Abstract
The efficient one-pot conversion of propargyl alcohols to their saturated carbonyl analogues is carried out for the first time using metal nanoparticle catalysts, dodecanethiolate-capped Pd nanoparticles. Kinetic studies reveal that the reaction progresses through a semi-hydrogenation intermediate (allyl alcohols) followed by isomerization to carbonyls.
The ease of preparation of propargyl alcohols1,2 and the broad utility of their reaction products have resulted in a vast library of effective reactions involving these compounds.3–5 For example, propargyl alcohols can undergo full hydrogenation to saturated alcohols in the presence of an activated metal such as Pt or Pd in a pressurized, hydrogen rich environment.3 Moreover, the semi-hydrogenation of propargyl alcohols, in which a triple bond is reduced to a double bond without any further hydrogenation, can be achieved by employing mildly poisoned catalysts, such as Lindlar’s catalyst.4 Even the isomerization of propargyl alcohols to α,β-unsaturated carbonyls have been attained utilizing ruthenium catalysts as reported by Trost et al.5
However, the catalytic reactions of propargyl alcohols are still somewhat deficient due to the lack of an effective one-pot synthetic method allowing for the conversion of propargyl alcohols directly to saturated carbonyls, which are important intermediates for pharmaceutical and industrial applications. To our knowledge, the one-pot conversion of the propargylic alcohol subgroup to its saturated carbonyl has only been achieved by Pd(OAc)2-catalysed hydrogenation of methyl 4-hydroxy-4-phenyl-2-butynoate, using excess HCO2H/NBu3 as the hydrogen source, to the corresponding γ-ketoester in a moderate yield of 65%.6 No example involving the direct conversion of simple propargyl alcohols to small saturated carbonyls has been reported. Until now, therefore, two-step synthetic methods such as the full hydrogenation followed by alcohol oxidation or the isomerization followed by selective reduction of C=C bond are considered to be the most effective ways for this particular conversion.7,8
In our previous studies, the synthesis of dodecanethiolate-protected palladium nanoparticles generated from sodium S-dodecylthiosulfate precursors yielded nanoparticles with a lower ligand surface coverage than those synthesized with dodecanethiol ligands.9 Used as catalysts, these palladium nanoparticles were capable of isomerizing 2-propen-1-ol (allyl alcohol) to propanal with a rather high selectivity over the hydrogenation to 1-propanol.9 However, these catalytic materials revealed limitations when employing allyl alcohol substrates with more than one substituent around the C=C bond.10 Recently, the optimization of these palladium nanoparticles was achieved by performing systematic variations of the synthetic parameters, such as reactant concentration and temperature.11 This allowed the further manipulation of the surface ligand density and the core size of the dodecanethiolate-capped palladium nanoparticles. The optimized palladium nanoparticles with the lower surface ligand coverage could produce isomerization products in high yields even for the substituted allyl alcohols.11
In this article, for the first time, we report the successful one-pot conversion of simple propargyl alcohols to their saturated carbonyl analogues using metal nanoparticle catalysts.12,13 The effects of hydrogen gas concentration, reaction temperature, and substrate structures were investigated. Moreover, the kinetic profile of this reaction was obtained to understand the reaction mechanism.
Palladium catalysts employed in this study are two different nanoparticles for which the detailed synthesis and characterization are documented in ESI.†11 A palladium nanoparticle catalyst, ~Pd1289(SC12H25)164, which has an average core size of ~3.4 nm with a moderately low ligand surface coverage (~0.34 ligands/surface Pd atoms),11,14 was initially applied to the catalytic reaction of 2-propyn-1-ol (propargyl alcohol) in the presence of 10 mmol H2 gas at 0 °C (Table 1). The reaction was analysed with 1H NMR after 6 h, which inferred the formation of a mixture of 2-propen-1-ol (semi-hydrogenation product), 1-propanol (full-hydrogenation product), and propanal (tandem semi-hydrogenation and isomerization product; in short tandem product) with fairly similar yields. Although there was no clear selectivity over any product, the generation of the tandem product was clearly observed from the reaction using these thiolate-poisoned palladium nanoparticle catalysts. The other palladium nanoparticle catalyst utilized in this reaction,~Pd116(SC12H25)59, featured an average core size of~1.5 nm.11 However, due to the higher surface ligand coverage (~0.73 ligands/surface Pd atoms) of these palladium nanoparticle catalysts, they exhibited poor catalytic activities and produced mainly semi-hydrogenation products in a low yield of 28% without any evidence of the tandem product.
Table 1.
The conversion of 2-propyn-1-ol to propanal by dodecanethiolate-capped PdNP catalysts at 0 °Ca
| Catalystsb | TGA % Pd |
TEM Diameter (nm) |
Ligands/surface atomsb | Catalysis yields (%)c | TOFd | ||
|---|---|---|---|---|---|---|---|
| Semi-hydrogenation | Full-hydrogenation | Tandem reaction | |||||
| Pd1289L164 | 80.6 | 3.38 ± 0.95 | 0.34 | 31 | 32 | 37 | 123 |
| Pd116L59 | 51.0 | 1.51 ± 0.46 | 0.75 | 28 | 5 | 0 | 0 |
Reaction conditions: 50 µL 2-propyn-1-ol; 5 mol% Pd; ~10 mmol H2; 2 mL CDCl3; 6 h.
Calculations were based on theoretical models provided by Hostetler et al. (ref. 14) along with TGA and TEM data.
Yields were determined by 1H NMR.
Initial TOFs are based on the mole isomerized per mol Pd atoms per hour.
A palladium nanoparticle with a lower surface ligand density, ~Pd1289(SC12H25)164, was, therefore, chosen as a catalyst for the optimization of reaction conditions. An increase in reaction temperature to room temperature (~25 °C) resulted in a higher reaction conversion indicated by the absence of 2-propen-1-ol, the semi-hydrogenation product, along with the upsurge in yields of both propanal and 1-propanol (Table 2, entry 1). However, the selectivity between 1-propanol (the full hydrogenation product) and propanal (the tandem product) remained mostly the same even at the increased temperature (46 : 54 to 42 : 58).
Table 2.
The optimization of the conversion of propargyl alcohols to carbonyl analogous using Pd1289L164 catalysts at 25 °C: the effects of temperature, H2 molar equivalents, and the substratesa
| Catalysis yields (%)b | ||||||||
|---|---|---|---|---|---|---|---|---|
| Entry | Propargyl alcohol | R1 | R2 | mmol H2 | Semi-hydrogenation | Full-hydrogenation | Tandem reaction | TOFc |
| 1 | 2-propyn-1-ol | H | H | 10 | 0 | 42 | 58 | 290 |
| 2 | 2-propyn-1-ol | H | H | 2 | 0 | 30 | 70 | 350 |
| 3 | 3-butyn-2-ol | CH3 | H | 2 | 0 | 20 | 76 | 380 |
| 4 | 2-butyn-1-ol | H | CH3 | 2 | 43 | 13 | 41 | 205 |
| 5 | 3-phenyl-2-propyn-1-ol | H | Ph | 2 | 24 | 1 | 39 | 195 |
Reaction condition: 50 µL of propargyl alcohol; 5 mol% Pd, 2 mL CDCl3; 4 h.
Yields were determined by 1H NMR.
Initial TOFs are based on the mole isomerized per mole Pd atoms per hour.
Prior studies involving the isomerization of allyl alcohols by palladium nanoparticle catalysts in the presence of hydrogen gas proposed that the catalytic reaction transpires through a Pd–alkyl mechanism (see ESI† for the proposed mechanism of the tandem reaction).15 Pd–H species generated upon the adsorption of hydrogen on the nanoparticle surface can ultimately insert a hydride to the π bond of allyl alcohol.15 The conversion to saturated carbonyls (the isomerization products) requires the β-H elimination of a Markovnikov intermediate (a branched Pd–alkyl intermediate). This β-H elimination step can theoretically be more favoured over the insertion of another hydride when Pd nanoparticles have hydride undersupplied surface. Therefore, the effect of hydrogen gas concentration was examined for the catalytic reaction of 2-propyn-1-ol after decreasing the hydrogen gas equivalent for a five-fold while maintaining all other reaction conditions. The results showed that the selectivity towards propanal increases with the decrease in concentration of hydrogen gas (Table 2, entry 2). A further decrease in hydrogen gas concentration, however, resulted in an incomplete reaction. Similar results were also observed from the catalytic reaction of 3-butyn-2-ol with the variation of hydrogen gas concentration (see ESI†).
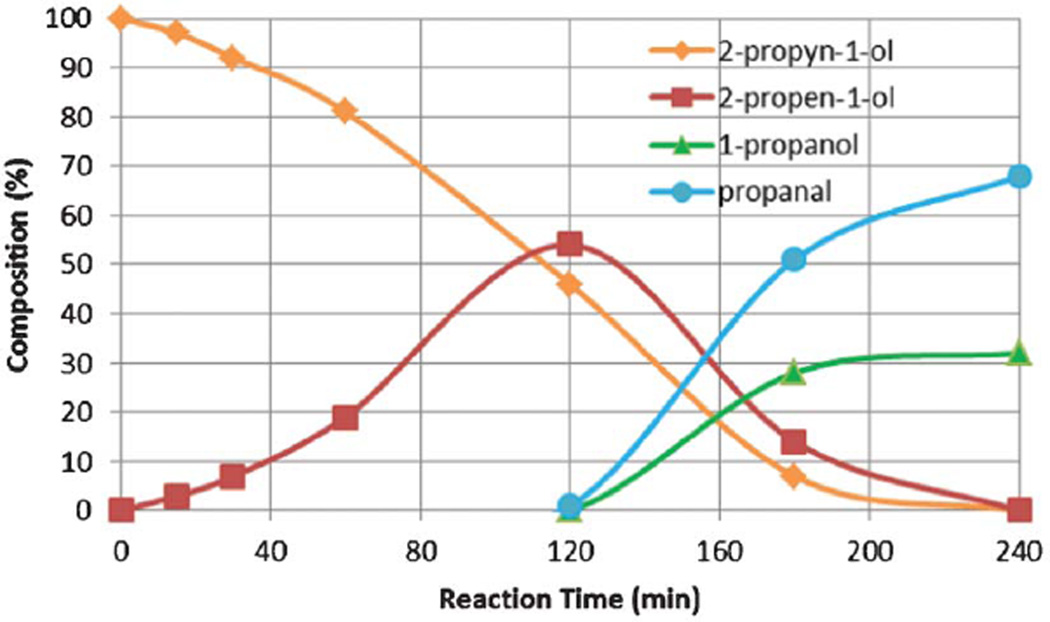
The kinetic profile of the conversion of 2-propyn-1-ol is illustrated in Fig. 1. As the data dictates, 2-propyn-1-ol was first converted to its semi-hydrogenation product, 2-propen-1-ol, without producing any full hydrogenation or the tandem products in the early stage of the reaction. It took about 2 h for the concentration of 2-propyn-1-ol to decrease below the concentration of the semi-hydrogenation product, 2-propen-1-ol, in the reaction mixture. After this, the allyl alcohol intermediate onset its conversion to either propanal or 1-propanol. The stronger and thermodynamically driven adsorption of alkyne on the palladium surface compared to alkene has well been documented in other publications.16,17 The kinetic profile in Fig. 1 was consistent with the high adsorption constant of the 2-propyn-1-ol, only leading to the isomerization of 2-propen-1-ol after consuming more than a half of 2-propyn-1-ol. Subsequent isomerization and hydrogenation of 2-propen-1-ol took place in logarithmic reaction gradients confirming a first order reaction.15
Fig. 1.
The kinetic profile of the catalytic conversion of propagyl alcohol to 1-propanal. Kinetic data was obtained by monitoring the reaction progress using 1H NMR.
To examine the stability of catalysts, the Pd nanoparticles were isolated from the reaction mixture by methanol-induced precipitation from the homogeneous solution. The precipitates were redissolved in organic solvents for characterization by TEM (see ESI†) and UV-Vis spectroscopy. TEM results showed the high population of small Pd nanoparticles without any notable morphological change. UV-Vis results also confirmed the absence of the absorption bands corresponding to both Pd(II) species and oxidized Pd. The earlier report from our group has also shown the high recyclability of dodecanethiolate-capped Pd nanoparticles in a similar reaction condition.15
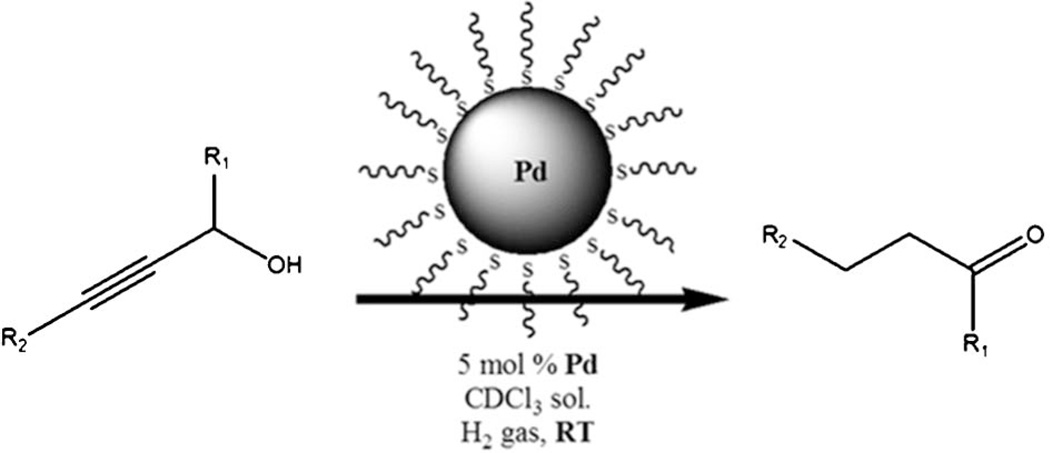
The ~Pd1289(SC12H25)164 catalyst was further tested against three additional commercially available substituted propargyl alcohols as summarized in Table 2 (entries 3–5) and Scheme 1. The conversion of 3-butyn-2-ol (entry 3) resulted in yields far congruent to its unsubstituted equivalent. Alkyl substituents at the R1 position (Scheme 1) seemed rather beneficial towards isomerization to saturated carbonyls since their presence essentially increases the thermodynamic stability of the enol intermediate.10 As for 2-butyn-1-ol (entry 4) which contains a methyl substituent at the R2 position, the overall conversion from the semi-hydrogenation product (2-buten-1-ol) to butanal and 1-butanol was far more difficult to achieve. This is likely due to the higher stability of 2-buten-1-ol (a di-substituted alkene) compared to that of other mono-substituted semi-hydrogenation intermediates. Nonetheless, the selectivity towards the isomerization product (butanal) over the full hydrogenation product (1-butanol) still remained the same (>3 times more favoured than the full hydrogenation product). Furthermore, the catalytic reaction of 3-phenyl-2-propyn-1-ol (entry 5), with a phenyl group at the R2 position, resulted in almost 35% of unreacted substrate. The accessibility of 3-phenyl-2-propyn-1-ol to the surface reaction sites on Pd nanoparticle was likely lower due to the steric interference of ligand surrounding nanoparticles.18 The more rigid nature of the substrate should also have contributed to the slower reaction by the limited access to the surface reaction sites. Although the catalytic reactivity was quite lower for this substrate, its selectivity with regards to the tandem reaction (39%) over the full-hydrogenation (1%) was much higher.
Scheme 1.
The conversion of propargyl alcohols (R1 = H or CH3; R2 = H, CH3, or Ph) to their carbonyl analogues using PdNP catalysts.
Conclusions
It has been shown that palladium nanoparticles synthesized from sodium S-dodecylthiosulfate precursors were able to convert small propargyl alcohols to their saturated carbonyl analogues under a one-pot condition. The catalytic reactions were performed under fairly mild conditions and without any other reagents besides palladium catalysts and H2 gas. Overall, the results clearly showed the significance of highly active homogeneous nanoparticle catalysts, which are capable of providing a high selectivity towards atypical products that are not possible through the traditional groups of catalysts. Future studies will focus on improving selectivity towards the tandem products by controlling the structure/density of capping ligands and introducing chemical additives for secondary surface poisoning.
Acknowledgments
The authors gratefully acknowledge the financial support from ACS-PRF (49407-UR7) and CSULB (RSCA, MGSS, and RSA).
Footnotes
Electronic supplementary information (ESI) available: The synthetic procedure for Pd nanoparticles, the characterization methods, the procedure for the catalytic reactions, the mechanism of the tandem reaction, the catalytic reaction results of 3-butyn-2-ol, TEM results before and after the catalytic reaction, and TGA results. See DOI: 10.1039/c3ra42119h
Notes and references
- 1.Li C-J. Acc. Chem. Res. 2010;4:581–590. doi: 10.1021/ar9002587. [DOI] [PubMed] [Google Scholar]
- 2.Ito J, Asal R, Nishiyama H. Org. Lett. 2010;12:3860–3862. doi: 10.1021/ol1015338. [DOI] [PubMed] [Google Scholar]
- 3.de Vries JG, Elsevier CJ, editors. Handbook of Homogenous Hydrogenation. Vol. 1. Weinheim: Wiley-VCH; 2007. pp. 375–409. [Google Scholar]
- 4.(a) Lindlar H, Dubuis R. Org. Synth. 1973;5:880–883. [Google Scholar]; (b) Bridier B, López N, Pérez-Ramírez J. Dalton Trans. 2010;39:8412–8419. doi: 10.1039/c0dt00010h. [DOI] [PubMed] [Google Scholar]
- 5.(a) Trost BM, Livingston RC. J. Am. Chem. Soc. 1995;117:9586–9587. [Google Scholar]; (b) Trost BM, Livingston RC. J. Am. Chem. Soc. 2008;130:11970–11978. doi: 10.1021/ja804105m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arcadi A, Bernocchi E, Burini A, Cacchi S, Marinelli F, Pietroni B. Tetrahedron. 1988;44:481–490. [Google Scholar]
- 7.(a) Kwon MS, Kim N, Park CM, Lee JS, Kang KY, Park J. Org. Lett. 2005;7:1077–1079. doi: 10.1021/ol047381w. [DOI] [PubMed] [Google Scholar]; (b) Zheng J, Lin S, Zhu X, Jiang B, Yang Z, Pan Z. Chem. Commun. 2012;48:6235–6237. doi: 10.1039/c2cc31948a. [DOI] [PubMed] [Google Scholar]
- 8.Ide MS, Hao B, Neurock M, Davis RJ. ACS Catal. 2012;2:671–683. [Google Scholar]
- 9.Sadegmoghaddam E, Lam C, Choi D, Shon Y-S. J. Mater. Chem. 2011;21:307–312. [Google Scholar]
- 10.Sadegmoghaddam E, Gaïeb K, Shon Y-S. Appl. Catal., A. 2011;405:137–141. [Google Scholar]
- 11.Gavia DJ, Shon Y-S. Langmuir. 2012;28:14502–14508. doi: 10.1021/la302653u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.(a) Crespo-Quesada M, Cárdenas-Lizana F, Dessimoz A-L, Kiwi-Minsker L. ACS Catal. 2012;2:1773–1786. [Google Scholar]; (b) Crespo-Quesada M, Yarulin A, Jin M, Xia Y, Kiwi-Minsker L. J. Am. Chem. Soc. 2011;133:12787–12794. doi: 10.1021/ja204557m. [DOI] [PubMed] [Google Scholar]
- 13.(a) van Laren MW, Elsevier CJ. Angew. Chem. Int. Ed. 1999;38:3715–3717. doi: 10.1002/(sici)1521-3773(19991216)38:24<3715::aid-anie3715>3.3.co;2-f. [DOI] [PubMed] [Google Scholar]; (b) Spee MPR, Boersma J, Meijer MD, Slagt MQ, van Koten G, Geus JW. J. Org. Chem. 2001;66:1647–1656. doi: 10.1021/jo001246p. [DOI] [PubMed] [Google Scholar]
- 14.Hostetler MJ, Wingate JE, Zhong C-J, Harris JE, Vachet RW, Clark MR, Londono JD, Green SJ, Stokes JJ, Wignall GD, Glish GL, Porter MD, Evans ND, Murray RW. Langmuir. 1998;14:17–30. [Google Scholar]
- 15.Sadegmoghaddam E, Gu H, Shon Y-S. ACS Catal. 2012;2:1838–1845. doi: 10.1021/cs300270d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Molnar A, Sarkany A, Varga M. J. Mol. Catal. A: Chem. 2001;173:185. [Google Scholar]
- 17.Crespo-Quesada M, Dykeman RR, Laurenczy G, Dyson PJ, Kiwi-Minsker L. J. Catal. 2011;279:66. [Google Scholar]
- 18.(a) Bhattacharjee S, Bruening ML. Langmuir. 2008;24:2916–2920. doi: 10.1021/la703055d. [DOI] [PubMed] [Google Scholar]; (b) Kidambi S, Dai J, Li J, Bruening ML. J. Am. Chem. Soc. 2004;126:2658–2659. doi: 10.1021/ja038804c. [DOI] [PubMed] [Google Scholar]