Abstract
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease characterized by the production of multiple autoantibodies. Antibodies against Ficolin-3 were previously identified in the sera of some SLE patients, but their prevalence and significance have not been yet investigated. The aims of this study were to determine the prevalence of anti-ficolin-3 antibodies among SLE patients and to investigate their potential as diagnostic and/or prognostic biomarkers in SLE. In this retrospective study, sera from SLE patients (n = 165) were selected from a preexisting declared biological collection. Samples from healthy controls (n = 48) were matched with SLE sera. Disease activity was determined according to the SLEDAI score. Anti-ficolin-3, anti-dsDNA and anti-C1q antibodies levels were measured in sera by ELISA. First, a highly significant difference was found in the anti-ficolin-3 levels between SLE patients and healthy subjects. Anti-ficolin-3 antibodies were detected as positive in 56 of 165 (34%) SLE patients. The titer of anti-ficolin-3 antibodies was correlated with the SLEDAI score (r = 0.38, p<0.0001). The presence of anti-ficolin-3 antibodies was associated with anti-C1q and anti-dsDNA antibodies. Regarding associations with clinical manifestations, the presence of active lupus nephritis was significantly associated with the presence of anti-ficolin-3 antibodies (p≤0.001). This association with renal involvement was higher with anti-ficolin-3 or anti-C1q antibodies than with other auto-antibodies. Interestingly, the combination of anti-ficolin-3 and anti-C1q antibodies demonstrated higher specificity than any other serological biomarker. These results suggest that anti-ficolin-3 antibodies could be useful for the diagnosis of active nephritis in SLE patients.
Introduction
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease with a prevalence of 4/10,000 among Northern Europeans and a predisposition of women of childbearing age [1]. SLE is characterized by the presence of circulating autoantibodies directed against self-antigens, such as dsDNA, nuclear antigens and several cytoplasmic components [2]. The accumulation of the resulting immune complexes mediates a systemic inflammatory response that is the primary cause of tissue damage. Recently, defects in apoptotic cells clearance leading to secondary cell necrosis and subsequent release of intracellular autoantigens has been proposed as one of the mechanisms of induction of these autoantibodies [3] [4]. According to this proposal, molecules of importance in the uptake of dying cells could have a role in host protection against autoimmune diseases, including SLE [5] [6] [7] [8].
Apart from its well characterized anti-microbial role, the complement system has also a pivotal role in maintaining host integrity by eliminating apoptotic and damaged cells as well as by clearing circulating immune complexes [9]. Indeed, deficiencies in early complement components are often associated with increased susceptibility to both infections and autoimmune diseases such as SLE, supporting a protective role for the complement system. Mice deficient for C1q, the recognition protein of the classical complement pathway, are predisposed to SLE-like diseases [10] and human studies report that hereditary homozygous deficiencies of C1q are strongly associated with susceptibility to SLE, with a defect in apoptotic cells uptake by macrophages in SLE patients [11]. Recent reports suggest a role for mannan-binding lectin (MBL), a recognition protein of the lectin complement pathway, in the pathogenesis of SLE [12]. Indeed, gene polymorphisms leading to reduced levels of serum MBL were found associated with a predisposition to SLE [13]. However, although MBL-null mice exhibit defective clearance of apoptotic cells, they fail to develop symptoms of autoimmune diseases, suggesting the ability of other molecules to compensate for MBL deficiency [14].
The potential role of the recently identified complement-activating lectin-like proteins ficolins (ficolin-1, -2 and -3, also referred as M-, L- and H-ficolins) has been suggested, since these proteins appear to mediate immune effector functions similar to those of MBL and C1q [14] [15]. Among these proteins, Ficolin-3 deserves special attention in the context of autoimmunity since it was initially characterized as a serum antigen target (Hakata antigen) for an autoantibody present in a Japanese patient with SLE [16]. Ficolin-3 has poor lectin-like activity, as shown by glycan array screening, but it binds to acetylated albumin with high affinity [17] [18]. It has been shown to specifically recognize few bacterial polysaccharides [19] [20]. Interestingly, binding of ficolin-3 to apoptotic material has clearly been shown and has been postulated to play a role in the clearance of apoptotic debris via phagocytosis [21] [22]. Recently, an association of high levels of ficolin-3 with specific manifestations in SLE, but not with disease activity, was reported [23]. To date, only one study reported the presence of anti-ficolin-3 antibodies in SLE sera using a precipitation reaction for antibodies detection, and its correlation with disease activity [24].
Our study aims to investigate the presence of anti-ficolin-3 antibodies in SLE patients and to evaluate their potential as diagnostic and/or prognostic SLE biomarkers.
Materials and Methods
Study
This study is a multicentric non-interventional retrospective study conducted in French University Hospitals (Grenoble University Hospital and Marseille University Hospital, France) over a 3-year period (2012–2014).
Samples
All venous blood samples were obtained from patients referred for routine detection of auto-antibodies and sera were conserved in a declared biobank DC 2012–1704 for Marseille and DC 2014–2268 for Grenoble, with respect of ethical directives.
Samples from SLE patients, satisfying the revised 1997 American College of Rheumatology (ACR) classification criteria for SLE [25] (n = 165), were selected to be further analyzed in this study. Samples from healthy blood donors (n = 48) obtained from “Etablissement Français du Sang”, matched for sex and age, served as controls. This study has been approved by local authorities (Institutional Review Board) according to standards currently applied in France (Commission Nationale de l’Informatique et des Libertés”, CNIL N°1841851vO). The study has also been registered to clinicaltrial.gov (N° NCT02625831). The study was done in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines.
For SLE patients, clinical and biological manifestations as well as treatments received and disease activity evaluated using the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) [26] at the time of sampling were recorded. The SLE patients were divided into two groups for further analyses: a group with quiescent or “low disease activity” (n = 88) and a group with active or “high disease activity” (n = 77), according to the physician in charge and respectively with a SLEDAI score ≤4 and >4. All patients with renal involvement had an active lupus nephritis (LN) documented by kidney biopsy (classes I, II, III, IV or V of the ISN/RPS classification) [27]. Samples were collected just before kidney biopsy. Active proliferation was defined by the presence of at least one glomerulus showing cellular or fibro-cellular crescent or endocapillary proliferation on kidney biopsy (classes III and IV).
Anti-ficolin-3 antibodies assessment
Recombinant ficolin-3 was purified from the culture supernatant of a stably transfected CHO cell line by affinity chromatography based on its binding capacity to acetylated BSA, as previously described [28]. Detection of anti-ficolin-3 antibodies was adapted from an ELISA previously described for measurement of anti-MBL antibodies [29]. Microtiter plates (96 wells) were coated overnight at 4°C with 2 μg/ml of recombinant ficolin-3 in 15 mM Na2CO3, 35 mM NaHCO3 (pH 9.6). The plates were washed three times with Phosphate Buffer Saline (PBS) containing 0.1% Tween-20 (w/v) (PBS-T) and blocked for 2 h at room temperature (RT) with PBS-T containing 1% BSA (w/v). Serum samples were added to the wells and incubated overnight at 4°C. After washing as above, horseradish peroxidase (HRP)-conjugated goat polyclonal anti-human IgGs (The Binding Site, dilution 1/15 000) were added for 1 h at RT. Subsequently, tetramethylbenzidine substrate was added to each well, the reaction was stopped by addition of H2SO4 and optical densities (ODs) were measured at 450 nm. The threshold value of 70 AU was calculated using the 98th percentile on the OD reading for the control group. A value was considered significantly elevated when the concentration was above 70 AU (arbitrary units). The concentration was defined as (OD sample/OD control) x 100 AU.
Ficolin-3 assessment
To quantify ficolin-3 in healthy blood donors and SLE patients’ sera, an in-house ELISA was adapted from Michalski et al. [30]. Microtiter plates were coated with 2.5 μg/ml of mouse monoclonal anti-human ficolin-3 (mAb 334, Hycult Biotechnologies) diluted in PBS (1:400) overnight at 4°C. Plates were washed 3 times with PBS-T and blocked for 2 h with PBS-T-BSA 1% (w/v) at RT. After 3 washings with PBS-T, serum samples (1:50 dilution in PBS) or recombinant ficolin-3 were added to each well (100μl/well) at different concentrations to establish the standard curve. After 3 h incubation at 37°C, the plates were washed 4 times with PBS-T, and an in-house rabbit polyclonal anti-human ficolin-3 (1:2000 dilution in PBS-T) was added and incubated 1 h at 37°C. After 4 washings with PBS-T, HRP-conjugated rabbit polyclonal anti-ficolin-3 (1:5000 diluted in PBS-T) was added and incubated 1 h at 37°C. Detection was achieved as described above.
Anti-dsDNA and anti-C1q antibodies assessment
Anti-double-stranded (ds)DNA antibodies were detected by ELISA (BIORAD) with normal value defined as <50 U/ml. For anti-C1q antibodies, microtiter plates (96 wells) were coated overnight at 4°C with 100 μl/well of purified C1q collagen stalks at 10 μg/ml in 20 mM H3BO3, 7.5 mM NaCl (pH 8.2) [31]. The plates were washed three times with PBS-T and blocked for 30 min at 37°C with PBS-T containing 2% BSA (w/v). Serum samples were added to the wells and incubated 30 min at 37°C. After washings as above, HRP-conjugated goat polyclonal anti-human IgG (The Binding Site, dilution 1:60,000) were added for 30 min at 37°C. Detection was achieved as described above. The threshold value was calculated using the 98th percentile on the OD reading for the control group. The concentration of IgG reactive to anti-C1q was expressed in arbitrary units (AU). The concentration was defined as (OD sample/OD cut-off) x 100 AU. A value was considered significantly elevated when the concentration was above 100 AU.
Complement fractions assessment
Complement levels were measured by nephelometry (BNII, Siemens), according to manufacturer’s instructions, with the normal range for C4 being >100 mg/l and for C3 >880 mg/l.
Statistics
Data analyses were carried out using Statview software version 5.0. Comparisons of continuous variables between subgroups were done using Mann-Whitney non parametric U-test. Correlations between continuous variables were assessed calculating Spearman’s rank correlation coefficients. Comparisons of categorical variables, expressed as counts (%), were done using Chi²- or Fisher’s exact-tests. P-values lower than 0.05 were considered statistically significant.
Results
Characteristics of SLE patients
Details on the characteristics of the 165 SLE patients and matched healthy controls are provided in Table 1. The median age of the patients was 42 years (range 16–84 years), 88% were women. At inclusion, the median SLEDAI score was 4 (range 0–24). 88 patients were classified in the “low disease activity” group and 77 in the “high disease activity” group, as previously defined (see Methods). In this latter group, 36 (47%) patients had biopsy-proven active lupus nephritis.
Table 1. Demographic and clinical variables for SLE patients.
| Demographic variables | Control (n = 48) | SLE (n = 165) |
|---|---|---|
| Age, range, years | 25–70 | 16–84 |
| Age, mean (median) (S.D), years | 41 (38) (13.3) | 44 (42) (15.5) |
| Gender, female, n (%) | 42 (88) | 145 (88) |
| Clinical features, n (%) | NA | |
| Kidney | 38 (23) | |
| Joint | 34 (21) | |
| Haematological | 10 (6) | |
| Cutaneous | 16 (10) | |
| Cardiac | 5 (3) | |
| Pulmonary | 2 (1) | |
| Neurological | 4 (2) |
It should be noted that some patients may have lupus flare involving several organs. NA: not applicable.
Prevalence of anti-ficolin-3 antibodies in SLE patients
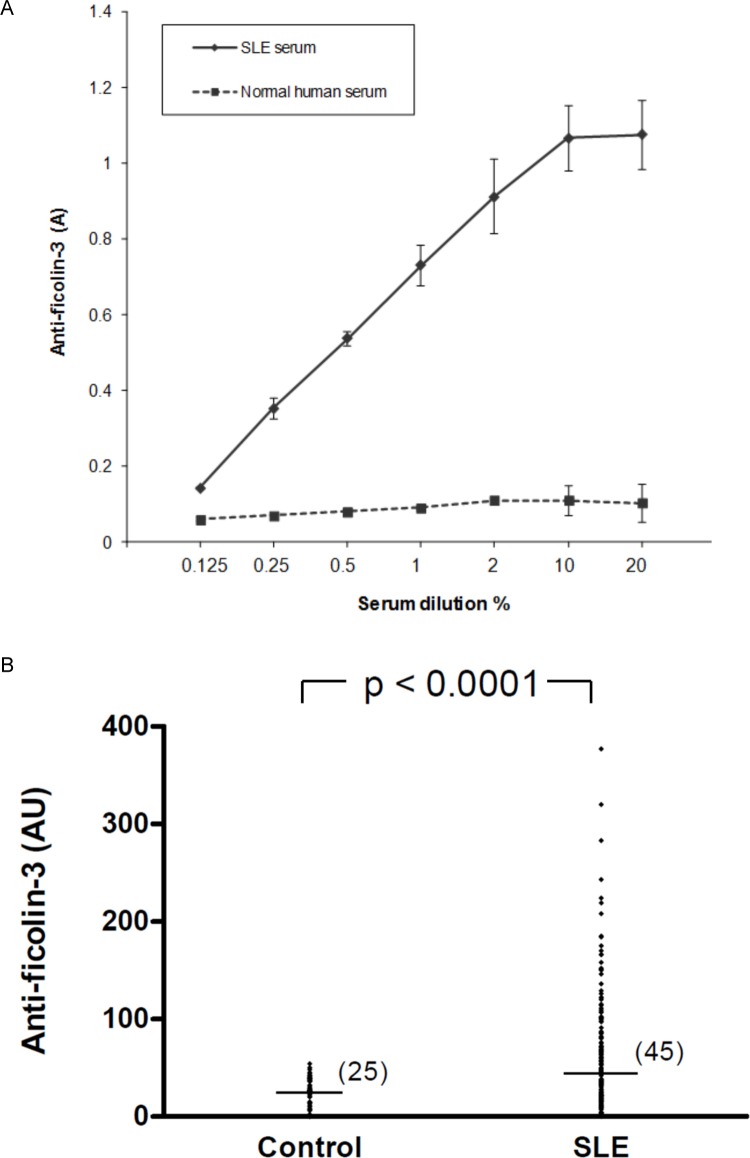
The presence of anti-ficolin-3 antibodies was first investigated in sera obtained from one SLE patient and one healthy blood donor. A dose-dependent binding of IgG to solid-phase ficolin-3 was demonstrated, while serum from a healthy blood donor did not show significant reactivity (Fig 1A). Those sera were used as positive and negative standards for further experiments. As a control, ELISA wells were coated with BSA and no detectable binding was observed for both sera.
Fig 1. Detection of anti-ficolin-3 antibodies in patients with SLE.
A) Binding of anti-ficolin-3 antibodies to immobilized ficolin-3. Microtiter plates were coated with ficolin-3. Sera from SLE patients and healthy controls were added in serial dilutions. Results represent the mean +/- standard deviation. B) Anti-ficolin-3 antibodies in serum samples. Anti-ficolin-3 antibodies were measured in 48 samples from healthy controls and in 165 samples from patients with SLE. Horizontal lines in each group indicate the median values. Statistical analyses were performed by Mann-Whitney test. A, absorbance; AU, Arbitrary units.
Subsequently, anti-ficolin-3 antibodies were tested in serum samples from the 165 SLE patients and from 48 healthy controls. Titers of anti-ficolin-3 antibodies were significantly higher in SLE patients than in healthy controls (median 45 vs 25 AU, p<0.0001, Mann-Whitney U-test) (Fig 1B). Using a cutoff value of 70 AU (see Methods) anti-ficolin-3 antibodies were detected as positive in 56 (34%) of 165 SLE patients. No anti-ficolin-3 antibodies were found in patients with rheumatoid arthritis (n = 14), Sjögren’s syndrome (n = 12), chronic renal failure (n = 12) and IgA nephropathy such as Berger disease (n = 12) (S1 Fig).
Association between anti-ficolin-3 antibodies levels and clinical and biological SLE activity
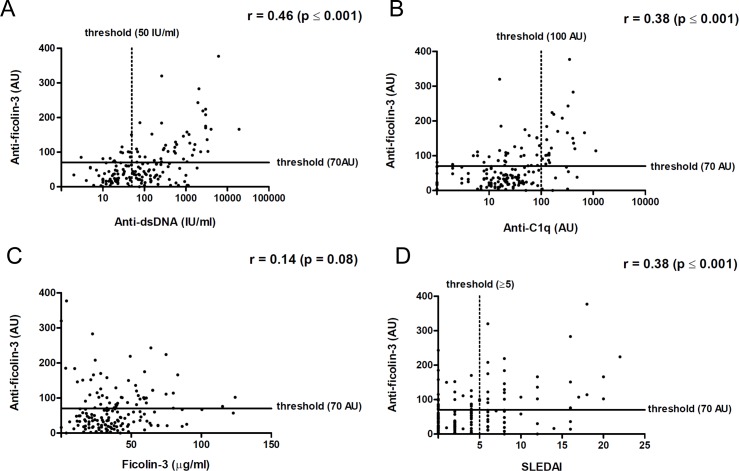
In SLE patients, the titers of anti-ficolin-3 antibodies were significantly associated with the titers of anti-dsDNA antibodies (r = 0.46, p<0.0001, Spearman test) (Fig 2A) as well as with the titers of anti-C1q antibodies (r = 0.38, p<0.0001, Spearman test) (Fig 2B). The presence of anti-ficolin-3 antibodies was not correlated to ficolin-3 serum levels (r = 0.14, p = 0.08, Spearman test) (Fig 2C). Furthermore, a statistical difference in serum ficolin-3 concentrations was observed between SLE patients and healthy individuals (p = 0.015, Mann-Whitney U-test) (S2 Fig).
Fig 2. Association between anti-ficolin-3 antibodies titers, biological markers and disease activity in patients with SLE.
Association between anti-ficolin-3 titers and anti-dsDNA antibodies (A), anti-C1q antibodies (B) and ficolin-3 concentrations (C) in SLE. D) Association between anti-ficolin-3 titers and SLE Disease Activity Index (SLEDAI). Statistical analyses were performed by Spearman’s rank correlation test.
A significant association between anti-ficolin-3 antibodies titers and disease activity (SLEDAI) was observed (r = 0.38, p<0.0001, Spearman test) (Fig 2D). Similar results were obtained using a clinical SLEDAI, which does not take anti-dsDNA antibodies and complement titers into account. A significant association was also observed between SLEDAI score and titers of anti-dsDNA antibodies (r = 0.62, p<0.0001, Spearman test) or titers of anti-C1q antibodies (r = 0.38, p<0.0001, Spearman test). Of note, ficolin-3 levels were not associated with lupus activity evaluated by SLEDAI score (r = 0.296, p = 0.083, Spearman test) (S3 Fig).
Association between SLE-related clinical features and autoantibodies positivity
Among various clinical manifestations presented by our patients (Table 2), the presence of anti-ficolin-3 antibodies was significantly associated with lupus nephritis (p≤0.001, Chi2 test). Similarly, the presence of anti-C1q antibodies was also significantly associated with lupus nephritis (p≤0.001, Chi2 test). Anti-dsDNA antibodies and low complement C3 and/or C4 were less associated with kidney involvement (respectively p = 0.012 and p = 0.015, Chi2 test).
Table 2. Associations between SLE-related clinical features of 77 patients with active SLE and titers of anti-ficolin-3, anti-C1q and anti-dsDNA antibodies.
| SLE damages | Number of patients with flare | Anti-ficolin-3 | Anti-C1q | Anti-dsDNA | Low complement | ||||
|---|---|---|---|---|---|---|---|---|---|
| Chi-2 | p | Chi-2 | p | Chi-2 | p | Chi-2 | p | ||
| Kidney | 36 | 10.92 | *** ≤ 0.001 | 12.72 | *** ≤ 0.001 | 6.36 | * 0.012 | 5.87 | * 0.015 |
| Joint | 33 | 3.90 | * 0.048 | 10.90 | *** ≤ 0.001 | 5.53 | * 0.019 | 10.84 | *** ≤ 0.001 |
| Haematological | 8 | 0.002 | 0.970 | 1.62 | 0.203 | 0.10 | 0.756 | 2.60 | 0.107 |
| Cutaneous | 16 | 7.57 | ** 0.006 | 1.73 | 0.188 | 6.47 | * 0.011 | 0.03 | 0.861 |
| Cardiac | 5 | 0.19 | 0.665 | 0.14 | 0.710 | 0.002 | 0.965 | 0.14 | 0.709 |
| Pulmonary | 2 | 2.11 | 0.147 | 4.27 | * 0.039 | 0.54 | 0.463 | 0.003 | 0.955 |
| Neurological | 4 | 0.001 | 0.979 | 0.11 | 0.743 | 2.19 | 0.139 | 1.23 | 0.268 |
It should be noted that some patients may have lupus flare involving several organs.
*P≤0.05
**P≤0.01
***P≤0.001 (Chi2-test).
Anti-ficolin-3 antibodies and active lupus nephritis
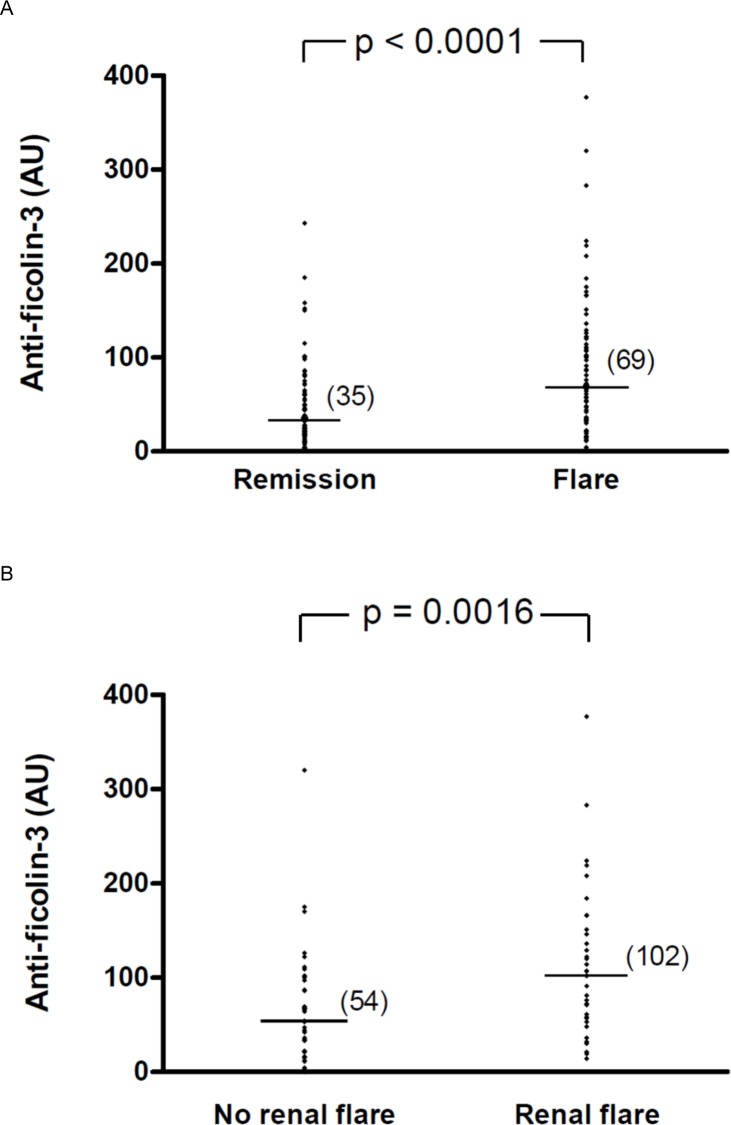
Anti-ficolin-3 antibodies were detected as positive in 38 (49%) of the 77 SLE patients characterized as having “high disease activity”. Titers of anti-ficolin-3 antibodies were significantly higher in SLE patients with “high disease activity” than in those with “low disease activity” (median 69 vs 35 AU, p<0.0001, Mann-Whitney U-test), (Fig 3A). Anti-ficolin-3 antibodies were detected as positive in 25 (69%) of 36 SLE patients with active disease and lupus nephritis. Characteristics of the patients are shown in Table 3. Interestingly, titers of anti-ficolin-3 antibodies were significantly higher in this subset of SLE patients with active disease and lupus nephritis than in patients with active disease but without renal involvement (median 102 vs 54 AU, p = 0.0016, Mann-Whitney U-test) (Fig 3B).
Fig 3. Serum anti-ficolin-3 antibodies titers in SLE patients with active disease (flare) or in disease remission.
A) Anti-ficolin-3 titers in SLE patients with active disease (SLEDAI >4) (n = 77) or in disease remission (SLEDAI ≤4) (n = 88). B) Anti-ficolin-3 titers in SLE patients with active disease with renal involvement (n = 36) or without renal involvement (n = 41). Horizontal lines in each group indicate the median values. Statistical analyses were performed by Mann-Whitney test.
Table 3. Characteristics of SLE patients with lupus nephritis.
| Demographic data | Age, mean +/- SD | 36 +/- 14.6 |
| Male/Female, n (%) | 12 (33) / 24 (67) | |
| White/African/Asian, n (%) | 30 (83)/5 (14)/1 (3) | |
| Renal abnormalities | Urinary casts (heme-granular or RBC casts) | 24 (69) |
| n (%) | High blood pressure | 6 (17) |
| Proteinuria | 34 (94) | |
| Abnormal creatinine | 13 (36) | |
| ISN/RPS 2003 class | I | 2 (6) |
| n (%) | II | 1 (3) |
| III | 4 (11) | |
| IV | 23 (64) | |
| V | 6 (17) | |
| Therapy | Corticoids | 21 (58) |
| n (%) | Azathioprine | 3 (8) |
| Plaquenil | 13 (36) | |
| Methotrexate | 1 (3) | |
| Rituximab | 2 (6) | |
| Mycophenolate mofetil | 3 (8) |
RBC, red blood cells
Lupus nephritis and combined biomarkers
As anti-ficolin-3, anti-C1q, anti-dsDNA and low complement C3 and/or C4 were associated with renal disease activity, we characterized the diagnostic performances of these biomarkers, alone or in combination, in our cohort. Sensitivity and specificity of anti-ficolin-3 antibodies for SLE renal involvement were respectively 70% and 68%. Predictive positive and negative values were respectively 66% and 72%. While anti-dsDNA antibodies alone indicated a better sensitivity than the other biomarkers, interestingly the combination of anti-ficolin-3 and anti-C1q antibodies showed the higher specificity for renal involvement (Table 4).
Table 4. Performances of SLE biomarkers.
| Biomarkers | Renal flare | No renal flare | Sensitivity (%) | Specificity (%) | NPV (%) | PPV (%) |
|---|---|---|---|---|---|---|
| Anti-ficolin-3 Abs | 25/36 (70%) | 13/41 (32%) | 70 | 68 | 72 | 66 |
| Anti-dsDNA Abs | 33/36 (92%) | 28/41 (68%) | 92 | 32 | 81 | 54 |
| Anti-C1q Abs | 19/36 (53%) | 6/41 (15%) | 53 | 85 | 67 | 76 |
| Low complement | 24/36 (67%) | 16/41 (39%) | 66 | 61 | 68 | 60 |
| Anti-ficolin-3 and anti-C1q Abs | 18/36 (50%) | 3/41 (7%) | 50 | 93 | 68 | 86 |
| Anti-ficolin-3 and anti-C1q Abs and low complement | 15/36 (42%) | 3/41 (7%) | 42 | 93 | 64 | 83 |
Abs, antibodies; NPV, negative predictive value; PPV, positive predictive value.
We then analyzed the possible link between the presence of anti-ficolin-3 antibodies and immunohistological characteristics of the 36 kidney biopsies. Patients with active proliferative lupus nephritis (i.e. Classes III and IV) had significantly more positive anti-ficolin-3 and anti-C1q antibodies than those with non-proliferative lupus nephritis (respectively p = 0.001 and p = 0.006, Fisher’s exact test) (Table 5). Other biological data such as proteinuria, abnormal creatinine, low complement and anti-DNA antibodies were not or little significantly different between the two groups.
Table 5. Biological characteristics of patients with active and inactive lupus nephritis.
| LN with non active proliferation n = 9 (%) | LN with active proliferation n = 27 (%) | P | |
|---|---|---|---|
| Proteinuria | 9 (100) | 25 (93) | 1.00 |
| Abnormal creatinine | 1 (11) | 12 (44) | 0.11 |
| Low complement | 3 (33) | 21 (78) | 0.04 * |
| Anti-dsDNA Abs | 7 (78) | 26 (96) | 0.15 |
| Anti-C1q Abs | 1 (11) | 18 (67) | 0.006 ** |
| Anti-ficolin-3 Abs | 2 (22) | 23 (85) | 0.001 *** |
*P≤0.05
**P≤0.01
***P≤0.001 (Fisher’s exact-test)
Discussion
Our study is to our knowledge the first one assessing the presence of antibodies targeting ficolin-3 and measuring their titers using an ELISA method in SLE patients. Indeed, only one previous study reported the presence of auto-antibodies against ficolin-3 in lupus sera, but authors used a double immunodiffusion technique which was not quantitative [24]. In that study, only 4 among 110 SLE patients (3.6%) were found to have anti-ficolin-3 antibodies. Conversely, in our cohort, 57/165 (34%) patients were found positive. This discrepancy likely relies on the higher sensitivity of our ELISA over older techniques.
The present study is also the first to investigate association between anti-ficolin-3 antibodies and clinical and serologic parameters of SLE using a large cohort of SLE patients. First, we found that the anti-ficolin-3 autoantibodies levels were significantly higher in SLE patients as compared to healthy subjects. Interestingly, these antibodies were found negative in other autoimmune diseases such as rheumatoid arthritis and Sjögren’s syndrome. Then we assessed the presence of anti-ficolin-3 antibodies in sera of SLE patients during inactive and active phases of the disease. The levels of anti-ficolin-3 were significantly higher in patients with active than quiescent disease and we observed a statistical correlation of antibodies levels and the global activity of the disease evaluated by the SLEDAI score.
These findings are of importance. Indeed, while many tests exist for the detection of autoantibodies in the sera of patients with SLE, such as anti-dsDNA and anti-C1q [32], only few of them are used in clinical practice. Most of the studies indicated that anti-dsDNA antibodies could be useful markers of the overall activity of SLE [33]. Some considered the elevated levels of anti-dsDNA as indicative of active forms of SLE [34]. In accordance with those observations, titers of anti-dsDNA were correlated with lupus activity in our SLE cohort. However, some studies suggested that anti-dsDNA antibodies have to be used with caution to evaluate the SLE activity [35] [36]. Because the associations between antibody profile and clinical symptoms are unclear, it is crucial to find new relevant diagnostic and/or prognostic biomarkers.
Similarities in structure and function exist between collagen-like defense molecules such as MBL, C1q and ficolin-3. Anti-MBL antibodies were found in SLE patients’ sera but no significant relationship was observed with clinical characteristics of SLE [29] [37]. Several studies reported that anti-C1q levels did not correlate with SLEDAI score in SLE patients [36] [38]. However, in our study, we found an association between titers of anti-C1q and SLE disease activity, in accordance with the study of Moura et al. [39]. The occurrence of anti-C1q antibodies in patients with active lupus nephritis remains controversial but the absence of anti-C1q antibodies seems to exclude active renal disease [40] [41].
Lupus nephritis, a severe manifestation of SLE, affects up to 30 to 50% patients in the course of the disease. Interestingly, in our cohort we observed a very high prevalence (70%) of anti-ficolin-3 antibodies in the subset of SLE patients with lupus nephritis. Regarding clinical associations, anti-ficolin-3 antibodies positivity was significantly related to renal involvement. There was no significant association between the positivity of anti-ficolin-3 antibodies and other clinical characteristics of SLE such as joint, hematological, neurological, cardiac or pulmonary damages. This is in accordance with our results showing significantly higher anti-ficolin-3 titers in patients with active nephritis compared to those in patients with active disease but without renal involvement. Moreover, the possibility that renal dysfunction might lead to higher level of antibody has been eliminated since anti-ficolin-3 antibodies were found negative in dialyzed patients with chronic renal failure. Importantly, we found that anti-ficolin-3 antibodies were more closely associated with active lupus nephritis than any other auto-antibodies. The development of nephritis is a major determinant of disease morbidity and mortality. Thus, the identification of non-invasive biomarkers in lupus nephritis is a priority. We observed that the combination of anti-ficolin-3 and anti-C1q antibodies outperformed anti-C1q, anti-dsDNA or low complement alone, demonstrating a higher specificity than either serological biomarker. Until now, anti-C1q in combination with anti-dsDNA and low complement was reported as the strongest serological association with renal involvement. Orbai et al. and Yang et al. showed that the combination of anti-C1q and anti-dsDNA was associated with higher renal disease activity [42] [43]. Even if nephritis assessment based on standard parameters such as proteinuria and creatinine remains essential, the combination of anti-ficolin-3 and anti-C1q antibodies could open new perspectives in evaluation of lupus nephritis.
Although a causal relationship between the presence of anti-ficolin-3 antibodies and lupus nephritis remains to be determined, the possible role of anti-ficolin-3 antibodies in the pathogenesis of lupus is a key issue. On one hand, anti-ficolin-3 antibodies may contribute to the formation of circulating immune complexes that are deposited in the kidney or to the local formation of immune complexes on the glomerular basement membrane. On the other hand, anti-ficolin-3 antibodies from SLE patients could specifically target ficolin-3 bound to apoptotic cells, thereby altering the clearance of dead cells, which is an important hypothesis of the pathogenesis of SLE. This role of anti-ficolin-3 antibodies is plausible, because ficolin-3 seems not to be affected in SLE. Indeed, we found increased serum levels of ficolin-3 in patients with SLE and no association of ficolin-3 levels and lupus activity, as shown by Andersen et al. and Hein et al. [44] [23]. Therefore, the role of anti-ficolin-3 antibodies in the SLE pathogenesis remains to be investigated and an interesting point resides in identifying the auto-antibodies specificities against ficolin-3. Indeed, anti-ficolin-3 antibodies could recognize either the collagen-like or the fibrinogen-like domains of ficolin-3 with different outcomes. A significant participation of complement activation was reported in the pathogenesis of lupus nephritis. The alternative and lectin pathways were involved in the progression of glomerular injury of patients with lupus nephritis [45] and in situ deposition of complement components in renal biopsy specimens has been shown [46]. Ficolin-3, which is a strong activator of the lectin pathway, could also contribute to renal pathogenesis. Moreover, Pang et al. [47] showed that in the presence of anti-C1q antibodies, immune complexes would not be removed from the circulation and might prone to deposit in some organs, such as kidneys, and induce inflammatory injury. The potential role of anti-ficolin-3 antibodies was not discussed in the literature and it would be of interest to study the association between anti-ficolin-3 antibodies and specific pathological lesions on kidney biopsies. In this regard, a link between the presence of anti-ficolin-3 antibodies and the immunohistological characteristics of the kidney biopsies was observed. However further investigations would be necessary to confirm these findings in a larger cohort of patients with lupus nephritis.
In conclusion, this is the first report on the significant presence of anti-ficolin-3 antibodies in the serum of SLE patients. From a clinical point of view, these data support the usefulness of anti-ficolin-3 as an additional serological biomarker for the diagnosis of active lupus with renal manifestation, but additional longitudinal studies are needed to validate the diagnostic and/or prognostic role of this new SLE parameter.
Supporting Information
Microtiter plates were coated with ficolin-3. Sera from healthy controls (n = 48), Sjögren’s Syndrome (SS) (n = 12), Rheumatoid Arthritis (RA) (n = 14), chronic renal failure (n = 12), IgA nephropathy such as Berger disease (n = 12) and SLE patients were added in serial dilutions. Horizontal lines in each group indicate the median values. AU, arbitrary units.
(TIF)
Ficolin-3 was measured in 48 samples from healthy controls and in 165 samples from patients with SLE. Horizontal lines in each group indicate the median values. Statistical analyses were performed by Mann-Whitney test.
(TIF)
Statistical analyses were performed by Spearman’s rank correlation test.
(TIF)
Acknowledgments
We would like to thank Dr Jean Gagnon and Pierre Audoin for their helpful comments on the manuscript
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Rahman A, Isenberg DA. Systemic lupus erythematosus. N Engl J Med. 2008;358: 929–939. 10.1056/NEJMra071297 [DOI] [PubMed] [Google Scholar]
- 2.Arbuckle MR, McClain MT, Rubertone MV, Scofield RH, Dennis GJ, James JA, et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med. 2003;349: 1526–1533. 10.1056/NEJMoa021933 [DOI] [PubMed] [Google Scholar]
- 3.Muñoz LE, Lauber K, Schiller M, Manfredi AA, Herrmann M. The role of defective clearance of apoptotic cells in systemic autoimmunity. Nat Rev Rheumatol. 2010;6: 280–289. 10.1038/nrrheum.2010.46 [DOI] [PubMed] [Google Scholar]
- 4.Herrmann M, Voll RE, Zoller OM, Hagenhofer M, Ponner BB, Kalden JR. Impaired phagocytosis of apoptotic cell material by monocyte-derived macrophages from patients with systemic lupus erythematosus. Arthritis Rheum. 1998;41: 1241–1250. [DOI] [PubMed] [Google Scholar]
- 5.Kravitz MS, Pitashny M, Shoenfeld Y. Protective molecules—C-reactive protein (CRP), serum amyloid P (SAP), pentraxin3 (PTX3), mannose-binding lectin (MBL), and apolipoprotein A1 (Apo A1), and their autoantibodies: prevalence and clinical significance in autoimmunity. J Clin Immunol. 2005;25: 582–591. 10.1007/s10875-005-7828-2 [DOI] [PubMed] [Google Scholar]
- 6.Gullstrand B, Lefort MH, Tydén H, Jönsen A, Lood C, Johansson A, et al. Combination of autoantibodies against different histone proteins influences complement-dependent phagocytosis of necrotic cell material by polymorphonuclear leukocytes in systemic lupus erythematosus. J Rheumatol. 2012;39: 1619–1627. 10.3899/jrheum.111511 [DOI] [PubMed] [Google Scholar]
- 7.Liphaus BL, Kiss MHB. The role of apoptosis proteins and complement components in the etiopathogenesis of systemic lupus erythematosus. Clin São Paulo Braz. 2010;65: 327–333. 10.1590/S1807-59322010000300014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jung J-Y, Suh C-H. Incomplete clearance of apoptotic cells in systemic lupus erythematosus: pathogenic role and potential biomarker. Int J Rheum Dis. 2015;18: 294–303. 10.1111/1756-185X.12568 [DOI] [PubMed] [Google Scholar]
- 9.Dunkelberger JR, Song W-C. Complement and its role in innate and adaptive immune responses. Cell Res. 2010;20: 34–50. 10.1038/cr.2009.139 [DOI] [PubMed] [Google Scholar]
- 10.Botto M, Dell’Agnola C, Bygrave AE, Thompson EM, Cook HT, Petry F, et al. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nat Genet. 1998;19: 56–59. 10.1038/ng0598-56 [DOI] [PubMed] [Google Scholar]
- 11.Botto M, Walport MJ. C1q, autoimmunity and apoptosis. Immunobiology. 2002;205: 395–406. 10.1078/0171-2985-00141 [DOI] [PubMed] [Google Scholar]
- 12.Panda AK, Parida JR, Tripathy R, Pattanaik SS, Ravindran B, Das BK. Mannose binding lectin: a biomarker of systemic lupus erythematosus disease activity. Arthritis Res Ther. 2012;14: R218 10.1186/ar4057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsutsumi A, Takahashi R, Sumida T. Mannose binding lectin: genetics and autoimmune disease. Autoimmun Rev. 2005;4: 364–372. 10.1016/j.autrev.2005.02.004 [DOI] [PubMed] [Google Scholar]
- 14.Fraser DA, Tenner AJ. Directing an appropriate immune response: the role of defense collagens and other soluble pattern recognition molecules. Curr Drug Targets. 2008;9: 113–122. [DOI] [PubMed] [Google Scholar]
- 15.Akaiwa M, Yae Y, Sugimoto R, Suzuki SO, Iwaki T, Izuhara K, et al. Hakata antigen, a new member of the ficolin/opsonin p35 family, is a novel human lectin secreted into bronchus/alveolus and bile. J Histochem Cytochem Off J Histochem Soc. 1999;47: 777–786. [DOI] [PubMed] [Google Scholar]
- 16.Yae Y, Inaba S, Sato H, Okochi K, Tokunaga F, Iwanaga S. Isolation and characterization of a thermolabile beta-2 macroglycoprotein (‘thermolabile substance’ or “Hakata antigen”) detected by precipitating (auto) antibody in sera of patients with systemic lupus erythematosus. Biochim Biophys Acta. 1991;1078: 369–376. [DOI] [PubMed] [Google Scholar]
- 17.Gout E, Garlatti V, Smith DF, Lacroix M, Dumestre-Pérard C, Lunardi T, et al. Carbohydrate recognition properties of human ficolins: glycan array screening reveals the sialic acid binding specificity of M-ficolin. J Biol Chem. 2010;285: 6612–6622. 10.1074/jbc.M109.065854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lacroix M, Dumestre-Pérard C, Schoehn G, Houen G, Cesbron J-Y, Arlaud GJ, et al. Residue Lys57 in the collagen-like region of human L-ficolin and its counterpart Lys47 in H-ficolin play a key role in the interaction with the mannan-binding lectin-associated serine proteases and the collectin receptor calreticulin. J Immunol Baltim Md 1950. 2009;182: 456–465. [DOI] [PubMed] [Google Scholar]
- 19.Tsujimura M, Ishida C, Sagara Y, Miyazaki T, Murakami K, Shiraki H, et al. Detection of serum thermolabile beta-2 macroglycoprotein (Hakata antigen) by enzyme-linked immunosorbent assay using polysaccharide produced by Aerococcus viridans. Clin Diagn Lab Immunol. 2001;8: 454–459. 10.1128/CDLI.8.2.454-459.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Swierzko A, Lukasiewicz J, Cedzynski M, Maciejewska A, Jachymek W, Niedziela T, et al. New functional ligands for ficolin-3 among lipopolysaccharides of Hafnia alvei. Glycobiology. 2012;22: 267–280. 10.1093/glycob/cwr119 [DOI] [PubMed] [Google Scholar]
- 21.Honoré C, Hummelshoj T, Hansen BE, Madsen HO, Eggleton P, Garred P. The innate immune component ficolin 3 (Hakata antigen) mediates the clearance of late apoptotic cells. Arthritis Rheum. 2007;56: 1598–1607. 10.1002/art.22564 [DOI] [PubMed] [Google Scholar]
- 22.Kuraya M, Ming Z, Liu X, Matsushita M, Fujita T. Specific binding of L-ficolin and H-ficolin to apoptotic cells leads to complement activation. Immunobiology. 2005;209: 689–697. 10.1016/j.imbio.2004.11.001 [DOI] [PubMed] [Google Scholar]
- 23.Andersen T, Munthe-Fog L, Garred P, Jacobsen S. Serum levels of ficolin-3 (Hakata antigen) in patients with systemic lupus erythematosus. J Rheumatol. 2009;36: 757–759. 10.3899/jrheum.080361 [DOI] [PubMed] [Google Scholar]
- 24.Yoshizawa S, Nagasawa K, Yae Y, Niho Y, Okochi K. A thermolabile beta 2-macroglycoprotein (TMG) and the antibody against TMG in patients with systemic lupus erythematosus. Clin Chim Acta Int J Clin Chem. 1997;264: 219–225. [DOI] [PubMed] [Google Scholar]
- 25.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40: 1725 [DOI] [PubMed] [Google Scholar]
- 26.Gladman DD, Ibañez D, Urowitz MB. Systemic lupus erythematosus disease activity index 2000. J Rheumatol. 2002;29: 288–291. [PubMed] [Google Scholar]
- 27.Weening JJ, D’Agati VD, Schwartz MM, Seshan SV, Alpers CE, Appel GB, et al. The Classification of Glomerulonephritis in Systemic Lupus Erythematosus Revisited. J Am Soc Nephrol. 2004;15: 241–250. 10.1097/01.ASN.0000108969.21691.5D [DOI] [PubMed] [Google Scholar]
- 28.Jacquet M, Lacroix M, Ancelet S, Gout E, Gaboriaud C, Thielens NM, et al. Deciphering complement receptor type 1 interactions with recognition proteins of the lectin complement pathway. J Immunol Baltim Md 1950. 2013;190: 3721–3731. 10.4049/jimmunol.1202451 [DOI] [PubMed] [Google Scholar]
- 29.Takahashi R, Tsutsumi A, Ohtani K, Goto D, Matsumoto I, Ito S, et al. Anti-mannose binding lectin antibodies in sera of Japanese patients with systemic lupus erythematosus. Clin Exp Immunol. 2004;136: 585–590. 10.1111/j.1365-2249.2004.02477.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Michalski M, Szala A, St Swierzko A, Lukasiewicz J, Maciejewska A, Kilpatrick DC, et al. H-ficolin (ficolin-3) concentrations and FCN3 gene polymorphism in neonates. Immunobiology. 2012;217: 730–737. 10.1016/j.imbio.2011.12.004 [DOI] [PubMed] [Google Scholar]
- 31.Sasaki T, Yonemasu K. Chemical studies on the isolated collagen-like and globular fragment of complement component C1q. Comparative studies on bovine and human C1q. Biochim Biophys Acta. 1983;742: 122–128. [DOI] [PubMed] [Google Scholar]
- 32.Julkunen H, Ekblom-Kullberg S, Miettinen A. Nonrenal and renal activity of systemic lupus erythematosus: a comparison of two anti-C1q and five anti-dsDNA assays and complement C3 and C4. Rheumatol Int. 2012;32: 2445–2451. 10.1007/s00296-011-1962-3 [DOI] [PubMed] [Google Scholar]
- 33.Isenberg DA, Manson JJ, Ehrenstein MR, Rahman A. Fifty years of anti-ds DNA antibodies: are we approaching journey’s end? Rheumatol Oxf Engl. 2007;46: 1052–1056. 10.1093/rheumatology/kem112 [DOI] [PubMed] [Google Scholar]
- 34.Haque SF. Anti-double stranded DNA antibodies are valuable markers of disease activity in systemic autoimmune disorders. Novel Science International Journal of Medical Science. 2012: 1–6. [Google Scholar]
- 35.Launay D, Schmidt J, Lepers S, Mirault T, Lambert M, Kyndt X, et al. Comparison of the Farr radioimmunoassay, 3 commercial enzyme immunoassays and Crithidia luciliae immunofluorescence test for diagnosis and activity assessment of systemic lupus erythematosus. Clin Chim Acta Int J Clin Chem. 2010;411: 959–964. 10.1016/j.cca.2010.03.016 [DOI] [PubMed] [Google Scholar]
- 36.Meyer OC, Nicaise-Roland P, Cadoudal N, Grootenboer-Mignot S, Palazzo E, Hayem G, et al. Anti-C1q antibodies antedate patent active glomerulonephritis in patients with systemic lupus erythematosus. Arthritis Res Ther. 2009;11: R87 10.1186/ar2725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seelen MA, Trouw LA, van der Hoorn JWA, Fallaux-van den Houten FC, Huizinga TWJ, Daha MR, et al. Autoantibodies against mannose-binding lectin in systemic lupus erythematosus. Clin Exp Immunol. 2003;134: 335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trad B, Ben Hassine H, Khalifa M, Idriss N, Slama F, Bahri F, et al. Anti-C1q antibodies and systemic lupus erythematosus in the Tunisian population. Pathol Biol (Paris). 2013;61: 113–116. 10.1016/j.patbio.2013.01.007 [DOI] [PubMed] [Google Scholar]
- 39.Moura CG, Lima I, Barbosa L, Athanazio D, Reis E, Reis M, et al. Anti-C1q antibodies: association with nephritis and disease activity in systemic lupus erythematosus. J Clin Lab Anal. 2009;23: 19–23. 10.1002/jcla.20280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Katsumata Y, Miyake K, Kawaguchi Y, Okamoto Y, Kawamoto M, Gono T, et al. Anti-C1q antibodies are associated with systemic lupus erythematosus global activity but not specifically with nephritis: a controlled study of 126 consecutive patients. Arthritis Rheum. 2011;63: 2436–2444. 10.1002/art.30401 [DOI] [PubMed] [Google Scholar]
- 41.Daha NA, Banda NK, Roos A, Beurskens FJ, Bakker JM, Daha MR, et al. Complement activation by (auto-) antibodies. Mol Immunol. 2011;48: 1656–1665. 10.1016/j.molimm.2011.04.024 [DOI] [PubMed] [Google Scholar]
- 42.Orbai A-M, Truedsson L, Sturfelt G, Nived O, Fang H, Alarcón GS, et al. Anti-C1q antibodies in systemic lupus erythematosus. Lupus. 2015;24: 42–49. 10.1177/0961203314547791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang X, Tan Y, Yu F, Zhao M. Combination of anti-C1q and anti-dsDNA antibodies is associated with higher renal disease activity and predicts renal prognosis of patients with lupus nephritis. Nephrol Dial Transplant Off Publ Eur Dial Transpl Assoc—Eur Ren Assoc. 2012;27: 3552–3559. 10.1093/ndt/gfs179 [DOI] [PubMed] [Google Scholar]
- 44.Hein E, Nielsen LA, Nielsen CT, Munthe-Fog L, Skjoedt M-O, Jacobsen S, et al. Ficolins and the lectin pathway of complement in patients with systemic lupus erythematosus. Mol Immunol. 2015;63: 209–214. 10.1016/j.molimm.2014.07.003 [DOI] [PubMed] [Google Scholar]
- 45.Sato N, Ohsawa I, Nagamachi S, Ishii M, Kusaba G, Inoshita H, et al. Significance of glomerular activation of the alternative pathway and lectin pathway in lupus nephritis. Lupus. 2011;20: 1378–1386. 10.1177/0961203311415561 [DOI] [PubMed] [Google Scholar]
- 46.Nisihara RM, Magrini F, Mocelin V, Messias-Reason IJ. Deposition of the lectin pathway of complement in renal biopsies of lupus nephritis patients. Hum Immunol. 2013;74: 907–910. 10.1016/j.humimm.2013.04.030 [DOI] [PubMed] [Google Scholar]
- 47.Pang Y, Yang X-W, Song Y, Yu F, Zhao M-H. Anti-C1q autoantibodies from active lupus nephritis patients could inhibit the clearance of apoptotic cells and complement classical pathway activation mediated by C1q in vitro. Immunobiology. 2014;219: 980–989. 10.1016/j.imbio.2014.07.004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Microtiter plates were coated with ficolin-3. Sera from healthy controls (n = 48), Sjögren’s Syndrome (SS) (n = 12), Rheumatoid Arthritis (RA) (n = 14), chronic renal failure (n = 12), IgA nephropathy such as Berger disease (n = 12) and SLE patients were added in serial dilutions. Horizontal lines in each group indicate the median values. AU, arbitrary units.
(TIF)
Ficolin-3 was measured in 48 samples from healthy controls and in 165 samples from patients with SLE. Horizontal lines in each group indicate the median values. Statistical analyses were performed by Mann-Whitney test.
(TIF)
Statistical analyses were performed by Spearman’s rank correlation test.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.