Abstract
Objective
To assess short- and long-term reproducibility of intrahepatic lipid (IHL) quantification by 1H-MRS and CT.
Methods
Sixteen obese subjects underwent 1H-MRS using a single-voxel PRESS sequence at 3T and non-contrast single-slice CT of the liver. Measurements were repeated after 6 weeks and 6 months. Clinical parameters (weight, activity, serum lipids) were collected. Short-term (baseline-6-weeks) and long-term (baseline-6-month) reproducibility of IHL was assessed by coefficient of variance (CV), standard deviation (SD), and intraclass correlation coefficient (ICC).
Results
Short-and long-term reproducibility of 1H-MRS were as follows: CV 5.9–18.8%, SD 0.7–1.9 and ICC 0.998–0.995 (95% CI 0.942–0.999). Short- and long-term reproducibility of CT were as follows: CV 4.4–14.2%, SD 2.4–8.7 and ICC 0.766–0.982 (95% CI 0.271–0.994). There was no significant change in clinical parameters (p>0.3).
Conclusions
1H-MRS and CT are reproducible methods for short- and long-term quantification of IHL content. Our results can guide sample size calculations for interventional and longitudinal studies.
Keywords: Proton MR spectroscopy, computed tomography, intrahepatic lipids, reproducibility, obesity
Introduction
A major complication of the obesity epidemic is nonalcoholic fatty liver disease (NAFLD) which encompasses a spectrum ranging from simple steatosis to nonalcoholic steatohepatitis (NASH). NASH may progress to liver fibrosis and cirrhosis [1, 2] and is predicted to be the main indication for liver transplantation by 2020 [3].
While liver biopsy with histopathologic grading is the reference standard for assessing hepatic steatosis, it is limited by its invasiveness, sampling errors and interobserver variability [4–6]. Therefore, there is an emerging need for the accurate and noninvasive quantification of intrahepatic lipid (IHL) content. Proton magnetic resonance spectroscopy (1H-MRS) has been recognized as an accurate technique for IHL quantification which correlates closely with histopathology [7, 8] and may be better than histology to detect longitudinal changes in IHL content [9]. Therefore, IHL content by 1H-MRS has been incorporated as an endpoint in longitudinal clinical studies [9–13]. Non-contrast computed tomography (CT) is a widely available imaging modality that allows for easy assessment of IHL content[14–16]. Shortcomings include ionizing radiation exposure and limited ability for accurate IHL quantification [17, 18].
When incorporating IHL as an endpoint in clinical trials using imaging techniques, it is important to assess the precision of the technique and thus the reliability of any estimated change. Most studies on variability for IHL quantification only performed measurements within the same day after repositioning of the subjects or within weeks, and there is limited data on longer-term reproducibility [19–22]. These data are relevant for sample size calculations in longitudinal and interventional studies where IHL content is used as an outcome measure.
The purpose of our study was therefore to assess short- and long-term reproducibility of IHL quantification by 1H-MRS and CT in obese subjects.
Materials and Methods
This prospective study was approved by our Institutional Review Board and was Health Insurance Portability and Accountability Act compliant. Written informed consent was obtained from all subjects after the nature of the procedures had been fully explained.
Subjects
The study group included healthy overweight/obese premenopausal women and men of similar mean age who were part of placebo groups in two 6-month clinical trials [10, 23]. Inclusion criteria included ages 18 to 45 years, BMI ≥25 kg/m2 and eumenorrhea in women. Exclusion criteria for the study included pregnancy, breastfeeding, diabetes mellitus or other chronic illness, estrogen or glucocorticoid use, and contraindications to MRI such as claustrophobia, presence of a pacemaker or metallic implant. All subjects were examined at our Clinical Research Center. BMI was calculated by using the formula BMI = W/(H)2, where W is weight in kilograms and H is height in meters. A complete history was recorded and a physical examination was performed. None of the patients had a history of liver disease.
Each participant underwent 1H-MRS and non-contrast CT of the liver for assessment of IHL content after an 8-hour overnight fast. We assessed BMI, physical activity, and fasting serum lipids (total, LDL, and HDL cholesterol, triglycerides) at time of imaging to ensure stability of these measures. Activity was assessed using the Paffenbarger questionnaire, a self-administered questionnaire that ascertains current levels of activity. All studies were repeated after 6 weeks and after 6 months using identical protocols and equipment. Subjects were instructed to maintain a stable diet and exercise regimen over the 6-month period.
Clinical characteristics and IHL data have been previously reported in a subset of subjects [10, 23–25]; however no data on IHL reproducibility have been described in any of the subjects.
1H-MR spectroscopy of liver
Study subjects were examined with the same 1H-MRS pulse sequences and equipment at all visits. 1H-MRS was performed using a 3.0 Tesla (Siemens Trio; Siemens Medical Systems, Erlangen, Germany) MRI system. Subjects were positioned supine and feet first in the magnet bore and a body matrix phased array coil was positioned over the abdomen. A tri-plane gradient echo localizer pulse sequence was performed to localize the liver. Subsequently, a breath-hold True Fast Imaging with Steady Precession (True FISP) sequence of the liver (TR, 3.8 ms; TE, 1.9 ms; slice thickness, 10 mm) was obtained. A voxel measuring 20 × 20 × 20 mm (8 mL) was placed within the peripheral portion of the right hepatic lobe, avoiding the diaphragm, vessels or artifact. For each voxel placement, automated optimization of gradient shimming was performed. The voxel placement was registered using screen captures to enable similar placement in the follow-up examinations at 6-weeks and 6-months. Single breath-hold single-voxel 1H-MRS data were acquired in mid-expiration using point-resolved single voxel spectroscopy (PRESS) pulse sequence without water suppression with the following parameters: TR, 1,500 ms; TE, 30 ms; 8 averages; 1024 data points; and receiver bandwidth, 2000 Hz. Acquisition time was 18 seconds [24].
1H-MR Spectroscopy Data Analysis
Fitting of all 1H-MRS data was performed using LCModel (version 6.3-0K) [26]. Data were transferred from the scanner to a Linux workstation and metabolite quantification was performed using eddy current correction and water scaling. A customized fitting algorithm for intrahepatic lipid analysis (LCModel “liver”) provided estimates for the lipid signals at 0.9, 1.3, and 2.0 and water signal at 4.7. All spectra had SD of the peak fit of <5% which represents the acceptable error of fit of the software. Values provided by 1H-MRS denote relative quantity of water to lipids in the volume of interest and are expressed as fat fraction using the following formula: lipid/(lipids+water) %.
Computed tomography
Following the 1H-MRS, all subjects underwent single slice CT of the upper abdomen (LightSpeed, General Electric, Milwaukee, WI) to determine liver density. Patients were placed supine and feet first in the CT scanner. Scan parameters for each image were standardized (144 table height, 80kV, 70 mA, 2 seconds gantry rotation, 1 cm slice thickness, 48 FOV). Single slice CT through the abdomen encompassing the liver was obtained. To assess hepatic fat content, the attenuation of the liver was determined within two circular regions of interest placed in the dorsal aspect of each organ. Attempts were made to avoid vessels, artifacts, and other areas of inhomogeneity. Hepatic fat content was studied as the liver attenuation absolute values.
Statistical Analysis
JMP Statistical Database Software (version 11.0; SAS Institute, Cary, NC) and MedCalc software (version 14; Mariakerke, Belgium) was used for statistical analyses. Short- and long-term reproducibility of 1H-MRS and CT was assessed by determining coefficient of variance (CV), standard deviation (SD), and intraclass correlation coefficient (ICC) with 95% confidence intervals (CI) of baseline and 6-week data and baseline and 6-month data. We also assessed reproducibility between the 6-week and 6-month data. In addition, we assessed intrahepatic variability using CT measurements performed in the right and left hepatic lobe at each visit. We assessed short- and long-term stability of BMI, exercise status, serum lipids, fasting glucose, and ALT by paired t-test. P < 0.05 was used to denote significance. Data are presented as mean ± SD.
Results
Clinical characteristics of study subjects at baseline, 6 weeks, and 6 months are shown in Table 1. The study group included 10 women and 6 men. The age of study participants ranged from 26 to 44 years, with a mean age of 37±6 years. Two subjects were overweight (BMI ≥ 25 kg/m2 and < 30 kg/m2) and 14 subjects were obese (BMI ≥ 30 kg/m2). BMI of study participants ranged from 26.2 to 46.7 kg/m2, with a mean BMI of 36.1±5.5 kg/m2.
Table 1.
Clinical characteristics and intrahepatic lipid content of study subjects (n=16)
| Variable | Baseline | 6 weeks | 6 months |
|---|---|---|---|
| Age (years) | 37±6 | ||
| Weight (kg) | 102.1±18.8 | 102.5±19.1 | 102.1±19.0 |
| BMI (kg/m2) | 36.1±5.5 | 36.3±5.5 | 36.5±5.7 |
| Moderate activity (hours/week) | 14.8±12.8 | 17.6±17.8 | 12.7±14.2 |
| Vigorous activity (hours/week) | 6.9±8.3 | 4.7±8.0 | 3.0±4.8 |
| Total cholesterol (mg/dl) | 163±31 | 167±24 | 167±31 |
| HDL cholesterol (mg/dl) | 40±14 | 39±11 | 41±15 |
| LDL cholesterol (mg/dl) | 100±28 | 103±21 | 102±31 |
| Triglycerides (mg/dl) | 117±59 | 137±61 | 125±70 |
| Alanine Aminotransferase (ALT) (mg/dl) | 28±21 | 25±14 | 24±15 |
| 1H-MRS Fat Fraction (%) | 12.6±12.5 | 12.7±12.6 | 10.4±11.4 |
| CT hepatic density (HU) | 56.7±11.5 | 54.8±10.6 | 64.6±13.7 |
Data presented as mean ± SD.
There was no significant change in clinical parameters between the baseline and 6-week visits (weight p=0.4, BMI p=0.4, moderate activity p =0.6, vigorous activity =0.1, serum total cholesterol p=0.5, HDL cholesterol p=0.5, LDL cholesterol p=1.0, triglycerides p=0.1). There was also no significant change in clinical parameters between the baseline and 6-month visits (weight p=0.2, BMI p=0.2, moderate activity p=0.4, vigorous activity p=0.1, serum total cholesterol p=0.5, HDL cholesterol p=0.5, LDL cholesterol p=0.7, triglycerides p=0.3).
1H-MR spectroscopy of liver
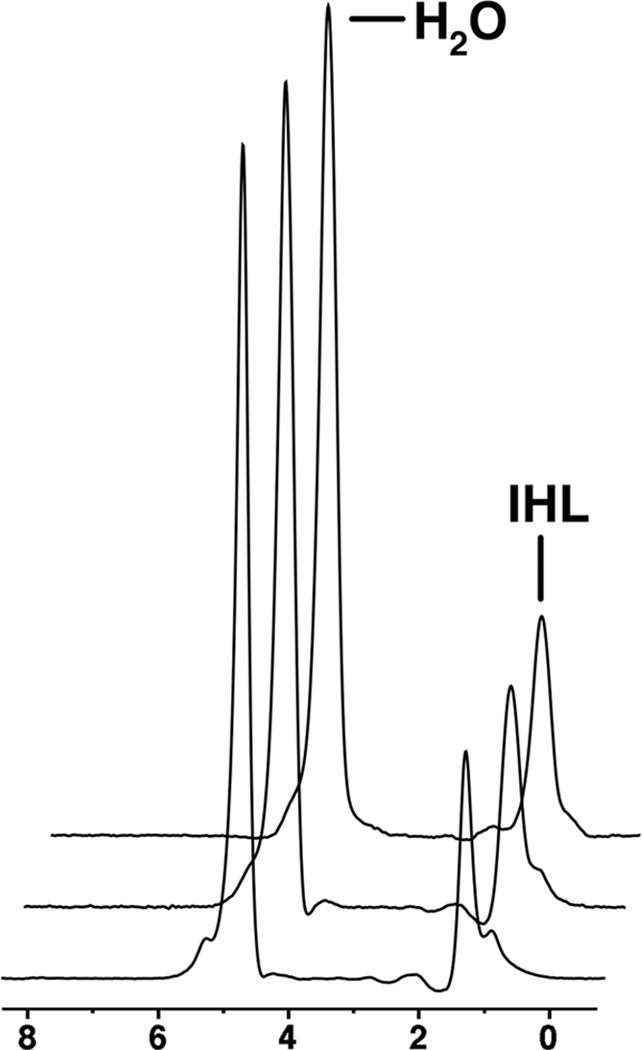
Short-term reproducibility of 1H-MRS for IHL quantification between baseline and 6-week scans was as follows: CV: 5.9%, SD: 0.7, ICC: 0.998 (95% CI 0.995 to 0.999) (Figure 1).
Figure 1.
1H-MRS of the right hepatic lobe in a 36 year-old man obtained at baseline (A), 6 weeks (B), and 6 months (C). Intrahepatic lipid (IHL) content by 1H-MR spectroscopy shows low variation between scans (fat fraction 23.7% at baseline, 24.3% at 6 weeks and 24.7% at 6 months). For purposes of visual comparison, the amplitudes of unsuppressed water are scaled identically.
Long-term reproducibility between baseline and 6-month scans as follows: CV: 18.8%, SD: 1.9, ICC: 0.982 (95% CI 0.942 to 0.995) (Figure 1 and Table 2).
Table 2.
Short- and long-term reproducibility of intrahepatic lipid quantification by proton magnetic resonance spectroscopy (1H-MRS).
| Coefficient of Variation (CV) |
Standard Deviation (SD) |
Intraclass Correlation Coefficient (ICC) (95% confidence interval) |
|
|---|---|---|---|
| Baseline to 6-week reproducibility | 5.9% | 0.7 | 0.998 (0.995–0.999) |
| Baseline to 6-month reproducibility | 18.8% | 1.9 | 0.982 (0.942 to 0.995) |
Reproducibility between the 6-week and 6-month scans was as follows: CV: 14.1%, SD: 1.3, ICC: 0.99 (95% CI 0.972 to 0.998).
Computed tomography of the liver
Short-term reproducibility of CT for IHL quantification between baseline and 6-week scans was as follows: CV: 4.4%, SD: 2.4, ICC: 0.982 (95% CI 0.946 to 0.994).
Long-term reproducibility between baseline and 6-month scans as follows: CV: 14.2%, SD: 8.7, ICC: 0.766 (95% CI 0.271 to 0.925) (Table 3).
Table 3.
Short- and long-term reproducibility of intrahepatic lipid quantification by computed tomography (CT).
| Coefficient of Variation (CV) |
Standard Deviation (SD) |
Intraclass Correlation Coefficient (ICC) (95% confidence interval) |
|
|---|---|---|---|
| Baseline to 6-week reproducibility | 4.4% | 2.4 | 0.982 (0.946–0.994) |
| Baseline to 6-month reproducibility | 14.2% | 8.7 | 0.766 (0.271 to 0.925) |
Reproducibility between the 6-week and 6-month scans was as follows: CV: 13.3%, SD 8.0, ICC: 0.770 (95% CI 0.245 to 0.930).
Assessment of intrahepatic variability between ROIs in the right and left hepatic lobe was as follows: baseline CV: 2.7%, SD: 1.5, ICC: 0.991 (95% CI 0.973 to 0.997); 6-week CV: 3.3%, SD: 1.8, ICC: 0.986 (95% CI 0.954 to 0.995) and 6-month CV: 2.7%, SD: 1.7, ICC: 0.992 (95% CI 0.974 to 0.997).
Discussion
Our study shows that reproducible IHL measurements can be obtained by 1H-MRS and CT with acceptable short-term and long-term CVs and high ICCs.
NAFLD represents a spectrum of disorders characterized by the accumulation of IHL without significant alcohol use. It ranges from simple steatosis to nonalcoholic steatohepatitis (NASH) which can progress to hepatic cirrhosis and hepatocellular carcinoma. In the majority of patients, NAFLD is associated with obesity, type 2 diabetes, and hyperlipidemia which are established risk factors for the development of NAFLD [1, 2, 27]. With the progressive obesity epidemic, the prevalence of NAFLD and its associated complications is increasing dramatically and NAFLD is currently the most common cause of chronic liver disease worldwide [1, 2, 28].
Therapeutic strategies for treating NAFLD and its complications are continuously evolving and range from lifestyle modification (diet and exercise) [29, 30], pharmacological therapies [9, 31–33], to bariatric surgery [34, 35]. In order to monitor the effect of these interventions on hepatic steatosis, accurate and non-invasive methods for IHL quantification are required. While liver biopsy is the reference standard for assessing hepatic steatosis, it is associated with sampling errors and interobserver variability. In addition, the invasiveness and potential complications, such as bleeding and pain, make biopsies impractical for longitudinal assessments [4–6].
Multiple imaging methods like US, CT, MRI, and 1H-MRS have been used to detect and quantify hepatic steatosis non-invasively [11, 14, 24, 36–38]. Non-contrast CT is an easy and widely available method for estimation of IHL content [14–16], with the advantage for IHL quantification in studies obtained for different purposes, such as virtual colonography [14]. Liver attenuation values decrease by about 1.6 HU for every milligram of triglyceride deposited per gram of liver tissue [37]. Although CT is quite accurate for the diagnosis of advanced steatosis [39], it has been found to less accurate for detecting mild steatosis and is limited by ionizing radiation [17, 18].
1H-MRS is the most sensitive imaging technique for IHL quantification and has been shown to correlate closely with histology [7, 8]. In fact, 1H-MRS may be better than histology to detect longitudinal changes in IHL content [9]. 1H-MRS has traditionally involved long acquisition times and has been performed under free breathing [7, 22, 40], which can introduce motion artifact and degradation of spectral quality. We therefore performed 1H-MRS using a single breath-hold sequence which was previously validated [24].
Knowledge of the normal variability of IHL content between imaging sessions is of importance for longitudinal studies performed to investigate effects of lifestyle, pharmacological, or surgical interventions on hepatic steatosis. As measurements may vary within a subject, knowledge of such differences is essential to accurately determine the clinical significance of pathophysiological changes following these interventions.
Most studies on 1H-MRS for IHL quantification have focused on the intra-day reproducibility by repositioning subjects between scans with reported CVs between 3.6 to 10% [19–22, 41]. Van Werven et al performed 1H-MRS in 12 subjects within 4 weeks and found CV of 9.5% and ICC of 0.998 [22]. However, there are no data on longer-term variability of IHL quantification. We therefore scanned a group of obese but otherwise healthy subjects three times over a period of 6 months under controlled conditions and identical imaging protocols. Our study shows that reproducible IHL measurements can be obtained by 1H-MRS and CT with acceptable short-term and long-term CVs and high ICCs.
In our study, the CVs of 1H-MRS obtained after 6 weeks and 6 months were 5.9% and 18.8%, respectively. Our 6-week CV is lower than that of studies obtained when subjects were re-scanned the same day after repositioning or re-scanned after 4 weeks. We report for the first time 6-month reproducibility data of IHL quantification with a CV of 18.8% and ICC of 0.982 (95% CI: 0.942 to 0.995). For CT we found similar CVs of 4.4% for 6-week reproducibility and 14.2% for 6-month reproducibility. While ICC was high for short-term reproducibility (0.982 with 95% CI of 0.946 to 0.994), ICC for 6-month reproducibility was lower (0.766 with 95% CI of 0.271 to 0.925). Our results provide data by which study designs can be optimized and study sample size powered for the detection of changes in IHL during interventional and longitudinal trials.
Factors affecting the variability of IHL quantification include natural biologic variations in metabolite concentrations and variations related to BMI, physical activity, diet, or medication use, as well as non-biologic factors related to equipment instability, voxel placement, and patient repositioning. We used identical 1H-MRS and CT protocols with reproducible voxel/ROI localization to minimize imperfect relocalization as a substantial source of measurement variability. In addition, we attempted to control biological factors such as weight, diet and exercise between scans intervals and performed all imaging after an overnight fast. However, patients did not follow a standardized diet which may account for physiologic variations in IHL content. Variability at 6 months was higher by 1H-MRS and CT which both demonstrated a decrease in IHL content compared to baseline and 6 weeks. This may be due to increased biologic variations in IHL content rather than factors related to equipment or scanning technique.
Our study had several limitations. First is our small sample size. Second, our study group was comprised of overweight/obese but otherwise healthy volunteers with normal liver enzymes, and our data may not be able to be extrapolated to patients with NASH or cirrhosis. Third, we only performed IHL measurements by 1H-MRS in the right hepatic lobe. However, there was low intrahepatic variability on CT measurements performed in the right and left hepatic lobes. Strengths of our study include the detailed assessment of IHL content by 1H-MRS and CT over 6 months under controlled condition and using identical imaging protocols.
In conclusion, 1H-MRS and CT are reproducible techniques for short- and long-term quantification of IHL content. Our results can guide sample size calculations for interventional and longitudinal studies where assessments are repeated over time in different imaging sessions.
Acknowledgments
This study was supported by NIH grants R01 HL-077674, K23 RR-23090, K24 HL-092902, KL2 TR00110 and UL1 RR-025758
Footnotes
Clinical trials number: NCT00131378
The authors have no conflict of interest to declare.
References
- 1.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 2.Lewis JR, Mohanty SR. Nonalcoholic fatty liver disease: a review and update. Dig Dis Sci. 2010;55:560–578. doi: 10.1007/s10620-009-1081-0. [DOI] [PubMed] [Google Scholar]
- 3.Charlton MR, Burns JM, Pedersen RA, Watt KD, Heimbach JK, Dierkhising RA. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology. 2011;141:1249–1253. doi: 10.1053/j.gastro.2011.06.061. [DOI] [PubMed] [Google Scholar]
- 4.Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med. 2001;344:495–500. doi: 10.1056/NEJM200102153440706. [DOI] [PubMed] [Google Scholar]
- 5.Ratziu V, Charlotte F, Heurtier A, et al. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005;128:1898–1906. doi: 10.1053/j.gastro.2005.03.084. [DOI] [PubMed] [Google Scholar]
- 6.Regev A, Berho M, Jeffers LJ, et al. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol. 2002;97:2614–2618. doi: 10.1111/j.1572-0241.2002.06038.x. [DOI] [PubMed] [Google Scholar]
- 7.Longo R, Pollesello P, Ricci C, et al. Proton MR spectroscopy in quantitative in vivo determination of fat content in human liver steatosis. J Magn Reson Imaging. 1995;5:281–285. doi: 10.1002/jmri.1880050311. [DOI] [PubMed] [Google Scholar]
- 8.Szczepaniak LS, Babcock EE, Schick F, et al. Measurement of intracellular triglyceride stores by H spectroscopy: validation in vivo. Am J Physiol. 1999;276:E977–E989. doi: 10.1152/ajpendo.1999.276.5.E977. [DOI] [PubMed] [Google Scholar]
- 9.Le TA, Chen J, Changchien C, et al. Effect of colesevelam on liver fat quantified by magnetic resonance in nonalcoholic steatohepatitis: a randomized controlled trial. Hepatology. 2012;56:922–932. doi: 10.1002/hep.25731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bredella MA, Gerweck AV, Lin E, et al. Effects of GH on body composition and cardiovascular risk markers in young men with abdominal obesity. J Clin Endocrinol Metab. 2013;98:3864–3872. doi: 10.1210/jc.2013-2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cowin GJ, Jonsson JR, Bauer JD, et al. Magnetic resonance imaging and spectroscopy for monitoring liver steatosis. J Magn Reson Imaging. 2008;28:937–945. doi: 10.1002/jmri.21542. [DOI] [PubMed] [Google Scholar]
- 12.Ryan MC, Itsiopoulos C, Thodis T, et al. The Mediterranean diet improves hepatic steatosis and insulin sensitivity in individuals with non-alcoholic fatty liver disease. J Hepatol. 2013;59:138–143. doi: 10.1016/j.jhep.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 13.Sathyanarayana P, Jogi M, Muthupillai R, Krishnamurthy R, Samson SL, Bajaj M. Effects of combined exenatide and pioglitazone therapy on hepatic fat content in type 2 diabetes. Obesity (Silver Spring) 2011;19:2310–2315. doi: 10.1038/oby.2011.152. [DOI] [PubMed] [Google Scholar]
- 14.Hahn L, Reeder SB, Del Rio AM, Pickhardt PJ. Longitudinal Changes in Liver Fat Content in Asymptomatic Adults: Hepatic Attenuation on Unenhanced CT as an Imaging Biomarker for Steatosis. AJR Am J Roentgenol. 2015;205:1167–1172. doi: 10.2214/AJR.15.14724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kodama Y, Ng CS, Wu TT, et al. Comparison of CT methods for determining the fat content of the liver. AJR Am J Roentgenol. 2007;188:1307–1312. doi: 10.2214/AJR.06.0992. [DOI] [PubMed] [Google Scholar]
- 16.Ricci C, Longo R, Gioulis E, et al. Noninvasive in vivo quantitative assessment of fat content in human liver. J Hepatol. 1997;27:108–113. doi: 10.1016/s0168-8278(97)80288-7. [DOI] [PubMed] [Google Scholar]
- 17.Saadeh S, Younossi ZM, Remer EM, et al. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology. 2002;123:745–750. doi: 10.1053/gast.2002.35354. [DOI] [PubMed] [Google Scholar]
- 18.Schwenzer NF, Springer F, Schraml C, Stefan N, Machann J, Schick F. Non-invasive assessment and quantification of liver steatosis by ultrasound, computed tomography and magnetic resonance. J Hepatol. 2009;51:433–445. doi: 10.1016/j.jhep.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 19.Johnson NA, Walton DW, Sachinwalla T, et al. Noninvasive assessment of hepatic lipid composition: Advancing understanding and management of fatty liver disorders. Hepatology. 2008;47:1513–1523. doi: 10.1002/hep.22220. [DOI] [PubMed] [Google Scholar]
- 20.Szczepaniak LS, Nurenberg P, Leonard D, et al. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab. 2005;288:E462–E468. doi: 10.1152/ajpendo.00064.2004. [DOI] [PubMed] [Google Scholar]
- 21.Thomas EL, Hamilton G, Patel N, et al. Hepatic triglyceride content and its relation to body adiposity: a magnetic resonance imaging and proton magnetic resonance spectroscopy study. Gut. 2005;54:122–127. doi: 10.1136/gut.2003.036566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Werven JR, Hoogduin JM, Nederveen AJ, et al. Reproducibility of 3.0 Tesla magnetic resonance spectroscopy for measuring hepatic fat content. J Magn Reson Imaging. 2009;30:444–448. doi: 10.1002/jmri.21837. [DOI] [PubMed] [Google Scholar]
- 23.Bredella MA, Lin E, Brick DJ, et al. Effects of GH in women with abdominal adiposity: a 6-month randomized, double-blind, placebo-controlled trial. Eur J Endocrinol. 2012;166:601–611. doi: 10.1530/EJE-11-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bredella MA, Ghomi RH, Thomas BJ, et al. Breath-hold 1H-magnetic resonance spectroscopy for intrahepatic lipid quantification at 3 Tesla. J Comput Assist Tomogr. 2010;34:372–376. doi: 10.1097/RCT.0b013e3181cefb89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bredella MA, Torriani M, Ghomi RH, et al. Adiponectin is inversely associated with intramyocellular and intrahepatic lipids in obese premenopausal women. Obesity (Silver Spring) 2011;19:911–916. doi: 10.1038/oby.2010.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30:672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- 27.Pagano G, Pacini G, Musso G, et al. Nonalcoholic steatohepatitis, insulin resistance, and metabolic syndrome: further evidence for an etiologic association. Hepatology. 2002;35:367–372. doi: 10.1053/jhep.2002.30690. [DOI] [PubMed] [Google Scholar]
- 28.Adams LA, Lindor KD. Nonalcoholic fatty liver disease. Ann Epidemiol. 2007;17:863–869. doi: 10.1016/j.annepidem.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 29.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005–2023. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- 30.Whitsett M, VanWagner LB. Physical activity as a treatment of non-alcoholic fatty liver disease: A systematic review. World J Hepatol. 2015;7:2041–2052. doi: 10.4254/wjh.v7.i16.2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harrison SA, Torgerson S, Hayashi P, Ward J, Schenker S. Vitamin E and vitamin C treatment improves fibrosis in patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2003;98:2485–2490. doi: 10.1111/j.1572-0241.2003.08699.x. [DOI] [PubMed] [Google Scholar]
- 32.Ratziu V, Sheikh MY, Sanyal AJ, et al. A phase 2, randomized, double-blind, placebo-controlled study of GS-9450 in subjects with nonalcoholic steatohepatitis. Hepatology. 2012;55:419–428. doi: 10.1002/hep.24747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanyal AJ, Chalasani N, Kowdley KV, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675–1685. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mathurin P, Hollebecque A, Arnalsteen L, et al. Prospective study of the long-term effects of bariatric surgery on liver injury in patients without advanced disease. Gastroenterology. 2009;137:532–540. doi: 10.1053/j.gastro.2009.04.052. [DOI] [PubMed] [Google Scholar]
- 35.Mummadi RR, Kasturi KS, Chennareddygari S, Sood GK. Effect of bariatric surgery on nonalcoholic fatty liver disease: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2008;6:1396–1402. doi: 10.1016/j.cgh.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 36.Cassidy FH, Yokoo T, Aganovic L, et al. Fatty liver disease: MR imaging techniques for the detection and quantification of liver steatosis. Radiographics. 2009;29:231–260. doi: 10.1148/rg.291075123. [DOI] [PubMed] [Google Scholar]
- 37.Pereira K, Salsamendi J, Casillas J. The Global Nonalcoholic Fatty Liver Disease Epidemic: What a Radiologist Needs to Know. J Clin Imaging Sci. 2015;5:32. doi: 10.4103/2156-7514.157860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Williamson RM, Perry E, Glancy S, et al. The use of ultrasound to diagnose hepatic steatosis in type 2 diabetes: intra- and interobserver variability and comparison with magnetic resonance spectroscopy. Clin Radiol. 2011;66:434–439. doi: 10.1016/j.crad.2010.09.021. [DOI] [PubMed] [Google Scholar]
- 39.Lee SW, Park SH, Kim KW, et al. Unenhanced CT for assessment of macrovesicular hepatic steatosis in living liver donors: comparison of visual grading with liver attenuation index. Radiology. 2007;244:479–485. doi: 10.1148/radiol.2442061177. [DOI] [PubMed] [Google Scholar]
- 40.Lee SS, Park SH, Kim HJ, et al. Non-invasive assessment of hepatic steatosis: prospective comparison of the accuracy of imaging examinations. J Hepatol. 2010;52:579–585. doi: 10.1016/j.jhep.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 41.Machann J, Stefan N, Schick F. (1)H MR spectroscopy of skeletal muscle, liver and bone marrow. Eur J Radiol. 2008;67:275–284. doi: 10.1016/j.ejrad.2008.02.032. [DOI] [PubMed] [Google Scholar]