analysis
Net reproductive rate (Ro) of A. bipunctata was greater on a diet of E. kuehniella eggs than on R. maidis nymphs (t = 3.16; P = 0.003) with the D. citri diet intermediate (P > 0.05, Table 4). No differences were seen among estimates of intrinsic rate of increase (rm), finite rate of increase (λ), generation time (T) or doubling time (Dt) (P > 0.05) all indicating populations increasing on all three diets (Table 4).
Table 4. Means and 95% CL of the life table parameters Ro (net reproductive rate), rm (intrinsic rate of increase), λ (finite rate of increase), T (generation time, days) and Dt (doubling time, days) of A. bipunctata on diets of E. kuehniella eggs, D. citri nymphs, and R. maidis nymphs.
| Diet | Ro | 95% CL | rm | 95% CL | λ | 95% CL | T | 95% CL | Dt | 95% CL |
|---|---|---|---|---|---|---|---|---|---|---|
| E. kuehniella | 150.2 a | 113.24–187.15 | 0.37 a | 0.28–0.45 | 1.44 a | 1.32–1.56 | 13.61 a | 10.76–16.46 | 1.88 a | 1.44–2.32 |
| D. citri | 103.2 ab | 73.00–133.37 | 0.33 a | 0.29–0.37 | 1.39 a | 1.34–1.45 | 13.96 a | 12.00–15.91 | 2.08 a | 1.84–2.32 |
| R. maidis | 86.3 b | 64.56–107.95 | 0.33 a | 0.26–0.40 | 1.39 a | 1.29–1.49 | 13.41 a | 10.59–16.23 | 2.08 a | 1.66–2.50 |
Means within a column sharing the same letter are not significantly different (P > 0.05)
Adalia bipunctata consumption of D. citri nymphs on citrus trees
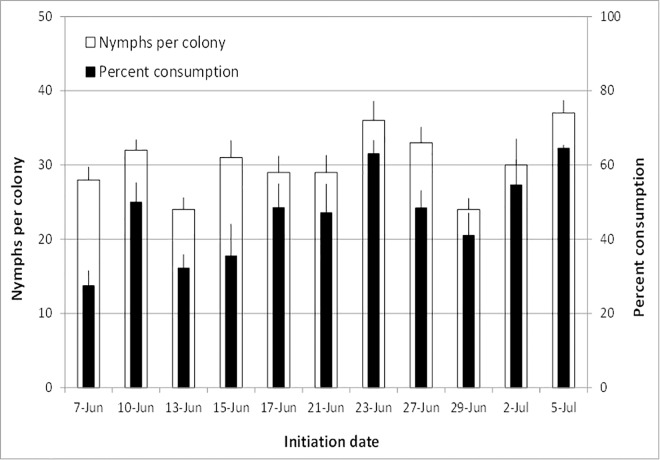
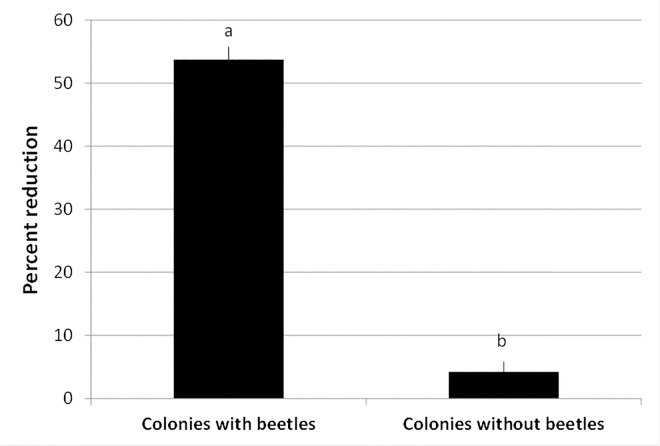
Adalia bipunctata beetles suppressed D. citri nymphs in all the tested colonies (n = 71, Fig 3). There was a significantly positive relationship between colony size, which ranged between 24–37 nymphs, and consumption rate (r = 0.77, P = 0.006, Fig 3). Overall, 54% reduction in nymphs was observed in colonies with beetles, which was significantly more than 4% in colonies without beetles (F = 379.69; df = 1, 18; P < 0.0001, Fig 4). On average, 136 ± 13 nymphs were consumed by each beetle.
Fig 3. Mean (±SEM) number of D. citri nymphs in colonies developing on 4-year old ‘Hamlin’ orange trees, and percentage of nymphs consumed by A. bipunctata ladybeetles caged at one beetle per colony.
Beetles were moved to new colonies every 2–4 days depending upon quantity of nymphs available to them. Percent consumption by beetles was corrected using Abbott’s formula (1925) to account for natural reduction in control colonies without beetles.
Fig 4. Total percentage reduction of nymphs (mean ± SEM) from all colonies of D. citri with and without A. bipunctata beetles.
Reduction in colonies with beetles was corrected using Abbott’s formula (1925) to account for natural reduction in control colonies without beetles (P<0.05).
Discussion
Neither A. bipunctata larvae nor adults expressed preference between D. citri or R. maidis nymphs in choice tests. Apparently, cues of a visual, chemical or tactile nature involved in the orientation of A. bipunctata toward these prey did not influence selection. Initially, more D. citri than R. maidis were consumed in no-choice tests, although 90% or more of the 20 nymphs offered were consumed after 12 h regardless of prey species. These findings suggest that both D. citri and R. maidis were equally preferred and suitable as food for A. bipunctata.
The apparent suitability of D. citri and R. maidis for A. bipunctata translated into successful larval survival to adulthood averaging 93% on either prey species, and not significantly different from the 100% survival on eggs of E. kuehniella. However, larval development on nymphs of D. citri was prolonged by one day compared to R. maidis nymphs though not compared to Ephestia eggs. Late instar D. citri nymphs have well developed wing buds that may serve as deterrence as well as reduce consumable body contents compared to R. maidis nymphs or Ephestia eggs which can be completely consumed. Beetle larvae may have spent more time and energy in handing D. citri nymphs containing mixed population of young and mature instars compared to R. maidis nymphs or Ephestia eggs, resulting in delayed development. However, suitability of young instar D. citri nymphs compared to R. maidis nymphs was not compromised in the choice or no-choice tests where both species were consumed at the same rate over 12 h. These were young instars with undeveloped wing buds and thus probably easy for the larvae to handle and consume. Although larvae on a diet of D. citri nymphs took somewhat longer, to develop, they were able to meet their nutritional requirements as indicated by adults that were not different in weight from those developed on R. maidis nymphs or Ephestia eggs. However, adults developed on R. maidis nymphs weighed less than those from Ephestia eggs. In addition to some possible nutritional advantage, eggs are easy to handle by the predators compared to nymphs which are mobile and provide some resistance when attacked.
Females lived longer than males irrespective of diet although diet did not affect longevity of either gender. Fecundity decreased with female age, and was greatest during first four weeks on all three diets. Reproductive performance on the two nymphal diets was comparable and better compared to Ephestia eggs except reduced fecundity on R. maidis. De Clercq et al. [23] also observed improved fertility of A. bipunctata on an aphid diet compare to Ephestia eggs. Reproductive performance of H. convergens on nymphs of D. citri, T. citricida and A. spiraecola was comparable and better compared to Ephestia eggs [18]. However, fecundity and fertility of the C. coeruleus, E. childreni, H. axyridis, and O. v-nigrum fed with frozen D. citri nymphs and Ephestia eggs did not differ [12] indicating that freezing could have reduced the nutritional value of the nymphs.
All life table parameters predicted increasing populations of A. bipunctata on all three diets without significant differences except Ro which was reduced on the R. maidis nymphs compare to Ephestia eggs. Estimated intrinsic rate of increase was higher and generation time shorter in the present study than reported for A. bipunctata on the green peach aphid Myzus persicae (Sulzer) [31] or H. convergens on the nymphs of D. citri, A. spiraecola, T. citricida or Ephestia eggs [18]. The generation time of A. bipunctata observed in the present study was also much shorter than that of O. v-nigrum, H. axyridis, E. childreni, C. sanguinea and C. coeruleus [12]. These results indicate that A. bipunctata is another important candidate predator of D. citri as well as of R. maidis. The addition of commercially reared predators may counteract the pesticide-caused reductions of natural enemies in the orchard environment.
Acknowledgments
We are thankful to the Higher Education Commission of Pakistan, for funding the PhD research work of Azhar A. Khan part of which was conducted at UF-SWFREC.
Data Availability
All relevant data are provided in the paper.
Funding Statement
This work was supported by the Higher Education Commission of Pakistan, for funding the PhD research work of Azhar A Khan part of which was conducted at UF-SWFREC. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Catling HD. Distribution of psyllid vectors of citrus greening disease with notes on the biology and bionomics of Diaphorina citri. FAO Plant Prot. Bull. 1970; 18: 8–15. [Google Scholar]
- 2.Kuwayama S. Die psylliden Japans. Transactions of the Sopporo Natural History Society 2 (parts I and II): 1908: 149–189. (D. citri: p. 160–161, Plate III, Fig 16).
- 3.Hussain MA, Nath LD. The citrus psylla “Diaphorina citri K.” (Homoptera: Psyllidae). Memoirs of the Department of Agriculture in India, Entomological Series. 1927; 10(2):1–27. [Google Scholar]
- 4.Lima AC. Insetos do Brasil, Homopteros. Ser. Didat. 4 Esc. Nac. Agron. 1947; 3:327 [Google Scholar]
- 5.Halbert SE. Entomology Section. Triology 1998; 37: 6–7. [Google Scholar]
- 6.Halbert SE. Pest alert: citrus greening/huanglongbing. Florida Dept. of Agr. and Consumer Serv., Dept. of Plant Ind. 2005.
- 7.Texeira DC. Ayres AJ, Kitajima EW, Tanaka FAO, Danet JL, Jagouiex-Eveillard S, et al. First report of a huanglongbing-like disease of citrus in S˜ao Paulo state, Brazil, and association of a new Liberibacter species, “Candidatus Liberibacter americanus,” with the disease. Plant Dis. 2005; 89:107. [DOI] [PubMed] [Google Scholar]
- 8.Teixeira DC, Dane JL, Eveillard S, Martins EC, de Jesus Junior WC, Yamamoto PD, et al. Citrus huanglongbing in S˜ao Paulo state, Brazil: PCR detection of the “Candidatus” Liberibacter species associated with the disease. Mol. Cell. Probes 2005; 19:173–79 [DOI] [PubMed] [Google Scholar]
- 9.McCoy CW. Citrus: current status of biological control in Florida In: Hoy M.A. and Herzog D.C., Editors, (1985). Biological Control in Agricultural IPM Systems, Academic, Orlando, FL, pp. 1985; 481–499. [Google Scholar]
- 10.Michaud JP. Natural mortality of Asian citrus psyllid (Homoptera: Psyllidae) in central Florida. Biol. Control 2004; 29: 260–269. [Google Scholar]
- 11.Qureshi JA, Stansly PA. Exclusion techniques reveal significant biotic mortality suffered by Asian citrus psyllid Diaphorina citri (Hemiptera: Psyllidae) populations in Florida citrus. Biological Control. 2009; 50: 129–136. [Google Scholar]
- 12.Michaud JP, Olsen LE. Suitability of Asian citrus psyllid, Diaphorina citri, as prey for ladybeetles. Bio Control 2004; 49: 417–431. [Google Scholar]
- 13.Monzo C, Stansly PA. Thresholds for vector control and compatibility with beneficial fauna in citrus with high incidence of Huanglongbing. pp. 1137–1144. In: Proceedings XIIth Intl. Citrus Congress, Acta Hort. 1065, ISHS 2015.
- 14.Qureshi JA, Stansly PA. Integrated approaches for managing the Asian citrus psyllid Diaphorina citri (Homoptera: Psyllidae) in Florida, pp. 110–115. In: Proceedings Florida State Horticultural Society 3–4 June 2007, Palm Beach, FL.
- 15.Qureshi JA, Stansly PA. Dormant season foliar sprays of broad spectrum insecticides: An effective component of integrated management for Diaphorina citri (Hemiptera: Psyllidae) in citrus orchards. Crop Protection. 2010; 29: 860–866. [Google Scholar]
- 16.Tiwari SS, Mann RS, Rogers ME, Stelinski L. Insecticide resistance in field populations of Asian citrus psyllid in Florida. Pest Manage. Sci. 2011; 67: 1258–1268. [DOI] [PubMed] [Google Scholar]
- 17.Kanga LHB, Eason J, Haseeb M, Qureshi JA, Stansly PA. Monitoring for insecticide resistance in Asian citrus psyllid populations in Florida. Journal of Economic Entomology, 2015; 1–5: doi: 10.1093/jee/tov348 [DOI] [PubMed] [Google Scholar]
- 18.Qureshi JA, Stansly PA. Three Homopteran pests of citrus as prey for the convergent ladybeetle: Suitability and Preference. Envirn. Ent. 2011; 40(6): 1503–1510 [DOI] [PubMed] [Google Scholar]
- 19.Juan-Blasco M, Qureshi JA, Urbaneja A, Stansly PA. Predatory mite Amblyseius swirskii (Acari: Phytoseiidae) for biological control of Asian citrus psyllid, Diaphorina citri (Hemiptera: Psyllidae). Florida Entomologist. 2012; 95: 543–551. [Google Scholar]
- 20.Hodek I. (Ed.). Biology of Coccinellidae. Dr. W. Junk publishers, The Hague, Holland, 1973; pp260.
- 21.Majerus MEN. Ladybirds. Harper Collins, London, 1994; pp367.
- 22.Hodek I, Honek A. (Ed.). Ecology of Coccinellidae Vol-54 Kluwer Academic Publishers, Dordrecht, The Netherlands: 1996. [Google Scholar]
- 23.Wyss E, Villiger M, Hemptinne JL, Mullers CH. Effects of augmentative releases of eggs and larvae of the two-spot ladybird beetle, Adalia bipunctata, on the abundance of the rosy apple aphid, Disaphis plantaginea, in organic apple orchards. Entomol. Exp. Appl. 1999; 90: 167–173. [Google Scholar]
- 24.De Clercq P, Bonte M, Speybroeck KV, Bolckmans K, Deforce K. Development and reproduction of Adalia bipunctata (Coleoptera: Coccinellidae) on eggs of Ephestia kuehniella (Lepidoptera: Phycitidae) and pollen. Pest Manag. Sci. 2005; 61: 1129–1132. [DOI] [PubMed] [Google Scholar]
- 25.Zahoor MK, Suhail A, Iqbal J, Zulfaqar Z, Anwar M. Biodiversity of predaceous Coccinellids and their role as bioindicators in an Agro-ecosystem. Int. J. Agri. Biol. 2003; 5(4): 555–559. [Google Scholar]
- 26.Khan I, Din S, Khalil SK, Rafi MA. Survey of predatory Coccinellids (Coleoptera: Coccinellidae) in the Chitral District, Pakistan. J. Insect Sci. 2006; 7:07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frazer BD, Gilbert N. Coccinellids and aphids; a quantitative study of the impact of adult ladybirds (Coleoptera:Coccinellidae) preying on field populations of pea aphids (Homoptera: Aphididae). J. Entomol. Soc. BC. 1976; 73: 33–56. [Google Scholar]
- 28.SAS Institute. Release 2012. SAS Institute, Cary, North Carolina, United States of America.
- 29.Maia HNM, Luiz AJB, Campanhola C. Statistical inference on associated fertility life table parameters using jackknife technique: computational aspects. J. Econ. Entomol. 2000; 93: 511–518. [DOI] [PubMed] [Google Scholar]
- 30.Abbott WS. A method for computing the effectiveness of an insecticide J. Econ. Entomol., 1925; 18 265–267 [Google Scholar]
- 31.Lanzoni A, Accinelli G, Bazzocchi GG, Burgio G. Biological traits and life table of the exotic Harmonia axyridis compared with Hippodamia variegata, and Adalia bipunctata (Col., Coccinellidae). Blackwell Verlag, Berlin. JEN 2004; 128(4): 298–306. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are provided in the paper.