Abstract
This study reports on genetic susceptibility to ectopic calcification in the LG/J and SM/J advanced intercross mice. Using 347 mice in 98 full-sibships, destabilization of medial meniscus (DMM) was performed to induce joint injury. We performed quantitative trait locus (QTL) analysis to map ectopic calcification phenotypes to discrete genomic locations. To validate the functional significance of the selected QTL candidate genes, we compared mRNA expression between parental LG/J and SM/J inbred strains. We found that joint destabilization instigated ectopic calcifications as detected and quantified by micro-CT. Overall, we detected 20 QTLs affecting synovial and meniscus calcification phenotypes with 11 QTLs linked to synovial calcification. Functional and bioinformatic analyses of single nucleotide polymorphism identified functional classifications relevant to angiogenesis (Myo1e, Kif26b, Nprl3, Stab2, Fam105b), bone metabolism/calcification (Tle3, Tgfb2, Lipc, Nfe2l1, Ank, Fam105b), arthritis (Stab2, Tbx21, Map4k4, Hoxb9, Larp6, Col1a2, Adam10, Timp3, Nfe2l1, Trpm3), and ankylosing-spondylitis (Ank, Pon1, Il1r2, Tbkbp1) indicating that ectopic calcification involves multiple mechanisms. Furthermore, the expression of 11 candidate genes was significantly different between LG/J and SM/J. Correlation analysis showed that Aff3, Fam81a, Syn3, and Ank were correlated with synovial calcification. Taken together, our findings of multiple genetic loci suggest the involvement of multiple genes contributing to ectopic calcification.
Keywords: ectopic calcification, QTL analysis, gene expression, genetics, advanced intercross line, osteoarthritis
INTRODUCTION
Pathological mineralization of synovial connective tissues leads to chondromatosis or “loose bodies” in the knee1; 2. Ectopic nodules appear as multiple intra-articular calcifications1; 3. Their rupture and detachment can disturb the nutritive supply to the joint and can result in mechanical injury ultimately leading to osteoarthritis (OA)3; 4.
Tissue mineralization is a complex phenomenon involving many physiological processes. Normally, bones, teeth, and otoconia are the principal mineralized human tissues while non-mineralized soft tissues are constantly awash in interstitial fluids supersaturated with calcium and phosphate with mineralization inhibited5. Disturbance of inhibitory mechanisms may permit pathological mineralization, e.g., atherosclerosis6, renal calculus and gout7; 8, skin ossifications9, craniosynostosis10, and OA-related ectopic calcification of joints. Some evidence suggests that synovial chondromatosis has a genetically simple or Mendelian basis11; 12. Segregation has been reported in several pedigrees13, while casual loci have been mapped to human chromosomes 5p11 and 8q12. A monogenic cause for ectopic calcification has been shown to be due to a segregating dominant allele of ANK on chromosome 514. However, many cases appear to have a multifactorial basis. While it is likely that environmental components of risk are substantial, recurrence rate among first-degree relatives is 10–30%15, indicative of genetic components. Constituting the majority of cases, genetic locations contributing to this polygenic form are unknown, let alone the physiological processes the gene variants affect. Identification of important susceptibility loci has highlighted disturbed physiological processes leading to calcification of joints and other tissues such as heterotrophic mineralization of musculoskeletal tissues, skin and blood vessels6; 9.
Here, we report the development of synovial and meniscal ectopic calcifications in response to knee trauma in an F44 advanced intercross of LG/J and SM/J mice (Wustl:LG, SM-G44). We mapped synovial and meniscal ectopic calcification phenotypes to discrete genomic locations. For each positional candidate gene, we compared genetic sequence variations between LG/J and SM/J alleles and performed functional, bioinformatic, and expression analyses to gain insight into the mechanisms underlying these phenotypes.
MATERIALS AND METHODS
Mice and knee injury model
All experiments were approved by the Animal Studies Committee. The F44 advanced intercross differs in a wide-range of traits and is a model for studying the genetics of complex traits segregating many interacting genes of small effect16–19. The F44 sample included 347 mice in 98 full-sibships. All mice were raised at our mouse facility operating at constant temperature of 21°C and on a 12-hour light/dark cycle at high standards of sanitation. Offspring were housed with their mothers until weaning at 3-weeks of age, and then separated into sex-specific cages of 4–5 mice/cage with each cage individually ventilated. All mice were fed on irradiated rodent chow (Purina 5053, Purina Mills St. Louis, MO) with food and water provided ad libitum.
OA was induced through DMM surgery in which the medial meniscotibial ligament (MMTL) was transected in 10-week old mice as described by us20 and others21. Mice were anesthetized using an intra-peritoneal injection of rodent cocktail (100 mg/kg ketamine, 20 mg/kg xylazine and 10 mg/kg acepromazine) before their right knee MMTL was resected to displace the medial meniscus. The contralateral left knee served as a sham, receiving the exact same surgery as DMM but without severing the MMTL. The primary reason for using the contralateral sham knee in these experiments is because we are using F44 generation families and the control knee must have exactly the same genotype as the DMM knee. Mice were sacrificed by CO2 asphyxiation at indicated time points. Knees were harvested and subjected to histological and micro-CT analyses. After surgery, mice were transferred to their original cages with original inmates in a group of 4–5 mice. All mice were weight bearing after 2 hours when they recovered from anesthesia. No running wheels or casts were provided therefore all the mice experienced equal levels of activity. All surgeries were performed by one surgeon.
Micro-CT analysis and phenotypic scoring
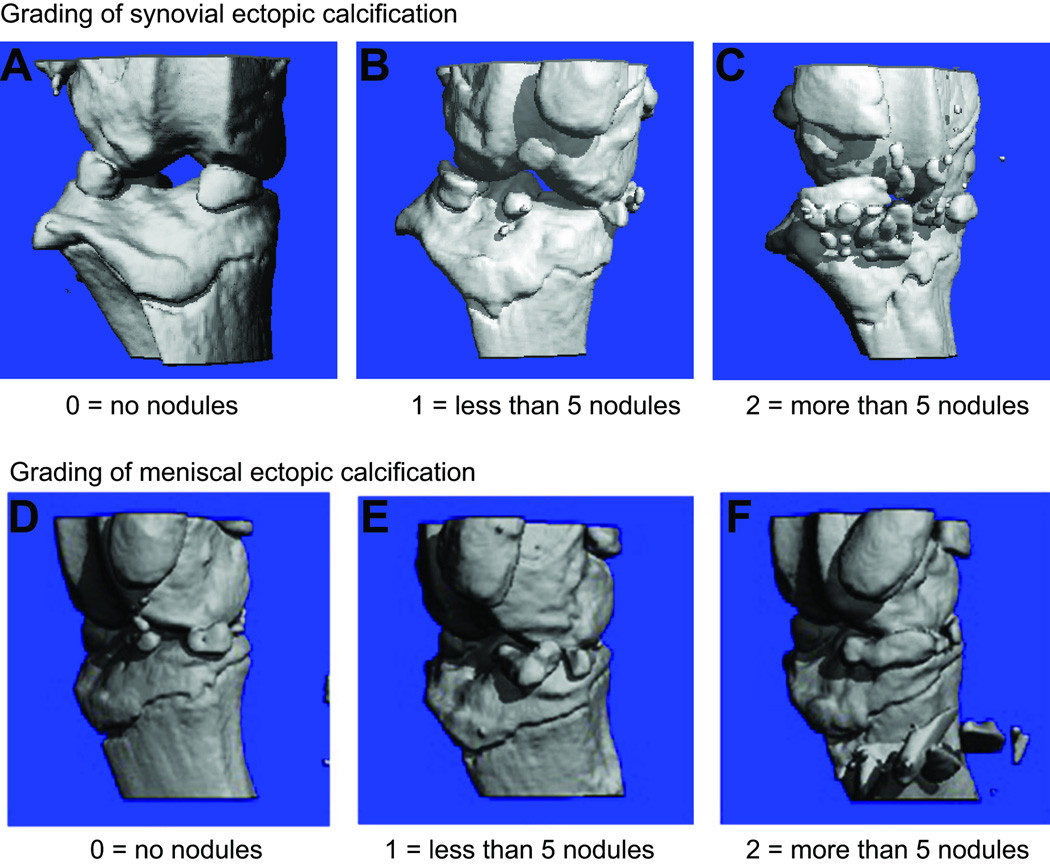
Ectopic calcifications were visualized by micro-computed tomography (micro-CT) scanner (Scanco-Medical) as described previously20. Each mouse was scored for presence/absence of synovial and/or meniscal ectopic calcifications as well as according to a graded scale: 0 = no nodules, 1 = <5 nodules, 2 = ≥5 nodules (Fig 1). We coded meniscal and synovial calcifications as separate phenotypes for the following reasons: The mouse meniscus is normally partially calcified, to a degree sufficient to render them visible on micro-CT. So, in this case, we are scoring the extent of normal calcification. The synovial tissue, on the other hand, is normally un-mineralized soft tissue. Hence it is quite possible that the two phenotypes are mechanistically unrelated, de novo calcification versus enhanced calcification. Further, measured phenotypes for meniscus calcifications and synovial calcifications are uncorrelated in a chi-square test of association (Cramer’s V = 0.040, p = 0.905, 3 df); they are distributed as independent traits.
Fig. 1. Scoring scheme to evaluate ectopic calcification.
We used binary and graded scoring schemes to evaluate the intensity of ectopic synovial (A–C) and meniscal (D–F) calcifications. Knees with no nodules were scored as absent (A, D) while knees with any calcification were scored as present (B, C, E, F) using binary scoring scheme. In the graded scheme 0 means no nodules (A, D), 1 means <5 nodules (B, E) and 2 means ≥5 nodules (C, F).
Histological analysis
Harvested knees were fixed overnight in 4% paraformaldehyde and methacrylate embedded. For proteoglycan detection, 10-um coronal sections were stained with Alcian blue and Safranin-O, and counterstained with fast green. Mineralization was detected with 1% silver nitrate as per the von Kossa method. For immunohistochemistry of collagen types I and collagen type II, knees were fixed in 10% neutral buffered-formalin, decalcified with 10% formic acid in 5% formaldehyde for 48-hours, and embedded in paraffin. Coronal sections (5-um in thickness) were taken through the joint and evaluated for immunofluorescence as described elsewhere22.
QTL mapping
Individuals of the F44 and their F43 parents were genotyped at 4,588 single nucleotide polymorphisms (SNPs) distributed across the 19 autosomes of the mouse genome23. A genetic map was constructed based on the physical ordering of SNPs along each autosome (Supplementary Table 1) according to NCBI build 37/mm9 (http://genome.ucsc.edu/) using R/qtl. Using the Hapi software24, ordered heterozygous genotypes were estimated twice from family data. Heterozygous genotypes were input initially as “LG/J:SM/J” and then “SM/J LG/J”. Loci with discrepant calls between the two analyses were treated as having missing phase information.
Based on each individual’s marker genotypes, we utilized Haley-Knott regression25 to impute additional genotypes every 1 centimorgan (cM), assigning additive, dominance, and imprinting genotypic scores at each marker and imputed position18. Then two mapping models were compared using the Mixed Procedure in SAS v9.1 (SAS Institute, Cary, NC). The full-model included sex plus genomic location effects and their interactions with sex as fixed effects, and family assignment and its interaction with sex as random effects. The −2 ln(likelihood) of the reduced and full-models were compared to a null-model including a sex effect but no specific genetic effect, using χ2 test with 3 and 6 degrees-of-freedom, respectively. Probabilities were transformed into LPR = −log10(Pr). Bonferroni-adjusted genome-wise and chromosome-wise significance thresholds were calculated (Supplementary Table 1). Since meniscal and synovial calcifications were scored twice according to our binary and graded classifications, QTLs of a given full- or reduced-model were considered valid only when they achieved at least chromosome-wise significance on one scale and at least point-wise significance at 10% level on the other scale. For loci significant under the full mapping model and with interaction terms significant at the point-wise level (LPR≥1.3), the data were partitioned by sex and refitted to the reduced-model. We used the standard of a peak LPR score ±1 LPR to define 95% positional support intervals. When two QTLs for the same phenotype appeared on the same chromosome within 50 cM of each other, the two positions were considered individually and jointly. A 2-QTL model was accepted if it fitted better at 5% significance than the best 1-QTL model using a likelihood ratio test.
SNP analysis
Using UCSC genome browser annotations of the NCBI37/mm9 assembly (http://genome.ucsc.edu/), we identified SNPs in the exons, introns, and untranslated regions of each candidate. SNPs occurring within 2500 base-pairs up- and down-stream of transcription start and stop sites were considered. Evolutionary conservation scores for each SNP were obtained from the PhastCons30Placental table (UCSC browser). Nonsynonymous SNPs were evaluated for potential functional significance by PolyPhen-2, SIFT, and LRT algorithms19; 26–29.
Candidate genes and functional classifications
We analyzed candidate genes by GeneGo MetaCore (https://portal.genego.com) to assess the gene ontology. We then performed a PubMed search for each candidate using its name plus these terms joined by the Boolean operator OR: arthritis, bone, calcification, cartilage, mineralization, osteocyte, synovium. Search results were perused for relevance to OA processes or skeletal tissue physiology. Further information regarding the molecular function, cellular component, and biological process were probed using Mouse Genome Informatics (MGI) gene ontology database (http://www.informatics.jax.org). We also used gene expression omnibus (http://www.ncbi.nlm.nih.gov/geo) to search for mutant alleles of our positional candidates with effects on skeletal tissues and to examine information regarding their expression patterns.
Enrichment ankylosing spondylitis-associated genes
We tested whether our QTL support intervals were enriched for candidates with human orthologs associated with ankylosing spondylitis. We compiled a list of 213 genes associated with ankylosing spondylitis using the Genotator database (http://www.genotator.hms.harvard.edu/geno). For each actual QTL detected, we positioned a support interval of equal size randomly in the mouse genome without resampling positions, counting the number of genes within intervals that occur on the disease association list. The process was repeated 1,000 times to arrive at the expected number of such occurrences.
Validation of candidate genes in parental LG/J and SM/J inbred strains
To validate the functional significance of the selected QTL candidate genes, we set out to compare mRNA expression levels in knee joint tissues between parental LG/J and SM/J inbred strains for 78 candidate genes. Using five mice from each parental strain, we performed DMM in the right knees as described above. At 8-week post-surgery, knees were harvested and scanned to evaluate ectopic calcification by the aforementioned micro-CT analysis. The knee joints were fixed in 10% neutral buffered formalin and decalcified using a mixture of 10% formic acid and 5% formaldehyde solution for 2-days at 4°C. Then the joints were incubated in 0.01 M ethylenediaminetetraacetic acid for another 6 hours at room temperature. Samples were paraffin embedded, mounted in blocks, and sagittally sectioned at 5 µm intervals using standard methods. The sections were mounted on polylysine-coated slides (Fisher Scientific, Silver Spring, MD) before staining. We used the Affymetrix QuantiGene Plex assay (Panomics Inc., Fremont, CA) to measure the mRNA expression in tissue lysate prepared from formalin-fixed paraffin embedded histological sections29. Briefly, 25 sections of sham- and DMM-operated knees were macrodissected to collect the entirety of the knee capsule tissues including subchondral bone of the tibia and femur, while removing excess paraffin and extraneous long bone material, prior to tissue homogenization. Tissue homogenates were prepared in a mixture of homogenizing solution and proteinase K. The mRNA expression of candidate genes was measured in tissue lysates by the Affymetrix QuantiGene Plex assay (Panomics Inc., Fremont, CA) as described previously29. The expression of candidate genes was normalized by obtaining their residuals from a multiple regression using Gapdh and Hprt1 readings as independent variables. Residuals from replicates were then averaged to obtain normalized readings from each knee per individual mouse. LG/J and SM/J gene expression levels were compared using correlated t-tests in which the expression level of the sham-operated knee was subtracted from the DMM-knee of each individual. We also computed the Pearson’s correlation coefficients between ectopic calcification scores and levels of gene expression for those candidate genes whose expression levels were significantly different between LG/J and SM/J at the ≤10% level of significance.
RESULTS
Phenotypic analysis
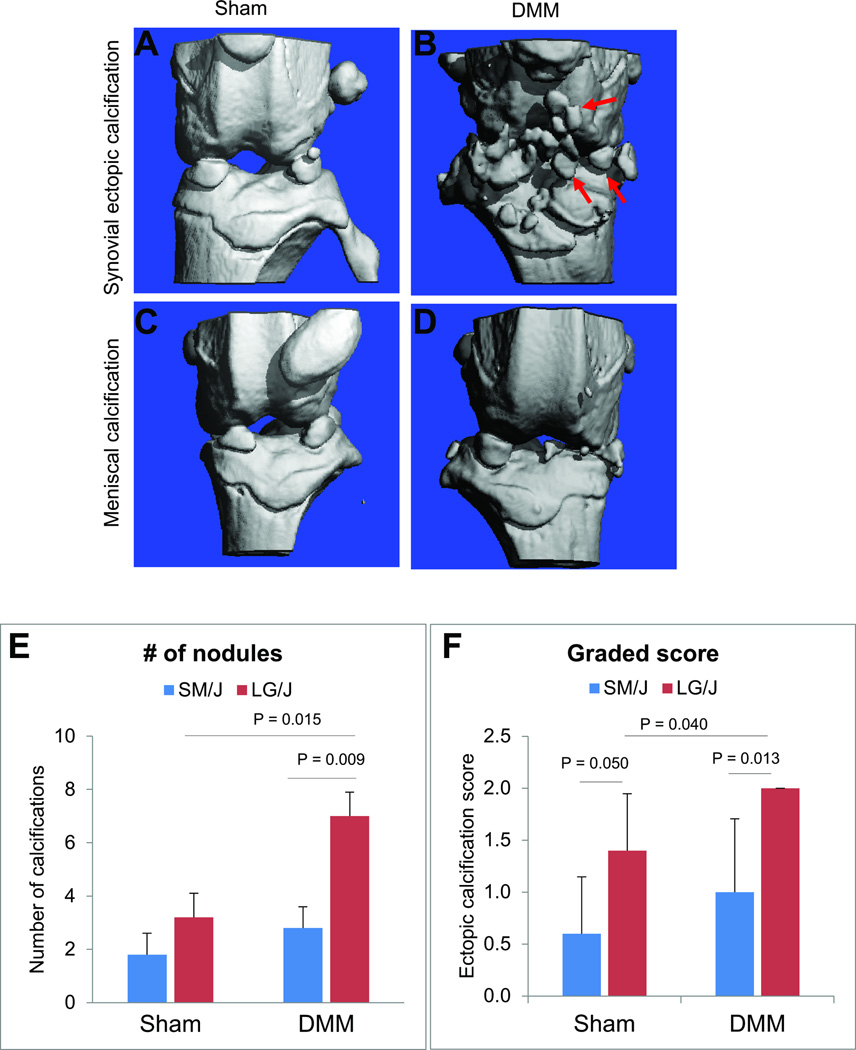
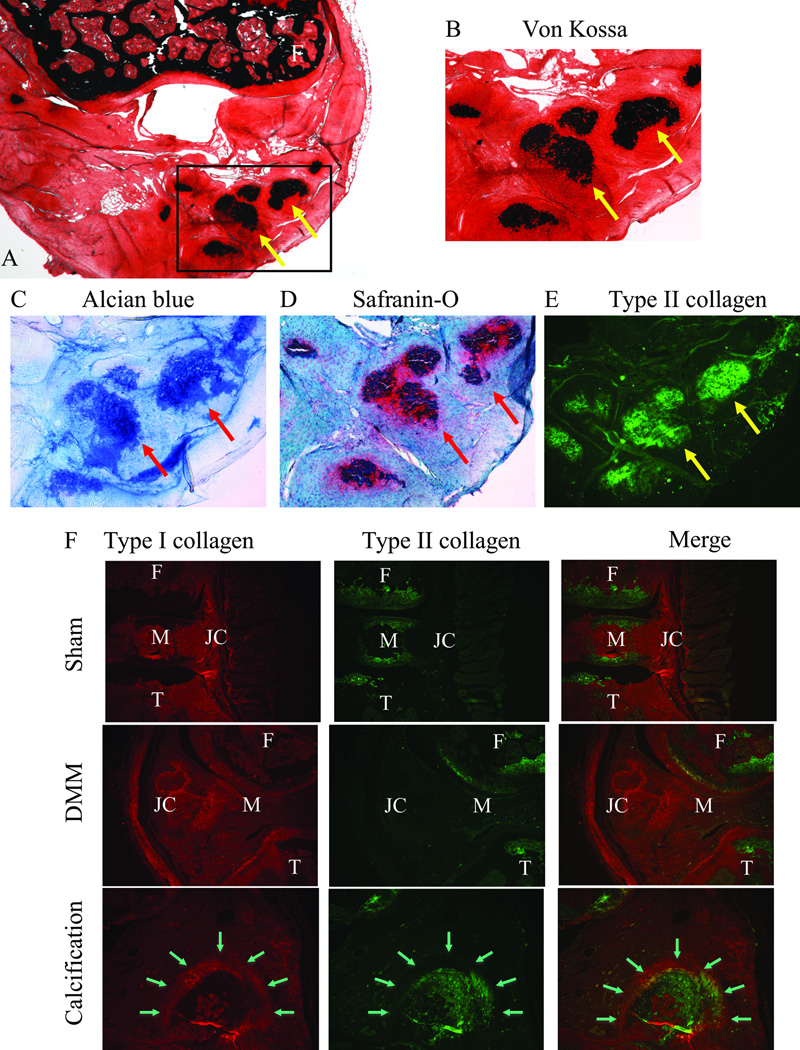
Ectopic nodules were analyzed at 8-weeks post-injury. Micro-CT analysis revealed calcified nodules around joint capsule, synovium, or meniscus only in the knees that underwent DMM surgery (Fig. 2B and 2D). No nodules were present in sham-operated knees (Fig. 2A and 2C). It was shown that LG/J mouse strain developed significantly more ectopic calcification in the right operated knee compared to contralateral left sham-operated knee as well as when compared to the DMM-operated knee of SM/J mouse strain (Fig. 2E and 2F). Our histological analysis confirmed the mineralized nature of ectopic nodules as was evident by von Kossa staining (Fig. 3A–B). These nodules stained positively for Alcian blue and Safranin-O, indicating abundant expression of glycosaminoglycan substituted proteoglycan components (Fig. 3C–D) of the type primarily present in cartilage along with collagen type II expression (Fig. 3E). These regions also stained positively for both collagen types I and II (Fig. 3F). The medial joint capsule of DMM-treated knees showed hypertrophy and expressed higher type II collagen compared to sham. Type II collagen expression was predominately on the periphery of the nodules while type I collagen was expressed mainly in the center.
Fig. 2. Typical phenotypic outcomes in sham and DMM knees.
F44 advanced intercross line mice were subjected to DMM at 10-week of age. Micro-CT analysis on the harvested knees was performed 8-week post-surgery. Micro-CT images showed ectopic synovial (A–B) and meniscal (C–D) calcifications exclusively in the knees subjected to DMM. No significant calcifications were observed in contralateral sham knees (A, C). We observed that ectopic synovial calcifications were significantly higher in the knees subjected to DMM of LG/J compared to contralateral sham-operated knee as well as DMM-operated knee of SM/J strain (E–F).
Fig. 3. Histological characteristics of ectopic calcifications.
Lower magnification of anterior joint capsule stained with Von Kossa (A). Yellow arrows indicate ectopic calcification. High magnification of ectopic calcifications stained with von Kossa (B), Alcian blue (C), Safranin-O (D), and immunohistochemistry of type II collagen (E) are also shown. Immunohistochemistry of type I and II collagen on medial knee joint (sham and DMM) and ectopic calcification (arrows) are shown (F). F: Femur, M: Meniscus, T: Tibia, and JC: Joint capsule.
QTL analysis
Overall, we detected 20 QTLs affecting synovial and meniscal calcification phenotypes with 11 QTLs linked to synovial calcification and 10 QTLs affecting meniscal calcification (Table 1). Calcification QTLs are identified by C followed by two numbers separated by a decimal. The first number indicates QTL’s chromosome; the second number identifies particular QTLs on the same chromosome. Mouse chromosomes are acrocentric, and QTLs on the same chromosome are numbered according to their relative position from the centromere. Among all the QTLs, only C19.1 exerted a pleiotropic effect on both phenotypes. While all other QTLs attained at least chromosome-wise significance, C1.2 affecting synovium achieved genome-wise significance with an LPR≥4.35. In contrast, C8.1 was only tentatively accepted since it occurred in a proximal region on chromosome 8 in which SM/J alleles were infrequent after 44 generations of genetic drift; so genotypic means for homozygotes at this locus were estimated from very few individuals.
Table 1.
Summary of quantitative trait loci and phenotypic effects.
| QTL | Chr | Tissue | LPR | Peak Mbpa |
Proximal Mbp |
Distal Mbp |
Interval length |
Effectsb | Sex | a/SDc | d/SD | i/SD | a SEd | d SE | i SE | Genese |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C1.1 | 1 | synovium | 3.99 | 38.746 | 38.094 | 38.932 | 0.838 | a, as, ds | M | −1.223 | −0.834 | 0.338 | 0.134 | 0.167 | 0.068 | 5 |
| F | 0.028 | 0.548 | −0.039 | 0.096 | 0.116 | 0.071 | ||||||||||
| C1.2 | 1 | synovium | 5.20 | 40.035 | 39.657 | 40.460 | 0.803 | a, i, ds, is | M | −0.478 | −0.502 | 0.004 | 0.054 | 0.076 | 0.046 | 6 |
| F | −0.334 | 0.139 | −0.393 | 0.047 | 0.067 | 0.047 | ||||||||||
| C1.3 | 1 | synovium | 3.08 | 155.851 | 155.549 | 156.758 | 1.209 | is | M | 0.054 | −0.102 | 0.511 | 0.052 | 0.088 | 0.050 | 7 |
| F | 0.009 | −0.084 | −0.334 | 0.058 | 0.088 | 0.049 | ||||||||||
| C1.4 | 1 | synovium | 3.77 | 181.640 | 180.846 | 182.258 | 1.412 | a, d, as | M | −0.131 | 0.114 | −0.045 | 0.078 | 0.120 | 0.082 | 9 |
| F | 0.266 | −0.232 | −0.227 | 0.079 | 0.102 | 0.062 | ||||||||||
| C1.5 | 1 | meniscus | 3.15 | 189.133 | 187.601 | 189.319 | 1.717 | i | B | −0.158 | −0.269 | −0.204 | 0.042 | 0.058 | 0.037 | 6 |
| C1.6 | 1 | meniscus | 3.06 | 192.193 | 191.851 | 192.530 | 0.679 | a, d, as, ds | M | −0.069 | −0.048 | −0.186 | 0.075 | 0.111 | 0.056 | 1 |
| F | −0.702 | 0.893 | −0.075 | 0.072 | 0.118 | 0.062 | ||||||||||
| C4.1 | 4 | synovium | 4.04 | 38.157 | 37.705 | 38.537 | 0.832 | a, d, as, ds | M | 0.025 | 0.079 | 0.006 | 0.047 | 0.063 | 0.044 | 0 |
| F | −0.524 | −0.379 | −0.072 | 0.037 | 0.052 | 0.039 | ||||||||||
| C5.1 | 5 | meniscus | 3.71 | 74.537 | 74.281 | 75.687 | 1.406 | a, d | B | 0.589 | 0.477 | 0.115 | 0.057 | 0.069 | 0.037 | 10 |
| C5.2 | 5 | meniscus | 4.06 | 136.041 | 135.867 | 136.190 | 0.322 | a, d, ds, is | M | 0.175 | 0.120 | 0.376 | 0.127 | 0.256 | 0.123 | 6 |
| F | 0.139 | −1.142 | −0.128 | 0.137 | 0.259 | 0.107 | ||||||||||
| C6.1 | 6 | synovium | 3.48 | 4.608 | 4.177 | 5.219 | 1.042 | a, d | B | −0.114 | −0.152 | −0.006 | 0.021 | 0.028 | 0.019 | 8 |
| C6.2 | 6 | meniscus | 3.17 | 61.571 | 59.615 | 63.355 | 3.740 | i, is | M | −0.171 | −0.203 | −0.428 | 0.043 | 0.060 | 0.043 | 4 |
| F | 0.017 | 0.148 | 0.016 | 0.052 | 0.070 | 0.051 | ||||||||||
| C8.1 | 8 | meniscus | 3.20 | 11.324 | 10.972 | 12.150 | 1.178 | as, ds | M | −0.625 | −0.365 | 0.001 | 0.185 | 0.209 | 0.121 | 10 |
| F | 0.331 | 0.709 | −0.218 | 0.153 | 0.201 | 0.129 | ||||||||||
| C9.1 | 9 | synovium | 3.25 | 61.044 | 60.553 | 61.490 | 0.936 | d | B | −0.046 | −0.160 | 0.011 | 0.021 | 0.031 | 0.022 | 3 |
| C9.2 | 9 | synovium | 3.51 | 70.435 | 69.607 | 70.785 | 1.177 | a | B | 0.128 | −0.075 | −0.003 | 0.019 | 0.029 | 0.021 | 12 |
| C10.1 | 10 | meniscus | 3.19 | 86.056 | 85.478 | 86.812 | 1.334 | ds | M | 0.183 | −0.469 | 0.072 | 0.041 | 0.056 | 0.043 | 6 |
| F | −0.027 | 0.345 | 0.152 | 0.047 | 0.068 | 0.052 | ||||||||||
| C11.1 | 11 | synovium | 3.73 | 96.433 | 96.047 | 97.102 | 1.055 | i, ds, is | M | 0.180 | −0.135 | −0.111 | 0.045 | 0.065 | 0.046 | 28 |
| F | 0.051 | 0.291 | 0.468 | 0.044 | 0.062 | 0.044 | ||||||||||
| C13.1 | 13 | synovium | 3.20 | 32.496 | 32.257 | 33.510 | 1.253 | a, d, i | B | 0.324 | −0.237 | 0.163 | 0.034 | 0.043 | 0.030 | 13 |
| C14.1 | 14 | meniscus | 3.57 | 120.301 | 120.062 | 120.872 | 0.810 | a, is | M | −0.292 | −0.333 | −0.460 | 0.069 | 0.095 | 0.053 | 3 |
| F | −0.514 | −0.148 | 0.142 | 0.081 | 0.116 | 0.062 | ||||||||||
| C15.1 | 15 | meniscus | 3.51 | 27.470 | 27.316 | 27.624 | 0.307 | d | B | −0.119 | 0.761 | 0.099 | 0.045 | 0.075 | 0.040 | |
| C19.1 | 19 | synovium | 3.58 | 51.468 | 50.737 | 51.891 | 1.154 | a, d | B | 0.308 | −0.477 | −0.109 | 0.036 | 0.051 | 0.033 | 3 |
| C19.1 | 19 | meniscus | 3.10 | 51.517 | 50.982 | 51.894 | 0.911 | d, as, is | M | 0.121 | 0.418 | 0.139 | 0.116 | 0.165 | 0.102 | 1 |
| F | −0.332 | 0.245 | −0.213 | 0.114 | 0.159 | 0.101 |
QTL = quantitative trait locus; LPR = -log (probability); a. Mbp = genome coordinates in millions of base pairs; b. a, d, i = additive, dominance, imprinting effects; as, ds, is = additive, dominance, imprinting by sex interaction effects; c. SD = standard deviation; c. SD = standard deviation; d. SE = standard error; e. number of known genes within interval; M = male, F = female, B = both (pooled sexes); The standardized effect sizes in boldface are statistically significant at ≤5% level
Sex effects were important, with 13 loci demonstrating significantly different effect sizes dependent on sex. An additive effect is half the difference between the means calcification scores of the two homozygotes. To compare effect sizes across phenotypes, we standardized them by dividing by their respective standard deviations (SD) obtained via one-way analysis of variance with sex as the independent factor. LG/J at C1.1 conferred the largest additive protective effect with a/SD = −1.223. This effect was only significant in males and was the largest overall effect. C5.1 conferred the largest additive increase in susceptibility with a/SD = 0.589 and affected both sexes equally. Among homozygotes at C8.1, the LG/J allele protected males (a/SD = −0.625) but increased ectopic calcifications in females (a/SD = 0.331). Of 14 significant additive effects, the LG/J allele was found to be protective against developing ectopic calcification at eight QTLs and susceptible to it at six locations (Table 1).
We detected 13 significant dominance effects (describing the difference between calcification score of heterozygotes and homozygotes), 10 of which reduced calcification scores indicating the general dominance of protective alleles. The largest reduction of susceptibility was conferred on males by C1.2 with d/SD = −0.502. In terms of dominance, females were most susceptible at C1.6 (d/SD = 0.893). Males were protected at C10.1 (d/SD = −0.469), while females were more prone to develop ectopic calcifications (d/SD = 0.345).
Given our experimental design, we were able to investigate imprinting effects at heterozygous loci, which measured phenotypic differences between heterozygotes receiving their LG/J and SM/J alleles from parents of opposite sex. We observed 10 cases of significant imprinting, similar in number to additive and dominance effects that are more commonly tested in genetic studies. Inheriting the LG/J allele from the dam reduced susceptibility in six of 10 cases of significant imprinting, most notably for males at C14.1 (i/SD = −0.460). At C1.3, inheriting LG/J from the sire protected females (i/SD = −0.334) but made males more susceptible (i/SD = 0.511).
Candidate gene nomination and prioritization
The average QTL support interval size was 1.15 megabases, containing on average seven or eight genes in the F44 advanced intercross line. From 141 positional candidates clustered in 20 QTL regions (Supplementary Table 2), we compiled two lists of priority candidate genes. An initial list of 35 candidates was determined through bibliographic searches to be associated with normal or disease states affecting skeletal tissues or to be involved in ectopic mineralization of soft tissues. A second list of 66 candidates was based on genes containing non-synonymous SNPs and/or highly conserved SNPs at noncoding positions (Table 2). Of these, 25 candidates harbored nonsynonymous SNPs and were further scrutinized using predictive algorithms for the likelihood that the resulting amino acid changes have functional consequences. The following genes contained the greatest number of coding SNPs in their respective QTL intervals: Aff3 (C1.1), Il1rl2 (C1.2), Zfp648 (C1.3), Rrp15 (C1.5), Hip1 (C5.2), Uaca (C9.1), Stab2 (C10.1), Oxgr1 (C14.1), Fam105a (C15.1), and Sorcs1 (C19.1) (Table 2; Supplementary Table 3). Stab2, Oxgr1, and Fam105a were predicted by either LRT, PolyPhen-2, and/or SIFT algorithms as encoding amino-acid polymorphisms of likely functional consequence, and as such were among the most highly ranked candidates. Il1rl2 and Stab2 are also distinguished by carrying numerous nonsynonymous SNPs, six and 14, respectively. MGI databases indicated that Aff3 is expressed in the axial skeleton, with one known mutant producing malformed vertebrae. Similarly, one mutant form of Hip1 caused axial malformations but yielded no specific arthritic phenotypes in joints of the appendicular skeleton. Of the other nonsynonymous SNP-carrying candidates, very little information is available regarding expression and functional roles in skeletal tissues. Fifty-eight genes from our list of 66 candidates contained only highly conserved noncoding SNPs. According to the MGI database, Rasl11b is expressed in pelvic skeletal tissue, Pon1 and Pon2 are expressed in the femur, and Gmds is expressed in appendicular skeleton. QTL interval C4.1 contained no known genes, and its phenotypic effects were likely due to regulatory sequences.
Table 2.
Top 66 candidate genes based on SNP composition
| Total SNPs | Coding SNPs | Noncoding SNPs | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| QTL | tissue | Gene* | RefGeneID | SNPs | HCa | Total coding |
HC | nsynb | HC | Predictionc | synd | HC | Total noncoding |
HC | 2500 bp up stream |
HC | 5' UTR |
HC | Introns | HC | 3' UTR |
HC | 2500 bp down stream |
HC |
| C1.1 | synovium | Aff3 | NM_010678 | 587 | 16 | 6 | 2 | 2 | 0 | . | 4 | 2 | 581 | 14 | 0 | 0 | 0 | 0 | 557 | 14 | 2 | 0 | 22 | 0 |
| Lonrf2 | NM_001029878 | 7 | 2 | 0 | 0 | 0 | 0 | . | 0 | 0 | 7 | 2 | 0 | 0 | 1 | 1 | 2 | 0 | 1 | 1 | 3 | 0 | ||
| Chst10 | NM_145142 | 174 | 2 | 1 | 0 | 0 | 0 | . | 1 | 0 | 173 | 2 | 8 | 0 | 0 | 0 | 138 | 2 | 8 | 0 | 19 | 0 | ||
| C1.2 | synovium | Rfx8 | NM_001145660 | 44 | 1 | 0 | 0 | 0 | 0 | . | 0 | 0 | 44 | 1 | 8 | 0 | 0 | 0 | 36 | 1 | 0 | 0 | 0 | 0 |
| Map4k4 | NM_001252200 | 380 | 12 | 7 | 6 | 0 | 0 | . | 7 | 6 | 373 | 6 | 10 | 0 | 0 | 0 | 358 | 6 | 2 | 0 | 3 | 0 | ||
| Il1rl2 | NM_133193 | 273 | 4 | 13 | 3 | 6 | 0 | . | 7 | 3 | 260 | 1 | 14 | 0 | 1 | 0 | 220 | 1 | 0 | 0 | 25 | 0 | ||
| C1.3 | synovium | Teddm1 | NM_178244 | 1 | 1 | 0 | 0 | 0 | 0 | . | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 |
| Zfp648 | NM_001204908 | 1 | 0 | 1 | 0 | 1 | 0 | . | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| C1.4 | synovium | Kif26b | NM_001161665 | 368 | 7 | 0 | 0 | 0 | 0 | . | 0 | 0 | 368 | 7 | 0 | 0 | 0 | 0 | 368 | 7 | 0 | 0 | 0 | 0 |
| Smyd3 | NM_027188 | 6 | 1 | 0 | 0 | 0 | 0 | . | 0 | 0 | 6 | 1 | 0 | 0 | 0 | 0 | 6 | 1 | 0 | 0 | 0 | 0 | ||
| C1.5 | meniscus | Rrp15 | NM_026041 | 46 | 3 | 1 | 0 | 1 | 0 | . | 0 | 0 | 45 | 3 | 3 | 0 | 0 | 0 | 40 | 3 | 1 | 0 | 1 | 0 |
| D1Pas1 | NM_033077 | 6 | 1 | 0 | 0 | 0 | 0 | . | 0 | 0 | 6 | 1 | 4 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | ||
| Spata17 | NM_028848 | 149 | 6 | 0 | 0 | 0 | 0 | . | 0 | 0 | 149 | 6 | 2 | 0 | 0 | 0 | 140 | 6 | 0 | 0 | 7 | 0 | ||
| Gpatch2 | NM_026367 | 412 | 14 | 1 | 0 | 0 | 0 | . | 1 | 0 | 411 | 14 | 1 | 0 | 0 | 0 | 410 | 14 | 0 | 0 | 0 | 0 | ||
| C1.6 | meniscus | Prox1 | NM_008937 | 99 | 7 | 1 | 0 | 0 | 0 | . | 1 | 0 | 98 | 7 | 5 | 0 | 0 | 0 | 91 | 6 | 0 | 0 | 2 | 1 |
| C5.1 | meniscus | Rasl11b | NM_026878 | 26 | 4 | 3 | 2 | 0 | 0 | . | 3 | 2 | 23 | 2 | 12 | 0 | 1 | 0 | 6 | 2 | 0 | 0 | 4 | 0 |
| Scfd2 | NM_001114660 | 492 | 7 | 0 | 0 | 0 | 0 | . | 0 | 0 | 492 | 7 | 0 | 0 | 0 | 0 | 456 | 7 | 10 | 0 | 26 | 0 | ||
| Fip1l1 | NM_001159573 | 40 | 1 | 0 | 0 | 0 | 0 | . | 0 | 0 | 40 | 1 | 0 | 0 | 0 | 0 | 34 | 1 | 2 | 0 | 4 | 0 | ||
| Lnx1 | NM_001159578 | 376 | 4 | 3 | 1 | 0 | 0 | . | 3 | 1 | 375 | 3 | 9 | 0 | 1 | 0 | 356 | 3 | 7 | 0 | 2 | 0 | ||
| C5.2 | meniscus | Pom121 | NM_148932 | 1 | 1 | 0 | 0 | 0 | 0 | . | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| Hip1 | NM_146001 | 23 | 0 | 2 | 0 | 1 | 0 | . | 1 | 0 | 21 | 0 | 1 | 0 | 0 | 0 | 20 | 0 | 0 | 0 | 0 | 0 | ||
| Ccl26 | NM_001013412 | 8 | 3 | 1 | 1 | 0 | 0 | . | 0 | 0 | 7 | 2 | 2 | 0 | 1 | 1 | 2 | 0 | 1 | 1 | 1 | 0 | ||
| C6.1 | synovium | Casd1 | NM_145398 | 19 | 1 | 0 | 0 | 0 | 0 | . | 0 | 0 | 19 | 1 | 2 | 1 | 0 | 0 | 15 | 0 | 0 | 0 | 2 | 0 |
| Sgce | NM_001130190 | 99 | 5 | 0 | 0 | 0 | 0 | . | 0 | 0 | 99 | 5 | 2 | 0 | 0 | 0 | 89 | 5 | 4 | 0 | 4 | 0 | ||
| Ppp1r9a | NM_181595 | 48 | 1 | 0 | 0 | 0 | 0 | . | 0 | 0 | 48 | 1 | 3 | 0 | 0 | 0 | 38 | 1 | 6 | 0 | 1 | 0 | ||
| Pon1 | NM_011134 | 130 | 5 | 3 | 1 | 0 | 0 | . | 3 | 1 | 127 | 4 | 0 | 0 | 0 | 0 | 126 | 4 | 0 | 0 | 1 | 0 | ||
| Pon2 | NM_183308 | 62 | 4 | 3 | 3 | 0 | 0 | . | 3 | 3 | 59 | 1 | 5 | 0 | 0 | 0 | 49 | 1 | 2 | 0 | 3 | 0 | ||
| C6.2 | meniscus | Grid2 | NM_008167 | 1556 | 17 | 2 | 1 | 0 | 0 | . | 2 | 1 | 1554 | 16 | 0 | 0 | 1 | 1 | 1552 | 15 | 0 | 0 | 1 | 0 |
| C8.1 | meniscus | Irs2 | NM_001081212 | 3 | 3 | 1 | 1 | 0 | 0 | . | 0 | 0 | 2 | 2 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 |
| Col4a2 | NM_009932 | 637 | 11 | 23 | 10 | 3 | 0 | . | 20 | 10 | 614 | 1 | 0 | 0 | 0 | 0 | 604 | 1 | 5 | 0 | 5 | 0 | ||
| Rab20 | NM_011227 | 241 | 4 | 3 | 1 | 1 | 0 | . | 2 | 1 | 238 | 3 | 25 | 0 | 1 | 1 | 197 | 1 | 1 | 1 | 14 | 0 | ||
| Carkd | NM_001190357 | 198 | 0 | 2 | 0 | 1 | 0 | . | 1 | 0 | 196 | 0 | 32 | 0 | 2 | 0 | 135 | 0 | 3 | 0 | 24 | 0 | ||
| Cars2 | NM_024248 | 405 | 0 | 6 | 0 | 2 | 0 | . | 4 | 0 | 399 | 0 | 24 | 0 | 1 | 0 | 347 | 0 | 0 | 0 | 27 | 0 | ||
| Ankrd10 | NM_001281975 | 149 | 7 | 2 | 1 | 1 | 1 | . | 1 | 0 | 147 | 6 | 16 | 0 | 1 | 1 | 115 | 5 | 0 | 0 | 15 | 0 | ||
| C9.1 | synovium | Uaca | NM_028283 | 235 | 5 | 20 | 4 | 3 | 0 | . | 17 | 4 | 215 | 1 | 1 | 0 | 0 | 0 | 198 | 0 | 0 | 0 | 16 | 1 |
| Tle3 | NM_001083927 | 46 | 4 | 3 | 2 | 0 | 0 | . | 3 | 2 | 43 | 2 | 1 | 0 | 0 | 0 | 33 | 2 | 3 | 0 | 6 | 0 | ||
| C9.2 | synovium | Fam81a | NM_029784 | 109 | 2 | 0 | 0 | 0 | 0 | . | 0 | 0 | 109 | 2 | 7 | 0 | 0 | 0 | 101 | 2 | 0 | 0 | 1 | 0 |
| Myo1e | NM_181072 | 513 | 15 | 7 | 5 | 1 | 1 | . | 6 | 4 | 506 | 10 | 0 | 0 | 0 | 0 | 502 | 10 | 2 | 0 | 2 | 0 | ||
| Fam63b | NM_172772 | 5 | 1 | 1 | 1 | 1 | 1 | LRT | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 1 | 0 | 0 | 0 | ||
| Adam10 | NM_007399 | 9 | 1 | 0 | 0 | 0 | 0 | . | 0 | 0 | 9 | 1 | 0 | 0 | 0 | 0 | 9 | 1 | 0 | 0 | 0 | 0 | ||
| C10.1 | meniscus | Syn3 | NM_013722 | 687 | 14 | 3 | 1 | 0 | 0 | . | 3 | 1 | 684 | 13 | 0 | 0 | 0 | 0 | 635 | 13 | 47 | 0 | 2 | 0 |
| Timp3 | NM_011595 | 139 | 6 | 2 | 2 | 0 | 0 | . | 2 | 2 | 137 | 4 | 26 | 0 | 0 | 0 | 104 | 4 | 4 | 0 | 3 | 0 | ||
| Stab2 | NM_138673 | 826 | 5 | 25 | 3 | 14 | 1 | LRT | 11 | 2 | 801 | 2 | 37 | 0 | 0 | 0 | 762 | 2 | 0 | 0 | 2 | 0 | ||
| C11.1 | synovium | Hoxb9 | NM_008270 | 18 | 5 | 0 | 0 | 0 | 0 | . | 0 | 0 | 18 | 5 | 6 | 0 | 0 | 0 | 3 | 2 | 1 | 0 | 8 | 3 |
| Skap1 | NM_001033186 | 2063 | 160 | 7 | 4 | 3 | 1 | Polyphen2 | 4 | 3 | 2056 | 156 | 0 | 0 | 0 | 0 | 1960 | 156 | 62 | 0 | 34 | 0 | ||
| Snx11 | NM_001163389 | 60 | 2 | 3 | 1 | 2 | 1 | . | 1 | 0 | 57 | 1 | 8 | 0 | 0 | 0 | 36 | 0 | 4 | 0 | 9 | 1 | ||
| Cbx1 | NM_007622 | 108 | 4 | 0 | 0 | 0 | 0 | . | 0 | 0 | 108 | 4 | 15 | 0 | 1 | 0 | 78 | 4 | 2 | 0 | 12 | 0 | ||
| Nfe2l1 | NM_001130450 | 66 | 9 | 3 | 3 | 0 | 0 | . | 3 | 3 | 63 | 6 | 19 | 2 | 0 | 0 | 20 | 3 | 3 | 0 | 21 | 1 | ||
| Copz2 | NM_019877 | 164 | 3 | 1 | 1 | 0 | 0 | . | 1 | 1 | 163 | 2 | 28 | 0 | 0 | 0 | 112 | 2 | 3 | 0 | 20 | 0 | ||
| Cdk5rap3 | NM_030248 | 93 | 6 | 9 | 4 | 3 | 1 | . | 6 | 3 | 84 | 2 | 15 | 0 | 0 | 0 | 50 | 0 | 4 | 0 | 15 | 2 | ||
| Pnpo | NM_134021 | 70 | 2 | 2 | 1 | 1 | 0 | . | 1 | 1 | 68 | 1 | 22 | 1 | 0 | 0 | 21 | 0 | 10 | 0 | 15 | 0 | ||
| Sp2 | NM_001080964 | 123 | 6 | 5 | 5 | 0 | 0 | . | 5 | 5 | 118 | 1 | 7 | 0 | 0 | 0 | 89 | 1 | 4 | 0 | 18 | 0 | ||
| Sp6 | NM_031183 | 68 | 3 | 1 | 0 | 0 | 0 | . | 1 | 0 | 67 | 3 | 14 | 2 | 0 | 0 | 36 | 1 | 5 | 0 | 12 | 0 | ||
| Scrn2 | NM_146027 | 37 | 3 | 6 | 2 | 2 | 0 | . | 4 | 2 | 31 | 1 | 8 | 0 | 0 | 0 | 7 | 0 | 0 | 0 | 16 | 1 | ||
| Lrrc46 | NM_027026 | 70 | 5 | 5 | 1 | 2 | 0 | . | 3 | 1 | 65 | 4 | 15 | 0 | 2 | 0 | 32 | 2 | 1 | 0 | 15 | 2 | ||
| Mrpl10 | NM_026154 | 93 | 4 | 5 | 3 | 2 | 1 | . | 3 | 2 | 88 | 1 | 15 | 0 | 0 | 0 | 50 | 0 | 12 | 0 | 11 | 1 | ||
| Osbpl7 | NM_001081434 | 181 | 10 | 12 | 10 | 1 | 0 | . | 11 | 10 | 169 | 0 | 20 | 0 | 1 | 0 | 126 | 0 | 2 | 0 | 20 | 0 | ||
| Tbx21 | NM_019507 | 74 | 4 | 4 | 2 | 0 | 0 | . | 4 | 2 | 70 | 2 | 0 | 0 | 0 | 0 | 46 | 2 | 7 | 0 | 17 | 0 | ||
| C13.1 | synovium | Gmds | NM_146041 | 81 | 2 | 0 | 0 | 0 | 0 | . | 0 | 0 | 81 | 2 | 1 | 0 | 0 | 0 | 79 | 2 | 0 | 0 | 1 | 0 |
| C14.1 | meniscus | Hs6st3 | NM_015820 | 844 | 25 | 1 | 0 | 0 | 0 | . | 1 | 0 | 843 | 25 | 0 | 0 | 0 | 0 | 843 | 25 | 0 | 0 | 0 | 0 |
| Oxgr1 | NM_001001490 | 48 | 2 | 5 | 2 | 3 | 2 | LRT; SIFT | 2 | 0 | 43 | 0 | 7 | 0 | 0 | 0 | 32 | 0 | 1 | 0 | 3 | 0 | ||
| Mbnl2 | NM_175341 | 691 | 27 | 2 | 2 | 0 | 0 | . | 2 | 2 | 689 | 25 | 0 | 0 | 1 | 0 | 668 | 25 | 1 | 0 | 19 | 0 | ||
| C15.1 | meniscus | Ank | NM_020332 | 520 | 2 | 3 | 2 | 0 | 0 | . | 3 | 2 | 519 | 1 | 2 | 0 | 0 | 0 | 513 | 1 | 2 | 0 | 2 | 0 |
| Fam105b | NM_001013792 | 82 | 2 | 0 | 0 | 0 | 0 | . | 0 | 0 | 81 | 1 | 33 | 0 | 0 | 0 | 47 | 1 | 0 | 0 | 1 | 0 | ||
| Fam105a | NM_001242424 | 254 | 3 | 6 | 2 | 3 | 0 | LRT; Polyphen2 | 3 | 2 | 253 | 2 | 0 | 0 | 0 | 0 | 187 | 2 | 25 | 0 | 41 | 0 | ||
| C19.1 | meniscus/synovium | Sorcs1 | NM_001252501 | 1885 | 48 | 4 | 0 | 2 | 0 | . | 2 | 0 | 1881 | 48 | 10 | 0 | 0 | 0 | 1841 | 48 | 10 | 0 | 20 | 0 |
QTL = quantitative trait locus; SNP = single nucleotide polymorphism; a. Highly conserved; b. nonsynonymous; c. LRT, SIFT, and/or PolyPhen-2 algorithm predictions of SNP; d. synonymous; Top nominated genes based on SNP composition and algorithm per interval are in boldface.
Ankylosing spondylitis-associated gene enrichment
From our QTL analysis, we found that four candidate genes have human orthologs that are associated with ankylosing spondylitis (Ank, Pon1, Il1r2, Tbkbp1). From our simulation described above, we expect to observe on average 1.66 such genes by random chance. The non-parametric probability of the result is P = 0.059, suggesting that whatever processes underlie ankylosing-spondylitis in human are likely important in ectopic calcification in our mouse model of knee injury.
Gene expression differences between parental LG/J and SM/J inbred strains
We observed that the expression levels of 11 candidate genes were significantly different between LG/J and SM/J mice at the 10% level or lower: Aff3 (P = 0.03), Gpatch2 (P = 0.07), Pon2 (P = 0.06), Uaca (P = 0.10), Fam81a (P = 0.02), Syn3 (P = 0.03), Copz2 (P = 0.07), Skap1 (P = 0.03), Mrpl10 (P = 0.04), Nfe2l1 (P = 0.05) and Ank (P = 0.09) (Table 3). Correlation analysis showed that Aff3 (r = 0.56; P = 0.02), Fam81a (r = 0.46; P = 0.06), Syn3 (r = 0.43; P = 0.08), and Ank (r = 0.40, P = 0.10) were correlated with synovial calcification phenotype.
Table 3.
Gene transcripts significantly differentially expressed between parental LG/J and SM/J inbred mouse strains
| Chromosome | Gene | LG/J | SM/J | P value | Standard error |
QTL interval |
HC noncoding SNPs |
correlation with calcification |
P value |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Aff3 | 0.048 | −0.055 | 0.03 | 0.038 | C1.1 | 14 | 0.56 | 0.02* |
| 1 | Gpatch2 | 0.008 | −0.101 | 0.07 | 0.053 | C1.5 | 14 | 0.39 | 0.11 |
| 6 | Pon2 | 0.021 | −0.054 | 0.06 | 0.036 | C6.1 | 1 | 0.38 | 0.12 |
| 9 | Uaca | 0.019 | −0.087 | 0.10 | 0.059 | C9.1 | 1 | 0.18 | 0.48 |
| 9 | Fam81a | 0.057 | −0.061 | 0.02 | 0.039 | C9.2 | 2 | 0.46 | 0.06* |
| 10 | Syn3 | 0.092 | −0.018 | 0.03 | 0.040 | C10.1 | 13 | 0.43 | 0.08* |
| 11 | Copz2 | 0.075 | −0.052 | 0.07 | 0.061 | C11.1 | 2 | −0.27 | 0.26 |
| 11 | Skap1 | 0.073 | −0.040 | 0.03 | 0.043 | C11.1 | 156 | 0.17 | 0.51 |
| 11 | Mrpl10 | 0.036 | −0.055 | 0.04 | 0.035 | C11.1 | 1 | −0.14 | 0.58 |
| 11 | Nfe2l1 | 0.035 | −0.078 | 0.05 | 0.046 | C11.1 | 6 | −0.28 | 0.27 |
| 15 | Ank | 0.084 | −0.017 | 0.09 | 0.050 | C15.1 | 1 | 0.40 | 0.10* |
QTL = quantitative trait locus; HC = highly conserved; SNP = single nucleotide polymorphism
DISCUSSION
We performed genome-wide mapping of QTLs affecting synovial and meniscus calcifications in an F44 advanced intercross line derived from LG/J and SM/J mice. We mapped 20 QTLs in our experimental population affecting ectopic calcification with 11 and 10 QTLs respectively underlying synovial and meniscal calcifications. The LG/J allele usually conferred an increased protective effect against ectopic calcification relative to the SM/J allele. Sex-specific genetic effects predominated, with 13 loci demonstrating significantly different effect sizes dependent on sex. At the expense of detection power, advanced intercross lines provided a mapping advantage over F2 or backcross generations in that QTL numbers and locations are more precisely localized. The average interval size was 1.15 megabases, typically containing 7–8 genes. We discuss candidate genes in terms of functional classifications encompassing angiogenesis, bone metabolism, calcification, arthritis, and ankylosing spondylitis.
Angiogenesis
Several genes associated with angiogenesis were identified in our QTLs intervals (Myo1e, Kif26b, Nprl3, Stab2, Fam105b). Angiogenesis is associated with ectopic calcification in a variety of tissues30 and exerts both direct and indirect effects on ectopic calcification through a two-step process: firstly, angiogenic factors and cytokines released by endothelial cells induce the differentiation of osteogenic progenitor cells and secondly, blood vessels provide oxygen and nutrients to initiate and augment bone growth31. While chondrocalcinosis is not always associated with increased angiogenesis32, it is believed that synovial and osteochondral angiogenesis are both features of arthritis and occur in parallel.
Bone metabolism and calcification
Deposition of calcium crystals in cartilage and meniscus is often associated with higher risk of OA33. Calcium crystals with a low propensity to induce acute inflammation may contribute to chronic synovitis and angiogenesis in chondrocalcinosis.
Tle3 (C9.1) varied at two highly conserved intronic SNPs, and is known to be involved in osteoblast differentiation34. Likewise, Tgfb2 (C1.5) is known to play significant roles in bone metabolism and turnover35, though alleles did not vary at evolutionarily conserved sites. While Lipc (C9.2) is expressed in osteocytes, and is associated with bone mineral density variation and coronary calcification36, it harbored no highly conserved SNPs. Nfe2l1 (C11.1), which contained six conserved noncoding SNPs, plays a role in bone development37.
Ankylosing spondylitis
In our study, ANK, which is associated with synovial chondromatosis in humans, was linked to meniscus calcification at C15.1. Finding Ank in an interval is very intriguing, yet according to our SNP criteria, it is outranked by Fam105b (associated with angiogenesis as above), a gene about which little is known but among our most highly ranked candidates based on SNP polymorphisms. Nonetheless, Ank plays an important role in inorganic pyrophosphate transport and inhibition of matrix mineralization38, and its human variants have been associated with joint chondrocalcinosis and ankylosing spondylitis39.
Interestingly, in addition to Ank, we found three other candidates (Pon1, Il1r2, and Tbkbp1) associated with human ankylosing spondylitis40, OA and RA41. Members of the IL1 gene cluster also play roles in growth plate and bone remodeling42. We noted for Il1r2 and Tbkbp1, and more generally for other candidates lacking conserved and/or nonsynonymous SNPs, that we defined alleles to include transcribed sequences plus the flanking 2,500 base-pairs linked but more distant regulatory sequences remain unexplored.
Arthritis
Though we did not find bibliographic evidence for its association with OA phenotypes, Stab2 (C10.1) binds to hyaluronic acid in the extracellular matrix43. Between the LG/J and SM/J, Stab2 varied at 14 nonsynonymous positions. Tbx21 (C11.1), differing at two highly conserved intronic SNPs, plays a role in T-cell function underlying numerous autoimmune disorders including rheumatoid arthritis (RA) and systemic sclerosis44. Map4k4 (C1.3) contained six highly conserved intronic SNPs, and human variants have been associated with RA and arterial calcification45. Hoxb9 (C11.1) is associates with human hip dysplasia and OA46 and varied at two conserved intronic and three downstream base positions. Larp6 (C9.1) functions in large part by directly binding to Col1a2 during its translation47, however, both Col1a2 (C6.1) and Larp6 carried no significant SNPs. In contrast, Adam10 (C9.2,) a member of aggrecanase family involved in proteolytic cleavage of protein ectodomains including those of Notch receptors48, as well as aggrecan varied at a single conserved intronic position. A role for metalloproteases was further implied by C10.1 candidate Timp3, containing four conserved intronic SNPs, which interacts with metalloproteases49 and plays a key role in inflammation and OA50. Playing a role in normal bone development, Nfe2l1 (C11.1) contains six conserved noncoding SNPs. In humans, NFE2l1 is an important transcription factor implicated in the regulation of genes differentially expressed between normal and RA patients41. Trpm3 is a calcium permeable channel associated with human RA through dysregulated synoviocyte secretion of hyaluronic acid51. It has one nonsynonymous SNP in addition to 32 highly conserved intronic SNPs.
Despite the fact that several genes identified from calcification phenotype to be associated with OA, to our knowledge, no QTL for OA in the mouse model have been reported other than a single locus found in STR/ort mice52, the main candidate being Sfrp1, a WNT antagonist. This QTL is located on chromosome 4 in a region distinct from our QTL on chromosome 4, C4.1.
To further address the functional relevance of candidate genes underlying post-traumatic ectopic calcification, we measured calcification phenotypes and expression levels of several candidate genes in the advance intercross parental strains, LG/J and SM/J. Our mRNA expression analysis showed that the expression of 11 candidates was significantly different between LG/J and SM/J, with four being correlated with the degree of ectopic calcification. Given the predominant effect of sex in our analyses, we suggest future mechanistics studies should have an explicit focus on the role of sex in modulating biological processes underlying ectopic calcification. Thus, continuing this approach coupled with mechanistic studies will further narrow the scope of our candidate genes toward the aim of identifying precise physiological mechanisms underlying ectopic calcification of joints following traumatic knee injury.
Our findings of multiple genetic loci affecting ectopic calcification suggest the involvement of multiple genes contributing to its pathogenesis. Our data also suggest pathogenic mechanisms leading to post-traumatic calcification in the joint space versus the synovium have distinct and overlapping components. As mentioned above, we coded meniscal and synovial calcifications as separate phenotypes because they are mechanistically unrelated: de novo calcification in one versus enhanced calcification in the other. In addition, these phenotypes were found to be uncorrelated in a chi-square test of association. Considering the distinct nature of these two tissue environments as well as the outcome from statistical test, we coded meniscus and synovial calcifications as distinct phenotypes yielding a total of 20 QTL loci. We considered the possibility that the calcifications should be coded as a single phenotype but their lack of phenotypic correlation and their distinct normal and pathological states led us to treat them separately.
Lastly, we believe the present analysis to be the first detailed genetic examination of meniscal or synovial calcification phenotypes resulting from knee-trauma in mice. We are not aware of any studies that have examined these meniscal or synovial calcification phenotypes in other mouse strains. However, we strongly believe that DMM surgery per se is not the sole inducer of these phenotypes as our unpublished observations in some of the genetic mouse strains have shown that non-invasive compressive loading also results in the formation of calcified nodules in the synovium and meniscus (MF Rai, D. Xin, LJ Sandell, unpublished results. We have previously reported that certain LGXSM recombinant inbred lines capable of healing ear wounds and repairing articular cartilage defects are protected against induced knee OA20,22. Interestingly, the healer mice develop higher degree of ectopic calcification than a mouse strain that does not repair above tissues and is susceptible to post-traumatic osteoarthritis. Therefore, we suggest that formation of ectopic calcification is an independent phenomenon and does not depend on the status of articular cartilage. Taken together with the lack of effect of sham surgery, we interpret our experimental analysis to reflect the calcifying response of the murine knee to generalized trauma and specifically here to joint instability caused by meniscal destabilization.
Supplementary Material
Acknowledgments
These studies were supported by an R01AR063757 (Sandell, Cheverud) and by P30-AR057235 (Musculoskeletal Research Center, Sandell, P.I.) from the National Institute of Arthritis, Musculoskeletal and Skin Diseases (NIAMS). Dr. Rai is supported through NIH Pathway to Independence Award (1K99AR064837) from NIAMS. Dr. Schmidt is supported through NIH T32 Metabolic Skeletal Disorders Training program (T32-AR-060719) from NIAMS. The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the NIAMS. We acknowledge with thanks Dr. Amy Williams (Massachusetts Institute of Technology, Cambridge, MA) for providing us with an advanced version of the minimum recombination option (hapi-mr) of the Hapi program, enabling us to phase our larger mouse families comprising up to 16 offspring. We also acknowledge with thanks the important technical support by Crystal Idleburg and Kara Janiszak.
Footnotes
Authors’ contributions statement
All authors were involved in drafting and revision of the manuscript and all authors approved the final version to be published. Dr. Rai had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design: Sandell, Rai, Cheverud
Acquisition of data: Rai, Schmidt, Hashimoto
Analysis and interpretation of data: Rai, Schmidt, Hashimoto, Sandell, Cheverud
Conflict of interest
Dr. Sandell is Editor in Chief of Journal of Orthopaedic Research. Dr. Sandell owns stock or stock options in ISTO Technologies and receives royalties from Merck/Millipore for a type IIA collagen N-propeptide enzyme-linked immunosorbent assay. All other authors have nothing to disclose. None of the authors has any competing interests with regard to this manuscript.
DATA SUBMISSION
Gene expression profiles of the samples have been deposited into the National Center of Biotechnology Information Gene Expression Omnibus (GEO, (http://www.ncbi.nlm.nih.gov/projects/geo) repository and are accessible through GEO series accession number GSE64793.
REFERENCES
- 1.Crotty JM, Monu JU, Pope TL., Jr Synovial osteochondromatosis. Radiologic clinics of North America. 1996;34:327–342. xi. [PubMed] [Google Scholar]
- 2.Barwell R. Clinical Lectures on Movable Bodies in Joints. Br Med J. 1876;1:184–185. doi: 10.1136/bmj.1.789.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Felson DT, Anderson JJ, Naimark A, et al. The prevalence of chondrocalcinosis in the elderly and its association with knee osteoarthritis: the Framingham Study. J Rheumatol. 1989;16:1241–1245. [PubMed] [Google Scholar]
- 4.Wilkins E, Dieppe P, Maddison P, et al. Osteoarthritis and articular chondrocalcinosis in the elderly. Ann Rheum Dis. 1983;42:280–284. doi: 10.1136/ard.42.3.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steitz SA, Speer MY, McKee MD, et al. Osteopontin inhibits mineral deposition and promotes regression of ectopic calcification. Am J Pathol. 2002;161:2035–2046. doi: 10.1016/S0002-9440(10)64482-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ji Y, Christopherson GT, Kluk MW, et al. Heterotopic ossification following musculoskeletal trauma: modeling stem and progenitor cells in their microenvironment. Adv Exp Med Biol. 2011;720:39–50. doi: 10.1007/978-1-4614-0254-1_4. [DOI] [PubMed] [Google Scholar]
- 7.Ciancio G, Bortoluzzi A, Govoni M. Epidemiology of gout and chondrocalcinosis. Reumatismo. 2011;63:207–220. doi: 10.4081/reumatismo.2011.207. [DOI] [PubMed] [Google Scholar]
- 8.Moochhala SH. Extracellular pyrophosphate in the kidney: how does it get there and what does it do? Nephron Physiology. 2012;120:33–38. doi: 10.1159/000341597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Q, Uitto J. Mineralization/anti-mineralization networks in the skin and vascular connective tissues. Am J Pathol. 2013;183:10–18. doi: 10.1016/j.ajpath.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Richtsmeier JT, Flaherty K. Hand in glove: brain and skull in development and dysmorphogenesis. Acta neuropathologica. 2013;125:469–489. doi: 10.1007/s00401-013-1104-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hughes AE, McGibbon D, Woodward E, et al. Localisation of a gene for chondrocalcinosis to chromosome 5p. Hum Mol Genet. 1995;4:1225–1228. doi: 10.1093/hmg/4.7.1225. [DOI] [PubMed] [Google Scholar]
- 12.Baldwin CT, Farrer LA, Adair R, et al. Linkage of early-onset osteoarthritis and chondrocalcinosis to human chromosome 8q. Am J Hum Genet. 1995;56:692–697. [PMC free article] [PubMed] [Google Scholar]
- 13.Rodriguez-Valverde V, Zuniga M, Casanueva B, et al. Hereditary articular chondrocalcinosis. Clinical and genetic features in 13 pedigrees. Am J Med. 1988;84:101–106. doi: 10.1016/0002-9343(88)90015-0. [DOI] [PubMed] [Google Scholar]
- 14.Pendleton A, Johnson MD, Hughes A, et al. Mutations in ANKH cause chondrocalcinosis. Am J Hum Genet. 2002;71:933–940. doi: 10.1086/343054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balsa A, Martin-Mola E, Gonzalez T, et al. Familial articular chondrocalcinosis in Spain. Ann Rheum Dis. 1990;49:531–535. doi: 10.1136/ard.49.7.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hrbek T, de Brito RA, Wang B, et al. Genetic characterization of a new set of recombinant inbred lines (LGXSM) formed from the inter-cross of SM/J and LG/J inbred mouse strains. Mamm Genome. 2006;17:417–429. doi: 10.1007/s00335-005-0038-7. [DOI] [PubMed] [Google Scholar]
- 17.Cheverud JM, Routman EJ, Duarte FA, et al. Quantitative trait loci for murine growth. Genetics. 1996;142:1305–1319. doi: 10.1093/genetics/142.4.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheverud JM, Lawson HA, Fawcett GL, et al. Diet-dependent genetic and genomic imprinting effects on obesity in mice. Obesity (Silver Spring) 2011;19:160–170. doi: 10.1038/oby.2010.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheverud JM, Lawson HA, Bouckaert K, et al. Fine-mapping quantitative trait loci affecting murine external ear tissue regeneration in the LG/J by SM/J advanced intercross line. Heredity (Edinb) 2014;112:508–518. doi: 10.1038/hdy.2013.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hashimoto S, Rai MF, Janiszak KL, et al. Cartilage and bone changes during development of post-traumatic osteoarthritis in selected LGXSM recombinant inbred mice. Osteoarthritis Cartilage. 2012;20:562–571. doi: 10.1016/j.joca.2012.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glasson SS, Blanchet TJ, Morris EA. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthritis Cartilage. 2007;15:1061–1069. doi: 10.1016/j.joca.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 22.Rai MF, Hashimoto S, Johnson EE, et al. Heritability of articular cartilage regeneration and its association with ear wound healing in mice. Arthritis Rheum. 2012;64:2300–2310. doi: 10.1002/art.34396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lawson HA, Cady JE, Partridge C, et al. Genetic effects at pleiotropic loci are context-dependent with consequences for the maintenance of genetic variation in populations. PLoS Genet. 2011;7:e1002256. doi: 10.1371/journal.pgen.1002256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williams AL, Housman DE, Rinard MC, et al. Rapid haplotype inference for nuclear families. Genome biology. 2010;11:R108. doi: 10.1186/gb-2010-11-10-r108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haley CS, Knott SA. A simple regression method for mapping quantitative trait loci in line crosses using flanking markers. Heredity (Edinb) 1992;69:315–324. doi: 10.1038/hdy.1992.131. [DOI] [PubMed] [Google Scholar]
- 26.Kumar P, Henikoff S, Ng PC. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nature protocols. 2009;4:1073–1081. doi: 10.1038/nprot.2009.86. [DOI] [PubMed] [Google Scholar]
- 27.Adzhubei IA, Schmidt S, Peshkin L, et al. A method and server for predicting damaging missense mutations. Nature methods. 2010;7:248–249. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chun S, Fay JC. Identification of deleterious mutations within three human genomes. Genome Res. 2009;19:1553–1561. doi: 10.1101/gr.092619.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rai MF, Schmidt EJ, McAlinden A, et al. Molecular insight into the association between cartilage regeneration and ear wound healing in genetic mouse models: targeting new genes in regeneration. 2013;G3(3):1881–1891. doi: 10.1534/g3.113.007302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ronchetti I, Boraldi F, Annovi G, et al. Fibroblast involvement in soft connective tissue calcification. Frontiers in genetics. 2013;4:22. doi: 10.3389/fgene.2013.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Collett GD, Canfield AE. Angiogenesis and pericytes in the initiation of ectopic calcification. Circ Res. 2005;96:930–938. doi: 10.1161/01.RES.0000163634.51301.0d. [DOI] [PubMed] [Google Scholar]
- 32.Piovesan EJ, Young Blood MR, Kowacs PA, et al. Prevalence of migraine in Noonan syndrome. Cephalalgia : an international journal of headache. 2007;27:330–335. doi: 10.1111/j.1468-2982.2007.01282.x. [DOI] [PubMed] [Google Scholar]
- 33.Neame RL, Carr AJ, Muir K, et al. UK community prevalence of knee chondrocalcinosis: evidence that correlation with osteoarthritis is through a shared association with osteophyte. Ann Rheum Dis. 2003;62:513–518. doi: 10.1136/ard.62.6.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kokabu S, Nguyen T, Ohte S, et al. TLE3, transducing-like enhancer of split 3, suppresses osteoblast differentiation of bone marrow stromal cells. Biochem Biophys Res Commun. 2013;438:205–210. doi: 10.1016/j.bbrc.2013.07.054. [DOI] [PubMed] [Google Scholar]
- 35.Filvaroff E, Erlebacher A, Ye J, et al. Inhibition of TGF-beta receptor signaling in osteoblasts leads to decreased bone remodeling and increased trabecular bone mass. Development. 1999;126:4267–4279. doi: 10.1242/dev.126.19.4267. [DOI] [PubMed] [Google Scholar]
- 36.Yamada Y, Ando F, Shimokata H. Association of candidate gene polymorphisms with bone mineral density in community-dwelling Japanese women and men. Int J Mol Med. 2007;19:791–801. [PubMed] [Google Scholar]
- 37.Kim J, Xing W, Wergedal J, et al. Targeted disruption of nuclear factor erythroid-derived 2-like 1 in osteoblasts reduces bone size and bone formation in mice. Physiol Genomics. 2010;40:100–110. doi: 10.1152/physiolgenomics.00105.2009. [DOI] [PubMed] [Google Scholar]
- 38.Gurley KA, Reimer RJ, Kingsley DM. Biochemical and genetic analysis of ANK in arthritis and bone disease. Am J Hum Genet. 2006;79:1017–1029. doi: 10.1086/509881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pimentel-Santos FM, Ligeiro D, Matos M, et al. ANKH and susceptibility to and severity of ankylosing spondylitis. J Rheumatol. 2012;39:131–134. doi: 10.3899/jrheum.110681. [DOI] [PubMed] [Google Scholar]
- 40.Australo-Anglo-American Spondyloarthritis C. Reveille JD, Sims AM, et al. Genome-wide association study of ankylosing spondylitis identifies non-MHC susceptibility loci. Nat Genet. 2010;42:123–127. doi: 10.1038/ng.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li G, Han N, Li Z, et al. Identification of transcription regulatory relationships in rheumatoid arthritis and osteoarthritis. Clin Rheumatol. 2013;32:609–615. doi: 10.1007/s10067-012-2143-9. [DOI] [PubMed] [Google Scholar]
- 42.Simsa-Maziel S, Zaretsky J, Reich A, et al. IL-1RI participates in normal growth plate development and bone modeling. Am J Physiol Endocrinol Metab. 2013;305:E15–E21. doi: 10.1152/ajpendo.00335.2012. [DOI] [PubMed] [Google Scholar]
- 43.Sokolowska M, Chen LY, Eberlein M, et al. Low molecular weight hyaluronan activates cytosolic phospholipase A2alpha and eicosanoid production in monocytes and macrophages. J Biol Chem. 2014;289:4470–4488. doi: 10.1074/jbc.M113.515106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kondo Y, Iizuka M, Wakamatsu E, et al. Overexpression of T-bet gene regulates murine autoimmune arthritis. Arthritis Rheum. 2012;64:162–172. doi: 10.1002/art.33335. [DOI] [PubMed] [Google Scholar]
- 45.Aouadi M, Tesz GJ, Nicoloro SM, et al. Orally delivered siRNA targeting macrophage Map4k4 suppresses systemic inflammation. Nature. 2009;458:1180–1184. doi: 10.1038/nature07774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rouault K, Scotet V, Autret S, et al. Do HOXB9 and COL1A1 genes play a role in congenital dislocation of the hip? Study in a Caucasian population. Osteoarthritis Cartilage. 2009;17:1099–1105. doi: 10.1016/j.joca.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 47.Blackstock CD, Higashi Y, Sukhanov S, et al. Insulin-like Growth Factor-1 Increases Synthesis of Collagen Type I via Induction of the mRNA-binding Protein LARP6 Expression and Binding to the 5' Stem-loop of COL1a1 and COL1a2 mRNA. J Biol Chem. 2014;289:7264–7274. doi: 10.1074/jbc.M113.518951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schlondorff J, Blobel CP. Metalloprotease-disintegrins: modular proteins capable of promoting cell-cell interactions and triggering signals by protein-ectodomain shedding. J Cell Sci. 1999;112(Pt 21):3603–3617. doi: 10.1242/jcs.112.21.3603. [DOI] [PubMed] [Google Scholar]
- 49.Jackson MT, Moradi B, Smith MM, et al. Matrix metalloproteinase (MMP)-2, MMP-9 and MMP-13 are activated by Activated Protein C (APC) in human osteoarthritic cartilage chondrocytes. Arthritis & rheumatology. 2014 doi: 10.1002/art.38401. [DOI] [PubMed] [Google Scholar]
- 50.Gill SE, Gharib SA, Bench EM, et al. Tissue inhibitor of metalloproteinases-3 moderates the proinflammatory status of macrophages. Am J Respir Cell Mol Biol. 2013;49:768–777. doi: 10.1165/rcmb.2012-0377OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ciurtin C, Majeed Y, Naylor J, et al. TRPM3 channel stimulated by pregnenolone sulphate in synovial fibroblasts and negatively coupled to hyaluronan. BMC Musculoskelet Disord. 2010;11:111. doi: 10.1186/1471-2474-11-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jaeger K, Selent C, Jaehme W, et al. The genetics of osteoarthritis in STR/ort mice. Osteoarthritis Cartilage. 2008;16:607–614. doi: 10.1016/j.joca.2007.09.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.