Abstract
Aims
Therapy-related consequences of treatment for type 1 diabetes mellitus (T1DM), such as weight gain and hypoglycaemia, act as a barrier to attaining optimal glycaemic control, indirectly influencing the incidence of vascular complications and associated morbidity and mortality. This study quantifies the individual and combined contribution of changes in hypoglycaemia frequency, weight and HbA1c to predicted quality-adjusted life-years (QALYs) within a T1DM population.
Materials and methods
We describe the Cardiff Type 1 Diabetes (CT1DM) Model, originally informed by the Diabetes Control and Complications Trial (DCCT) and updated with the Epidemiology of Diabetes Interventions and Complications (EDIC) study and Swedish National Diabetes Registry for microvascular and cardiovascular complications respectively. We report model validation results and the QALY impact of HbA1c, weight and hypoglycaemia changes.
Results
Validation results demonstrated coefficients of determination for clinical endpoints of R2 = 0.863 (internal R2 = 0.999; external R2 = 0.823), costs R2 = 0.980 and QALYs R2 = 0.951. Achieving and maintaining a 1% HbA1c reduction was estimated to provide 0.61 additional discounted QALYs. Weight changes of ±1kg, ±2kg or ±3kg led to discounted QALY changes of ±0.03, ±0.07 and ±0.10 respectively, while modifying hypoglycaemia frequency by -10%, -20% or -30% resulted in changes of -0.05, -0.11 and -0.17. The differences in discounted costs, life-years and QALYs associated with HbA1c 6% versus 10% were -£19,037, 2.49 and 2.35 respectively.
Conclusions
Using a model updated with contemporary epidemiological data, this study presents an outcome-focused perspective to assessing the health economic consequences of differing levels of glycaemic control in T1DM with and without weight and hypoglycaemia effects.
Introduction
Type 1 diabetes mellitus (T1DM) is a chronic autoimmune disorder associated with significant excess morbidity and mortality [1, 2]. It is estimated that 8.5% of those diagnosed with diabetes in the UK have T1DM; equating to 284,405 people and representing a 0.4% prevalence rate [3].
Treatment of T1DM typically requires multiple daily injections of insulin with therapeutic guidelines advocating the use of patient optimised management strategies and individualised targets [4]. However, despite such guidelines, fewer than 30% of UK T1DM adults reach treatment targets for glucose control, with the disease reducing adult life expectancy in the UK by approximately 13 years [5].
Therapy-related consequences of treatment, such as weight gain and hypoglycaemia are known to act as a potential barrier to attaining optimal glycaemic control [6] and may therefore indirectly influence the incidence of vascular complications. Furthermore, the independent impact of hypoglycaemia and weight gain upon quality of life has been well documented [7–9]. Consequently, changes in HbA1c, weight and the frequency of hypoglycaemia are important, inter-related determinants of the cost effectiveness of therapeutic interventions. This is of particular relevance to the management of T1DM as the risk of recurrent hypoglycaemia in insulin treated patients is high [10] and the prevalence of obesity amongst T1DM patients has increased significantly over recent years [11].
Previous health economic analysis has characterised the relative impact of weight change, hypoglycaemia frequency and unit changes in HbA1c upon predicted quality-adjusted life years (QALYs) in type 2 diabetes mellitus [12]; however, such analyses have not been undertaken in T1DM. Consequently, the principle objective of this study was to quantify the individual and combined contribution of changes in hypoglycaemia frequency, weight and HbA1c to predicted quality-adjusted life years (QALYs) in a T1DM population. A secondary objective was to quantify the health economic value associated with improvements in glycaemic control in T1DM.
Materials and Methods
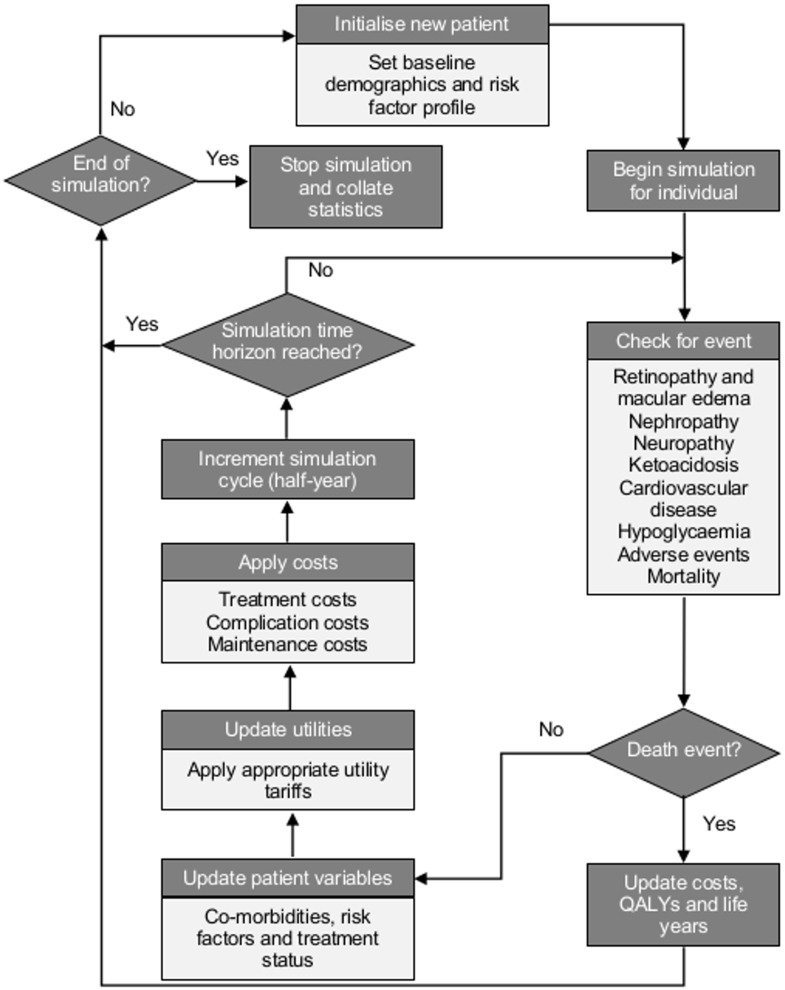
The Cardiff Type 1 Diabetes (CT1DM) Model is a fixed-time-increment stochastic simulation model designed to evaluate the lifetime impact of therapeutic changes on individual simulated patients. The model was originally designed in 2009 and based on the original CORE T1DM model [13] with disease progression data being predominantly drawn from the Diabetes Control and Complications Trial (DCCT) [1] for microvascular complications and Framingham [14] for cardiovascular complications. Consistent with both established and recently published T1DM models [15]] the model has been updated to include long-term epidemiological evidence from the DCCT follow-up study—the Epidemiology of Diabetes Interventions and Complications (EDIC) [2] study and also the T1DM specific Swedish National Diabetes Registry [16] cardiovascular risk equations. Fig 1 shows the model’s flow diagram.
Fig 1. Flow diagram of the Cardiff Type 1 Diabetes Model simulation process.
Microvascular event rates
The estimation of transition probabilities for microvascular health states was undertaken using a similar approach to that reported by Lung et al. [17] in which time-dependent parametric Weibull regression equations were fitted to cumulative incidence data from DCCT and EDIC.
Specifically, regression models were fitted to cumulative incidence of retinopathy and macular edema using EDIC [18] with simulated patients capable of progressing from no retinopathy to background diabetic retinopathy (BDR), to proliferative diabetic retinopathy (PDR) and to severe vision loss (SVL) with macular edema a separate health state associated with increased risk of SVL.
Patients can progress from no nephropathy to micro-albuminuria [19], from which they may either return to no nephropathy or progress to macro-albuminuria with or without impaired glomerular filtration rate (GFR) and finally to end stage renal disease (ESRD) [20]. Upon progression to ESRD patients can receive transplant [21], experience graft failure and return to dialysis [22], or die either whilst receiving dialysis [23] or from the functioning graft health state [22].
Patients may progress from no neuropathy to diabetic peripheral neuropathy [24] with rates controlling progression to foot ulcers, deep foot infections, amputations and event specific mortality taken from a previously published Markov model [25]. Specific details of the rates and Weibull regression models used to control simulated patients thought the various microvascular health states together with the risk factor variables that influence the likelihood of progression are detailed in Appendix 1.
Cardiovascular disease
Modelling cardiovascular disease (CVD) was implemented using an equation derived from the Swedish National Diabetes Registry in which 3,661 subjects with T1DM were followed up for five years [16]. Risk of CVD (defined as fatal or non-fatal myocardial infarction or stroke events) from this study was significantly associated with age, duration of T1DM, total cholesterol to high-density lipoprotein (HDL) cholesterol ratio, systolic blood pressure (SBP) and the following binary variables: current smoker, presence of macroalbuminuria and a history of prior CVD. The Swedish Equation for CVD does not partition events into fatal or non-fatal; consequently we assume a fixed proportion (39.19%) are fatal [26].
Hypoglycaemia
Hypoglycaemia is modelled utilising therapy-related event rates, categorised as a daytime or nocturnal non-severe hypoglycaemic event (NSHE) or as a severe hypoglycaemic event (SHE). The occurrence of an event can be associated with a cost [27] and a decrease in quality of life [7].
Ketoacidosis
Ketoacidosis is modelled as an acute event health state. Patients may have a ketoacidosis event during any cycle of the modelled time horizon and may have multiple events over a lifetime. The rate of ketoacidosis incidence applied in the model is 1.585 per 100,000 persons and is taken from the Swedish study by Wang et al. [28].
Baseline characteristics and time-dependent risk factors
The default baseline characteristics used by the model are detailed in Table 1 and are consistent with profiles reported in the recent guideline for T1DM issued by the National Institute for Health and Care Excellence (NICE) [4]. The likelihood of clinical events is influenced by a number of risk factors that are time-dependent and, consistent with other models [13], we assume in the absence of any specific intervention that HbA1c will increase annually by 0.045% [29]. Furthermore, while the model has the capability of allowing other modifiable risk factors to change over time we hold weight, blood pressure and lipid parameters constant with respect to time for this study.
Table 1. Baseline cohort characteristics and model inputs.
| Variable | Mean | Standard Deviation | Source | ||||
| Age (years) | 42.98 | 19.14 | [4] | ||||
| Duration (years) | 16.92 | 13.31 | |||||
| Proportion male | 0.57 | - | |||||
| HbA1c (%) | 8.60 | 4.00 | |||||
| SBP(mmHg) | 128.27 | 16.07 | |||||
| DBP(mmHg) | 73.55 | 15.25 | [30] | ||||
| Total-C (mg/dL) | 176.50 | 33 | [4] | ||||
| HDL-C (mg/dL) | 50.25 | 13 | |||||
| BMI (kg/m2) | 27.09 | 5.77 | |||||
| Proportion smoker | 0.22 | . | |||||
| NSHE | 29 | 6.48 | [31] | ||||
| SHE | 0.46 | 0.064 | |||||
| Event | Utility Decrement | Source | Event Cost (£) | SE | Maintenance Cost (£) | SE | Source |
| Baseline | 0.810 | [32] | - | - | - | - | - |
| CVD (non-fatal) | -0.076† | [32, 33] | 4688.69 | 468.87 | 585.75 | 58.57 | [4] |
| CVD (fatal) | - | 3824.34 | 382.43 | ||||
| BDR | -* | [34] | - | - | - | - | |
| PDR | -0.086** | - | - | - | - | ||
| Severe vision loss | -0.185*** | 5585 | 558.5 | 5396 | 540 | ||
| Macular edema | - | Assumed | - | - | - | - | |
| Micro-albuminuria | - | Assumed | - | - | - | - | |
| Macro-albuminuria | -0.017‡ | [35] | - | - | - | - | |
| Impaired GFR | -0.017 | Assumed | |||||
| Dialysis | -0.330 | [32] | 30480 | 3048 | 30480 | 3048 | [4] |
| Transplant | -0.076 | [36] | 20373 | 2037.3 | 7609 | 760.9 | |
| Neuropathy | -0.055 | [35] | 361.6 | 36.16 | 361.6 | 36.16 | |
| Ketoacidosis | - | Assumed | 952 | 95.2 | - | - | |
| PVD | - | Assumed | |||||
| Uncomplicated FU | -0.083✶ | [37] | 4070 | 407 | 5483 | 54.83 | [4] |
| Deep foot infection | -0.083 | Assumed | 7328 | 732.8 | 7328 | 732.8 | |
| FU/critical ischaemia | -0.083 | Assumed | 10336 | 1.033.60 | 10336 | 1036.6 | |
| Minor amputation | -0.116ⅎ | [35] | 11290 | 1129 | 11290 | 1129 | |
| Major amputation | -0.116 | 11290 | 1129 | 11290 | 1129 | ||
| NSHE₸ | -0.014 | [7] | - | - | - | - | |
| SHE₸ | -0.047 | 333 | - | - | - | [27] | |
| BMI | -0.006 | [38] | - | - | - | - | [4] |
| Hyperlipidaemia | 38.22 - | 3.82 | 38.22 | 3.82 | |||
| ACE inhibitor therapy | 18.54 | 1.85 | 18.54 | 1.85 | |||
ACE: angiotensin-converting-enzyme; BDR: background diabetic retinopathy; BMI: body mass index; C: cholesterol; CVD: cardiovascular disease; DBP: diastolic blood pressure; FU: foot ulcer; GFR: glomerular filtration rate; HbA1c: haemoglobin A1c; HDL: high-density lipoprotein; NSHE: nocturnal non-severe hypoglycaemic event; PDR: proliferative diabetic retinopathy; PVD: peripheral vascular disease; SBP: systolic blood pressure; SHE: severe hypoglycaemic event.
† CVD was calculated as 60% MI, 32% angina and 8% stroke, where a utility decrement of 0.06 for MI and 0.22 for stroke were taken from Lung et al. A utility decrement of 0.07 for angina was taken from Lee et al.
* BDR taken as a 6/6–6/9 vision on the visual acuity scale.
** PDR taken as a 6/12–6/18 vision on the visual acuity scale.
*** Severe vision loss taken as 6/60–6/120 vision on the visual acuity scale.
‡ value was taken as diabetic kidney disease.
✶ value was taken as a generic ulcer, assumed equal for uncomplicated and complicated foot ulcer as well as foot ulcer with critical ischaemia.
ⅎ value taken was for generic amputation, assumed equal for minor and major.
₸ Disutility presented as mean per event although the model implements the regression equations reported in [7] linking frequency and severity of hypoglycaemia to utility via the fear of hypoglycaemia score.
Costs and utilities
The model considers the direct costs associated with the treatment and management of T1DM in addition to the costs associated with complications and adverse events. Complication related costs are partitioned into three components: fatal, non-fatal and maintenance costs. Fatal or non-fatal costs are applied within the cycle in which that event occurred. Maintenance costs for those surviving are applied in all subsequent years until either the subject dies or the simulated time horizon is reached. Table 1 reports UK specific costs (inflated to 2013/14 values using the PSSRU Hospital and Community Health Services (HCHS) index [39].
The occurrence of diabetes-related events is also associated with reduction in quality of life; the utility decrements used by the model are also reported in Table 1. The decrements applied are consistent with the default values used in the CORE Diabetes Model [40] and applied in recent guidelines [4]. Different disutility values may be specified for the year in which the event occurs and the years that follow. The model handles utility decrements for multiple events by applying the individual decrements additively. Disutility values should be entered in to the model as positive values.
Parameter uncertainty
The output from individual patient level simulation models exhibit variability due to first-order uncertainty (random walk) and parameter (second-order) uncertainty. The model consequently simulates cohorts of up to 10,000 individuals to eliminate first-order uncertainty and the assessment of parameter uncertainty is undertaken by repeatedly simulating cohorts (up to 1,000) with input parameters for each cohort sampled from either normal (patient specific), gamma (costs) or beta (utilities) distributions. Due to the lack of published covariance information, sampled input data are independently generated. Uncertainty is quantified via cost-effectiveness acceptability curves and incremental cost-effectiveness ratio (ICER) scatterplots.
Model validation
The availability of candidate external validation studies suitable for assessing the model’s predictive performance are relatively limited in T1DM; principally due to the key sources of epidemiological data (DCCT/EDIC) forming the basis of the model’s disease progression rates. The Supplementary Material (S1 Appendix) contains verification of the internal validation of the model’s equations to source data; we also present internal validation results for the model’s endpoint predictions when these equations are utilised within the model. To assess the external consistency of the model’s predictions we assessed the clinical events, costs and QALYs predicted by the model with a number of other new and established T1DM models; in particular: the Sheffield patient level simulation model [41]; the CRC discrete event simulation [42, 43]; the Treeage based model from McQueen et al. [44]; the patient level CORE Diabetes simulation model [45–48] and the patient-level simulation described by Wolowacz et al. [49]
In each case the CT1DM Model was initiated with baseline cohort, cost and health utility profiles consistent with those reported or cited in each publication and model output compared over the relevant time horizons. When comparing output, a number of candidate statistical tests for comparing model output with observed outcomes exist; however, there is little consensus upon the best approach [31]. Formal hypothesis testing is complicated by the fact that the disease model we are seeking to evaluate is only an approximation to the actual disease; consequently testing the null hypothesis of no difference between the validation study observation and model predictions makes little sense. However, to understand where model fit was poor, we also assessed goodness of fit between predicted (C1TDM Model) and observed (internal validation study endpoints and endpoints, costs and QALYs from other T1DM models) using the mean absolute percentage error (MAPE). These were calculated by comparing X (the predicted output) with Y (observed output): X1, X2, …, Xn and Y1, Y2, …, Yn where n is the sample size (the number of validation endpoints). We define the residuals Z as the paired difference between the two sets of results (predicted and observed): Z = Y − X, i = 1, 2, …, n. Calculation of the MAPE was computed using:
Finally, and consistent with other validation studies published in the health economic literature, we present scatterplots of observed versus predicted endpoints along with the coefficient of determination (R2).
Analysis
The analysis undertaken for this study utilised a simulated cohort of 10,000 individuals modelled over a 80 year time horizon. To ensure convergence of results each simulated cohort was replicated 1,000 times with all summary statistics and measures of precision relating to the mean and standard error of these 1,000 replicates. The model was initialised with a population profile consistent with recently published UK based clinical guidelines [4] as presented in Table 1; rates of hypoglycaemia were taken from the UK Hypoglycaemia Study Group [31]. Costs and health utility decrements associated with macro- and microvascular complications, hypoglycaemia and weight change were sourced from the published literature, as indicated in Table 1.
The model was used firstly to evaluate the benefit (measured by a change in predicted QALYs) associated with a 1% improvement in HbA1c. Subsequently, the following treatment related changes were applied to the baseline cohort profile: NSHE rates were modified by ±10%, ±20% or ±30%); weight was then modified by ±1kg, ±2kg or ±3kg). These changes were evaluated singularly and in combination. All changes were applied over the first 6 months and maintained for the patient’s lifetime; total and incremental QALYs evaluated over a 80-year horizon and discounted at 3.5% annually.
Secondly, the model was used to evaluate the impact of unit (%) changes in HbA1c on per-patient cost savings, QALY gains and therefore the health economic value (defined as the amount of additional spend (£) justified to obtain the additional QALY gain incorporating costs savings predicted for each unit reduction in HbA1c using a willingness-to-pay threshold of £20,000).
Results
Validation
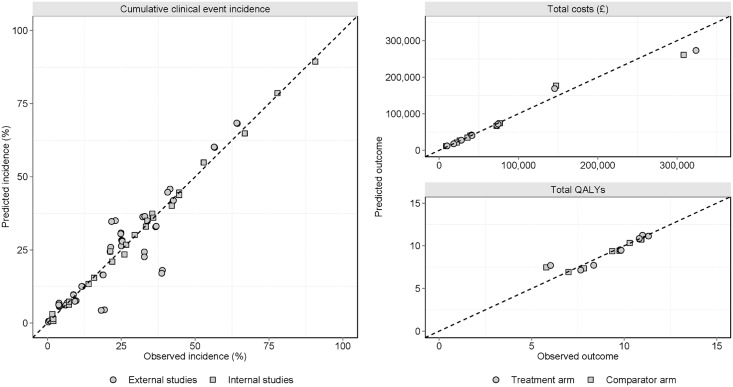
Observed versus predicted validation endpoints, costs and QALYs are presented graphically in Fig 2. Overall, the validation coefficient of determination for clinical endpoints was R2 = 0.863 (internal R2 = 0.999; external R2 = 0.823) and total costs R2 = 0.979; total QALYs R2 = 0.951. Regression analysis indicated that endpoint predictions and costs had non-significant intercept terms (p = 0.009 and p = 0.652 respectively) indicating no systematic over or under-prediction. MAPE to predicted endpoints was 135.6% overall (11.3% internal and 213.6% external); MAPE for total costs was 26.0% and total QALYs was 21.0%.
Fig 2. Model validation.
Observed versus predicted validation endpoints (internal and external) and validation to published T1DM model output (costs and quality adjusted life years). Overall validation coefficient of determination for clinical endpoints, R2 = 0.863; internal R2 = 0.999; external R2 = 0.823; total costs R2 = 0.979; total QALYs R2 = 0.951.
QALY gains associated with weight and hypoglycaemia
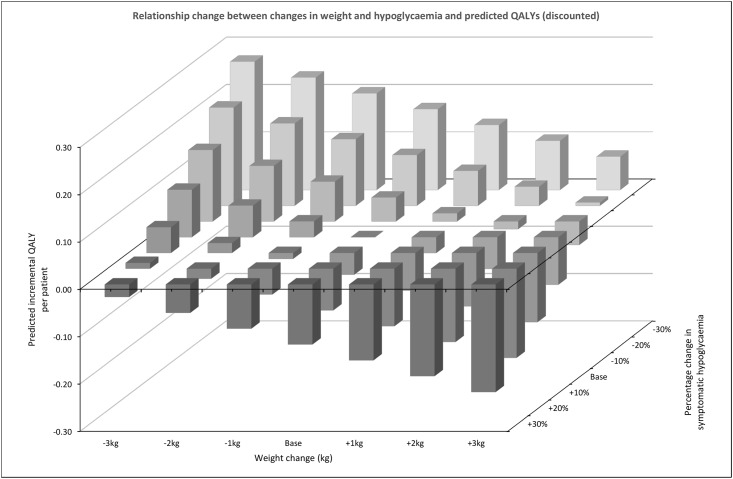
Running the simulation model with the baseline cohort profiles specified in Table 1 resulted in a mean predicted life expectancy of 69.25 (conditional upon a start age of 42.98) representing 26.3 and 16.0 additional undiscounted life years and QALYs (16.8 and 10.4 discounted life years and QALYs respectively). Achieving and maintaining a 1% reduction in HbA1c was associated with an estimated gain of 0.64 and 0.61 discounted life years and QALYs respectively (the similarity in life years and QALY gains is driven principally by discounting). Changes in weight by ±1kg, ±2kg or ±3kg were associated with changes in discounted QALYs of ±0.03, ±0.07 and ±0.10 respectively. Modifying hypoglycaemia frequency by -10%, -20% or -30% resulted in changes to discounted QALYs of +0.05, +0.11 and +0.17 respectively. Modifying hypoglycaemia frequency by +10%, +20% or +30% resulted in changes to discounted QALYs of -0.05, -0.09 and -0.13 respectively. The combined effect of increasing weight by 3kg and a 30% increase in the frequency of hypoglycaemia reduced quality-adjusted life expectancy by 0.23, see Fig 3.
Fig 3. Weight and hypoglycaemia QALY plot.
Assessing the impact of changes in weight and rates of hypoglycaemia events on per-patient lifetime quality-adjusted life year (QALY) difference. The reference point relates to a 1% reduction in HbA1c (%) with no associated changes in weight or hypoglycaemia, which was associated with a predicted QALY gains of 0.99. This figure illustrates the relative impact of weight change ±3 kg and hypoglycaemia changes ±30% on the QALY gained, beyond those already seen with the reference point.
The health economic value of improving glycaemic control
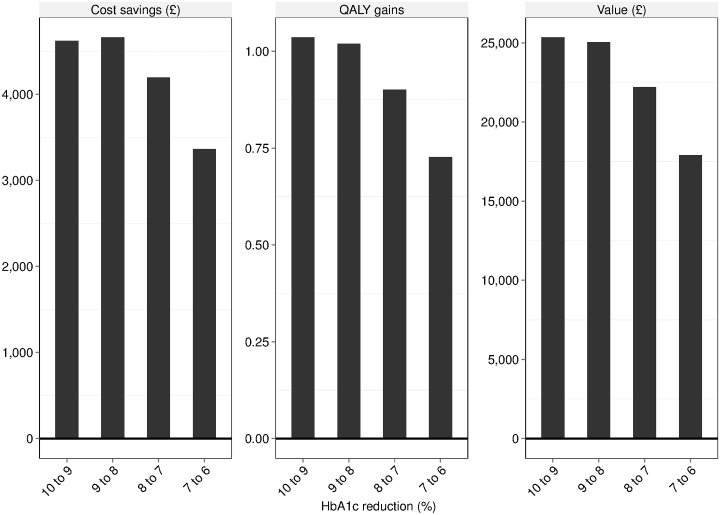
When initiated with cohort profiles specified in Table 1 the model estimates undiscounted lifetime per-patient costs of £72,586, 23.9 life-years and 14.0 QALYs (discounted values: £37,377; life expectancy of 15.8 years and QALYs of 9.5) at an HbA1c of 10%. When contrasted with the maintenance of HbA1c at 6% total undiscounted per-patient cost reduces to £39,508 (Δ = £33,078), 30.2 life years (Δ = 6.3) and 19.3 QALYs (Δ = 5.3). Discounted per-patient values were £18,340 (Δ = £19,037), 18.3 life years (Δ = 2.5) and 11.8 QALYs (Δ = 2.3). Fig 4 illustrates the impact of unit (%) changes in HbA1c on discounted per-patient cost savings, QALY gains and value; this plots highlights the greatest expected impact on costs, QALYs and consequently value is achieved with HbA1c reductions from 10% to 9% and 9%-8%.
Fig 4. Health economic value associated with various levels of glucose control.
Assessing the impact of unit (%) changes in HbA1c on per-patient cost savings, QALY gains and health economic value (defined as the amount of additional spend (£) justified to obtain the additional QALY gain predicted for each unit reduction in HbA1c at a willingness-to-pay threshold of £20, 000.
Discussion
The availability of long-term follow-up data from the original DCCT cohort in addition to the publication of T1DM specific cardiovascular risk equations has resulted in a renewed interest in the development and updating of T1DM health economic simulation models. The CT1DM Model was initially based on the original CORE Diabetes Model and the update described in this study is methodologically consistent with the approach taken by other modelling groups [17, 49, 50]. Consequently, the model is structurally consistent with other T1DM health economic models [15]. The model includes data drawn principally from the DCCT/EDIC and the Swedish National Diabetes Registry and the validation analysis presented indicates the model provides consistent predictions to both validation endpoints and cost-effectiveness output reported by other Type 1 diabetes models. The analysis presented in this study is also consistent with the research undertaken using the Cardiff Type 2 Diabetes Model in which key health economic issues related to diabetes have been assessed; for example, assessing the impact of risk factor changes on costs and outcomes [51]; the cost-effectiveness of treatment strategies at a population level [52]; the relative impact of weight, hypoglycaemia and HbA1c changes upon predicted QALYs [12] and the impact of variance reduction techniques on computation time [53].
Consistent with results reported in type 2 diabetes [12] this study highlights that the beneficial effects of improved glycaemic control on QALYs, achieved through the avoidance of diabetes-related complications, may be offset by characteristic treatment-specific adverse effects, such as weight gain and hypoglycaemia. The comparative weight and hypoglycaemic profiles of available therapies are therefore key to both their cost-effectiveness and effectiveness in clinical practice. Importantly, our evaluation is independent of any specific treatment and sought to quantify the value associated with attributes pertinent to the management of glucose control in T1DM, in particular, hypoglycaemia and weight change. We believe this to be an important consideration as it defines health economic value that is tailored to the patient profile rather than a specific therapeutic profile. As such, the health economic approach adopted here supports the ethos of personalized care and circumvents a key methodological challenge when evaluating competing technologies in T1DM; namely the synthesis of data across structurally heterogeneous clinical trials. For example, differing dose-titration algorithms, definitions of hypoglycaemia, variation in target levels and the number/timing of targets (for example, fasting blood glucose and/or post-prandial glucose) result in treatment effects that are specific to each individual trial. Our focus in this study seeks to provide an assessment of the health economic value of glucose lowering within the context of changes in hypoglycaemia frequency and weight change regardless of any specific particular intervention. We believe this approach offers an important complementary benefit over conventional analyses in that it seeks to quantify health economic value from the perspective of clinical outcomes achieved rather than an assessment of clinical and economic inputs.
Consistent with most modelling studies there are inevitably a number of important limitations with our model. We do not currently model recurrent CVD, which is reflective of a lack of relevant epidemiological data. However, it is unlikely that this omission will significantly influence the models results as the incidence of recurrent CVD in a type 2 diabetes population aged 40–97 years has been documented at a relatively low rate of 6% per year [54]. This has also been evaluated within the context of cost effectiveness in which the omission of subsequent events had no material impact upon predicted incremental cost effectiveness [55].
A further limitation relates to the analysis undertaken rather than the structural design of the model. While the model is capable of predicting time-dependent risk factor trajectories the analysis presented in this study has not sought to incorporate this feature. Our motivation for this was to ensure that we were able to present the marginal contributions to changes in costs and QALYs associated with changes in glycaemic control, weight and hypoglycaemia with all other factors held constant. Our analysis has sought to characterise the inter-relationship between changes in weight, hypoglycaemia and HbA1c and their individual impact upon life years and QALYs. In this application hypoglycaemia and weight are principally impacting health utility, while changes in HbA1c modifies the risk of complications and therefore influences both QALYS and life expectancy. A limitation of this analysis is that we do not quantify the inter-relationship between weight and blood pressure or cholesterol and therefore it is likely that the benefits of weight loss (or avoiding weight gain) are underestimated. Consequently, the interpretation of the analysis presented here should take this limitation into consideration, particularly as multifactorial risk factor management is a matter of routine clinical practice.
To conclude, we have presented an outcome-focused perspective to assessing the health economic consequences of differing levels of glycaemic control in T1DM with and without the effects of weight change and hypoglycaemia. The model reported uses contemporary data that enables the impact of a variety of risk factor management strategies on cost and outcomes to be assessed. Given the particular challenge that exists with respect to achieving optimal glucose control within the context of weight gain and hypoglycaemia acting as potential barriers this model provides an addition decision support tool for those seeking to ensure that current therapeutic approaches to the management of T1DM represent value for money.
Supporting Information
(DOCX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Funding for the study has been provided by AstraZeneca plc; no other funding sources were received for this study. The funder provided support in the form of salaries for authors JP and KB, but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific roles of these authors are articulated in the ‘author contributions’ section. PM, HB and JF are employed by HEOR Ltd., which received funding support in the form of a grant from AstraZeneca plc. HEOR Ltd. provided support in the form of salaries for authors PM, HB and JF, but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the Response to Reviewers and cover letter manuscript. The specific roles of these authors are articulated in the ‘author contributions’ section.
References
- 1.Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin dependent diabetes mellitus. The New England journal of medicine. 1993;329(14):977–86. [DOI] [PubMed] [Google Scholar]
- 2.Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. The New England journal of medicine. 2005;353(25):2643–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holman N, Young B, Gadsby R. Current prevalence of Type 1 and Type 2 diabetes in adults and children in the UK. Diabetic medicine: a journal of the British Diabetic Association. 2015;32(9):1119–20. [DOI] [PubMed] [Google Scholar]
- 4.National Institute for Health and Care Excellence. NICE guidelines [NG17]. Type 1 diabetes in adults: diagnosis and management 2015. Available from: https://www.nice.org.uk/guidance/ng17. [PubMed]
- 5.Amiel SA, Pursey N, Higgins B, Dawoud D. Diagnosis and management of type 1 diabetes in adults: summary of updated NICE guidance. BMJ. 2015;351. [DOI] [PubMed] [Google Scholar]
- 6.Snoek FJ. Barriers to good glycaemic control: the patient's perspective. International journal of obesity and related metabolic disorders: journal of the International Association for the Study of Obesity. 2000;24 Suppl 3:S12–20. [DOI] [PubMed] [Google Scholar]
- 7.Currie CJ, Morgan CL, Poole CD, Sharplin P, Lammert M, McEwan P. Multivariate models of health-related utility and the fear of hypoglycaemia in people with diabetes. Current Medical Research and Opinion. 2006;22(8):1523–34. [DOI] [PubMed] [Google Scholar]
- 8.Lauridsen JT, Lonborg J, Gundgaard J, Jensen HH. Diminishing marginal disutility of hypoglycaemic events: results from a time trade-off survey in five countries. Quality of life research: an international journal of quality of life aspects of treatment, care and rehabilitation. 2014;23(9):2645–50. [DOI] [PubMed] [Google Scholar]
- 9.Dennett SL, Boye KS, Yurgin NR. The impact of body weight on patient utilities with or without type 2 diabetes: a review of the medical literature. Value in health: the journal of the International Society for Pharmacoeconomics and Outcomes Research. 2008;11(3):478–86. [DOI] [PubMed] [Google Scholar]
- 10.Cryer PE. Hypoglycemia in Type 1 Diabetes Mellitus. Endocrinology and metabolism clinics of North America. 2010;39(3):641–54. 10.1016/j.ecl.2010.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conway B, Miller RG, Costacou T, Fried L, Kelsey S, Evans RW, et al. Temporal patterns in overweight and obesity in Type 1 diabetes. Diabetic medicine: a journal of the British Diabetic Association. 2010;27(4):398–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McEwan P, Evans M, Kan H, Bergenheim K. Understanding the inter-relationship between improved glycaemic control, hypoglycaemia and weight change within a long-term economic model. Diabetes, obesity & metabolism. 2010;12(5):431–6. [DOI] [PubMed] [Google Scholar]
- 13.Palmer AJ, Roze S, Valentine WJ, Minshall ME, Foos V, Lurati FM, et al. The CORE Diabetes Model: projecting long-term clinical outcomes, costs and costeffectiveness of interventions in diabetes mellitus (types 1 and 2) to support clinical and reimbursement decision-making. Current Medical Research and Opinion. 2004;20(S1):S5–S26. [DOI] [PubMed] [Google Scholar]
- 14.Anderson KM, Odell PM, Wilson PW, Kannel WB. Cardiovascular disease risk profiles. American heart journal. 1991;121(1 Pt 2):293–8. [DOI] [PubMed] [Google Scholar]
- 15.Henriksson M, Jindal R, Sternhufvud C, Bergenheim K, Sorstadius E, Willis M. A Systematic Review of Cost-Effectiveness Models in Type 1 Diabetes Mellitus. Pharmacoeconomics. 2016;34(6):569–85. 10.1007/s40273-015-0374-8 [DOI] [PubMed] [Google Scholar]
- 16.Cederholm J, Eeg-Olofsson K, Eliasson B, Zethelius B, Gudbjornsdottir S. A new model for 5-year risk of cardiovascular disease in Type 1 diabetes; from the Swedish National Diabetes Register (NDR). Diabetic medicine: a journal of the British Diabetic Association. 2011;28(10):1213–20. [DOI] [PubMed] [Google Scholar]
- 17.Lung TW, Clarke PM, Hayes AJ, Stevens RJ, Farmer A. Simulating lifetime outcomes associated with complications for people with type 1 diabetes. Pharmacoeconomics. 2013;31(6):509–18. 10.1007/s40273-013-0047-4 [DOI] [PubMed] [Google Scholar]
- 18.Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research G. Effect of intensive diabetes therapy on the progression of diabetic retinopathy in patients with type 1 diabetes: 18 years of follow-up in the DCCT/EDIC. Diabetes. 2015;64(2):631–42. 10.2337/db14-0930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Boer IH. Kidney disease and related findings in the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes care. 2014;37(1):24–30. 10.2337/dc13-2113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Boer IH, Rue TC, Cleary PA, Lachin JM, Molitch ME, Steffes MW, et al. Long-term renal outcomes of patients with type 1 diabetes mellitus and microalbuminuria: an analysis of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications cohort. Archives of internal medicine. 2011;171(5):412–20. 10.1001/archinternmed.2011.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gilg J, Pruthi R, Fogarty D. UK Renal Registry 17th Annual Report: Chapter 1 UK Renal Replacement Therapy Incidence in 2013: National and Centre-specific Analyses. Nephron. 2015;129 Suppl 1:1–29. 10.1159/000370271 [DOI] [PubMed] [Google Scholar]
- 22.Taber DJ, Meadows HB, Pilch NA, Chavin KD, Baliga PK, Egede LE. Pre‐existing diabetes significantly increases the risk of graft failure and mortality following renal transplantation. Clinical transplantation. 2013;27(2):274–82. 10.1111/ctr.12080 [DOI] [PubMed] [Google Scholar]
- 23.Steenkamp R, Rao A, Roderick P. UK Renal Registry 17th Annual Report: Chapter 5 Survival and Cause of Death in UK Adult Patients on Renal Replacement Therapy in 2013: National and Centre-specific Analyses. Nephron. 2015;129 Suppl 1:99–129. 10.1159/000370275 [DOI] [PubMed] [Google Scholar]
- 24.Martin CL, Albers JW, Pop-Busui R. Neuropathy and related findings in the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes Care. 2014;37(1):31–8. 10.2337/dc13-2114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ragnarson Tennvall G, Apelqvist J. Prevention of diabetes-related foot ulcers and amputations: a cost-utility analysis based on Markov model simulations. Diabetologia. 2001;44(11):2077–87. [DOI] [PubMed] [Google Scholar]
- 26.Rawshani A, Svensson A-M, Rosengren A, Eliasson B, Gudbjörnsdottir S. Impact of Socioeconomic Status on Cardiovascular Disease and Mortality in 24,947 Individuals With Type 1 Diabetes. Diabetes care. 2015:dc150145. [DOI] [PubMed] [Google Scholar]
- 27.Hammer M, Lammert M, Mejias SM, Kern W, Frier BM. Costs of managing severe hypoglycaemia in three European countries. J Med Econ. 2009;12(4):281–90. 10.3111/13696990903336597 [DOI] [PubMed] [Google Scholar]
- 28.Wang ZH, Kihl‐Selstam E, Eriksson JW. Ketoacidosis occurs in both Type 1 and Type 2 diabetes—a population‐based study from Northern Sweden. Diabetic Medicine. 2008;25(7):867–70. 10.1111/j.1464-5491.2008.02461.x [DOI] [PubMed] [Google Scholar]
- 29.The absence of a glycemic threshold for the development of long-term complications: the perspective of the Diabetes Control and Complications Trial. Diabetes. 1996;45(10):1289–98. [PubMed] [Google Scholar]
- 30.Saunders SA, Wallymhamed M, Macfarlane IA. Improvements in glycaemic control and cardiovascular risk factors in a cohort of patients with type 1 diabetes over a 5-year period. QJM: monthly journal of the Association of Physicians. 2009;102(1):29–34. 10.1093/qjmed/hcn125 [DOI] [PubMed] [Google Scholar]
- 31.Risk of hypoglycaemia in types 1 and 2 diabetes: effects of treatment modalities and their duration. Diabetologia. 2007;50(6):1140–7. [DOI] [PubMed] [Google Scholar]
- 32.Lung TW, Hayes AJ, Hayen A, Farmer A, Clarke PM. A meta-analysis of health state valuations for people with diabetes: explaining the variation across methods and implications for economic evaluation. Quality of life research: an international journal of quality of life aspects of treatment, care and rehabilitation. 2011;20(10):1669–78. [DOI] [PubMed] [Google Scholar]
- 33.Lee JM, Rhee K, O'Grady M J, Basu A, Winn A, John P, et al. Health utilities for children and adults with type 1 diabetes. Med Care. 2011;49(10):924–31. 10.1097/MLR.0b013e318216592c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lloyd A, Nafees B, Gavriel S, Rousculp MD, Boye KS, Ahmad A. Health utility values associated with diabetic retinopathy. Diabetic Medicine. 2008;25(5):618–24. 10.1111/j.1464-5491.2008.02430.x [DOI] [PubMed] [Google Scholar]
- 35.Coffey JT, Brandle M, Zhou H, Marriott D, Burke R, Tabaei BP, et al. Valuing health-related quality of life in diabetes. Diabetes Care. 2002;25(12):2238–43. [DOI] [PubMed] [Google Scholar]
- 36.Kiberd BA, Jindal KK. Screening to prevent renal failure in insulin dependent diabetic patients: an economic evaluation. BMJ: British Medical Journal. 1995;311(7020):1595–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Solli O, Stavem K, Kristiansen IS. Health-related quality of life in diabetes: The associations of complications with EQ-5D scores. Health and Quality of Life Outcomes. 2010;8:18 10.1186/1477-7525-8-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bagust A, Beale S. Modelling EuroQol health‐related utility values for diabetic complications from CODE‐2 data. Health Economics. 2005;14(3):217–30. [DOI] [PubMed] [Google Scholar]
- 39.Personal Social Services Research Unit. Unit Costs of Health and Social Care 2014. 2014.
- 40.Beaudet A, Clegg J, Thuresson PO, Lloyd A, McEwan P. Review of utility values for economic modeling in type 2 diabetes. Value in health: the journal of the International Society for Pharmacoeconomics and Outcomes Research. 2014;17(4):462–70. [DOI] [PubMed] [Google Scholar]
- 41.Kruger J, Brennan A, Thokala P, Basarir H, Jacques R, Elliott J, et al. The cost-effectiveness of the Dose Adjustment for Normal Eating (DAFNE) structured education programme: an update using the Sheffield Type 1 Diabetes Policy Model. Diabetic medicine: a journal of the British Diabetic Association. 2013;30(10):1236–44. [DOI] [PubMed] [Google Scholar]
- 42.McEwan P, Poole CD, Tetlow T, Holmes P, Currie CJ. Evaluation of the cost-effectiveness of insulin glargine versus NPH insulin for the treatment of type 2 diabetes in the UK. Current Medical Research and Opinion. 2007;23(sup1):S21–S31. [Google Scholar]
- 43.Pfohl M, Schadlich PK, Dippel FW, Koltermann KC. Health economic evaluation of insulin glargine vs NPH insulin in intensified conventional therapy for type 1 diabetes in Germany. J Med Econ. 2012;15 Suppl 2:14–27. 10.3111/13696998.2012.713879 [DOI] [PubMed] [Google Scholar]
- 44.McQueen RB, Ellis SL, Campbell JD, Nair KV, Sullivan PW. Cost-effectiveness of continuous glucose monitoring and intensive insulin therapy for type 1 diabetes. Cost effectiveness and resource allocation: C/E. 2011;9:13 10.1186/1478-7547-9-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Palmer AJ, Valentine WJ, Ray JA, Foos V, Lurati F, Smith I, et al. An economic assessment of analogue basal-bolus insulin versus human basal-bolus insulin in subjects with type 1 diabetes in the UK. Curr Med Res Opin. 2007;23(4):895–901. [DOI] [PubMed] [Google Scholar]
- 46.Tunis SL, Minshall ME, Conner C, McCormick JI, Kapor J, Yale JF, et al. Cost-effectiveness of insulin detemir compared to NPH insulin for type 1 and type 2 diabetes mellitus in the Canadian payer setting: modeling analysis. Curr Med Res Opin. 2009;25(5):1273–84. 10.1185/03007990902869169 [DOI] [PubMed] [Google Scholar]
- 47.Guillermin AL, Samyshkin Y, Wright D, Nguyen T, Villeneuve J. Modeling the lifetime costs of insulin glargine and insulin detemir in type 1 and type 2 diabetes patients in Canada: a meta-analysis and a cost-minimization analysis. J Med Econ. 2011;14(2):207–16. 10.3111/13696998.2011.561390 [DOI] [PubMed] [Google Scholar]
- 48.Valentine WJ, Aagren M, Haglund M, Ericsson A, Gschwend MH. Evaluation of the long-term cost-effectiveness of insulin detemir compared with neutral protamine hagedorn insulin in patients with type 1 diabetes using a basal-bolus regimen in Sweden. Scandinavian journal of public health. 2011;39(1):79–87. 10.1177/1403494810379290 [DOI] [PubMed] [Google Scholar]
- 49.Wolowacz S, Pearson I, Shannon P, Chubb B, Gundgaard J, Davies M, et al. Development and validation of a cost-utility model for Type 1 diabetes mellitus. Diabetic medicine: a journal of the British Diabetic Association. 2015;32(8):1023–35. [DOI] [PubMed] [Google Scholar]
- 50.Thokala P, Kruger J, Brennan A, Basarir H, Duenas A, Pandor A, et al. Assessing the cost-effectiveness of type 1 diabetes interventions: the Sheffield type 1 diabetes policy model. Diabetic medicine: a journal of the British Diabetic Association. 2014;31(4):477–86. [DOI] [PubMed] [Google Scholar]
- 51.McEwan P, Peters JR, Bergenheim K, Currie CJ. Evaluation of the costs and outcomes from changes in risk factors in type 2 diabetes using the Cardiff stochastic simulation cost-utility model (DiabForecaster). Curr Med Res Opin. 2006;22(1):121–9. [DOI] [PubMed] [Google Scholar]
- 52.McEwan P, Evans M, Bergenheim K. A population model evaluating the costs and benefits associated with different oral treatment strategies in people with type 2 diabetes. Diabetes, obesity & metabolism. 2010;12(7):623–30. [DOI] [PubMed] [Google Scholar]
- 53.McEwan P, Bergenheim K, Yuan Y, Tetlow AP, Gordon JP. Assessing the relationship between computational speed and precision: a case study comparing an interpreted versus compiled programming language using a stochastic simulation model in diabetes care. Pharmacoeconomics. 2010;28(8):665–74. 10.2165/11535350-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 54.Giorda CB, Avogaro A, Maggini M, Lombardo F, Mannucci E, Turco S, et al. Recurrence of cardiovascular events in patients with type 2 diabetes: epidemiology and risk factors. Diabetes Care. 2008;31(11):2154–9. 10.2337/dc08-1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McEwan P, Evans M, Bergenheim K, editors. 1238: Assessing the influence of modelling subsequent cardiovascular events into a type 2 diabetes cost-effectiveness model. 46th EASD Meeting; 2010; Stockholm, Sweden.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.