Abstract
Cognitive impairments are central to schizophrenia, but their clinical utility for tagging heterogeneity in lifetime outcome and response to treatment is not conclusive. By exploiting four cognitive domains consistently showing large deficits in studies, we tested whether cluster analysis would define separate subsets of patients and then whether the disease heterogeneity marked by these clusters would be related to lifetime outcome and response to treatment. A total of 112 schizophrenia patients completed a neuropsychological evaluation. The PANSS, GAF-S and GAF-F were rated at the onset and endpoint of the illness trajectory. A blind judgment of the lifetime response to treatment was made. The first cluster presented near-normal cognitive performance. Two other clusters of severely impaired patients were identified: one generally impaired in the four cognitive domains and another selectively impaired in visual episodic memory and processing speed, each relating to a different lifetime evolution of disease and treatment response. Although the two impaired clusters were clinically indistinguishable in symptom severity and functioning at disease onset, patients with selective cognitive impairments demonstrated better improvement at outcome, whereas the generally impaired patients were more likely to be treatment refractory. The findings have implications for the management of patients and for clinical trials since particular combinations of cognitive deficits in patients would influence their treatment response.
Keywords: Schizophrenia, Heterogeneity, Cognitive profiles, Functioning, Response to treatment
Introduction
Measurable indicators of disease progression or treatment response that can be translated into clinical practice are still scarce in psychiatry [1]. Moreover, etiological heterogeneity is a sturdy obstacle to research progress in schizophrenia [2]. This heterogeneity is manifest in the different trajectories characterizing prognosis in most studies [3–5] that observed four to five different outcome courses characterized by good responders to treatment, moderate or variable improvement and treatment resistance with a poor outcome. These typical outcomes would ideally need to be distinguished early at the disease onset since early efficient treatment is now recognized as a cornerstone to alleviating later disease severity [6].
Despite accumulated knowledge about cognitive deficits in schizophrenia, many questions remain about the capacity of impaired cognition to mark later disease course and response to treatment [7]. Such information might influence initial clinical decisions and recommendations to patients and families. Individual cognitive functions, such as verbal memory or processing speed, have been moderately associated with functional outcome [8–19] or symptomatology [20–23] with correlations usually in the ~0.30 range. Interestingly, a number of studies have attempted to use neurocognition to tag the heterogeneity in schizophrenia [13, 24–30], but few studies have tested the relationship between heterogeneity and lifetime outcome or response to treatment.
The relevance of studying cognitive impairment profiles in relation to outcome relies on at least four pieces of evidence in schizophrenia. First, impairments of different magnitudes are consistently reported in several cognitive domains in patients, with the largest differences from normal controls (effects size, ES, of 0.8–1.2) lying in verbal and visual episodic memory, working memory, processing speed, executive functions and attention [31, 32]. Our own studies produced convergent results [33]. Second, meta-analyses suggest little deterioration of cognition with disease progression since similarly large effect sizes are already present in first-episode patients [32]. Third, children at risk of schizophrenia harbor sizeable cognitive impairments in verbal and visual episodic memory, processing speed and working memory, indicating that the cognitive deterioration emerges a long time before the prodrome [34–40]. Fourth, in search of robust constructs explaining the disease, studies on the cognitive factorial structure consistently showed six to seven separate cognitive factors characterized by verbal memory, visual memory, processing speed, working memory, attention/vigilance and problem-solving/reasoning [41–43]. These factor analyses matched to a large degree the MATRICS consensual battery [44]. However, the extent to which such factor structures may characterize the heterogeneity in clinical trajectories and pharmacological response remains unresolved [7, 41].
The goal of this study was to define separate subgroups of schizophrenia patients based on their cognitive functioning and then tested whether the disease heterogeneity marked by these clusters would be related to lifetime outcome and response to treatment.
To do so, we selected four domains of cognitive impairments, verbal and visual episodic memory, working memory and processing speed, based on the following criteria: (1) large effect sizes in both young and chronic patients, (2) a consistent presence in the factorial structures and (3) an incidence many years before prodrome as shown by the presence of large effect sizes in children at risk. Furthermore, the considerable between-studies heterogeneity found in meta-analyses of cognitive studies [31, 32] also motivated our choice of these four cognitive domains to increase future comparability of our results to other samples.
Methods
Sample
Sample characteristics
The sample consisted of 112 unrelated schizophrenia patients described in Table 1. The inclusion criteria were having a definite DSM-IV schizophrenia diagnosis and having undergone a neuropsychological evaluation before age 55. The exclusion criteria were the following: schizoaffective diagnosis, brain disorder/trauma and metabolic disorders known to cause neuropsychological impairments. The sample consisted of stabilized outpatients referred by their treating psychiatrists in a university hospital or in regional psychiatric departments. Patients were explained about the study, and a signed consent was obtained, as reviewed by our Institutional Ethics Committee.
Table 1.
Characteristics of the sample
| Variables | Total sample (N = 112) | Mean (SD) or n (%) | Statistic F or χ2 | p valueb | |||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Mean (SD) or n (%) | Range | Generally impaired (N = 18) | Selectively impaired (N = 46) | Near-normal functioning (N = 48) | |||
| Age (years) at the time of assessment | 41.1 (9.6) | 20.1–54.9 | 43.3 (9.0) | 42.3 (8.5) | 39.1 (10.5) | 1.92 | 0.15b |
| Gender (male) | 81 (72 %) | – | 7 (39 %) | 36 (78 %) | 38 (79 %) | 11.99 | 0.003c |
| Positive family history (yes) | 67 (61 %) | – | 13 (72 %) | 26 (59 %) | 28 (60 %) | 1.06 | 0.59c |
| Socioeconomic statusd | 25.36 (7.8) | 22.1–59.9 | 23.0 (1.7) | 23.3 (3.4) | 28.4 (11.1) | 4.61 | 0.013b,e |
| Age of onset (years) | 24.6 (6.9) | 12–44 | 22.7 (5.7) | 24.8 (6.8) | 25.1 (7.5) | 0.82 | 0.44b |
| Illness duration (years) | 16.8 (9.3) | 1.2–37.2 | 20.6 (9.4) | 17.5 (9.1) | 14.7 (9.0) | 2.93 | 0.06b |
| Duration | 78/32 | – | 14/4 | 35/11 | 30/18 | 2.39 | 0.30c |
| ≥10 year/< 10 year | 70 %/30 % | 79 %/21 % | 76 %/24 % | 62 %/38 % | |||
| Medication dosea | 21.7 (18.2) | 0–135.2 | 27.9 (16.7) | 22.3 (22.8) | 19.0 (12.8) | 1.29 | 0.28b |
Antipsychotics doses converted to an olanzapine equivalence [56]
p values obtained from one-way ANOVAs with significance levels fixed at 0.05. Analyses were performed using SAS/STAT software, version 9.2, of the SAS System for Windows. Copyright© 2002–2008 SAS Institute Inc
p values obtained from a chi-square (χ2) test
We used the Blishen index [68] according to the highest socioeconomic status of the two parents. This index is based on education and income and on a Canadian census of 514 occupational categories according to the Canadian Classification and Dictionary of Occupations
The “near-normal functioning” group is significantly different from the “generally impaired” and the “selectively impaired” groups for the socioeconomic status (NN > SI, p = 0.007; NN > GI, p = 0.034). The “generally impaired” and the “selectively impaired” clusters have similar socioeconomic status (p = 0.89)
Bold values indicate statistical significant
Diagnostic methods
Our lifetime best-estimate procedure for psychiatric ascertainment of patients was based on information from a direct interview with the subjects and on all available medical records across lifetime according to a previous method showing reliability [45–48] and detailed in Supplemental Methods. The public universal health care in Québec makes available the out- and inpatient medical records across the patient’s life. Based on this information across the whole life, a consensus DSM-IV schizophrenia diagnosis was derived by a panel of four investigators including MM who were blind to previous diagnoses, family history and cognitive measures.
Measures
Cognitive battery
The following cognitive domains were assessed: (1) processing speed—Digit symbol Substitution Task from the WAIS-III and Category Fluency: animal naming; (2) verbal episodic memory—California Verbal Learning Test (CVLT-II) total recall trials 1–5 and delayed recall; (3) visual episodic memory—Rey Complex Figure (RCF) immediate and delayed recall; (4) working memory—Digit span from the WAIS-III and Spatial Span; and (5) executive functioning—Wisconsin Card Sorting Test: total errors and Tower of London (TOLDX): number of problems solved in minimum moves. To compose the cognitive domains, for each subtest, we calculated z scores using data from published standardized norms and then, for each cognitive domain, we calculated the average of the two subtests’ z scores.
We also assessed: Intelligence—WAIS-III; Attention—Continuous performance test; and Motor coordination—Purdue Pegboard (details of the test’s procedures were described in our previous publications [33, 34, 49]). The 16th percentile (−1 SD) was selected as the cutoff for a below-average performance [50].
Clinical measures of severity and symptoms
The Global Assessment of functioning (GAF) [51] on symptom and on function scale [52, 53] and the Positive and Negative Symptom Scale (PANSS) [54] were used. Capitalizing on the available lifetime information based on the review of all the in- and outpatient medical charts and interviews, we were able to rate the GAF symptom and function scale and the PANSS severity of symptoms (calculated in average items score as defined in Fig. 2), year by year from the onset to the disease endpoint when cognitive measures were taken, in a life-chart derived from Post et al. [55] that we used in previous studies [46–48] and detailed in Supplemental Methods.
Fig. 2.
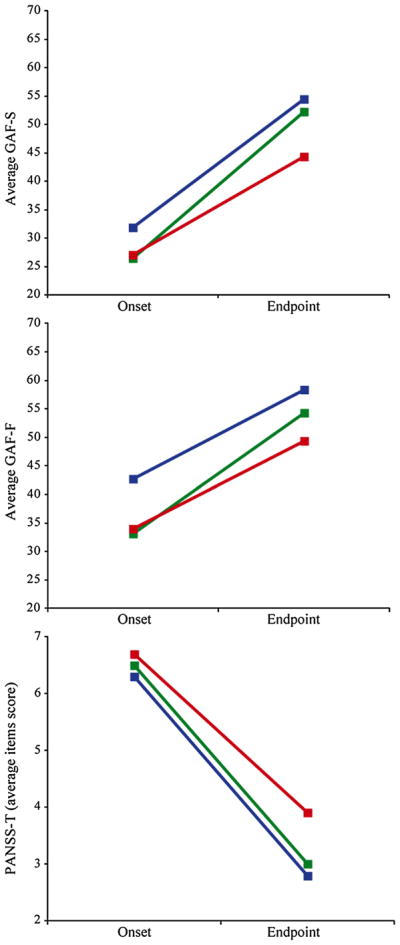
Change of GAF and PANSS from disease onset to endpoint for the two cognitively impaired clusters and the cluster with near-normal cognitive functioning. The GAF-S, the GAF-F and the PANSS were rated at disease onset and endpoint. PANSS scores were calculated in average item score. Briefly, the items of the positive and the negative subscales were rated, and a mean score for each subscale was calculated
Lifetime best estimate of response to treatment (BER)
A consensual clinical judgment on the response to treatment across life was made blind to cognitive measures by a team comprising a research psychiatrist (MM) and three experienced research professionals who had reviewed the lifetime information concerning the patient and filled in the life-chart. A consensus on a global judgment (poor, intermediate or good) of lifetime response was then reached. The sources of information were the in- and outpatient lifetime medical charts, ratings of the PANSS positive and negative scales and of the GAF symptom and functioning scales. All the available information across life was reviewed to assess the functioning at two different illness periods—the period of first admission or first episode of illness hereafter called disease onset, and the last 6–24 months of illness with the same medication hereafter called disease endpoint. Compliance to medication as well as a sufficient dose of antipsychotic was also considered in the judgment of response. The medical charts also provided the lifetime history of medication. Patients who were judged as good responders with the present method displayed at endpoint GAF-S and GAF-F scores ≥55 and a PANSS-T average item score<2; the poor responders were either patients taking clozapine or, for those not taking clozapine, presented at endpoint GAF-S and GAF-F scores <55 and a PANSS-T average item score ≥2. The intermediate responders had scores in between.
Of the 112 schizophrenic patients, 93 were administered new-generation antipsychotics, the others taking first-generation antipsychotics. Medication was transformed into olanzapine equivalent doses [56]. The response to treatment was judged undetermined in 23 patients, due either to short duration of illness (≤2 years; 5 subjects) or to low compliance or insufficient clinical information (18 subjects). The remaining 89 subjects were categorized as follows: 47 good, 14 intermediate and 28 poor responders (respectively, 52.8, 15.7 and 31.5 %). Such proportions were compatible with existing reports [3–5, 57, 58]. The undetermined responses were homogeneously distributed in the three clusters of patients (χ2 = 1.65, df = 2, p = 0.44).
Statistical analysis
Cluster analysis
To identify subsets of patients that would be homogeneous and separated from each other in terms of their cognitive functioning, four cognitive domains, i.e., verbal and visual episodic memory, working memory and processing speed, were entered into a cluster analysis based on a Gaussian mixture model, implemented in the R software with the package Mclust (available at http://cran.r-project.org/web/packages/mclust/index.html). Since we did not want to define a number of a priori subgroups of patients, we compared the adjustment of different clustering solutions with 1, 2, 3, 4 and 5 clusters using the Bayesian Information Criterion (BIC). The inspection of the BIC values showed a first maximum of −3,838.4 for the five-cluster solution and a second maximum BIC of −3,871.1 for the three-cluster solution. Given the very slight difference in BIC values, we opted for the simplest interpretable solution that fitted the data, i.e., the three-cluster definition. Then, each subject was assigned to a most probable cluster.
Comparison of the three clusters on demographic and clinical characteristics and on clinical change from disease onset to endpoint
The three clusters were compared on cognitive domains not entered in the cluster analysis, demographic and clinical/outcome variables with chi-square (χ2) test (when the cognitive functioning was categorized as impaired: under 16th percentile) and one-way ANOVAs (on a continuum of cognition). With ANCOVA, the three clusters of patients were also compared on the mean change of GAF symptom, GAF functioning and PANSS-T scores from onset to disease endpoint. For each clinical variable, age, gender and scores at the onset of the patients were entered as covariables in the analyses. Controlling for scores at onset ensures that differences on improvement found among clusters were not mainly due to differences in severity at onset. Post hoc ANCOVAs between pairs of clusters were performed when the Fisher statistic (F test) revealed overall significant differences among clusters. The significance level was fixed at 0.05. Analyses were performed using SAS/STAT software, version 9.2, of the SAS System for Windows. Copyright© 2002–2008 SAS Institute Inc.
Results
Cluster analysis identified three separate subsets of patients
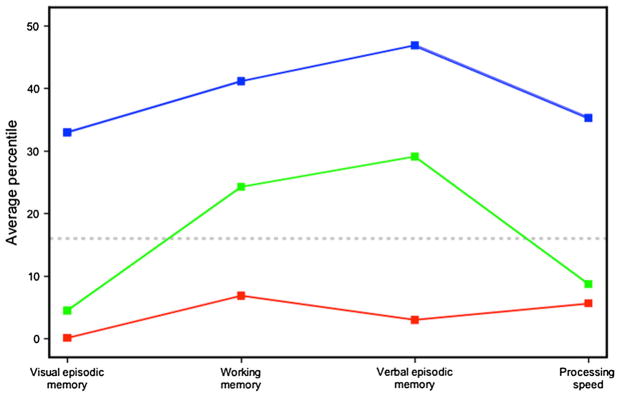
The cluster analysis defined three subsets of patients characterized by distinct neurocognitive profiles. The analysis detected two different clusters that were severely impaired: one generally impaired and another selectively impaired. A third cluster with near-normal cognitive functioning was also identified (Fig. 1). The cluster of patients generally impaired in the four cognitive domains suggested a severe and more diffuse cognitive dysfunction for 16 % of the sample having an average IQ of 68.1 (SD 6.4). The selectively impaired cluster classified 41 % of the patients characterized by selective deficits in visual episodic memory and processing speed, with near-average performance in working memory and verbal episodic memory. These selectively impaired patients had a mean IQ of 77.8 (SD 9.3). Finally, a cluster made of 43 % of the patients displayed an average performance in the four cognitive domains and a mean IQ of 92.8 (SD 11.2). An ANOVA showed a significant difference in IQ between the three clusters (p < 0.0001), with the near-normal functioning cluster having a higher mean IQ than the other two clusters. The generally impaired and selectively impaired clusters had similar IQ (p = 0.11).
Fig. 1.
Cluster analysis classified three separate subsets of schizophrenia patients. Cluster analysis identified three separate subsets of schizophrenia patients according to distinct profiles on their performance in the four cognitive domains: visual episodic memory, working memory, verbal episodic memory and processing speed. The first cluster (red line; 16 % of patients) of cognitively impaired patients had impairments, as defined under percentile 16th, in the four cognitive domains. A second cluster (green line; 41 % of patients) of cognitively impaired patients presented selective impairments in visual episodic memory and processing speed, with near-normative performance in working memory and verbal episodic memory. The third cluster (blue line; 43 % of patients) was characterized by near-normal performance on the four cognitive domains.
Characteristics of the clusters
The three clusters did not differ significantly in age of illness onset, age at neuropsychological evaluation, positive familial history of psychosis, illness duration or antipsychotic dose (Table 1). Using a 10-year cutoff of illness duration, the distribution of patients with shorter or longer durations of illness was homogeneous in the three clusters (χ2 = 2.39, df = 2, p = 0.30), suggesting that illness duration was little at play in the cluster classification. The clusters were different in socioeconomic status at endpoint (SES, p = 0.013): The near-normal functioning cluster had a higher SES than the other two clusters (see Table 1) that had similar SES. The three clusters were also compared on cognitive domains not entered in the cluster analysis, i.e., executive functions of problem-solving and planning, motor coordination and selective attention (Table 2). Patients in the two impaired clusters did not differ much except for selective attention. As expected, patients in the near-normal functioning cluster had better performances than the two other clusters on these cognitive domains.
Table 2.
Comparisons of the three clusters on the cognitive domains not entered in the cluster analysis
| Cognitive domain | Cluster | Meana | SE | Global p value | Post hoc analyses
|
||
|---|---|---|---|---|---|---|---|
| GI versus SI | GI versus NN | SI versus NN | |||||
| Executive function (problem-solving) | GI | 68.7 | 7.2 | ||||
| SI | 52.9 | 3.7 | 0.0002 | 0.056 NS | 0.0002 | 0.003 | |
| NN | 36.9 | 3.6 | |||||
| Executive function (planning) | GI | 2.6 | 0.65 | ||||
| SI | 2.8 | 0.37 | 0.0005 | 0.8 NS | 0.006 | 0.0003 | |
| NN | 4.8 | 0.36 | |||||
| Motor coordination | GI | 8.7 | 0.43 | ||||
| SI | 9.2 | 0.26 | <0.0001 | 0.28 NS | 0.0001 | <0.0001 | |
| NN | 10.8 | 0.25 | |||||
| Selective attention/vigilance | GI | 18.7 | 3.2 | ||||
| SI | 8.0 | 1.8 | <0.0001 | 0.004 | <0.0001 | 0.008 | |
| NN | 1.1 | 1.8 | |||||
In the table, GI stands for cluster of generally impaired patients, SI for cluster of selectively impaired patients and NN for cluster of near-normal functioning patients. The comparison of the three clusters was made on executive function (problem-solving and planning), motor coordination and selective attention by means of ANOVAs on percentile. Global p values were significant for all the cognitive domains. Post hoc analyses showed that the GI and the SI did not differ much except for selective attention, whereas the NN patients had the best overall performance when compared to the two impaired clusters
Mean scores are in percentile
Bold values indicate statistical significant
We focused on the higher proportion of women found in the generally impaired cluster (Table 1). First, a fair number of women were present in the two other clusters (Table 1). Second, we excluded the women and repeated the cluster analysis in men only: A similar three-cluster solution fitted the data best (results not shown) and included a near-normal functioning cluster (27 % of patients), a selectively impaired cluster (45 % of patients) and a generally impaired cluster (28 % of patients), suggesting that the generally impaired cluster we detected was not mainly a female cluster.
Differences among clusters in terms of clinical change, outcome and response to treatment
The three clusters of patients were compared on the change of their clinical scores from disease onset to endpoint (mean change of GAF symptom, GAF functioning and PANSS-T). Age, gender and baseline score of the patient on each clinical variable were entered as covariables in the analysis (Table 3). The clusters were found significantly different in terms of their improvement on disease severity (GAF symptom, p = 0.0002), functioning (GAF function, p = 0.013) and symptoms (PANSS-T, p = 0.005). Post hoc tests of between-group comparisons are shown in Table 3. Interestingly, patients of the two cognitively impaired clusters, generally impaired and selectively impaired, displayed different patterns of improvement over time. The selectively impaired patients had significantly better evolutions as indexed by their change scores on their GAF symptom (p = 0.0004), GAF functioning (p = 0.035) and PANSS-T (p = 0.003) than the generally impaired cluster. A visual inspection of Fig. 2 suggested that although these two impaired clusters had similar clinical severity and symptomatology at onset, the selectively impaired cluster had a better outcome. Not surprisingly, the near-normal functioning patients showed considerable improvement in their outcome (Table 3).
Table 3.
Clinical change of GAF and PANSS for patients of the three clusters from disease onset to endpoint
| Clinical variable | Cluster | Change over time (adjusted mean) | SE | F | Global p value | Post hoc analyses
|
||
|---|---|---|---|---|---|---|---|---|
| GI versus SI | GI versus NN | SI versus NN | ||||||
| GAF-S | GI | 15.0 | 2.2 | |||||
| SI | 25.1 | 1.6 | 9.23 | 0.0002 | 0.0004 | <0.0001 | 0.43 NS | |
| NN | 26.6 | 1.5 | ||||||
| GAF-F | GI | 12.9 | 2.0 | |||||
| SI | 18.3 | 1.5 | 4.60 | 0.013 | 0.035 | 0.003 | 0.22 NS | |
| NN | 20.6 | 1.4 | ||||||
| PANSS-T | GI | −2.7 | 0.3 | |||||
| SI | −3.9 | 0.2 | 5.68 | 0.005 | 0.003 | 0.002 | 0.9 NS | |
| NN | −3.9 | 0.2 | ||||||
In the table, GI stands for cluster of generally impaired patients, SI for cluster of selectively impaired patients and NN for cluster of near-normal functioning patients. The three clusters of patients were compared on the change in scores from disease onset to endpoint (mean change on GAF symptom, GAF functioning and PANSS-T) by means of ANCOVA. For each clinical variable, age, gender and scores at onset on each clinical variable were entered as covariables in the analysis. The clusters were found significantly different in terms of their improvement on disease severity (GAF symptom), functioning (GAF function) and symptoms (PANSS-T). Post hoc tests of between-group comparisons show that patients of the two cognitively impaired clusters, generally impaired and selectively impaired, displayed different patterns of improvement over time. The selectively impaired patients had significantly better evolutions of their GAF symptom (p = 0.0004), GAF functioning (p = 0.035) and PANSS-T (p = 0.003) than the generally impaired cluster. The bold italic emphasizes the post hoc comparison of the generally impaired cluster (GI) versus the selectively impaired cluster (SI)
Bold values indicate statistical significant
Congruent results were obtained with the lifetime best estimate of response to treatment: The patients in the selectively impaired cluster showed a significantly better lifetime response to treatment than the generally impaired patients (χ2 = 4.75, df = 1, p = 0.029; OR of 4.67 (95 % CI 1.09–19.9); Table 4 and Table S1). The exclusion of the intermediate responders from the analysis provided similar results (χ2 = 3.93, df = 1, p = 0.047, OR of 4.67).
Table 4.
Comparison of the generally impaired cluster to the selectively impaired cluster on the lifetime best estimate of response to treatment (N = 49)
| Best-estimate response (lifetime)
|
||
|---|---|---|
| Poor and intermediate | Good | |
| Generally impaired cluster | 76.9 % (n = 10) | 23.1 % (n = 3) |
| Selectively impaired cluster | 41.7 % (n = 15) | 58.3 % (n = 21) |
χ2 = 4.75, p = 0.029; OR = 4.67
The lifetime best-estimate response to treatment was judged consensually according to all available clinical information across the illness duration and was blind to cognitive functioning. Judgment was made on a three-point scale: poor responders, intermediate and good lifetime responders to antipsychotics, based on the GAF symptom, the GAF functioning and the PANSS, each of the three scales being rated on a life-chart as defined in the “Methods” section
From the original 112 patients, the best estimate of response could not be rated in 23 patients due to insufficient information or short duration of disease (<2 years) and was then judged as undetermined. As detailed in “Methods,” the undetermined responses were distributed homogeneously in the three clusters. This explains the lower number of subjects
Individual cognitive domains less associated with response to treatment than cognitive clustering
Each of the cognitive domains (e.g., verbal or visual episodic memory, working memory or processing speed) was not individually found associated with the best estimate of response to treatment either when considering cognitive functioning as a continuum or as categories (i.e., deficit <16th percentile) (Supplemental Table S2 and S3). This suggested that the cluster profiles were more related to disease outcome than each cognitive deficit alone.
Negative symptoms did not account for the effect of cognitive clusters on outcome
We controlled for the potential effect of negative symptoms on the observed relationship between the clusters cognitive profiles and clinical outcome [12]. Entering the PANSS negative score as a covariate in the analyses did not change the results (Supplemental Table S4).
Considering executive function as a fifth cluster did not explain additional variability
Impairment in executive functions (EFs) is also a core feature of schizophrenia and has been related to clinical features of the disease and overall outcome [30]. However, our criteria for selecting the cognitive domains were the presence of a cognitive deficit many years before disease onset. Since our cohort of high-risk children did not show significant impairments in EF [33], we did not include EF in our main analysis. Nevertheless, we introduced EF as a fifth cognitive domain (Tower of London and Wisconsin Card Sorting Test) in a secondary cluster analysis to verify whether adding this domain would help in our goal to define separate subgroups of schizophrenia patients based on their cognitive functioning. The inspection of the BIC values of this secondary analysis led us to select a four-cluster solution, which showed up as congruent with the three-cluster solution described above (see Figure S1): The majority of the selectively impaired patient cluster split into two clusters of selectively impaired patients, one with preserved EF and another with impaired EF. Most salient results in terms of external validation held with the five-cognitive-domain-cluster solution (data not shown) despite a reduced statistical power.
Validation of the clusters by a neuropsychologist’s clinical classification
Blind to the hierarchical cluster analysis, a clinical classification of each subject was performed by a neuropsychologist (EG) who inspected the neuropsychological tests to clinically redefine the patients’ cognitive profiles (see the clinical definitions in Supplemental Table S5) based on particular combinations of deficits in the four cognitive domains considered. This clinical classification yielded a group of 23 generally impaired, 33 selectively impaired and 47 near-normal functioning patients. We then tested the extent to which this clinical judgment would reclassify patients in the cluster they were assigned to by the probabilistic classification. The overlap between the two methods was satisfactory with a kappa of 0.85 (Supplemental Table S5). Only ten subjects were classified in a cluster other than the one assigned by the cluster analysis. Nine subjects who did not meet any of the Table S5 criteria were declared undetermined and withdrawn from the concordance analysis.
Discussion
Cluster analysis detected three separate subsets of patients
Without a priori categorizing the patients, we performed a hierarchical cluster analysis entering four cognitive domains known to be considerably and consistently impaired in patients, i.e., verbal episodic memory, visual episodic memory, working memory and processing speed. This analysis identified three clusters of patients. A salient finding of our study was the detection of two different subsets of severely impaired patients, each having an idiosyncratic prognosis: One cluster was generally impaired in the four cognitive domains, and the other was selectively impaired in visual episodic memory and processing speed. Consistent with previous cluster analyses in schizophrenia, we reproduced a cluster with near-normal cognitive functioning [24, 25, 27] including around 43 % of patients, and another cluster with general impairments in the four domains classifying around 16 % of patients [13, 24–29].
Different lifetime outcome for each of the cognitively impaired clusters: translational relevance for the practitioner and for clinical trials
We found that the two cognitively impaired clusters had very different profiles of disease outcome and lifetime response to treatment. At the onset of illness, clinicians would face two main heterogeneous subsets of cognitively impaired patients who would be clinically indistinguishable given their similar age of onset, clinical severity on the GAF-S and the PANSS and lower IQ (see Tables 1, 2; Fig. 2). Our findings suggest, however, that getting hold of their profile on four specific cognitive domains could help to anticipate divergent prognoses of these two impaired subsets of patients: The subgroup characterized by general impairments would likely have a worse outcome with less clinical improvement, residual symptoms and a poorer response to treatment. In contrast, patients with selective deficits in visual episodic memory and processing speed would display a better long-term clinical improvement in symptomatology and functional severity associated with a better lifetime response to treatment. Since early adapted treatment alleviates the future burden of the disease [6], an early cognitive portray might induce the clinician to conduct a personalized surveillance and eventually select different multimodal treatments. More research focused on this issue is warranted.
The implications also extend to clinical trial design. The prospects of an addition of dimensional criteria in DSM-V, and the fact that cognition may become the target of novel drugs [59], deserve methodological attention since patient profiles of cognitive deficits would by themselves influence the response to treatment. Our results suggest that the cognitive profiling of patients, for instance, along the present four cognitive domains, (1) should be considered at entry in pharmacological trials, since different combinations of deficits might be associated with disparate response to drug treatment and render intersite replications arduous and/or (2) should be accounted for in the analysis of drug efficacy.
Combinations of cognitive deficits may be more related to disease outcome than an individual deficit alone
It is noteworthy that in this study, the four cognitive domains individually had no association with disease outcome in terms of our lifetime best estimate of response. This is congruent with the little effect of individual deficits on clinical variables documented by previous studies with correlations usually in the ~0.30 range [8–17]. Our finding that combinations of deficits have a stronger association with the disease outcome than individual deficits may also be compatible with prevalent theories that schizophrenia would be underlain by more than one trait or brain dysfunction [60].
Strengths and limitations
The limitations and the strengths of our methods deserve to be raised. First, contrary to many previous cluster analysis studies [4, 61, 62], our study included only schizophrenia spectrum disorders, thus excluding schizoaffective diagnoses. This limits the generalizability of our results to schizophrenia, but in return, this also eliminates the potential confounding effect of a schizoaffective diagnosis on the cluster solution as the latter diagnosis was previously found overrepresented in cognitively preserved clusters [13, 29]. Second, in our main and secondary analysis we selected cognitive domains known to be severely impaired in schizophrenia in our studies and in others [31–33], hoping that different combinations of these deficits might define separate subsets of patients with a specific prognosis. Our findings must be interpreted with the limitation that cognitive deficits in other domains such as social cognition [63] were not entered in the cluster analysis. Third, the small sample size of the derived subgroups may have generated type 2 errors. Fourth, a strong feature of our study was our ability to measure, blind to cognitive functioning, the lifetime trajectories and history of pharmacological treatment based on several clinical scales rated from a follow-back of contemporary medical charts available across the illness duration, instead of a retrospective recollection from patients or family. Fifth, a potential limitation is that patients were tested at different ages, but we found little difference in illness duration among the three clusters. However, studies found little evidence of increased neuropsychological deficits with illness progression [64–67], and meta-analyses showed that large cognitive deficits are already present in first-episode patients [32]. Sixth, the relatively large presence of women in the generally impaired cluster may occur because our sampling is mostly in outpatient psychiatric services of a regional psychiatric hospital. This may have increased the proportion in the cohort of schizophrenic women with a severe presentation, assuming that affected women with less severe disease or impairments are more likely to be followed up in community services than in specialized psychiatric services.
In conclusion, specific combinations of cognitive deficits may define heterogeneity in schizophrenia as related to long-term clinical improvement and response to treatment. One must also conceive that other dimensions, genetic or physiological, will probably have to be added to cognition in a multimodal approach to improve estimate of clinical and pharmacological prognosis.
Supplementary Material
Acknowledgments
We would like to thank our research assistant team, Marie-Claude Boisvert, Louise Bélanger, Joanne Lavoie, Linda René, Valérie Beaupré-Monfette, and the patients who have participated in this study. We are grateful to Dr Marco Battaglia for his helpful comments. This study was supported by a Canada Research Chair (#950-200810) in psychiatric genetics of which Maziade is the holder, by Canadian Institute of Health Research Grants (#MOP-74430, #MOP-114988 and #MOP-119408) and by a CQDM Grant. The funding organizations had no role in the writing of this review.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00406-013-0463-7) contains supplementary material, which is available to authorized users.
Conflict of interest MM and MAR have been consultants for GlaxoSmithKline (GSK) and Eli Lilly and have received research funding from GSK, Eli Lilly and AstraZeneca that are not related to the material of this study. RHB has been a consultant and/or received funding from Eli Lilly, Pfizer, AstraZeneca and Janssen Ortho that are not related to the material of this study. EG, CM, NR, VJ, CE and TP report no financial relationships with commercial interests.
References
- 1.Perlis RH. Translating biomarkers to clinical practice. Mol Psychiatry. 2011;16:1076–1087. doi: 10.1038/mp.2011.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keshavan MS, Nasrallah HA, Tandon R. Schizophrenia, “just the facts” 6. Moving ahead with the schizophrenia concept: from the elephant to the mouse. Schizophr Res. 2011;127:3–13. doi: 10.1016/j.schres.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levine SZ, Leucht S. Elaboration on the early-onset hypothesis of antipsychotic drug action: treatment response trajectories. Biol Psychiatry. 2010;68:86–92. doi: 10.1016/j.biopsych.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 4.Levine SZ, Rabinowitz J. Trajectories and antecedents of treatment response over time in early-episode psychosis. Schizophr Bull. 2010;36:624–632. doi: 10.1093/schbul/sbn120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levine SZ, Rabinowitz J, Faries D, Lawson AH, Ascher-Svanum H. Treatment response trajectories and antipsychotic medications: examination of up to 18 months of treatment in the CATIE chronic schizophrenia trial. Schizophr Res. 2012;137(1–3):141–146. doi: 10.1016/j.schres.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 6.Perkins DO, Gu H, Boteva K, Lieberman JA. Relationship between duration of untreated psychosis and outcome in first-episode schizophrenia: a critical review and meta-analysis. Am J Psychiatry. 2005;162:1785–1804. doi: 10.1176/appi.ajp.162.10.1785. [DOI] [PubMed] [Google Scholar]
- 7.Dickinson D, Gold JM. Less unique variance than meets the eye: overlap among traditional neuropsychological dimensions in schizophrenia. Schizophr Bull. 2008;34:423–434. doi: 10.1093/schbul/sbm092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”? Schizophr Bull. 2000;26:119–136. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- 9.Green MF. What are the functional consequences of neuro-cognitive deficits in schizophrenia? Am J Psychiatry. 1996;153:321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- 10.Fett AK, Viechtbauer W, Dominguez MD, Penn DL, van Os J, Krabbendam L. The relationship between neurocognition and social cognition with functional outcomes in schizophrenia: a meta-analysis. Neurosci Biobehav Rev. 2011;35:573–588. doi: 10.1016/j.neubiorev.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Nuechterlein KH, Subotnik KL, Green MF, Ventura J, Asarnow RF, Gitlin MJ, Yee CM, Gretchen-Doorly D, Mintz J. Neurocognitive predictors of work outcome in recent-onset schizophrenia. Schizophr Bull. 2011;37(Suppl 2):S33–S40. doi: 10.1093/schbul/sbr084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Milev P, Ho BC, Arndt S, Andreasen NC. Predictive values of neurocognition and negative symptoms on functional outcome in schizophrenia: a longitudinal first-episode study with 7-year follow-up. Am J Psychiatry. 2005;162:495–506. doi: 10.1176/appi.ajp.162.3.495. [DOI] [PubMed] [Google Scholar]
- 13.Bell MD, Johannesen JK, Greig TC, Wexler BE. Memory profiles in schizophrenia: categorization validity and stability. Schizophr Res. 2010;118:26–33. doi: 10.1016/j.schres.2009.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Potter AI, Nestor PG. Iq subtypes in schizophrenia: distinct symptom and neuropsychological profiles. J Nerv Ment Dis. 2010;198:580–585. doi: 10.1097/NMD.0b013e3181ea4e43. [DOI] [PubMed] [Google Scholar]
- 15.Carrion RE, Goldberg TE, McLaughlin D, Auther AM, Correll CU, Cornblatt BA. Impact of neurocognition on social and role functioning in individuals at clinical high risk for psychosis. Am J Psychiatry. 2011;168:806–813. doi: 10.1176/appi.ajp.2011.10081209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knowles EE, David AS, Reichenberg A. Processing speed deficits in schizophrenia: reexamining the evidence. Am J Psychiatry. 2010;167:828–835. doi: 10.1176/appi.ajp.2010.09070937. [DOI] [PubMed] [Google Scholar]
- 17.Allott K, Liu P, Proffitt TM, Killackey E. Cognition at illness onset as a predictor of later functional outcome in early psychosis: systematic review and methodological critique. Schizophr Res. 2011;125:221–235. doi: 10.1016/j.schres.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 18.Wittorf A, Wiedemann G, Buchkremer G, Klingberg S. Prediction of community outcome in schizophrenia 1 year after discharge from inpatient treatment. Eur Arch Psychiatry Clin Neurosci. 2008;258:48–58. doi: 10.1007/s00406-007-0761-z. [DOI] [PubMed] [Google Scholar]
- 19.Wolwer W, Brinkmeyer J, Riesbeck M, Freimuller L, Klimke A, Wagner M, Moller HJ, Klingberg S, Gaebel W. Neuro-psychological impairments predict the clinical course in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2008;258(Suppl 5):28–34. doi: 10.1007/s00406-008-5006-2. [DOI] [PubMed] [Google Scholar]
- 20.Bell MD, Tsang HW, Greig T, Bryson G. Cognitive predictors of symptom change for participants in vocational rehabilitation. Schizophr Res. 2007;96(1–3):162–168. doi: 10.1016/j.schres.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 21.Seaton BE, Goldstein G, Allen DN. Sources of heterogeneity in schizophrenia: the role of neuropsychological functioning. Neuropsychol Rev. 2001;11:45–67. doi: 10.1023/a:1009013718684. [DOI] [PubMed] [Google Scholar]
- 22.Basso MR, Nasrallah HA, Olson SC, Bornstein RA. Neuropsychological correlates of negative, disorganized and psychotic symptoms in schizophrenia. Schizophr Res. 1998;31:99–111. doi: 10.1016/s0920-9964(98)00023-1. [DOI] [PubMed] [Google Scholar]
- 23.Rethelyi JM, Czobor P, Polgar P, Mersich B, Balint S, Jekkel E, Magyar K, Meszaros A, Fabian A, Bitter I. General and domain-specific neurocognitive impairments in deficit and non-deficit schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2012;262:107–115. doi: 10.1007/s00406-011-0224-4. [DOI] [PubMed] [Google Scholar]
- 24.Weickert TW, Goldberg TE, Gold JM, Bigelow LB, Egan MF, Weinberger DR. Cognitive impairments in patients with schizophrenia displaying preserved and compromised intellect. Arch Gen Psychiatry. 2000;57:907–913. doi: 10.1001/archpsyc.57.9.907. [DOI] [PubMed] [Google Scholar]
- 25.Kremen WS, Seidman LJ, Faraone SV, Toomey R, Tsuang MT. The paradox of normal neuropsychological function in schizophrenia. J Abnorm Psychol. 2000;109:743–752. doi: 10.1037//0021-843x.109.4.743. [DOI] [PubMed] [Google Scholar]
- 26.Ilonen T, Taiminen T, Karlsson H, Lauerma H, Leinonen KM, Wallenius E, Salokangas RK. Neuropsychological subtyping of schizophrenia. Psychiatry Res. 2004;129:191–199. doi: 10.1016/j.psychres.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 27.Hill SK, Ragland JD, Gur RC, Gur RE. Neuropsychological profiles delineate distinct profiles of schizophrenia, an interaction between memory and executive function, and uneven distribution of clinical subtypes. J Clin Exp Neuropsychol. 2002;24:765–780. doi: 10.1076/jcen.24.6.765.8402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kremen WS, Seidman LJ, Faraone SV, Toomey R, Tsuang MT. Heterogeneity of schizophrenia: a study of individual neuropsychological profiles. Schizophr Res. 2004;71:307–321. doi: 10.1016/j.schres.2004.02.022. [DOI] [PubMed] [Google Scholar]
- 29.Ammari N, Heinrichs RW, Miles AA. An investigation of 3 neurocognitive subtypes in schizophrenia. Schizophr Res. 2010;121:32–38. doi: 10.1016/j.schres.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 30.Raffard S, Bayard S. Understanding the executive functioning heterogeneity in schizophrenia. Brain Cogn. 2012;79:60–69. doi: 10.1016/j.bandc.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 31.Fioravanti M, Bianchi V, Cinti ME. Cognitive deficits in schizophrenia: an updated metanalysis of the scientific evidence. BMC Psychiatry. 2012;12:64. doi: 10.1186/1471-244X-12-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mesholam-Gately RI, Giuliano AJ, Goff KP, Faraone SV, Seidman LJ. Neurocognition in first-episode schizophrenia: a meta-analytic review. Neuropsychology. 2009;23:315–336. doi: 10.1037/a0014708. [DOI] [PubMed] [Google Scholar]
- 33.Maziade M, Rouleau N, Merette C, Cellard C, Battaglia M, Marino C, Jomphe V, Gilbert E, Achim A, Bouchard RH, Paccalet T, Paradis ME, Roy MA. Verbal and visual memory impairments among young offspring and healthy adult relatives of patients with schizophrenia and bipolar disorder: selective generational patterns indicate different developmental trajectories. Schizophr Bull. 2011;37:1218–1228. doi: 10.1093/schbul/sbq026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maziade M, Rouleau N, Gingras N, Boutin P, Paradis ME, Jomphe V, Boutin J, Letourneau K, Gilbert E, Lefebvre AA, Dore MC, Marino C, Battaglia M, Merette C, Roy MA. Shared neurocognitive dysfunctions in young offspring at extreme risk for schizophrenia or bipolar disorder in eastern Quebec multi-generational families. Schizophr Bull. 2009;35:919–930. doi: 10.1093/schbul/sbn058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cannon M, Caspi A, Moffitt TE, Harrington H, Taylor A, Murray RM, Poulton R. Evidence for early-childhood, pan-developmental impairment specific to schizophreniform disorder: results from a longitudinal birth cohort. Arch Gen Psychiatry. 2002;59:449–456. doi: 10.1001/archpsyc.59.5.449. [DOI] [PubMed] [Google Scholar]
- 36.Niendam TA, Bearden CE, Rosso IM, Sanchez LE, Hadley T, Nuechterlein KH, Cannon TD. A prospective study of childhood neurocognitive functioning in schizophrenic patients and their siblings. Am J Psychiatry. 2003;160:2060–2062. doi: 10.1176/appi.ajp.160.11.2060. [DOI] [PubMed] [Google Scholar]
- 37.Reichenberg A, Caspi A, Harrington H, Houts R, Keefe RS, Murray RM, Poulton R, Moffitt TE. Static and dynamic cognitive deficits in childhood preceding adult schizophrenia: a 30-year study. Am J Psychiatry. 2010;167:160–169. doi: 10.1176/appi.ajp.2009.09040574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jones P, Rodgers B, Murray R, Marmot M. Child development risk factors for adult schizophrenia in the British 1946 birth cohort. Lancet. 1994;344:1398–1402. doi: 10.1016/s0140-6736(94)90569-x. [DOI] [PubMed] [Google Scholar]
- 39.Osler M, Lawlor DA, Nordentoft M. Cognitive function in childhood and early adulthood and hospital admission for schizophrenia and bipolar disorders in Danish men born in 1953. Schizophr Res. 2007;92:132–141. doi: 10.1016/j.schres.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 40.Cannon TD, Bearden CE, Hollister JM, Rosso IM, Sanchez LE, Hadley T. Childhood cognitive functioning in schizophrenia patients and their unaffected siblings: a prospective cohort study. Schizophr Bull. 2000;26:379–393. doi: 10.1093/oxfordjournals.schbul.a033460. [DOI] [PubMed] [Google Scholar]
- 41.Dickinson D, Goldberg TE, Gold JM, Elvevag B, Weinberger DR. Cognitive factor structure and invariance in people with schizophrenia, their unaffected siblings, and controls. Schizophr Bull. 2011;37:1157–1167. doi: 10.1093/schbul/sbq018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nuechterlein KH, Barch DM, Gold JM, Goldberg TE, Green MF, Heaton RK. Identification of separable cognitive factors in schizophrenia. Schizophr Res. 2004;72:29–39. doi: 10.1016/j.schres.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 43.Genderson MR, Dickinson D, Diaz-Asper CM, Egan MF, Weinberger DR, Goldberg TE. Factor analysis of neuro-cognitive tests in a large sample of schizophrenic probands, their siblings, and healthy controls. Schizophr Res. 2007;94:231–239. doi: 10.1016/j.schres.2006.12.031. [DOI] [PubMed] [Google Scholar]
- 44.Nuechterlein KH, Green MF, Kern RS, Baade LE, Barch DM, Cohen JD, Essock S, Fenton WS, Frese FJ, 3rd, Gold JM, Goldberg T, Heaton RK, Keefe RS, Kraemer H, Mesholam-Gately R, Seidman LJ, Stover E, Weinberger DR, Young AS, Zalcman S, Marder SR. The matrics consensus cognitive battery, part 1: test selection, reliability, and validity. Am J Psychiatry. 2008;165:203–213. doi: 10.1176/appi.ajp.2007.07010042. [DOI] [PubMed] [Google Scholar]
- 45.Maziade M, Roy MA, Chagnon YC, Cliche D, Fournier JP, Montgrain N, Dion C, Lavallee JC, Garneau Y, Gingras N, Nicole L, Pires A, Ponton AM, Potvin A, Wallot H, Merette C. Shared and specific susceptibility loci for schizophrenia and bipolar disorder: a dense genome scan in eastern Quebec families. Mol Psychiatry. 2005;10:486–499. doi: 10.1038/sj.mp.4001594. [DOI] [PubMed] [Google Scholar]
- 46.Maziade M, Roy MA, Fournier JP, Cliche D, Merette C, Caron C, Garneau Y, Montgrain N, Shriqui C, Dion C, et al. Reliability of best-estimate diagnosis in genetic linkage studies of major psychoses: results from the Quebec pedigree studies. Am J Psychiatry. 1992;149:1674–1686. doi: 10.1176/ajp.149.12.1674. [DOI] [PubMed] [Google Scholar]
- 47.Maziade M, Roy MA, Martinez M, Cliche D, Fournier JP, Garneau Y, Nicole L, Montgrain N, Dion C, Ponton AM. Negative, psychoticism, and disorganized dimensions in patients with familial schizophrenia or bipolar disorder: continuity and discontinuity between the major psychoses. Am J Psychiatry. 1995;152:1458–1463. doi: 10.1176/ajp.152.10.1458. [DOI] [PubMed] [Google Scholar]
- 48.Roy MA, Lanctôt G, Mérette C, Cliche D, Fournier JP, Boutin P, Rodrigue C, Charron L, Turgeon M, Hamel M, Montgrain N, Nicole L, Pires A, Wallot H, Ponton AM, Garneau Y, Dion C, Lavallée JC, Potvin A, Szatmari P, Maziade M. Clinical and methodological factors related to reliability of the best-estimate diagnostic procedure. Am J Psychiatry. 1997;154:1726–1733. doi: 10.1176/ajp.154.12.1726. [DOI] [PubMed] [Google Scholar]
- 49.Maziade M, Rouleau N, Cellard C, Battaglia M, Paccalet T, Moreau I, Gagnon V, Gingras N, Marino C, Gilbert E, Roy MA, Merette C. Young offspring at genetic risk of adult psychoses: the form of the trajectory of IQ or memory may orient to the right dysfunction at the right time. PLoS ONE. 2011;6:e19153. doi: 10.1371/journal.pone.0019153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Slick DJ. Psychometric in neuropsychological assessment. In: Strauss E, Sherman EMS, Spreen O, editors. A compendium of neuropsychological tests: Administration, norms, and commentary. Oxford University Press; USA: 2006. [Google Scholar]
- 51.Endicott J, Spitzer RL, Fleiss JL, Cohen J. The global assessment scale. A procedure for measuring overall severity of psychiatric disturbance. Arch Gen Psychiatry. 1976;33:766–771. doi: 10.1001/archpsyc.1976.01770060086012. [DOI] [PubMed] [Google Scholar]
- 52.Aas IH. Global assessment of functioning (GAF): properties and frontier of current knowledge. Ann Gen Psychiatry. 2010;9:20. doi: 10.1186/1744-859X-9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pedersen G, Hagtvet KA, Karterud S. Generalizability studies of the global assessment of functioning-split version. Compr Psychiatry. 2007;48:88–94. doi: 10.1016/j.comppsych.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 54.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 55.Post RM, Roy-Byrne PP, Uhde TW. Graphic representation of the life course of illness in patients with affective disorder. Am J Psychiatry. 1988;145:844–848. doi: 10.1176/ajp.145.7.844. [DOI] [PubMed] [Google Scholar]
- 56.Gardner DM, Murphy AL, O’Donnell H, Centorrino F, Baldessarini RJ. International consensus study of antipsychotic dosing. Am J Psychiatry. 2010;167:686–693. doi: 10.1176/appi.ajp.2009.09060802. [DOI] [PubMed] [Google Scholar]
- 57.Marques TR, Arenovich T, Agid O, Sajeev G, Muthen B, Chen L, Kinon BJ, Kapur S. The different trajectories of antipsychotic response: antipsychotics versus placebo. Psychol Med. 2011;41:1481–1488. doi: 10.1017/S0033291710002035. [DOI] [PubMed] [Google Scholar]
- 58.Levine SZ, Lurie I, Kohn R, Levav I. Trajectories of the course of schizophrenia: from progressive deterioration to amelioration over three decades. Schizophr Res. 2011;126:184–191. doi: 10.1016/j.schres.2010.10.026. [DOI] [PubMed] [Google Scholar]
- 59.Butlen-Ducuing F, Haas M, Pani L, van Zwieten-Boot B, Broich K. DSM-5 and clinical trials in psychiatry: challenges to come? Nat Rev Drug Discovery. 2012;11:583–584. doi: 10.1038/nrd3811. [DOI] [PubMed] [Google Scholar]
- 60.Braff DL, Freedman R, Schork NJ, Gottesman I. Deconstructing schizophrenia: an overview of the use of endophenotypes in order to understand a complex disorder. Schizophr Bull. 2007;33:21–32. doi: 10.1093/schbul/sbl049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Levine SZ, Rabinowitz J, Case M, Ascher-Svanum H. Treatment response trajectories and their antecedents in recent-onset psychosis: a 2-year prospective study. J Clin Psychopharmacol. 2010;30:446–449. doi: 10.1097/JCP.0b013e3181e68e80. [DOI] [PubMed] [Google Scholar]
- 62.Stauffer V, Case M, Kollack-Walker S, Ascher-Svanum H, Ball T, Kapur S, Kinon BJ. Trajectories of response to treatment with atypical antipsychotic medication in patients with schizophrenia pooled from 6 double-blind, randomized clinical trials. Schizophr Res. 2011;130:11–19. doi: 10.1016/j.schres.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 63.Derntl B, Habel U. Deficits in social cognition: a marker for psychiatric disorders? Eur Arch Psychiatry Clin Neurosci. 2011;261(Suppl 2):S145–S149. doi: 10.1007/s00406-011-0244-0. [DOI] [PubMed] [Google Scholar]
- 64.Heaton RK, Gladsjo JA, Palmer BW, Kuck J, Marcotte TD, Jeste DV. Stability and course of neuropsychological deficits in schizophrenia. Arch Gen Psychiatry. 2001;58:24–32. doi: 10.1001/archpsyc.58.1.24. [DOI] [PubMed] [Google Scholar]
- 65.Holthausen EA, Wiersma D, Sitskoorn MM, Hijman R, Dingemans PM, Schene AH, van den Bosch RJ. Schizophrenic patients without neuropsychological deficits: subgroup, disease severity or cognitive compensation? Psychiatry Res. 2002;112:1–11. doi: 10.1016/s0165-1781(02)00184-1. [DOI] [PubMed] [Google Scholar]
- 66.Cobia DJ, Csernansky JG, Wang L. Cortical thickness in neuropsychologically near-normal schizophrenia. Schizophr Res. 2011;133:68–76. doi: 10.1016/j.schres.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Albus M, Hubmann W, Mohr F, Hecht S, Hinterberger-Weber P, Seitz NN, Kuchenhoff H. Neurocognitive functioning in patients with first-episode schizophrenia : results of a prospective 5-year follow-up study. Eur Arch Psychiatry Clin Neurosci. 2006;256:442–451. doi: 10.1007/s00406-006-0667-1. [DOI] [PubMed] [Google Scholar]
- 68.Blishen BR, Carroll WK, Moore C. The 1981 socioeconomic index for occupations in Canada. Can Rev Soc Anthrop. 1987;24:465–488. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.