Abstract
Background. The aim of the present study was to compare coronally advanced flap (CAF) plus amniotic membrane (AM) to CAF with connective tissue graft (CTG) in the treatment of Miller’s class I and II gingival recessions.
Methods. Eleven healthy subjects with thirty Miller’s class І and ІІ gingival recessions ≥3 mm, were selevted for this research and randomly assigned to two groups in a split-mouth design. In the control group gingival recessions were treated with CAF and CTG; however, in the test group the lesions were treated with (AM) and CAF. The clinical parameters, including recession depth (RD), recession width (RW), keratinized tissue width (WKT), probing depth (PD) and clinical attachment level (CAL), were measured at baseline and 1, 3 and 6 months postoperatively. Statistical significance was set at P < 0.01.
Results. Position changes of RD, RW, CAL, and MGJ were significant between baseline and one month after surgery (P < 0.01) in both the test and control groups and these values remained unchanged at 3- and 6-month follow-ups. There were no statistically significant differences in PD and WKT between baseline and 1-, 3- and 6-months intervals postoperatively. The mean root coverage values after 6 months were 75.5% and 63.1% for two groups, respectively. The mean recession depth reductions were 2.63±0.63 mm and 2±1.4 mm in the test and control groups, respectively.
Conclusion. The results of this research showed that application of AM instead of connective tissue decreased surgical operation time and patient discomfort but the amount of root coverage was not significantly different between the two methods.
Keywords: Amniotic membrane, gingival recession, connective tissue
Introduction
Gingival recession can be seen in people with both poor and good oral hygiene.1-3The incidence of gingival recession varies from 8% in children to 100% after 50 years of age.4-5Several aspects of gingival recession such as esthetic/cosmetic demands and root sensitivity make it clinically significant, requiring surgical correction. Another common indication for the treatment of root coverage procedures is to level off the marginal soft tissue with the aim of better plaque control.5 Pedicle grafts,7 free grafts,8 guided tissue regeneration,9 and subepithelial connective tissue grafts (SCTG)10,11 are the most commonly used surgical procedures in the treatment of root exposures.
SCTG technique is considered as the “gold standard” of root coverage procedures.10Although this method has high predictability and results in successful root coverage in the long term, its source is limited and significantly leads to patient discomfort.12 If we use an alternative source for the donor site, surgical stages will be reduced and the morbidity of patient following root coverage surgery would decrease.13
Amniotic membrane (AM) is an allograft that is derived from human amniotic tissue. It is the innermost layer of fetal membranes and has a thin epithelial layer, a thick basement membrane and an avascular stroma consisting mainly of collagen (Figure 1). Studies have found that amniotic membrane stimulates re-epithelialization, decreases inflammatory response and modulates angiogenesis. It has shown antibacterial properties and low immunogenicity.14-18Also, studies have demonstrated that AM is rich in some growth factors like basic fibroblast growth factor (b FGF), epidermal growth factor (EGF), transforming growth factor-α (TGF-α), transforming growth factor-β (TGF-β), hepatocyte growth factor (HGF) and keratinocyte growth factor (KGF).17-19
Figure 1.
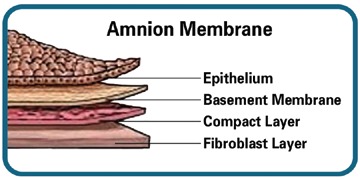
Histological structure of human amniotic membrane.
The usefulness of AM has been reported in the treatment of many medical problems.20-23 In dentistry Kothiwale et al24 used AM as guided tissue regeneration (GTR) for the management of human periodontal Grade II buccal furcation defects with two different bone substitute materials. Reduction of CAL, PD, bone fill and percentage gain in furcation area was significant in this treatment.24 Valez et al used amniotic membrane in implant surgery and showed that AM is effective in helping cicatrization and wound healing.25Ghahrodi et al used AM for root coverage treatments. The results of a study showed that connective tissue graft might be successfully replaced by connective tissue graft in surgeries for root coverage.26
Based on the biologic properties of amniotic membrane and its potential, we theorized that it would be effective in the management of root exposure. The aim of the present study was to compare the clinical effectiveness of amniotic membrane and coronally advanced flap to subepithelial connective tissue with a coronally advanced flap in Miller class І and II root exposures.
Methods
The study was approved by the research Ethics Committee of Tabriz University of Medical Sciences (5/4/9997) and registered with the local World Health Organization Registry Network (IRCT138808142670N1).
Study population
In this split-mouth, blind, randomized clinical trial eleven subjects with a mean age of 34 ± 12 years were selected. Thirty defects were randomly divided into two groups: control group (treated with CAF + SCTG) and test group (treated with CAF+AM) by flip of a coin. All the patients met the inclusion and exclusion criteria. Inclusion criteria included Miller class I and II buccal recessions with minimum 3 mm of RD on the premolars, canines and incisors in both the left and right quadrants of the same jaw in the mandible or maxilla. The width of keratinized gingiva measuring ≥2 mm25was a prerequisite for accomplishing the CPF. All the patients met the inclusion. Exclusion criteria consisted of systemic conditions affecting the periodontium, bleeding on probing in surgical sites, poor oral hygiene (plaque index ≥20%), pregnancy, steroid therapy, history of root canal therapy, history of root coverage surgery, symptoms of pulpal pain in selected teeth, PDL widening, periapical radiolucency, need for antibiotic prophylaxis, orthodontic therapy, high frenal and muscular attachments and smoking and teeth with buccal and lingual inclinations. The selected teeth included 4 central incisors, 4 lateral incisors, 12 canines, 8 first premolars and 2 second premolars. A total of 20 defects were in the maxilla and 10 defects in the mandible.
Pre-surgical procedures
The study procedures were explained to all the participants. The protocol of this clinical trial was approved by the Ethics Committee and Research Vice-chancellor of Tabriz University of Medical Sciences under the code IRCT138808142670N1. All the patients signed an informed consent form.
Phase I periodontal treatment was carried out, including thorough scaling and root planing (SRP) with ultrasonic scalers and hand instruments for all the patients. Four to six weeks after initial phase of the treatment, a reassessment was made to evaluate PD, CAL, BOP and mobility. For all the participants, an O´Leary plaque score28<20% and GI29 ≤1 was prerequisite before undertaking surgical phase of the therapy. Impressions were taken from the affected areas and acrylic stents with guiding grooves were fabricated to attain reproducible measurements. A standard UNC periodontal probe was used for measurements. All the parameters were measured at baseline and at 1-, 3- and 6-month postoperative interevals: recession width (RW), plaque index (PI), probing depth (PD), gingival index (GI), recession depth (RD), clinical attachment level (CAL), and width of keratinized tissue (WKT). RD was recorded from cemento-enamel junction (CEJ) to the gingival margin. RW was recorded as a line that connected the two points at the most coronal parts of the recession. A single examiner blinded to the study design and grouping recorded all the clinical measurements. Root coverage percentage was determined 1, 3 and 6 months after surgery.
Surgical procedure
One surgeon carried out all the surgical procedures with local anesthesia (2% lidocaine with 1:80000 epinephrine). Each subject received two horizontal incisions at the level of the CEJ followed by two oblique vertical incisions extending beyond the mucogingival junction (MGJ) and a partial-thickness flap was reflected. The surface epithelium of the adjacent interdental papilla was removed. In the control group, the CTG was prepared with a palate origin and sutured in the target site.9Then the partial-thickness flap was secured in a coronal position with a sling suture technique to cover the connective tissue graft (Figure 2). In the test group the human amniotic membrane, (AmniDress®, ImenChemiNarin Ltd Co, Tehran, Iran.) (Figure 3) was removed from cryo-box container approximately 5‒10 minutes prior to surgery according to the manufacture’s protocol. The cryo-preserved AM was trimmed for the recipient site size and transferred to the recipient site. Upon placement, the amniotic membrane adhered to the recipient root and proximal site, thus eliminating the need to suturing. The prepared flap was positioned coronally over the amniotic membrane and sutured in place with a sling technique. The surgical areas were covered with periodontal dressing (Figure 4).
Figure 2.
AmniDress®.
Figure 3.
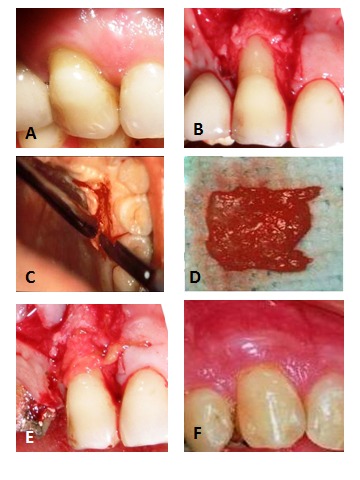
Control group: A) Recession defect on the right maxillary canine; B) Preparation of the recipient site; C & D) Harvesting the connective tissue graft; E) The connective tissue graft was placed over the exposed root surface; F) Complete root coverage at 6-month follow-up.
Figure 4.
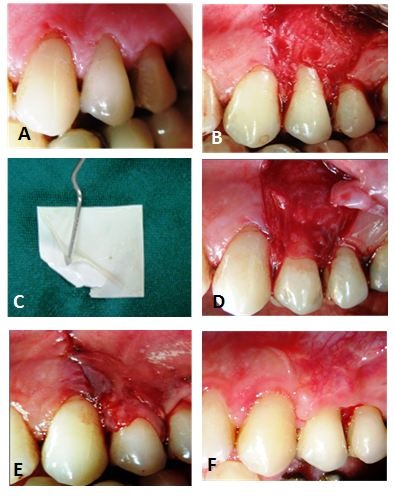
Test group: A) Recession defect on the vleft maxillary bicuspid; B) Preparation of the recipient site; C) Preparation of the amniotic membrane; D) The amniotic membrane was placed over the exposed root surface; E) The flap was positioned coronally over the amniotic membrane and secured with a sling sutures; F) Partial root coverage at 6-month follow-up.
Post-surgical considerations
All the patients were instructed to use chlorhexidine mouthrinse (0.2%) (Iran Najo, Tehran, Iran) twice a day for two weeks. To prevent postsurgical infection 500-mg amoxicillin capsules (Alhavi, Tehran, Iran) were prescribed three times a day for a week. Oral hygiene instructions included discontinuing tooth brushing near the surgical site. Sutures and periodontal dressing were removed 14 days after surgery. All the participants were seen two, four, twelve, and twenty-four weeks following surgery and in each session, the grafted defect sites were re-measured with a standard periodontal probe. Mechanical therapy was carried out for the patients during the study.
Statistical analysis
The parameters were reported as means ± standard divisions (SD). Inter-group differences were compared before and after the treatment, using ANOVA, and t-test was used to compare the results between the two groups at baseline and 1, 3, and 6 months postoperatively. P < 0.01 was considered statistically significant.
Results
Thirty Miller’s class I and II root exposures in eleven patients were selected for this study. The average changes for the study variables from baseline to the sixth month in the control and test groups are shown in Table 1. There were no statistically significant differences between the control and test groups at the baseline (P > 0.01). In both groups, improvement of PD and WKT from baseline to 1, 3 and 6 months were not statistically significant. The changes of RW, RD, CAL and the CEJ-MGJ distance were significant between baseline and first month after surgery, remaining stable during future follow-ups. The initial mean recession depth (RD) changed from 3.43 ± 0.63 mm to 0.8 ± 0.8 mm and 3.13±0.4 mm to 1.13 ± 1.26 mm (P < 0.01) for the control and test groups, respectively. There were no statistically significant differences between the two groups in RW, RD, CAL and the CEJ-MGJ distance (P > 0.01). However, root coverage percentage was higher in the control group compared to the test group (75.5% vs. 63.1%) and the differences were not statistically significant (P = 0.331). Complete root coverage was detected in 66.6% (10 defects) of the subjects in the control group compared to 46.6% (7 defects) of the subjects in the test group 6 months after surgery.
Table 1. The measured variables (mean ± SD) in the test and control groups at baseline and1, 3, and 6 months after surgery .
| Baseline | 1 month | 3 month | 6 month | ||
| PD (mm) | Control | 1±0.33 | 1.16±0.36 | 0.9±0.34 | 0.86±0.3 |
| Test | 1.17±0.56 | 1.17±0.31 | 1.07±0.26 | 1.03±0.3 | |
| RW (mm) | Control | 4.5±0.5 | 2.2±1.07* | 2.1±1.04* | 2.1±1.04 |
| Test | 4.33±0.84 | 1.73±1.55* | 1.7±1.54* | 1.66±1.67 | |
| RD (mm) | Control | 3.43±0.63 | 0.83±0.82* | 0.76±0.73* | 0.8±0.8 |
| Test | 3.13±0.4 | 1.2±1.13* | 1.13±1.13* | 1.13±1.26 | |
| CAL (mm) | Control | 4.43±0.9 | 1.99±0.96* | 1.66±0.78* | 1.66±0.86 |
| Test | 4.3±0.62 | 2.37±1.19* | 2.2±1.13* | 2.16±1.31 | |
| WKT (mm) | Control | 3.53±1.2 | 3.3±1.07 | 3.3±0.71 | 3.53±0.83 |
| Test | 3.13±0.3 | 3.07±0.18 | 3.2±0.32 | 3.23±0.32 | |
| CEJ-MGJ (mm) | Control | 7±1.16 | 4.33±1.05* | 4.4±0.87* | 4.4±0.87 |
| Test | 6.26±0.5 | 4.26±1.16 | 4.33±1.06 | 4.36±1.13 | |
| ROOT COVERAGE (%) | Control | 74.49±26 | 6.09±7.24 | 75.54±26.2 | |
| Test | 61.32±36.4 | 63.55±36.2 | 63.18±40.6 |
PD: probing depth, RW: recession width, RD: recession width, CAL: clinical attachment level, WKT: width of keratinized tissue, CEJ-MGJ: cemento-enamel junction to muco-gingival junction
*Indicates statistical significance.
Discussion
The aim of this study was to compare the use of AM for the CTG, together with a CAF, in the management of Miller Class I and II root exposures. Both treatment procedures reduced RD, RW and CAL at 6 months significantly. There were no statistically significant changes in clinical parameters between the test and control groups, although the control group achieved clinically superior results.
The recorded GI and PI in the test and control group at baseline were ≤20% and ≤1, respectively, remaining stable during future follow-ups. A similar result was obtained in relation to PD, consistent with other studies.30-32The enhancement of CAL from baseline to the first month after surgery was statistically significant in the study groups. No significant changes were found between the first month and 3 or 6 months after surgery. The CAL gain in the control and test groups averaged 2.76 ± 0.86 mm and 2.1 ± 1.31 mm, respectively. Other researchers reported CAL gain ranging from 2.3 mm to 5.1 mm, with an insignificant change in probing depth, following the use of CAF + CTG.30-32 Nevertheless, determination of the nature of the resultant attachment without histological evaluation is not possible. The WKT did not increase in the control and test groups, although MGJ moved coronally. There are a few studies available on changes in WKT after root coverage surgery. It has been shown that WKT may decrease slightly 5‒12 months after coronally advanced flap surgery.33-34 But long-term studies have indicated that gingival dimensions may increase.35-36Chambrone in a Cochrane systematic review included clinical trials with a study duration of ≥6 months and found that subepithelial connective tissue graft procedures significantly increased WKT.34Due to the short duration of this study, more prolonged follow-ups are suggested.
In this study, the RD reduction was 0.33 mm more in the control group compared to the test group (P = 0.39). RD decreased from 3.43 ± 0.63 mm to 0.8 ± 0.8 mm and 3.13 ± 1.4 mm to 1.13 ± 1.26 mm in the control and test group, respectively. Harris et al30 reported superior results in RD reduction (3.68 mm 3 months after surgery) using SCTG and CAF. However, in Harris study, the baseline RD was less than that in this study. Huang et al showed that the amount of recession depth changes in coronally advanced flap technique is positively related to the baseline recession depth.37
Average of RW reduction was 2.4 ± 1.04 mm in the control group and 2.66 ± 1.82 mm in the test group, respectively. A reduction of 2.7 ± 1.2 mm in RW after 6 months was found in Wang et al38 research.
Harris et al30demonstrated that recession width decreased from 3.5± 1 mm to 0.7 ± 1.2 mm and from 3.6 ± 1.2 mm to 0.4 ± 1 mm after three months and one year, respectively. The mean root coverage was 63.18% and 75.54% in the control and test groups after 6 months, respectively, with no significant differences between the two groups. The mean root coverage in the control group is consistent with the results of previous studies, indicating a mean root coverage of 86% (53‒98%).39This discrepancy may partly relate to differences in study designs, research duration, follow-up intervals and statistical analyses. Ghahroudi et al26 in a research similar to our study compared the efficacy of AM and CTG in the management of root exposure. Average root coverage rates after 6 months in the two groups were 67% and 54%, respectively. The mean percentage of RC in our study was greater than that in this study; this difference might be attributed to differences in the design of the present study. The present study design was split-mouth, which could reduce the effects of confounding factors.
Two important factors in the success of surgical root coverage include bridging and creeping attachment.27Borghetti and Gardella40pointed out that any improvement in the coverage of exposed root 1 month after surgery can be related to the creeping attachment. In the present study, we found 0.23 mm and 0.16 mm of creeping attachment in two groups between the first and the sixth months. Two prognostic factors affecting the success of coronally advanced flap (CAF) are tension flap and flap thickness.41In this study, after coronal placement of the flap, the extension of the vertical incision limited flap tension, and the flap was fixed with interrupted sutures in its new position.
Conclusion
Obviously, SCTG with CAF is the gold standard for the treatment of Miller class I and II gingival recession defects, and amniotic membrane with CAF may be relatively comparable with gold standard. This new allograft decreases the duration of surgery and morbidity of the patient. In fact, satisfaction with esthetic results of amniotic membrane is higher.
Acknowledgments
The authors would like to acknowledge the Dental and Periodontal Research Center of Tabriz University of Medical Sciences for their support in the development and realization of the present study.
Authors’ contributions
AL, NA, MF, ME were responsible for the main design and concept. AE and FS performed the literature search. AL and MF contributed in data acquisition and analysis in this research. MF and NA drafted the manuscript. All the authors have read and approved the final manuscript.
Funding
The work was supported by the Dental and Periodontal Research Center, Tabriz University of Medical Sciences, Tabriz, Iran.
Competing interests
The authors declare that they have no competing interests with regards to authorship and/or publication of this article.
Ethics approval
The study was approved by the Research Ethics Committee of Tabriz University of Medical Sciences (5/4/9997) and registered with the local World Health Organization Registry Network (IRCT138808142670N1).
References
- 1.Sangnes G. Traumatization of teeth and gingiva related to habitual tooth cleaning procedures. J Clin Periodontol. 1976;3:94–103. doi: 10.1111/j.1600-051X.1976.tb01855.x. [DOI] [PubMed] [Google Scholar]
- 2.Murtomaa H, Meurman JH, Rytömaa I, Turtola L. Periodontal status in university students. J Clin Periodontol. 1987;14:462–5. doi: 10.1111/j.1600-051X.1987.tb02252.x. [DOI] [PubMed] [Google Scholar]
- 3.Löe H, Anerud A, Boysen H. The natural history of periodontal disease in man: prevalence, severity, and extent of gingival recession. J Periodontol. 1992;63:489–95. doi: 10.1902/jop.1992.63.6.489. [DOI] [PubMed] [Google Scholar]
- 4.Woofter C. The prevalence and etiology of gingival recession. Periodontal Abstr. 1969;17:45–50. [PubMed] [Google Scholar]
- 5.Lafzi A, Abolfazli N, Eskandari A. Assessment of the etiologic factors of gingival recession in a group of patients in northwest Iran. J Dent Res Dent Clin Dent Prospects. 2009;3(3):90–3. doi: 10.5681/joddd.2009.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller PD Jr. Regenerative and reconstructive periodontal plastic surgery. Dent Clin North Am. 1988;32:287–306. [PubMed] [Google Scholar]
- 7.Harris RJ. The connective tissue and partial thickness double pedicle graft: a predictable method of obtaining root coverage. J Periodontol. 1992;63:477–86. doi: 10.1902/jop.1992.63.5.477. [DOI] [PubMed] [Google Scholar]
- 8.Sullivan HC, Atkins JC. Free autogenous gingival grafts, IIIUtilization of grafts in the treatment of gingival recessions. Periodontics. 1968;6:121–129. [PubMed] [Google Scholar]
- 9.Pini Prato G, Tinti C, Vincenzi G, Magnani C, Cortellini P, Clauser C. Guided Tissue Regeneration Versus Mucogingival Surgery in the Treatment of Human Buccal Gingival Recession. J Periodontol. 1992;63:919–28. doi: 10.1902/jop.1992.63.11.919. [DOI] [PubMed] [Google Scholar]
- 10.Langer B, Langer L. Subepithelial connective tissue graft technique for root coverage. J Periodontol. 1985;56:715. doi: 10.1902/jop.1985.56.12.715. [DOI] [PubMed] [Google Scholar]
- 11.Moslemi N, Heidari M, Mousavi Jazi M, Daneshmonfared M. Application of an acellular dermal matrixallograft (CenoDerm) for treatment of multiple gingival recession defects: A case report with one-year follow-up. J Dent Res Dent Clin Dent Prospects. 2014;8(3):180–3. doi: 10.5681/joddd.2014.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wennström JL. Mucogingival therapy. Annals of Periodontology. 1996;1:671–701. doi: 10.1902/annals.1996.1.1.671. [DOI] [PubMed] [Google Scholar]
- 13.Aichelmann-Reidy ME, Yukna RA, Evans GH, Nasr HF, Mayer ET. Clinical evaluation of acellular allograft dermis for the treatment of human gingival recession. J Periodontol. 2001;72:998–1005. doi: 10.1902/jop.2001.72.8.998. [DOI] [PubMed] [Google Scholar]
- 14.Burgos H. Angiogenic factor from human term placentaPurification and partial characterization. Eur J Clin Invest. 1986;16:486–93. doi: 10.1111/j.1365-2362.1986.tb02166.x. [DOI] [PubMed] [Google Scholar]
- 15.Talmi YP, Sigler L, Inge E, Finkelstein Y, Zohar Y. Antibacterial properties of human amniotic membranes. Placenta. 1991;12:285–8. doi: 10.1016/0143-4004(91)90010-d. [DOI] [PubMed] [Google Scholar]
- 16.Solomon A, Wajngarten M, Alviano F, Anteby I, Elchalal U, Pe'er J, Levi-Schaffer F. Suppression of inflammatory and fibrotic responses in allergic inflammation by the amniotic membrane stromal matrix. Clin Exp Allergy. 2005;35:941–8. doi: 10.1111/j.1365-2222.2005.02285.x. [DOI] [PubMed] [Google Scholar]
- 17.Koizumi NJ, Inatomi TJ, Sotozono CJ, Fullwood NJ, Quantock AJ, Kinoshita S. Growth factor mRNA and protein in preserved human amniotic membrane. Curr Eye Res. 2000;20:173–7. doi: 10.1076/0271-3683%28200003%292031-9FT173. [DOI] [PubMed] [Google Scholar]
- 18.Alviano F, Fossati V, Marchionni C, Arpinati M, Bonsi L, Franchina M, etal etal. Term amniotic membrane is a high throughput source for multipotentmesenchymal stem cells with the ability to differentiate into endothelial cells in vitro. BMC Dev Biol. 2007;7:11. doi: 10.1186/1471-213X-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toda A, Okabe M, Yoshida T, Nikaido T. The potential of amniotic membrane/ amnion-derived cells for regeneration of various tissues. J Pharmacol Sci. 2007;105:215–28. doi: 10.1254/jphs.cr0070034. [DOI] [PubMed] [Google Scholar]
- 20.Trelford-Sauder M, Dawe EJ, Trelford JD. Use of allograft amniotic membrane for control of intra-abdominal adhesions. J Med. 1978;9:273–84. [PubMed] [Google Scholar]
- 21.Tancer ML, Katz M, Veridiano NP. Vaginal epithelialization with human amnion. Obstet Gynecol. 1979;54:345–9. [PubMed] [Google Scholar]
- 22.Paridaens D, Beekhuis H, Van Den Bosch W, Remeyer I, Melles G. Amniotic membrane transplantation in the management of conjunctiva malignant melanoma and primary acquired melanosis with atypia. Br J Ophthalmol. 2001;85:658–61. doi: 10.1136/bjo.85.6.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Branski LK, Herndon DN, Celis MM, Norbury WB, Masters OE, JeschkeMG JeschkeMG. Amnion in the treatment of pediatric partial-thickness facial burns. Burns. 2008;34:393–9. doi: 10.1016/j.burns.2007.06. [DOI] [PubMed] [Google Scholar]
- 24.Kothiwale SV, Anuroopa P, Gajiwala AL. A clinical and radiological evaluation of DFDBA with amniotic membrane versus bovine derived xenograft with amniotic membrane in human periodontal grade II furcation defects. Cell Tissue Bank. 2009;10:317–26. doi: 10.1007/s10561-009-9126-3. [DOI] [PubMed] [Google Scholar]
- 25.Velez I, Parker WB, Siegel MA, Hernandez M. Cryopreserved amniotic membrane for modulation of periodontal soft tissue healing: a pilot study. J Periodontol. 2010;81:1797–804. doi: 10.1902/jop.2010.100060. [DOI] [PubMed] [Google Scholar]
- 26.Ghahroudi AA, Khorsand A, Rokn AR, Sabounchi SS, Shayesteh YS, Soolari A. Comparison of amnion allograft with connective tissue graft for root coverage procedures: a double-blind, randomized, controlled clinical trial. J Int Acad Periodontol. 2013;15:101–12. [PubMed] [Google Scholar]
- 27.Allen EP, Miller PO. Coronal positioning of existing gingivaShort term results in the treatment of shallow marginal tissue recession. J Periodontol. 1989;60:316–9. doi: 10.1902/jop.1989.60.6.316. [DOI] [PubMed] [Google Scholar]
- 28.O'Leary TJ, Drake RB, Naylor JE . The plaque control record. J Periodontol. 1972;43:38. doi: 10.1902/jop.1972.43.1.38. [DOI] [PubMed] [Google Scholar]
- 29.Leo H. The gingival index, the plaque index and the retention index systems. J Periodontol. 1967;38:610–6. doi: 10.1902/jop.1967.38.6.610. [DOI] [PubMed] [Google Scholar]
- 30.Harris RJ. Connective tissue grafts combined with either double pedicle grafts or coronally positioned pedicle grafts: results of 266 consecutively treated defects in 200 patients. Int J Periodontics Restorative Dent. 2002;22:463–71. [PubMed] [Google Scholar]
- 31.Saletta D, Pini Prato GP, Pagliro V, Baldi C, Mauri M, NieriM NieriM. Coronally advanced flap procedure. Is the interdental papilla a prognostic factor for root coverage? J Periodontol. 2001;72:760–6. doi: 10.1902/jop.2001.72.6.760. [DOI] [PubMed] [Google Scholar]
- 32.Zuchelli G, Amore C, Sforza NM, Montebugnoli L, De Sanctis M. Bilaminar techniques for the treatment of recession defectsA comparative clinical study. J Clin Periodontol. 2003;30:862–70. doi: 10.1034/j.1600-051x.2003.00397.x. [DOI] [PubMed] [Google Scholar]
- 33.Paolantonio M, Di Murro C, Cattabriga A, Cattabriga M. Subpedicle connective tissue graft versus free gingival graft in the coverage of exposed root surfacesA 5year- clinical study. J Clin Periodontol. 1997;24:51–6. doi: 10.1111/j.1600-051x.1997.tb01184.x. [DOI] [PubMed] [Google Scholar]
- 34.Chambrone L, Sukekava F, Araújo MG, Pustiglioni FE, Chambrone LA, Lima LA. Root-coverage procedures for the treatment of localized recession-type defects: A Cochrane systematic review. J Periodontol. 2010;81:452–78. doi: 10.1902/jop.2010.090540. [DOI] [PubMed] [Google Scholar]
- 35.Romanos GE, Bernimoulin JP, Marggraf F. The double lateral bridging flap for root coverage of denuded root surfaces: longitudinal study and clinical evaluation after 5 to 8 years. J Periodontol. 1993;64:683–8. doi: 10.1902/jop.1993.64.8.683. [DOI] [PubMed] [Google Scholar]
- 36.Wennstrom JL, Zucchelli G. Increased gingival dimensionsA significant factor for successful outcome of root coverage procedures?A 2-year prospective clinical study. J Clin Periodontol. 1996;23:770–7. doi: 10.1111/j.1600-051x.1996.tb00608.x. [DOI] [PubMed] [Google Scholar]
- 37.Huang LH, Nieva RF, Wang HL. Factors affecting the outcomes of coronally advanced flap root coverage procedure. J Periodontol. 2005;76:1729–34. doi: 10.1902/jop.2005.76.10.1729. [DOI] [PubMed] [Google Scholar]
- 38.Wang HL, Bunyaratavej P, Labadie M, Shyr Y, MacNeil RL. Comparison of 2 clinical techniques for treatment of gingival recession. J Periodontol. 2001;72:1301–11. doi: 10.1902/jop.2001.72.10.1301. [DOI] [PubMed] [Google Scholar]
- 39.Harris RJ. Root coverage with connective tissue grafts: an evaluation of short and long-term results. J Periodontol. 2002;73(9):1054–9. doi: 10.1902/jop.2002.73.9.1054. [DOI] [PubMed] [Google Scholar]
- 40.Borghetti A, Gardella JP. Thick gingival autograft for the coverage of gingival recession: A clinical evaluation. Int J Periodontics Restorative Dent. 1990;10:216–29. [PubMed] [Google Scholar]
- 41.Hwang D, Wang HL. Flap thickness as a predictor of root coverage: a systematic review. J Periodontol. 2006;77:1625–34. doi: 10.1902/jop.2006.060107. [DOI] [PubMed] [Google Scholar]


