Abstract
There is significant interest in understanding inflammatory responses within the brain and spinal cord. Inflammatory responses that are centralized within the brain and spinal cord are generally referred to as “neuroinflammatory”. Aspects of neuroinflammation vary within the context of disease, injury, infection or stress. The context, course, and duration of these inflammatory responses are all critical aspects in the understanding of these processes and their corresponding physiological, biochemical and behavioral consequences. Microglia, innate immune cells of the central nervous system (CNS), play key roles in mediating these neuroinflammatory responses. Because the connotation of neuroinflammation is inherently negative and maladaptive, the majority of research focus is on the pathological aspects of neuroinflammation. There are, however, several degrees of neuroinflammatory responses, some of which are positive. In many circumstances including CNS injury, there is a balance of inflammatory and intrinsic repair processes that influences functional recovery. In addition, there are several other examples where communication between the brain and immune system involves neuroinflammatory processes that are beneficial and adaptive. The purpose of this review is to distinguish different variations of neuroinflammation in a context-specific manner and detail both positive and negative aspects of neuroinflammatory processes.
Keywords: neuroinflammation, microglia, astrocytes, lipopolysaccharide, sickness behavior
Introduction to Neuroinflammation
Neuroinflammation is defined as an inflammatory response within the brain or spinal cord. This inflammation is mediated by the production of cytokines, chemokines, reactive oxygen species, and secondary messengers. These mediators are produced by resident CNS glia (microglia and astrocytes), endothelial cells, and peripherally derived immune cells. There are immune, physiological, biochemical, and psychological consequences of these neuroinflammatory responses. Moreover, the degree of neuroinflammation depends on the context, duration, and course of the primary stimulus or insult (Figure 1). For instance, inflammation can lead to recruitment of immune cells, edema, tissue damage and potentially cell death. The term neuroinflammation, however, is not universally equivalent. Therefore, the primary goal here is to discuss different degrees of neuroinflammation and describe pathways that represent positive and negative aspects of neuroinflammatory processes in the context of the insult.
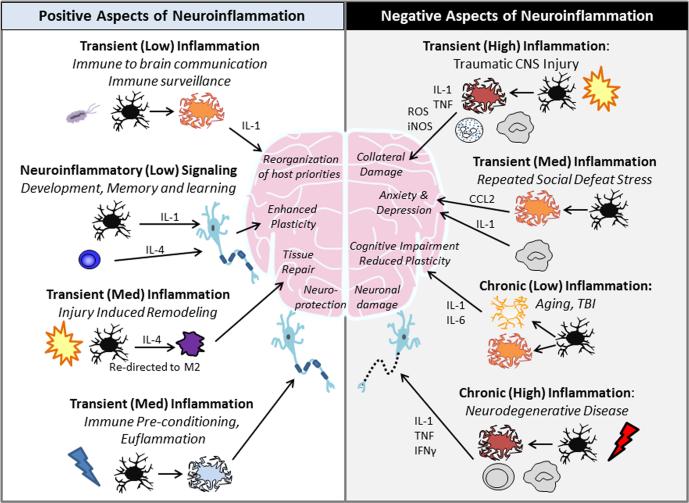
Figure 1. Positive and Negative Aspects of Neuroinflammation.
The intensity and duration of inflammation account for much of whether immune signals are supportive or destructive to the central nervous system. On the left, we show examples of brief and controlled inflammatory responses that are generally considered beneficial to the host organism. For instance, immune-to-brain signals after infection lead to the subsequent reorganization of host priorities and induction of sickness behaviors. Additionally, there is an important maintenance role of IL-1 and IL-4 on learning and memory. Following traumatic CNS injury, IL-4-driven repolarization of macrophages (M2) has been proven to be highly effective in promoting recovery and axonal regrowth. Immune preconditioning, or euflammation, provides a method for training the innate immune system toward a more neuro-protective phenotype. Conversely, on the right we demonstrate various maladaptive inflammatory responses. Chronic, uncontrolled inflammation is characterized by increased production of cytokines (IL-1 and TNF), reactive oxygen species (ROS), and other inflammatory mediators (inducible nitric oxide synthase). These markers are highly evident following trauma to the CNS, and are accompanied by significant recruitment and trafficking of peripheral macrophages and neutrophils to the site of injury. The transient inflammation after repeated social defeat stress also leads to monocyte and macrophage recruitment and causes anxiety and depression. Additionally, a low-level and chronic inflammatory response driven by IL-1 and IL-6 is caused by aging, follows the acute phase of CNS trauma, and leads to reduced neuronal plasticity and cognitive impairments. A higher degree of chronic inflammation is greatly damaging to the nervous system and is characteristic of neurodegenerative diseases.
Microglia: Central Players in Neuroinflammation
Microglia are the focal point for any discussion of neuroinflammation. This is because these innate immune cells perform the primary immune surveillance and macrophage-like activities of the CNS, including the production of cytokines and chemokines. Indeed, much of the innate immune capacity of the CNS is mediated by microglia. These cells are resident CNS cells that reside in both the white matter and gray matter of the brain and spinal cord. Overall, microglia comprise 10% of the CNS population. Microglia develop early in embryogenesis from myeloid precursor cells in the embryonic yolk sac, and migrate to the area of the CNS around embryonic day 8.5 (Ginhoux et al. 2010). In fact, microglia have the same progenitor as the rest of the long-lived tissues macrophages of the body (Alliot et al. 1999). Consistent with this idea, microglia are long-lived cells that have limited turnover from the myeloid cells of the bone marrow during the course of a lifetime (Ajami et al. 2007, Ginhoux et al. 2010). These cells are not replaced from myeloid cells of the bone marrow. Nonetheless, when depleted, microglia turnover is possible from a potential progenitor source within the CNS (Elmore et al. 2014). The rate at which microglia would normally be turned over in the absence of pharmacological or genetic depletion is still unclear, yet microglia appear to have a relatively low overall turnover rate (Ajami et al. 2007, Ginhoux et al. 2010). This low turnover would make them susceptible to the potentially pro-inflammatory effects of age, injury or stress.
Microglia have an active role in immune surveillance. For instance, elegant two photon imaging studies show that microglia use their processes to actively survey their microenvironment (Nimmerjahn et al. 2005, Davalos et al. 2005). Other immune related activities include the propagation of inflammatory signals that are initiated in the periphery (Dantzer et al. 2008). These responses are pivotal in the coordinated communication between the immune system and the brain. For example, in infection or disease, microglia become ‘activated’ and function as inflammatory cellular mediators. Activated microglia rapidly alter their transcriptional profile and produce inflammatory cytokines and chemokines. Depending on context, the production of cytokines and chemokines can facilitate the recruitment of leukocytes to the brain (Zhou et al. 2006). Active microglia also undergo cytoskeletal rearrangements that alter the pattern of receptor expression on the cell surface. In addition, these alterations allow for microglia to migrate towards sites of injury or infection (Russo & McGavern 2015) and potentially increase their phagocytic efficiency (Davalos et al. 2005). In general, microglial activation and the increased expression of cytokines are intended to protect the CNS and benefit the host organism. Nonetheless, amplified, exaggerated, or chronic microglial activation can lead to robust pathological changes and neurobehavioral complications such as depression and cognitive deficits (Norden & Godbout 2013).
The Degrees of Neuroinflammation and Neuroinflammatory Signaling
Neuroinflammatory responses are mediated by several key pro-inflammatory cytokines (IL-1β, IL-6, and TNFα), chemokines (CCL2, CCL5, CXCL1), secondary messengers (NO and prostaglandins) and reactive oxygen species (ROS). Many of these mediators are produced by activated resident CNS cells including microglia and astrocytes (Norden et al. 2016). In addition, endothelia cells and perivascular macrophages (Dunn et al. 2006) are also important in interpreting and propagating these inflammatory signals within the CNS. Neuroinflammation remains difficult to summarily define, and will be discussed here as the presence of cytokines/chemokines/secondary messengers within CNS tissue. Illustrating the broadness of this definition, there is evidence of active microglia and production of cytokines in early brain development (Salter & Beggs 2014). Moreover, active microglia provide support, synaptic pruning and immunological activities within the CNS (Schafer & Stevens 2013). In addition, a recent report indicates that IL-1 signaling is important in the repopulation of depleted microglia from a local progenitor source (Bruttger et al. 2015). Furthermore, enhanced neuroinflammatory signaling between T-cells and resident CNS cells are implicated in normal memory and learning (Derecki et al. 2010, Ziv et al. 2006). These examples represent a degree of neuroinflammatory cytokine production that acts on other cells to influence cellular biochemistry, physiology and development. Such instances do not represent pathological “neuroinflammation”, often a term with negative connotation, but rather demonstrate a way in which signals are communicated to or within the CNS.
Highly destructive or pathological neuroinflammation is associated with activation of CNS glia with significant cytokine and chemokine production, infiltration of peripheral immune cells, edema, increased blood-brain barrier (BBB) permeability and breakdown (Hawkins & Davis 2005, Michael et al. 2015, Monahan et al. 2008). There is also primary damage caused by the mechanical and physical damage of the infection, injury, or insult. In addition, there can be vascular occlusion, ischemia, cell death, and other secondary inflammatory components from these insults. This degree of neuroinflammation has been associated with CNS infection (Goldman et al. 2001), stroke (Liesz et al. 2011), or disease (Linker et al. 2002, Walter et al. 2007). In many of these examples the insults are life threating, can elicit neuroinflammatory processes with more pathological components, and are associated with negative functional outcomes. In addition, this high degree of inflammation has primary and secondary damage, and can also have chronic neuroinflammatory components that may never resolve. This degree of neuroinflammation is associated with immune responses induced by autoimmune disease like multiple sclerosis (MS), modeled in mice with experimental autoimmune encephalomyelitis (EAE) (Linker et al. 2002). In this example there is activation of CNS glia, cytokine and chemokine production, infiltration of peripheral immune cells, and presence of auto-reactive T-cells (Reboldi et al. 2009, Samoilova et al. 1998). Here there is significant autoimmune reaction against myelin basic protein which leads to demyelination of axons. In addition, over time this inflammation becomes chronic and results in loss of axons (Lovas et al. 2000). The immediate and chronic phases of this response have a notable impact on myelin physiology, including functional disability caused by the loss of myelin and axonal fragmentation. A notable chronic type of neuroinflammation is associated with Alzheimer's disease (Sokolova et al. 2009, Walter et al. 2007). AD disease progression consists of protein mis-folding, activation of CNS glia, infiltration of peripheral immune cells, neuronal damage and death, and neuronal atrophy over time (Bucciantini et al. 2002, Sokolova et al. 2009). These represent examples of the ebb and flow of chronic neuroinflammatory processes that contribute to tissue damage over time. Such tissue damage to axons and neurons in MS and AD has significant functional outcomes. This degree of neuroinflammation is chronic, progressive and increasingly destructive over time. Notably, these classic examples of neuroinflammation are reviewed elsewhere (Gold et al. 2006, Mrak et al. 1995, Tansey & Goldberg 2010) and are difficult to discuss in the context of the positive aspects of neuroinflammation. Additionally, the benefits of inducing phagocytic activity in neurodegeneration have been extensively reviewed by Sokolowski and Mandell (2011).
There can also be transient neuroinflammation that involves activation of resident glia and the production of several neuroinflammatory cytokines including IL-1β, TNFα, and IL-6. This induction of neuroinflammatory cytokines can be elicited as a part of coordinated CNS interpretation of peripheral infection or insult. In this context, activation of CNS glia and the production of cytokines and chemokines lead to physiological and behavioral responses that are beneficial to the host organism (Imeri & Opp 2009). It is important to highlight that this is a transient response and is not associated with significant infiltration of adaptive immune cells into the brain, blood-brain barrier breakdown or cell death. Interpretation of psychological stressors also has a similar neuroinflammatory profile, with glial activation and cytokine and chemokine production. Both of these interpretations occur in the absence of tissue pathology. In addition, others have harnessed this form of transient immune activation as a way to promote immune conditioning. For example, transient activation of the immune system prior to injury or infection (euflammation) is associated with reduced inflammatory profiles and increased neuroprotection (Tarr et al. 2014). Overall, this type of neuroinflammation represents the process of immune to brain signaling events that influences behavior.
Classic Examples of Neuroinflammation in the Context of Central Nervous System Trauma
There is a significant degree of neuroinflammation associated with brain and spinal cord injury. With CNS injury, there is activation of resident glia (microglia and astrocytes), moderate cytokine and chemokine production, and infiltration of peripheral immune cells (Werner & Engelhard 2007). In addition, there is cellular recruitment, increased blood brain barrier permeability and edema. Brain injury may consist of non-penetrating and penetrating injuries, both of which involve a direct, physical consequence on the CNS which leads to significant post-injury symptoms and long-term complications. Additionally, both brain and spinal cord injury involve an acute, beneficial initial inflammatory response (David & Kroner 2011, Woodcock & Morganti-Kossmann 2013) which becomes damaging should it persist too long. Penetrating brain injuries have more direct CNS tissue damage and more intermediate inflammation. Nonetheless, both penetrating and non-penetrating injuries can have secondary and chronic phases of inflammation that are considered maladaptive.
Neuroinflammation after Traumatic Brain Injury
One example of CNS injury is traumatic brain injury. TBI is defined by a complex assortment of heterogeneous physical insults to the head, resulting in biochemical injury to the brain and surrounding structures. TBI includes biphasic injuries and are described by their primary and secondary components (Maas et al. 2008, Osier et al. 2014). In brief, the primary injury caused by mechanical disruption of macro- and microscopic structures within and associated with the brain, resulting in tissue damage. Depending on the type of injury this can include overt tissue damage from a penetration injury or diffuse axonal injury from accelerating and rotational forces. Secondary injury is associated with tissue pathology and cell dysfunction leading to progressive damage extending long after the initial primary injury. For instance, the secondary injury with TBI is associated with cell death, neurotransmitter excitotoxicity, electrolyte imbalances, mitochondrial dysfunction, and ischemic injury (Maas et al. 2008).
Myriad studies indicate that both focal (penetrating) and diffuse (non-penetrating) TBI induce significant inflammatory processes in the brain mediated, in part, by resident microglia and astrocytes (McCrea et al. 2003, Lifshitz et al. 2007b, Tang et al. 1997, Woodcock & Morganti-Kossmann 2013). Following TBI, microglia respond to damaged cells, other activated glia, and peripherally derived stimuli after the breakdown of the blood brain barrier (Abdul-Muneer et al. 2013, Blixt et al. 2015). Active microglia rapidly induce the expression and release of cytokines and chemokines after injury (Kumar et al. 2015, Witcher et al. 2015). Microglial activation after injury is also associated with alterations in morphology and redistribution of cell-surface markers. For instance, a recent study identified “jellyfish” microglia by two-photon microscopy (Corps et al. 2015) that were detected after TBI was induced via pressure on a thin skull preparation. These microglia were highly activated, moved to the area of damage, and phagocytosed particulate matter associated with tissue damage (Corps et al. 2015). Active microglia also increase surface expression of Iba1 and CD68 and have a de-ramified morphology after diffuse TBI induced by midline fluid percussion injury (mFPI) (Bachstetter et al. 2013). Another study showed that morphological changes in microglia following diffuse TBI were dependent on intact p38α MAPK signaling, a pathway that also induces pro-inflammatory cytokine production (Bachstetter et al. 2011). Diffuse TBI induced by mFPI results in transiently elevated IL-1β, TNFα, and CD14 in the cortex and hippocampus of mice within 4 hours (Fenn et al. 2014). Moreover, microglia have increased IL-1β, CD14, iNOS, and arginase expression 24 hours after diffuse TBI compared with sham-injured controls (Fenn et al. 2015). Therefore, TBI results in the induction of both inflammatory (M1) and repair (M2) cascades associated with macrophage and microglia profiles (Kumar et al. 2013). Similar induction of both inflammatory and repair related mediators including iNOS, CD86, CD68, CD11b, IL-10, chitinase, TGF-β, and arginase are induced after focal brain injury by controlled cortical injury (Loane et al. 2014, Turtzo et al. 2014, Wang et al. 2013). Thus, both diffuse and focal head injuries drive a concordant inflammatory and repair response by microglia.
Along with resident microglia, there are other myeloid cells within the injured CNS that contribute to acute neuroinflammation. Differentiating the contribution of resident microglia from infiltrating macrophages can be difficult, particularly in the context of penetrating brain injury. This is because once monocytes differentiate into brain macrophages they are nearly indistinguishable from microglia by histology due to similarities in surface marker expression. Nonetheless, activation of microglia following TBI results in production of chemokines including CCL2, which attract monocytes and granulocytes to the site of injury (Semple et al. 2010). Notably, antagonism of the CCL2 receptor, CCR2, reduces inflammation and improves cognitive function 1 month after TBI induced by controlled cortical impact injury (CCI) (Morganti et al. 2015). Penetrating injury causes microglia/macrophages to associate with the axon initial segment within 3 hours (Baalman et al. 2015) and diffuse brain injury induces microglia to form tight clusters and long rod-like structures in the cortex within 7 days (Ziebell et al. 2012). Both of these cell types are rapidly activated and have central roles in mediating neuroinflammatory responses after injury.
Clinical evidence supports the idea that microglial and macrophage activation occurs rapidly following TBI. In autopsy of patients who have died following severe TBI, activated microglial morphologies were detected 2-10 days after injury (Velazquez et al. 2015). In addition, increased cerebrospinal fluid (CSF) levels of ferritin and soluble CD163, markers of macrophage and microglial activation, were elevated in pediatric patients within 3 days of severe TBI (Newell et al. 2015). Additionally, plasma levels of microglial activation products MMP-9 and galectin-3 were elevated within 8 hours of mild TBI in adults (Shan et al. 2016). Clinically, the detection of inflammatory proteins in CSF or blood may be useful biomarkers of microglia/macrophage-mediated inflammation.
In summary, microglia are rapidly activated and have both pro-inflammatory and neuroprotective roles immediately after TBI. The majority of anti-inflammatory interventions in rodent models of head injury reduces inflammation and improves functional recovery. Therefore, the reduction in acute neuroinflammation in TBI is interpreted to be beneficial. In several examples of TBI in rodents, minocycline, an anti-inflammatory agent and purported microglia inhibitor, reduced inflammation and improved functional recovery after TBI (Haber et al. 2013, Kovesdi et al. 2012). In addition, intravenous administration of methylene blue (MB), an anti-inflammatory agent (Oz et al. 2011), reduced expression of inflammatory mediators in the brain 1 day post-injury (Fenn et al. 2014). Notably, this reduction blocked the development of a TBI-associated depressive-like phenotype 7 days later. Another paper showed that MB treatment increased neuronal survival in a controlled cortical impact model of injury (Talley Watts et al. 2016). There are many examples of therapies that reduce the activation profiles of microglia and improve functional recovery (Kumar & Loane 2012). Another is issue is the degree to which inflammation fails to resolve, instead persisting chronically after injury. Evidence suggests that such a prolonged neuroinflammatory response mediated by microglia and macrophages is significantly detrimental after CNS injury. Therefore treatment strategies that can lower acute inflammation after TBI may have a long term benefit.
Neuroinflammation after Spinal Cord Injury
Another example of traumatic CNS injury is spinal cord injury (SCI). SCI is characterized both by the acute and focal contusion, as well as by an extensive secondary injury composed of ischemia, excitotoxicity, and inflammation (David et al. 2012). The initial, acute period of inflammation is characterized by an influx of neutrophils and monocytes into the damaged region of the spinal cord (Pineau et al. 2010). In the secondary phase of inflammation, there is continued degeneration that occurs over a period of months and is driven by lymphocytes (Ankeny et al. 2006). The cells of the CNS including microglia, astrocytes, neurons, and oligodendrocytes respond quickly to SCI, upregulating IL-1β and TNFα within hours (Yang et al. 2004, Yang et al. 2005, Bastien et al. 2015). There is activation of microglia in the cord, even distal from the site, associated with tissue reorganization and impediment of functional recovery (Hansen et al. 2013). Additionally, there is a significant contribution of peripheral immune cells to inflammation following SCI. For instance, there is rapid infiltration of neutrophils after injury, the presence of which is transient and disappears by 3 days post-injury (Fleming et al. 2006). Monocytes are recruited to the injured cord, as well, with lymphocytes found within the spinal cord after a far longer period of time, reaching their peak concentration in mice at 42 days (Ankeny et al. 2006, Sroga et al. 2003) and in humans after several months (Fleming et al. 2006). Thus, there is a significant and long lasting contribution of peripheral immune cells to the inflammatory environment within the spinal cord after injury.
The infiltrating peripheral myeloid cells play a large role in the secondary degeneration of SCI. A reduction in neutrophils present in the spinal cord has led to improved recovery (Bao et al. 2004, Bao et al. 2008, Gris et al. 2004), while depletion of macrophages via clodronate liposomes reduced damage to the tissue at the contusion site (Popovich et al. 1999). New evidence suggests that these myeloid cells are not inherently damaging, but that they may be driven toward a more inflammatory phenotype by the presence of cytokines such as TNFα and IL-1β (Genovese et al. 2008, Zong et al. 2012) and free radicals (Bao et al. 2004, Bao et al. 2008). Antagonism of the IL-1 receptor at early time points following SCI, and sequestration of TNFα via administration of soluble tumor necrosis factor receptor superfamily member 1A (TNFR1), reduced apoptosis (Ferguson et al. 2008, Nesic et al. 2001). Additionally, the increase in expression of TNFα caused spontaneous demyelination via activation of macrophages and microglia (Probert et al. 2000). Overall, the presence of peripherally derived myeloid cells in the spinal cord contributes to prolonged tissue damage after SCI.
Positive Aspects of Neuroinflammation
Cytokine-Mediated Sickness Behavior
Now that classic neuroinflammation has been discussed in the context of CNS injury, we can discuss that there are other degrees of neuroinflammation that are beneficial and adaptive. A key component to this argument is that the immune system and the brain communicate with one another and that this communication is bi-directional. For instance, challenges to the peripheral immune system are sensed by the CNS. This immune activation is conveyed to the brain using several neural and humoral pathways. This “inflammation” is interpreted and propagated within the brain. Key areas of communication include the neurovascular unit (endothelium), brain stem, and circumventricular organs of the brain (Hansen et al. 2001, Laflamme et al. 1999, Lacroix et al. 1998, Ching et al. 2007). This communication results in microglial propagation of cytokines and chemokines within the brain (Henry et al. 2009, Chen et al. 2012) and allows for functional communication between the immune system and the brain.
In the context of a peripheral immune challenge, one consequence of the propagation of neuroinflammatory cytokines is the induction of sickness behavior. A coordinated response mediated by the neurovascular unit (Serrats et al. 2010) and resident glia (Henry et al. 2009, Norden et al. 2014) propagates cytokine and secondary signals that cause the physiological and behavioral components of the sickness response including fever, hypophagia, lethargy, listlessness, decreased activity, and reduced social interaction (Dantzer et al. 2008). These behavioral changes are evolutionarily adaptive and necessary to reallocate the host's resources and to fight infection (Berg et al. 2004, Bluthe et al. 2000). Disruptions to this response have a negative effect on morbidity and mortality (Corona et al. 2013). Thus, this response is beneficial to the host organism when properly controlled and resolved.
Neuroimmune communication, signal propagation, and behavioral output are cytokine-mediated and several neuroinflammatory cytokines, such as IL-1β, TNFα, and IL-6, play a key role in the induction and maintenance of this response. It is important to clarify that the duration of cytokine exposure is short and the effect is transient, characterized by temporary activation of both microglia and astrocytes (Norden et al. 2016). This response is tightly regulated and quickly resolved, indicating that the CNS is designed to handle this level of neuroinflammation as a beneficial response (Norden & Godbout 2013). Such immune to brain communication occurs without breakdown of the BBB, entrance of peripheral immune cells, or overt neuropathology (Dantzer et al. 2008). Additionally, sickness behaviors are evolutionarily conserved, indicating their importance to recovery after infection. Thus, this is one broad example of how communication between the immune system and the brain results an acute inflammatory response that is adaptive and beneficial.
Immune Conditioning or Euflammation
Another positive aspect of inflammation is immune conditioning (Chen et al. 2013, Tarr et al. 2014). One example of immune conditioning is euflammation, defined as the induction of peripheral inflammation in the absence of sickness behavior. This is induced by repeated subthreshold Escherichia coli i.p. injections on three consecutive days which create a euflammation induction locus (EIL). The doses of E.coli incrementally increase, and are administered at 2.0×107, 25×107, and 100×107 CFUs. This low level challenge beneficially “conditions” the immune system and leukocytes within the EIL release less cytokines upon subsequent immune stimulation (Chen et al. 2013, Tarr et al. 2014). This reduction in inflammatory signal propagation is contrasted by increased phagocytic potential of macrophages within the EIL.
This immune condition of inflammation was associated with minimized sickness behavior compared to mice exposed to a single E.coli dose. Additionally, leukocytes plated from inside the EIL displayed decreased cytokine mRNA expression, and leukocytes from outside the EIL displayed increased cytokine mRNA expression, when stimulated with LPS (Tarr et al. 2014). Locomotor deficits and piloerection were absent after euflammation conditioning (Chen et al. 2013, Tarr et al. 2014). Additionally, anhedonia, anorexia, and adipsia, more subtle markers for sickness (Andonova et al. 1998, Borowski et al. 1998, Riediger et al. 2010), were all resolved faster in the pre-conditioned mice (Tarr et al. 2014). The difference in sickness behavior following euflammatory treatment was attributed to changes in the phenotype of myeloid cells and a reduction in their inflammatory profile. Cells associated with the immune response (CD11b+/MHC II+, CD11b+/TLR4+, CD11b+/CD86+) were reduced within the EIL, but are increased in the blood and the spleen (Tarr et al. 2014). In addition, cultured peritoneal leukocytes had reduced production of IL-1β, IL-6, and IL-10 mRNA when stimulated with LPS (Tarr et al. 2014). This is a phenotype similar to the development of endotoxin tolerance. Outside of the EIL, blood leukocytes instead increase their expression of IL-1β, TNFα, and IL-10, a phenotype similar to trained immunity. This duality is unique to euflammation, combining the profiles previously described as endotoxin tolerance (Biswas & Lopez-Collazo 2009) and trained immunity (Netea 2013).
Related to these points, there are other examples of immune conditioning relevant to CNS injury. In this context, studies have aimed to precondition the CNS and protect against traumatic CNS injury. This preconditioning can be induced in a wide variety of manners, such as via LPS injections, the use of anesthetics such as isofluorane, and exposure to hypo- and hyperthermia (Baughman et al. 1988, Ota et al. 2000, Tasaki et al. 1997). For example, repeated LPS exposures lead to preconditioning of the CNS. Conditioned microglial activation can lead to the ensheathing of cortical neurons and the subsequent reduction in inhibitory axosomatic synapses, a neuroprotective function after traumatic brain injury caused by cryo-injury (Chen et al. 2012). The mechanism of action for this function is still under investigation, but it is hypothesized to be similar to the protective benefits offered by the synaptic stimulation of NMDA receptors and their anti-apoptotic signaling pathway through CREB activation (Hardingham et al. 2002). This process is paralleled by the activated microglia transiently and preferentially reducing GABAergic signaling (Chen et al. 2012). Overall, immune preconditioning may drive a unique microglial profile that is more anti-inflammatory or repair that allow for protection from the hyper-inflammatory conditions associated with traumatic CNS injury.
Signaling in Memory
There are also example of the benefits of neuroinflammatory signaling in brain development and plasticity. As outlined in the introduction, this represents neuroinflammatory signaling as opposed to true neuroinflammation discussed for CNS injury. For instance, recent studies in learning and memory have detailed the involvement of a complex cytokine network during LTP (del Rey et al. 2013). The major cytokines involved in these studies, IL-1β, IL-6, and TNFα, are produced by several cell types, including immune cells. A similarly diverse cellular population responds to these immune signals. These cells include neurons, both within and outside the hippocampus, and astrocytes, which are involved in the maintenance of synapses (Elmariah et al. 2005, McAfoose & Baune 2009, Ullian et al. 2004). Both IL-1β and IL-6 are overexpressed in the hippocampus after LTP induction, and they produce opposing effects in that IL-1β bolsters learning while IL-6 inhibits it (Goshen & Yirmiya 2009, Schneider et al. 1998). In addition to these two primary cytokines, TNFα, IL-1 receptor antagonist (IL-1RA), and IL-18 are also implicated in learning and memory. All of these cytokines have important roles in learning processes, an often overlooked property of classically inflammatory molecules.
Cytokine expression in the hippocampus displays regional specificity. The hippocampal increases mentioned above are complimented by additional changes in expression in the pre-frontal cortex (PFC). The effect of IL-1 upon neuronal activity can be elucidated through the study of IL-1 receptor 3 (IL-1R3). This shortened protein is preferentially expressed in neurons, opposed to the classical IL-1R1 expression on immune cells (Qian et al. 2012). In conjunction with the IL-1 receptor accessory protein B (IL-1RACPb), both of which are found preferentially within tissue of the nervous system, IL-1R3 activation increases voltage-gated potassium current. The discovery of IL-1R3 has led to clearer explanations of how IL-1 signaling can induce neuronal activity in the absence of NF-κB pathway activation (Qian et al. 2012). In sum, IL-1R1 and IL-1R3 activity helps to illuminate the increasingly complex role of IL-1 signaling in learning and memory. Additionally, this demonstrates the importance of cytokine signaling in the CNS even during such basic, homeostatic conditions as LTP induction.
Another route to learning and memory alteration by cytokines may lie in their modulatory effect on neurotransmission. In this context the source for the cytokines, particularly IL-1β and IL-6, is glia including astrocytes (John et al. 2003). Astrocytes receive neuronal signals and reciprocating by activating NF-κB pathways to affect neuronal plasticity (del Rey et al. 2013, Freudenthal et al. 2005, Kaltschmidt et al. 2006, Meffert et al. 2003). IL-1 and IL-6 also exert opposite effects on molecular signaling: IL-1 enhances AMPA- or NMDA-mediated transmission, while IL-6 inhibits glutamate release and synaptic plasticity (Kawasaki et al. 2008). In addition, IL-1 stimulates aminergic release in the hippocampus (Kabiersch et al. 1988). There is also evidence that immune cells, especially T cells, communicate to resident cells and are critical to normal memory and learning processes (Ziv et al. 2006, Kipnis et al. 2012). Thus, neuroinflammation signaling in this context is interpreted have a beneficial role in brain development, memory, and learning processes.
Promotion of Repair Processes
As discussed above, traumatic injuries provide illuminating examples of true neuroinflammation involving contribution of both resident microglia and recruited leukocytes. Following SCI or TBI extensive inflammation is reported, composed of both resident microglia and peripheral macrophages recruited by chemokines. Most of the evidence indicates that the reduction of acute inflammation of infiltrating immune cells into injury sites is beneficial to the injured. Notably, an important observation from SCI and TBI is that active neuroinflammation also coincides with active intrinsic repair responses (Gensel et al. 2009, Kigerl et al. 2009). Thus, injury induces inflammatory responses and repair responses at the same time. The classical view of neuroinflammation in traumatic injury is that of chronic, unchecked microglial and macrophage activation, cytokine release, and tissue damage and degeneration. Nonetheless, there is a balance and an “alternative activation” (M2) profile of microglia/macrophages has been characterized since 1992 (Stein et al. 1992). Interestingly, this balance between classic (M1) and alternative (M2) activation states in the CNS is influenced by the method of entry of the infiltrating leukocytes to the CNS (Shechter et al. 2013). Clearly the acute neuroinflammatory response to injury must serve a purpose. One example of this is the recruitment of macrophages to combat excitotoxicity via glutamate uptake (Rimaniol et al. 2000). Secondly, the recruitment of immune cells helps to complete the wound healing process as infiltrating macrophages help to clear debris caused by the injury. Indeed, phagocytosis of the dead cells and myelin is required in order to foster a microenvironment conducive toward repair and regrowth (Barrette et al. 2008, Kotter et al. 2001, Popovich et al. 1999). For example, due to myelin's growth-inhibitory properties, its removal is paramount for axon regeneration to occur in SCI. M2-type macrophages, induced by IL-4 or IL-13, can simultaneously promote angiogenesis and suppress the inflammation caused by SCI. In addition, these M2 cells promote long-distance axon regeneration even within inhibitory environments (Kigerl et al. 2009, Sica et al. 2006). They are also able to foster the generation of oligodendrocytes via the activation of TLR4 receptors (Schonberg et al. 2007). To summarize, alternative activation of microglia and macrophages is paramount for intrinsic repair after SCI and these cells help to promote myelin clearance and oligodendrocyte regeneration.
As noted above, immune responses can benefit the tissue repair process after traumatic CNS injury. Several lines of evidence indicate that bolstering M2-repair responses of microglia and macrophages is beneficial. For instance, M2 re-direction of macrophages with Azithromycin promotes tissue sparing and locomotor recovery after SCI, as shown by the anti-inflammatory effects of azithromycin treatment in mice (Zhang et al. 2015b). Recent studies have shown that active microglia upregulate the receptor for IL-4, which directs activated microglia toward a more M2a or repair profile (Fenn et al. 2012). Thus, a shift in inflammatory balance towards a profile that is more supportive of repair would be beneficial in the context of CNS injury. In fact, IL-4 may be produced by CD4+ T-cells that enter the injury site (Walsh et al. 2015). IL-4-producing T-cells provide a neuroprotective signal on neurons directly (Walsh et al. 2015) and may also provide IL-4 to the resident microglia. For instance, a recent study using adult and aged (20 months) mice showed that microglia of adult mice enhance IL-4Rα on the cell surface after SCI (Fenn et al. 2014). This induction, however, was not detected on aged microglia after SCI. This was associated with less IL-4-dependent reprograming of microglia and reduced arginase induction in the microglia of aged mice compared with adult microglia after SCI (Fenn et al. 2014). In addition, microglia from aged mice had reduced expression of IL-1β and CCL2 in the injured cord and a differential recruitment of macrophages. In a related study, aged mice (14 months) had reduced IL-10 expression after SCI. This IL-10 reduction in the aged was associated with reduced presence of M2 regulatory macrophages, less tissue sparing, and worse functional recovery (Zhang et al. 2015a). In sum, cytokine pathways that re-direct activated microglia and macrophages toward a more M2-type profile help turn the tide of inflammation from damage toward repair.
Negative Aspects of Neuroinflammation
Stress
While the neuroinflammation derived from the communication between the immune and the brain is beneficial, certain circumstances can throw off this balance. A good example of this is the response to chronic or traumatic stressors. Traumatic or chronic stressors appear to promote a more neuroinflammatory profile that involves both resident microglia and bone marrow-derived macrophages (Wohleb et al. 2014a). A myriad of studies with rodents indicate that microglia play a major role in response to chronic or traumatic stressors, activating and releasing inflammatory signals (Frank et al. 2007, Frank et al. 2006, Tynan et al. 2010b, Wohleb et al. 2012, Wohleb et al. 2011a, Wohleb et al. 2013). For example, repeated social defeat induces activation of microglia and increased pro-inflammatory cytokine and chemokine expression in the brain (Wohleb et al. 2012, Wohleb et al. 2011a, Wohleb et al. 2013). These inflammatory inductions are paralleled by increased Iba1-immmunoreactivity of microglia, particularly in stress responsive regions of the brain (i.e., fear and threat appraisal centers) (Tynan et al. 2010b, Wohleb et al. 2011a). Other stressors including foot shock and unpredictable stress also provide evidence of the activation of resident microglia (Frank et al., 2007). The connection between stress appraisal and activation of microglia has been attributed to noradrenergic signaling (Gyoneva & Traynelis 2013, Johnson et al. 2005), and inflammasome activation and the release of ATP (Iwata et al. 2013).
In repeated social defeat (RSD), threat interpretation and microglial activation is also associated with the recruitment of inflammatory monocytes from the bone marrow to the brain. This is relevant because it provides a means by which the immune system can relay signals back to the brain and influence behavior. In RSD, sympathetic activation induced the production of pro-inflammatory (Ly6Chi/CD45+) monocytes (Wohleb et al. 2011b, Heidt et al. 2014, Powell et al. 2013) that are released into circulation. These cells traffic to the brain, including within specific stress-responsive brain regions (Wohleb et al. 2011b, Tynan et al. 2010a) that are spatially coupled to neurovascular facilitation of monocyte recruitment (Sawicki et al. 2014). The recruitment of these monocytes following chronic stress promotes the development of prolonged anxiety-like behavior (Wohleb et al. 2014b, Wohleb et al. 2013). Notably, when either monocyte release from the BM or monocyte recruitment to the brain is blocked, induction of anxiety is inhibited. For instance, β-adrenergic receptor antagonists, benzodiazepines, and antidepressants that modify neuronal adaptation prevent many of the neuroimmune and behavioral responses to psychosocial stress, including anxiety and social avoidance (Ramirez et al. 2016, Wohleb et al. 2011a, Wohleb et al. 2013). In addition, mice deficient in CCR2 or IL-1R1 do not develop anxiety after RSD (Wohleb et al. 2013). Overall, the prevention of macrophage trafficking to the brain blocks the induction of stress-related anxiety in mice (Wohleb et al. 2014a, Wohleb et al. 2013). Thus, stress-induced neuroinflammatory responses serve as an important link between immune dysfunction and development of mood disorders.
This is relevant because in clinical evidence indicates that individuals exposed to chronic stress show persistent cognitive and emotional dysregulation that contributes to deterioration of overall mental health and quality of life (Baum et al. 1993, McEwen 2013). For instance, studies with caregivers (Caswell et al. 2003, Mackenzie et al. 2007) and college-age students (Keinan et al. 1999) demonstrate that chronic stress is strongly associated with cognitive impairments and accelerated cognitive decline (Vitaliano et al. 2011). Additional studies also report associations between stress-induced neuroinflammatory activation and psychological disturbances. For example, elevated pro-inflammatory cytokines (Janelidze et al. 2011), increased microglia activation (Schnieder et al. 2014), and increased brain macrophages (Torres-Platas et al. 2014) were all detected within specific brain regions of depressed suicide victims. Thus, stress-induced neuropsychiatric disturbances may involve impaired neuroplasticity caused by microglial activation, monocyte recruitment, and enhanced neuroinflammatory signaling.
Aging
The normal aging process provides a key example of the disruption in the communication and balance between the brain and the immune system. In this case, there is a myriad of alterations in both peripheral immune cells and brain cells. Notably, the number of microglia in the dentate gyrus of aged mice is inversely correlated with the number of stem/progenitor cells (Gebara et al. 2013). In the brain, a higher inflammatory profile of microglia in rodents, canines, non-human primates, and humans has been reported. Several mediators associated with immunity are increased including MHC II, CD68, CD11b and CD11c, TLRs, and CD86 (Frank et al. 2006, Godbout et al. 2005, Griffin et al. 2006, Lucin & Wyss-Coray 2009, Stichel & Luebbert 2007). Aged microglia also have altered morphological profiles associated with de-ramification, shorter processes, and fewer branching arbors (Choi et al. 2007). In addition, there is an increase in inflammatory genes for cytokines such as IL-1β and IL-6 (Godbout et al. 2005, Sierra et al. 2007, Xie et al. 2003), and a corresponding decrease in anti-inflammatory genes of cytokines including IL-10 and IL-4 (Maher et al. 2005, Ye & Johnson 2001). Recent transcriptome analysis of the aged brain (Youm et al. 2013) sorted aged microglia confirmed a higher inflammatory profile with an mRNA signature of less TGFβ-related responsiveness.
One interpretation of this increased inflammatory profile of microglia with age is that these cells are primed or sensitized. This ‘priming’ profile of microglia is represented threefold: 1) increased expression of markers and mediators of inflammation, 2) decreased threshold and time for activation, and 3) increased response and inflammation following this activation (Henry et al. 2009, Norden & Godbout 2013). It is clear that the inflammatory status increases with age (Chung et al. 2009). Recent studies have also used positron emission tomography (PET) to evaluate microglial activation in humans. The ligand PK [11C](R)PK11195 binds to translocator protein (TSPO) receptors that are expressed in mitochondria of activated microglia. PET imaging using this compound showed increased TSPO in older individuals, indicating that microglial activation was elevated with age (Schuitemaker et al. 2012). Taken together, the increase of inflammatory mediators and altered phenotype of microglia with age indicates that microglia develop a primed phenotype and that this may contribute to the heightened inflammatory status of the aged brain.
A consequence of an increased primed profile in the aged brain is the hyper-activation of microglia in response to immune challenge. For instance, peripheral immune challenge with lipopolysaccharide or E.coli causes an exaggerated and prolonged neuroinflammation in aged rodents compared to adults. In aged mice, peripheral LPS challenge results in elevated mRNA levels of IL-1β, IL-6, and TNFα (Abraham et al. 2008, Godbout et al. 2005), which remained elevated for 24 and 72 hours (Godbout et al. 2008b). The prolonged exaggeration of IL-1β in microglia coincides with a similarly prolonged downregulation of fractalkine receptor (CX3CR1) (Wynne et al. 2010). Evidence points to primed microglia as the major arbiters of this inflammation, as MHC II+ microglia had a robust increase IL-1β protein expression following LPS stimulation (Henry et al. 2009). Similarly, microglia isolated from aged mice display elevated TNFα, IL-1β, IL-6, IL-12, TLR2, and indoleamine 2,3-dioxygenase (IDO) mRNA after LPS when compared with adult controls (Henry et al. 2009, Sierra et al. 2007). These changes in cytokine production are also present in aged mice following minor surgery and mild psychological stress (Buchanan et al. 2008, Rosczyk et al. 2008). Furthermore, the induction of IL-1β after immune challenge was greater in MHC II+ microglia of aged mice compared to MHC II− microglia (Henry et al. 2009). These studies indicate that these primed MHC II+ microglia produce inflammatory cytokines, to an exaggerated and prolonged degree, after peripheral immune stimulation.
As discussed above, cytokine-mediated sickness behavior is a necessary and beneficial response to infection. An unchecked, amplified, or prolonged response, however, affects behavioral and cognitive processes (Jurgens & Johnson 2010). In the studies discussed above, LPS stimulation, either peripheral or central, caused protracted neuroinflammation in the brain of aged rodents. This was accompanied by a protracted sickness response notably characterized by persistent anorexia, lethargy, and social withdrawal (Godbout et al. 2005, Abraham et al. 2008, Huang et al. 2008). An exaggerated sickness response was also detected in older rats following subcutaneous E.coli injection. The aged rats displayed an altered febrile response characterized by a blunted and delayed increase of core body temperature followed by a notable and protracted increase of inflammatory cytokines in the brain (Barrientos et al. 2009b). Similar to the extended sickness behaviors in aged BALB/c mice, the increase of inflammatory cytokines were driven by exaggerated microglial IL-1β (Henry et al. 2009). Indeed, i.c.v. infusion of IL-1 receptor antagonist (IL-1RA) reversed the extended LPS-induced sickness behavior in aged mice (Abraham & Johnson 2008). These findings indicate that the exaggerated sickness response in aged rodents was caused by the exaggerated and prolonged production of IL-1β by primed microglia.
Following an immune challenge, there is also evidence of the development of depressive-like behavioral complications and cognitive deficits in aged rodents. For example, in aged BALB/c mice, peripheral LPS stimulation caused prolonged depressive-like behavior 72 h after injection in aged mice after acute (Godbout et al. 2008a) and chronic (Kelley et al. 2013) immune challenge. In addition, injection of LPS caused an amplified cytokine response in the hippocampus of older mice that was paralleled by impaired hippocampal-dependent spatial memory (Chen et al. 2008). Moreover, infection by E.coli led to prolonged production of IL-1β in the hippocampus of aged rats (Barrientos et al. 2009a) and reduced long-term contextual memory examined by context-dependent fear conditioning and Morris water maze (Barrientos et al. 2006, Barrientos et al. 2009a). When aged mice were fed a diet supplemented with resveratrol, a potent anti-oxidant, LPS-induced neuroinflammation and working memory deficits were attenuated (Abraham & Johnson 2009). Increased cytokine production in the aged brain after peripheral innate immune challenge is also associated with impaired cognitive function. Taken together, when there is a primed or sensitized profile of microglia the normal communication between immune system and the brain is impaired. The normally acute neuroinflammatory signaling events are instead amplified and prolonged, and cognitive and behavioral deficits develop in aged mice that are not detected in healthy adults.
Chronic Microglial Activation after Traumatic Brain Injury
As discussed, acute neuroinflammation has both positive and negative influences of functional recovery and repair processes after injury. Thus, it can be difficult to state whether inflammation is negative or positive. The majority of clinical and experimental data, however, indicates that neuroinflammation has a negative effect when it is prolonged. For instance, a pro-inflammatory profile of microglia persists long after injury and is evident in diffuse, penetrating, and repeated head injuries. Furthermore, persistent changes in microglia and astrocytes after penetrating TBI, such as increased MHC II expression in rats 16 days post-injury, are caused by CCI (Holmin et al. 1995). CCI-injured rodents also display an increased inflammatory profile of microglia up to 1 year after injury (Loane et al. 2014). Microglia in the lesion border more highly express several pro-inflammatory mediators such as MHC II, CD68 and NADPH oxidase (NOX2) in the cortex and thalamus, persisting 12 months after injury. In the same brain regions, YM-1, an alternative activation marker (M2) was transiently up-regulated 1 week later, but was reduced over time and was undetected at 3 and 12 months after injury. This supports the notion that in the midst of heightened inflammatory status and progressing gliosis, reparative mechanisms are down-regulated. Additionally, CCI-initiated inflammatory marker expression elevation on microglia and macrophages is associated with increased lesion volume expansion and loss of neurons in the hippocampus (Loane et al. 2014). Such tissue damage and elevated inflammatory mediators are mediated by microglia and macrophages, persist over time, and are consistent with other models of focal or penetrating brain injury (Shultz et al. 2013, Huang et al. 2014).
There is evidence of a persistent, primed, pro-inflammatory microglial profile after diffuse head injury. Reliable experimental models of this non-penetrating, diffuse injury include mFPI, closed head impact (CHI), and blast injuries (Xiong et al. 2013). Injuries by mFPI are administered by a fluid-filled pulse directly at the brain midline without disrupting the dura, causing global undulation of brain tissue. This causes mild neuronal pathology including diffuse axonal injury (Lifshitz et al. 2007a) and transient neurological deficits (Morales et al. 2005) that mimic clinical complications after mild to moderate concussive head injuries in human patients (Lifshitz 2009). Moderate mFPI in rodents causes activated microglia and an altered microglial phenotype, such as increased MHC II and CD68 expression, that persists 1 week to 1 month post-injury. (Ziebell et al. 2012). Moreover, these microglia have a unique rod-like morphology and train arrangement along axon tracks and are predominantly detected in the primary sensory barrel fields of rats (Ziebell et al. 2012). A related study in mice has shown that mFPI increases mRNA and protein expression of MHC II on microglia in the brain up to 1 month post-injury (Fenn et al. 2014). Furthermore, there subtle changes in microglial morphology are evident in the parietal cortex and hippocampus, both of which are brain regions affected by the diffuse injury. These increases in MHC II expression on microglia 30 days post-injury were detected in mice that had returned to baseline behavior. Overall, these data provide evidence that there are pro-inflammatory microglial profiles of microglia that develop and are maintained after brain injury.
An inflammatory glial profile that persists after TBI is paralleled in human patients following moderate or severe TBI. For example, increased CD11b and CD68 microglial expression has been shown in blunt head trauma patients at short- and long-term time points, up to 16 years post-injury (Gentleman et al. 2004). Another study has demonstrated that densely-packed, reactive (CR3/43+ or CD68+) microglia are detectable 1-18 years after a single moderate or severe TBI (Johnson et al. 2013). Recent developments in magnetic resonance imaging (MRI) techniques have been used to examine microglial activation in human brains after TBI in vivo. As described above, PK [11C](R)PK11195 ligand is expressed on activated microglia. Higher PK binding was detected in TBI patients in the thalamus, occipital lobes, putamen and posterior limb of internal capsule (Ramlackhansingh et al. 2011). Notably, chronic microglial activation increases in cell populations further from a focal lesion, being most prominent within subcortical structures such as the thalamus and putamen. Furthermore, although the time post-injury for those studied ranged from 11 months to 17 years, no correlation was found between PK binding and the time since the head injury. These findings indicate that chronic microglial activation persists for months to years after the initial insult in human patients.
Evidence for Microglial Priming and Microglia Reactivity after Central Nervous System Injury
Discussed above in the aging section, a central component of microglia priming is microglial hyper reactivity following immune stimulation. Reactivity of microglia to secondary immune challenge is also evident after CNS injury. For example increased expression of CD68 in resident microglia is maintained after optic nerve (ON) crush injury. Induction of an immune response with peripheral LPS administration 28 days after ON crush significantly increased expression of IL-1β, TNF-α, and IL-6 (Palin et al. 2009). This heightened inflammatory response after LPS suggests that ON crush prompts development of primed CD68+ microglia. Moreover, MHC II mRNA and protein were elevated in microglia 1 month after injury induced by midline fluid percussion injury (Fenn et al. 2014). Secondary LPS challenge induced exaggerated expression of IL-1β, TNFα, and CCL2 specifically in microglia from TBI (30 dpi) mice compared to controls (Fenn et al. 2014, Muccigrosso et al. 2016). This exaggerated microglia activation following immune challenge in TBI mice was associated with prolonged social withdrawal and depressive-like behavior that was not detected in control mice injected with LPS (Fenn et al. 2014). Moreover, the LPS challenge also significantly impaired memory recall in TBI mice but not controls (Muccigrosso et al. 2016). Thus, CNS injury is a “priming event,” leaving microglia in an activated state, capable of hyper-responsiveness with subsequent immune activation.
Microglial priming may also be a factor in repeated TBI, where the initial injury is the priming event and subsequent injuries result in exaggerated inflammatory responses and progressive pathology (Mouzon et al. 2014, Weil et al. 2014, Aungst et al. 2014). This idea has not been investigated specifically, yet it is clear across the literature that even a single TBI has long-lasting glial affects and repeated TBI exacerbates the neuroinflammatory response. For example, Mouzon et al. (2014) showed morphologically reactive astrocytes and microglia (thick processes and hypertrophied cell soma) were evident in cortices and hippocampal regions 6 months after single and repeated closed head TBI (5 hits with 48h interval). Inflammation persisted at 12 and 18 months and was associated with spatial learning deficits that were worse following repeated traumas compared to single TBI (Mouzon et al. 2014). The cognitive changes 12 and 18 months after injury were associated with white matter damage and increased Iba1 labeling, but were not associated with neurodegenerative pathology. The time frame allotted in between injuries may also be an important factor. Another study looked at pathological outcomes after a single and repeated closed head TBI (2 hits) with a 3 or 20 day interval (Weil et al. 2014). One month after the last hit, repeated TBI with a shorter interval induced an enhanced neuroinflammatory response, more robust axonal degeneration and poorer performance in spatial learning and memory task. Therefore, multiple injuries can augment neuroinflammation and functional decline. Overall, microglia display increased reactivity to subsequent stressors after traumatic brain injury, leading to affective disorders, cognitive impairments, and increased cytokine production.
Concluding Remarks
The concept of neuroinflammation is broad and encompasses two large areas of biological science in the nervous and immune systems. We are just now beginning to parse out the positive and negative aspects of immune system-nervous system interaction, as illustrated in Figure 1. It is difficult to make generalized conclusions about the positivity or negativity of neuroinflammation when considering systems as nuanced as those discussed in this review. Additionally, due to the complexity of the subject, we are unable to probe each topic discussed here as deeply as is necessary. Perhaps the best way to sum up the state of the field is that its study requires careful consideration of context. Complete removal of inflammatory cells has been shown to be an unreliable treatment for degenerative diseases or mood disorders, while modification of the M1/M2 polarization has shown more promise. Rather than ask whether neuroinflammation is a friend or a foe, the science of neuroimmunology should instead study the individual situations and specific contexts in which the neuroinflammation is occurring.
Acknowledgements
This research was supported by an NIA grant (R01-AG-033028 to J.P.G.).
List of Abbreviations Used
- AD
Alzheimer's Disease
- BBB
blood-brain barrier
- BM
bone marrow
- CCI
controlled cortical impact
- CCL
chemokine (C-C motif) ligand
- CCR
chemokine (C-C motif) receptor
- CD
cluster of differentiation
- CFU
colony-forming unit
- CHI
closed head injury
- CNS
central nervous system
- EAE
experimental autoimmune encephalomyelitis
- EIL
euflammation induction locus
- i.c.v.
intracerebroventricular
- i.p.
intraperitoneal
- i.v.
intravenous
- IFN
interferon
- IL
interleukin
- iNOS
inducible nitric oxide synthase
- LPS
lipopolysaccharide
- LTP
long-term potentiation
- M1
classically activated macrophage
- M2
alternately activated macrophage
- MB
methylene blue
- mFPI
midline fluid percussion injury
- MHC
major histocompatibility complex
- MS
multiple sclerosis
- NOX
NADPH oxidase
- ON
optic nerve
- RSD
repeated social defeat
- SCI
spinal cord injury
- TBI
traumatic brain injury
- TGF
transforming growth factor
- TLR
toll-like receptor
- TNF
tumor necrosis factor
- TSPO
translocator protein
Footnotes
Conflicts of Interest
All authors declare that there are no conflicts of interest.
References
- Abdul-Muneer PM, Schuetz H, Wang F, Skotak M, Jones J, Gorantla S, Zimmerman MC, Chandra N, Haorah J. Induction of oxidative and nitrosative damage leads to cerebrovascular inflammation in an animal model of mild traumatic brain injury induced by primary blast. Free Radic Biol Med. 2013;60:282–291. doi: 10.1016/j.freeradbiomed.2013.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham J, Jang S, Godbout JP, Chen J, Kelley KW, Dantzer R, Johnson RW. Aging sensitizes mice to behavioral deficits induced by central HIV-1 gp120. Neurobiol Aging. 2008;29:614–621. doi: 10.1016/j.neurobiolaging.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham J, Johnson RW. Central inhibition of interleukin-1beta ameliorates sickness behavior in aged mice. Brain, behavior, and immunity. 2008 doi: 10.1016/j.bbi.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham J, Johnson RW. Consuming a diet supplemented with resveratrol reduced infection-related neuroinflammation and deficits in working memory in aged mice. Rejuvenation Res. 2009;12:445–453. doi: 10.1089/rej.2009.0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FM. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat Neurosci. 2007;10:1538–1543. doi: 10.1038/nn2014. [DOI] [PubMed] [Google Scholar]
- Alliot F, Godin I, Pessac B. Microglia derive from progenitors, originating from the yolk sac, and which proliferate in the brain. Brain Res Dev Brain Res. 1999;117:145–152. doi: 10.1016/s0165-3806(99)00113-3. [DOI] [PubMed] [Google Scholar]
- Andonova M, Goundasheva D, Georgiev P, Ivanov V. Effects of indomethacin on lipopolysaccharide-induced plasma PGE2 concentrations and clinical pathological disorders in experimental endotoxemia. Vet Hum Toxicol. 1998;40:14–18. [PubMed] [Google Scholar]
- Ankeny DP, Lucin KM, Sanders VM, McGaughy VM, Popovich PG. Spinal cord injury triggers systemic autoimmunity: evidence for chronic B lymphocyte activation and lupus-like autoantibody synthesis. J Neurochem. 2006;99:1073–1087. doi: 10.1111/j.1471-4159.2006.04147.x. [DOI] [PubMed] [Google Scholar]
- Aungst SL, Kabadi SV, Thompson SM, Stoica BA, Faden AI. Repeated mild traumatic brain injury causes chronic neuroinflammation, changes in hippocampal synaptic plasticity, and associated cognitive deficits. J Cereb Blood Flow Metab. 2014;34:1223–1232. doi: 10.1038/jcbfm.2014.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baalman K, Marin MA, Ho TS, Godoy M, Cherian L, Robertson C, Rasband MN. Axon initial segment-associated microglia. J Neurosci. 2015;35:2283–2292. doi: 10.1523/JNEUROSCI.3751-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachstetter AD, Rowe RK, Kaneko M, Goulding D, Lifshitz J, Van Eldik LJ. The p38alpha MAPK regulates microglial responsiveness to diffuse traumatic brain injury. J Neurosci. 2013;33:6143–6153. doi: 10.1523/JNEUROSCI.5399-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachstetter AD, Xing B, de Almeida L, Dimayuga ER, Watterson DM, Van Eldik LJ. Microglial p38alpha MAPK is a key regulator of proinflammatory cytokine up-regulation induced by toll-like receptor (TLR) ligands or beta-amyloid (Abeta). J Neuroinflammation. 2011;8:79. doi: 10.1186/1742-2094-8-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao F, Chen Y, Dekaban GA, Weaver LC. Early anti-inflammatory treatment reduces lipid peroxidation and protein nitration after spinal cord injury in rats. J Neurochem. 2004;88:1335–1344. doi: 10.1046/j.1471-4159.2003.02240.x. [DOI] [PubMed] [Google Scholar]
- Bao F, Chen Y, Schneider KA, Weaver LC. An integrin inhibiting molecule decreases oxidative damage and improves neurological function after spinal cord injury. Exp Neurol. 2008;214:160–167. doi: 10.1016/j.expneurol.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Barrette B, Hebert MA, Filali M, Lafortune K, Vallieres N, Gowing G, Julien JP, Lacroix S. Requirement of myeloid cells for axon regeneration. J Neurosci. 2008;28:9363–9376. doi: 10.1523/JNEUROSCI.1447-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos RM, Frank MG, Hein AM, Higgins EA, Watkins LR, Rudy JW, Maier SF. Time course of hippocampal IL-1 beta and memory consolidation impairments in aging rats following peripheral infection. Brain Behav Immun. 2009a;23:46–54. doi: 10.1016/j.bbi.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos RM, Higgins EA, Biedenkapp JC, Sprunger DB, Wright-Hardesty KJ, Watkins LR, Rudy JW, Maier SF. Peripheral infection and aging interact to impair hippocampal memory consolidation. Neurobiol Aging. 2006;27:723–732. doi: 10.1016/j.neurobiolaging.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Barrientos RM, Watkins LR, Rudy JW, Maier SF. Characterization of the sickness response in young and aging rats following E. coli infection. Brain Behav Immun. 2009b;23:450–454. doi: 10.1016/j.bbi.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastien D, Bellver Landete V, Lessard M, Vallieres N, Champagne M, Takashima A, Tremblay ME, Doyon Y, Lacroix S. IL-1alpha Gene Deletion Protects Oligodendrocytes after Spinal Cord Injury through Upregulation of the Survival Factor Tox3. J Neurosci. 2015;35:10715–10730. doi: 10.1523/JNEUROSCI.0498-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baughman VL, Hoffman WE, Miletich DJ, Albrecht RF, Thomas C. Neurologic outcome in rats following incomplete cerebral ischemia during halothane, isoflurane, or N2O. Anesthesiology. 1988;69:192–198. doi: 10.1097/00000542-198808000-00007. [DOI] [PubMed] [Google Scholar]
- Baum A, Cohen L, Hall M. Control and intrusive memories as possible determinants of chronic stress. Psychosom Med. 1993;55:274–286. doi: 10.1097/00006842-199305000-00005. [DOI] [PubMed] [Google Scholar]
- Berg BM, Godbout JP, Kelley KW, Johnson RW. Alpha-tocopherol attenuates lipopolysaccharide-induced sickness behavior in mice. Brain, behavior, and immunity. 2004;18:149–157. doi: 10.1016/S0889-1591(03)00113-2. [DOI] [PubMed] [Google Scholar]
- Biswas SK, Lopez-Collazo E. Endotoxin tolerance: new mechanisms, molecules and clinical significance. Trends Immunol. 2009;30:475–487. doi: 10.1016/j.it.2009.07.009. [DOI] [PubMed] [Google Scholar]
- Blixt J, Svensson M, Gunnarson E, Wanecek M. Aquaporins and blood-brain barrier permeability in early edema development after traumatic brain injury. Brain Res. 2015;1611:18–28. doi: 10.1016/j.brainres.2015.03.004. [DOI] [PubMed] [Google Scholar]
- Bluthe RM, Laye S, Michaud B, Combe C, Dantzer R, Parnet P. Role of interleukin-1beta and tumour necrosis factor-alpha in lipopolysaccharide-induced sickness behaviour: a study with interleukin-1 type I receptor-deficient mice. The European journal of neuroscience. 2000;12:4447–4456. [PubMed] [Google Scholar]
- Borowski T, Kokkinidis L, Merali Z, Anisman H. Lipopolysaccharide, central in vivo biogenic amine variations, and anhedonia. Neuroreport. 1998;9:3797–3802. doi: 10.1097/00001756-199812010-00006. [DOI] [PubMed] [Google Scholar]
- Bruttger J, Karram K, Wortge S, et al. Genetic Cell Ablation Reveals Clusters of Local Self-Renewing Microglia in the Mammalian Central Nervous System. Immunity. 2015;43:92–106. doi: 10.1016/j.immuni.2015.06.012. [DOI] [PubMed] [Google Scholar]
- Bucciantini M, Giannoni E, Chiti F, et al. Inherent toxicity of aggregates implies a common mechanism for protein misfolding diseases. Nature. 2002;416:507–511. doi: 10.1038/416507a. [DOI] [PubMed] [Google Scholar]
- Buchanan JB, Sparkman NL, Chen J, Johnson RW. Cognitive and neuroinflammatory consequences of mild repeated stress are exacerbated in aged mice. Psychoneuroendocrinology. 2008;33:755–765. doi: 10.1016/j.psyneuen.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caswell LW, Vitaliano PP, Croyle KL, Scanlan JM, Zhang J, Daruwala A. Negative associations of chronic stress and cognitive performance in older adult spouse caregivers. Exp Aging Res. 2003;29:303–318. doi: 10.1080/03610730303721. [DOI] [PubMed] [Google Scholar]
- Chen J, Buchanan JB, Sparkman NL, Godbout JP, Freund GG, Johnson RW. Neuroinflammation and disruption in working memory in aged mice after acute stimulation of the peripheral innate immune system. Brain, behavior, and immunity. 2008;22:301–311. doi: 10.1016/j.bbi.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Tarr AJ, Liu X, Wang Y, Reed NS, Demarsh CP, Sheridan JF, Quan N. Controlled progressive innate immune stimulation regimen prevents the induction of sickness behavior in the open field test. J Inflamm Res. 2013;6:91–98. doi: 10.2147/JIR.S45111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Jalabi W, Shpargel KB, et al. Lipopolysaccharide-induced microglial activation and neuroprotection against experimental brain injury is independent of hematogenous TLR4. J Neurosci. 2012;32:11706–11715. doi: 10.1523/JNEUROSCI.0730-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching S, Zhang H, Belevych N, He L, Lai W, Pu XA, Jaeger LB, Chen Q, Quan N. Endothelial-specific knockdown of interleukin-1 (IL-1) type 1 receptor differentially alters CNS responses to IL-1 depending on its route of administration. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:10476–10486. doi: 10.1523/JNEUROSCI.3357-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JH, Lee CH, Hwang IK, Won MH, Seong JK, Yoon YS, Lee HS, Lee IS. Age-related changes in ionized calcium-binding adapter molecule 1 immunoreactivity and protein level in the gerbil hippocampal CA1 region. J Vet Med Sci. 2007;69:1131–1136. doi: 10.1292/jvms.69.1131. [DOI] [PubMed] [Google Scholar]
- Chung HY, Cesari M, Anton S, Marzetti E, Giovannini S, Seo AY, Carter C, Yu BP, Leeuwenburgh C. Molecular inflammation: underpinnings of aging and age-related diseases. Ageing Res Rev. 2009;8:18–30. doi: 10.1016/j.arr.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corona AW, Norden DM, Skendelas JP, Huang Y, O'Connor JC, Lawson M, Dantzer R, Kelley KW, Godbout JP. Indoleamine 2,3-dioxygenase inhibition attenuates lipopolysaccharide induced persistent microglial activation and depressive-like complications in fractalkine receptor (CX(3)CR1)-deficient mice. Brain Behav Immun. 2013;31:134–142. doi: 10.1016/j.bbi.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corps KN, Roth TL, McGavern DB. Inflammation and neuroprotection in traumatic brain injury. JAMA Neurol. 2015;72:355–362. doi: 10.1001/jamaneurol.2014.3558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- David S, Greenhalgh AD, Lopez-Vales R. Role of phospholipase A2s and lipid mediators in secondary damage after spinal cord injury. Cell Tissue Res. 2012;349:249–267. doi: 10.1007/s00441-012-1430-8. [DOI] [PubMed] [Google Scholar]
- David S, Kroner A. Repertoire of microglial and macrophage responses after spinal cord injury. Nat Rev Neurosci. 2011;12:388–399. doi: 10.1038/nrn3053. [DOI] [PubMed] [Google Scholar]
- del Rey A, Balschun D, Wetzel W, Randolf A, Besedovsky HO. A cytokine network involving brain-borne IL-1beta, IL-1ra, IL-18, IL-6, and TNFalpha operates during long-term potentiation and learning. Brain Behav Immun. 2013;33:15–23. doi: 10.1016/j.bbi.2013.05.011. [DOI] [PubMed] [Google Scholar]
- Derecki NC, Cardani AN, Yang CH, Quinnies KM, Crihfield A, Lynch KR, Kipnis J. Regulation of learning and memory by meningeal immunity: a key role for IL-4. J Exp Med. 2010;207:1067–1080. doi: 10.1084/jem.20091419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn AJ, Swiergiel AH, Zhang H, Quan N. Reduced ingestion of sweetened milk induced by interleukin-1 and lipopolysaccharide is associated with induction of cyclooxygenase-2 in brain endothelia. Neuroimmunomodulation. 2006;13:96–104. doi: 10.1159/000096291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmariah SB, Oh EJ, Hughes EG, Balice-Gordon RJ. Astrocytes regulate inhibitory synapse formation via Trk-mediated modulation of postsynaptic GABAA receptors. J Neurosci. 2005;25:3638–3650. doi: 10.1523/JNEUROSCI.3980-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmore MR, Najafi AR, Koike MA, et al. Colony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron. 2014;82:380–397. doi: 10.1016/j.neuron.2014.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenn AM, Gensel JC, Huang Y, Popovich PG, Lifshitz J, Godbout JP. Immune activation promotes depression 1 month after diffuse brain injury: a role for primed microglia. Biol Psychiatry. 2014;76:575–584. doi: 10.1016/j.biopsych.2013.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenn AM, Henry CJ, Huang Y, Dugan A, Godbout JP. Lipopolysaccharide-induced interleukin (IL)-4 receptor-alpha expression and corresponding sensitivity to the M2 promoting effects of IL-4 are impaired in microglia of aged mice. Brain Behav Immun. 2012;26:766–777. doi: 10.1016/j.bbi.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenn AM, Skendelas JP, Moussa DN, Muccigrosso MM, Popovich PG, Lifshitz J, Eiferman DS, Godbout JP. Methylene blue attenuates traumatic brain injury-associated neuroinflammation and acute depressive-like behavior in mice. J Neurotrauma. 2015;32:127–138. doi: 10.1089/neu.2014.3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson AR, Christensen RN, Gensel JC, Miller BA, Sun F, Beattie EC, Bresnahan JC, Beattie MS. Cell death after spinal cord injury is exacerbated by rapid TNF alpha-induced trafficking of GluR2-lacking AMPARs to the plasma membrane. J Neurosci. 2008;28:11391–11400. doi: 10.1523/JNEUROSCI.3708-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming JC, Norenberg MD, Ramsay DA, Dekaban GA, Marcillo AE, Saenz AD, Pasquale-Styles M, Dietrich WD, Weaver LC. The cellular inflammatory response in human spinal cords after injury. Brain. 2006;129:3249–3269. doi: 10.1093/brain/awl296. [DOI] [PubMed] [Google Scholar]
- Frank MG, Baratta MV, Sprunger DB, Watkins LR, Maier SF. Microglia serve as a neuroimmune substrate for stress-induced potentiation of CNS pro-inflammatory cytokine responses. Brain Behav Immun. 2007;21:47–59. doi: 10.1016/j.bbi.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Frank MG, Barrientos RM, Biedenkapp JC, Rudy JW, Watkins LR, Maier SF. mRNA up-regulation of MHC II and pivotal pro-inflammatory genes in normal brain aging. Neurobiol Aging. 2006;27:717–722. doi: 10.1016/j.neurobiolaging.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Freudenthal R, Boccia MM, Acosta GB, Blake MG, Merlo E, Baratti CM, Romano A. NF-kappaB transcription factor is required for inhibitory avoidance long-term memory in mice. Eur J Neurosci. 2005;21:2845–2852. doi: 10.1111/j.1460-9568.2005.04126.x. [DOI] [PubMed] [Google Scholar]
- Gebara E, Sultan S, Kocher-Braissant J, Toni N. Adult hippocampal neurogenesis inversely correlates with microglia in conditions of voluntary running and aging. Front Neurosci. 2013;7:145. doi: 10.3389/fnins.2013.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese T, Mazzon E, Crisafulli C, Di Paola R, Muia C, Esposito E, Bramanti P, Cuzzocrea S. TNF-alpha blockage in a mouse model of SCI: evidence for improved outcome. Shock. 2008;29:32–41. doi: 10.1097/shk.0b013e318059053a. [DOI] [PubMed] [Google Scholar]
- Gensel JC, Nakamura S, Guan Z, van Rooijen N, Ankeny DP, Popovich PG. Macrophages promote axon regeneration with concurrent neurotoxicity. J Neurosci. 2009;29:3956–3968. doi: 10.1523/JNEUROSCI.3992-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentleman SM, Leclercq PD, Moyes L, Graham DI, Smith C, Griffin WS, Nicoll JA. Long-term intracerebral inflammatory response after traumatic brain injury. Forensic Sci Int. 2004;146:97–104. doi: 10.1016/j.forsciint.2004.06.027. [DOI] [PubMed] [Google Scholar]
- Ginhoux F, Greter M, Leboeuf M, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godbout JP, Chen J, Abraham J, Richwine AF, Berg BM, Kelley KW, Johnson RW. Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. FASEB J. 2005;19:1329–1331. doi: 10.1096/fj.05-3776fje. [DOI] [PubMed] [Google Scholar]
- Godbout JP, Moreau M, Lestage J, et al. Aging exacerbates depressive-like behavior in mice in response to activation of the peripheral innate immune system. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2008a;33:2341–2351. doi: 10.1038/sj.npp.1301649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godbout JP, Moreau M, Lestage J, et al. Aging exacerbates depressive-like behavior in mice in response to activation of the peripheral innate immune system. Neuropsychopharmacology. 2008b;33:2341–2351. doi: 10.1038/sj.npp.1301649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold R, Linington C, Lassmann H. Understanding pathogenesis and therapy of multiple sclerosis via animal models: 70 years of merits and culprits in experimental autoimmune encephalomyelitis research. Brain. 2006;129:1953–1971. doi: 10.1093/brain/awl075. [DOI] [PubMed] [Google Scholar]
- Goldman D, Song X, Kitai R, Casadevall A, Zhao ML, Lee SC. Cryptococcus neoformans induces macrophage inflammatory protein 1alpha (MIP-1alpha) and MIP-1beta in human microglia: role of specific antibody and soluble capsular polysaccharide. Infect Immun. 2001;69:1808–1815. doi: 10.1128/IAI.69.3.1808-1815.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshen I, Yirmiya R. Interleukin-1 (IL-1): a central regulator of stress responses. Front Neuroendocrinol. 2009;30:30–45. doi: 10.1016/j.yfrne.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Griffin R, Nally R, Nolan Y, McCartney Y, Linden J, Lynch MA. The age-related attenuation in long-term potentiation is associated with microglial activation. J Neurochem. 2006;99:1263–1272. doi: 10.1111/j.1471-4159.2006.04165.x. [DOI] [PubMed] [Google Scholar]
- Gris D, Marsh DR, Oatway MA, Chen Y, Hamilton EF, Dekaban GA, Weaver LC. Transient blockade of the CD11d/CD18 integrin reduces secondary damage after spinal cord injury, improving sensory, autonomic, and motor function. J Neurosci. 2004;24:4043–4051. doi: 10.1523/JNEUROSCI.5343-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyoneva S, Traynelis SF. Norepinephrine modulates the motility of resting and activated microglia via different adrenergic receptors. J Biol Chem. 2013;288:15291–15302. doi: 10.1074/jbc.M113.458901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber M, Abdel Baki SG, Grin'kina NM, Irizarry R, Ershova A, Orsi S, Grill RJ, Dash P, Bergold PJ. Minocycline plus N-acetylcysteine synergize to modulate inflammation and prevent cognitive and memory deficits in a rat model of mild traumatic brain injury. Exp Neurol. 2013;249:169–177. doi: 10.1016/j.expneurol.2013.09.002. [DOI] [PubMed] [Google Scholar]
- Hansen CN, Fisher LC, Deibert RJ, Jakeman LB, Zhang H, Noble-Haeusslein L, White S, Basso DM. Elevated MMP-9 in the lumbar cord early after thoracic spinal cord injury impedes motor relearning in mice. J Neurosci. 2013;33:13101–13111. doi: 10.1523/JNEUROSCI.1576-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen MK, O'Connor KA, Goehler LE, Watkins LR, Maier SF. The contribution of the vagus nerve in interleukin-1beta-induced fever is dependent on dose. American journal of physiology. Regulatory, integrative and comparative physiology. 2001;280:R929–934. doi: 10.1152/ajpregu.2001.280.4.R929. [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Fukunaga Y, Bading H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat Neurosci. 2002;5:405–414. doi: 10.1038/nn835. [DOI] [PubMed] [Google Scholar]
- Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- Heidt T, Sager HB, Courties G, et al. Chronic variable stress activates hematopoietic stem cells. Nat Med. 2014;20:754–758. doi: 10.1038/nm.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry CJ, Huang Y, Wynne AM, Godbout JP. Peripheral lipopolysaccharide (LPS) challenge promotes microglial hyperactivity in aged mice that is associated with exaggerated induction of both pro-inflammatory IL-1beta and anti-inflammatory IL-10 cytokines. Brain, behavior, and immunity. 2009;23:309–317. doi: 10.1016/j.bbi.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmin S, Mathiesen T, Shetye J, Biberfeld P. Intracerebral inflammatory response to experimental brain contusion. Acta Neurochir (Wien) 1995;132:110–119. doi: 10.1007/BF01404857. [DOI] [PubMed] [Google Scholar]
- Huang EY, Tsai TH, Kuo TT, Tsai JJ, Tsui PF, Chou YC, Ma HI, Chiang YH, Chen YH. Remote effects on the striatal dopamine system after fluid percussion injury. Behavioural brain research. 2014;267:156–172. doi: 10.1016/j.bbr.2014.03.033. [DOI] [PubMed] [Google Scholar]
- Huang Y, Henry CJ, Dantzer R, Johnson RW, Godbout JP. Exaggerated sickness behavior and brain proinflammatory cytokine expression in aged mice in response to intracerebroventricular lipopolysaccharide. Neurobiology of aging. 2008;29:1744–1753. doi: 10.1016/j.neurobiolaging.2007.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imeri L, Opp MR. How (and why) the immune system makes us sleep. Nat Rev Neurosci. 2009;10:199–210. doi: 10.1038/nrn2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata M, Ota KT, Duman RS. The inflammasome: pathways linking psychological stress, depression, and systemic illnesses. Brain Behav Immun. 2013;31:105–114. doi: 10.1016/j.bbi.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janelidze S, Mattei D, Westrin A, Traskman-Bendz L, Brundin L. Cytokine levels in the blood may distinguish suicide attempters from depressed patients. Brain Behav Immun. 2011;25:335–339. doi: 10.1016/j.bbi.2010.10.010. [DOI] [PubMed] [Google Scholar]
- John GR, Lee SC, Brosnan CF. Cytokines: powerful regulators of glial cell activation. Neuroscientist. 2003;9:10–22. doi: 10.1177/1073858402239587. [DOI] [PubMed] [Google Scholar]
- Johnson JD, Campisi J, Sharkey CM, Kennedy SL, Nickerson M, Greenwood BN, Fleshner M. Catecholamines mediate stress-induced increases in peripheral and central inflammatory cytokines. Neuroscience. 2005;135:1295–1307. doi: 10.1016/j.neuroscience.2005.06.090. [DOI] [PubMed] [Google Scholar]
- Johnson VE, Stewart JE, Begbie FD, Trojanowski JQ, Smith DH, Stewart W. Inflammation and white matter degeneration persist for years after a single traumatic brain injury. Brain. 2013;136:28–42. doi: 10.1093/brain/aws322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurgens HA, Johnson RW. Dysregulated neuronal-microglial cross-talk during aging, stress and inflammation. Experimental neurology. 2010 doi: 10.1016/j.expneurol.2010.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabiersch A, del Rey A, Honegger CG, Besedovsky HO. Interleukin-1 induces changes in norepinephrine metabolism in the rat brain. Brain Behav Immun. 1988;2:267–274. doi: 10.1016/0889-1591(88)90028-1. [DOI] [PubMed] [Google Scholar]
- Kaltschmidt B, Ndiaye D, Korte M, et al. NF-kappaB regulates spatial memory formation and synaptic plasticity through protein kinase A/CREB signaling. Mol Cell Biol. 2006;26:2936–2946. doi: 10.1128/MCB.26.8.2936-2946.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki Y, Zhang L, Cheng JK, Ji RR. Cytokine mechanisms of central sensitization: distinct and overlapping role of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in regulating synaptic and neuronal activity in the superficial spinal cord. J Neurosci. 2008;28:5189–5194. doi: 10.1523/JNEUROSCI.3338-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keinan G, Friedland N, Kahneman D, Roth D. The effect of stress on the suppression of erroneous competing responses. Anxiety, stress, and coping. 1999;12:455–476. doi: 10.1080/10615809908249321. [DOI] [PubMed] [Google Scholar]
- Kelley KW, O'Connor JC, Lawson MA, Dantzer R, Rodriguez-Zas SL, McCusker RH. Aging leads to prolonged duration of inflammation-induced depression-like behavior caused by Bacillus Calmette-Guerin. Brain Behav Immun. 2013;32:63–69. doi: 10.1016/j.bbi.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci. 2009;29:13435–13444. doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipnis J, Gadani S, Derecki NC. Pro-cognitive properties of T cells. Nat Rev Immunol. 2012;12:663–669. doi: 10.1038/nri3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotter MR, Setzu A, Sim FJ, Van Rooijen N, Franklin RJ. Macrophage depletion impairs oligodendrocyte remyelination following lysolecithin-induced demyelination. Glia. 2001;35:204–212. doi: 10.1002/glia.1085. [DOI] [PubMed] [Google Scholar]
- Kovesdi E, Kamnaksh A, Wingo D, Ahmed F, Grunberg NE, Long JB, Kasper CE, Agoston DV. Acute minocycline treatment mitigates the symptoms of mild blast-induced traumatic brain injury. Front Neurol. 2012;3:111. doi: 10.3389/fneur.2012.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Alvarez-Croda DM, Stoica BA, Faden AI, Loane DJ. Microglial/Macrophage Polarization Dynamics following Traumatic Brain Injury. J Neurotrauma. 2015 doi: 10.1089/neu.2015.4268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Loane DJ. Neuroinflammation after traumatic brain injury: opportunities for therapeutic intervention. Brain Behav Immun. 2012;26:1191–1201. doi: 10.1016/j.bbi.2012.06.008. [DOI] [PubMed] [Google Scholar]
- Kumar A, Stoica BA, Sabirzhanov B, Burns MP, Faden AI, Loane DJ. Traumatic brain injury in aged animals increases lesion size and chronically alters microglial/macrophage classical and alternative activation states. Neurobiol Aging. 2013;34:1397–1411. doi: 10.1016/j.neurobiolaging.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacroix S, Feinstein D, Rivest S. The bacterial endotoxin lipopolysaccharide has the ability to target the brain in upregulating its membrane CD14 receptor within specific cellular populations. Brain pathology. 1998;8:625–640. doi: 10.1111/j.1750-3639.1998.tb00189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laflamme N, Lacroix S, Rivest S. An essential role of interleukin-1beta in mediating NF-kappaB activity and COX-2 transcription in cells of the blood-brain barrier in response to a systemic and localized inflammation but not during endotoxemia. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1999;19:10923–10930. doi: 10.1523/JNEUROSCI.19-24-10923.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesz A, Zhou W, Mracsko E, et al. Inhibition of lymphocyte trafficking shields the brain against deleterious neuroinflammation after stroke. Brain. 2011;134:704–720. doi: 10.1093/brain/awr008. [DOI] [PubMed] [Google Scholar]
- Lifshitz J. Fluid percussion injury model. In: Chen J, Xu ZC, Xu X-M, Zhang JH, editors. Animal Models of Acute Neurological Injuries. Humana Press; 2009. p. 369. [Google Scholar]
- Lifshitz J, Kelley BJ, Povlishock JT. Perisomatic thalamic axotomy after diffuse traumatic brain injury is associated with atrophy rather than cell death. J Neuropathol Exp Neurol. 2007a;66:218–229. doi: 10.1097/01.jnen.0000248558.75950.4d. [DOI] [PubMed] [Google Scholar]
- Lifshitz J, Witgen B, Grady M. Acute cognitive impairment after lateral fluid percussion brain injury recovers by 1 month: evaluation by conditioned fear response. Beh Brain Res. 2007b;177:347. doi: 10.1016/j.bbr.2006.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linker RA, Maurer M, Gaupp S, et al. CNTF is a major protective factor in demyelinating CNS disease: a neurotrophic cytokine as modulator in neuroinflammation. Nat Med. 2002;8:620–624. doi: 10.1038/nm0602-620. [DOI] [PubMed] [Google Scholar]
- Loane DJ, Kumar A, Stoica BA, Cabatbat R, Faden AI. Progressive neurodegeneration after experimental brain trauma: association with chronic microglial activation. J Neuropathol Exp Neurol. 2014;73:14–29. doi: 10.1097/NEN.0000000000000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovas G, Szilagyi N, Majtenyi K, Palkovits M, Komoly S. Axonal changes in chronic demyelinated cervical spinal cord plaques. Brain. 2000;123(Pt 2):308–317. doi: 10.1093/brain/123.2.308. [DOI] [PubMed] [Google Scholar]
- Lucin KM, Wyss-Coray T. Immune activation in brain aging and neurodegeneration: too much or too little? Neuron. 2009;64:110–122. doi: 10.1016/j.neuron.2009.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas AI, Stocchetti N, Bullock R. Moderate and severe traumatic brain injury in adults. Lancet Neurol. 2008;7:728–741. doi: 10.1016/S1474-4422(08)70164-9. [DOI] [PubMed] [Google Scholar]
- Mackenzie CS, Smith MC, Hasher L, Leach L, Behl P. Cognitive functioning under stress: evidence from informal caregivers of palliative patients. J Palliat Med. 2007;10:749–758. doi: 10.1089/jpm.2006.0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher FO, Nolan Y, Lynch MA. Downregulation of IL-4-induced signalling in hippocampus contributes to deficits in LTP in the aged rat. Neurobiol Aging. 2005;26:717–728. doi: 10.1016/j.neurobiolaging.2004.07.002. [DOI] [PubMed] [Google Scholar]
- McAfoose J, Baune BT. Evidence for a cytokine model of cognitive function. Neurosci Biobehav Rev. 2009;33:355–366. doi: 10.1016/j.neubiorev.2008.10.005. [DOI] [PubMed] [Google Scholar]
- McCrea M, Guskiewicz KM, Marshall SW, Barr W, Randolph C, Cantu RC, Onate JA, Yang J, Kelly JP. Acute effects and recovery time following concussion in collegiate football players: The NCAA concussion study. JAMA. 2003;290:2556. doi: 10.1001/jama.290.19.2556. [DOI] [PubMed] [Google Scholar]
- McEwen BS. The Brain on Stress: Toward an Integrative Approach to Brain, Body and Behavior. Perspectives on psychological science : a journal of the Association for Psychological Science. 2013;8:673–675. doi: 10.1177/1745691613506907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meffert MK, Chang JM, Wiltgen BJ, Fanselow MS, Baltimore D. NF-kappa B functions in synaptic signaling and behavior. Nat Neurosci. 2003;6:1072–1078. doi: 10.1038/nn1110. [DOI] [PubMed] [Google Scholar]
- Michael BD, Griffiths MJ, Granerod J, et al. The interleukin-1 balance is associated with clinical severity, blood-brain barrier permeability, neuroimaging changes and outcome in encephalitis. J Infect Dis. 2015 doi: 10.1093/infdis/jiv771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monahan AJ, Warren M, Carvey PM. Neuroinflammation and peripheral immune infiltration in Parkinson's disease: an autoimmune hypothesis. Cell Transplant. 2008;17:363–372. [PubMed] [Google Scholar]
- Morales D, Marklund N, Lebold D, et al. Experimental models of traumatic brain injury: do we really need to build a better mousetrap? Neuroscience. 2005;136:971. doi: 10.1016/j.neuroscience.2005.08.030. [DOI] [PubMed] [Google Scholar]
- Morganti JM, Jopson TD, Liu S, Riparip LK, Guandique CK, Gupta N, Ferguson AR, Rosi S. CCR2 antagonism alters brain macrophage polarization and ameliorates cognitive dysfunction induced by traumatic brain injury. J Neurosci. 2015;35:748–760. doi: 10.1523/JNEUROSCI.2405-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouzon BC, Bachmeier C, Ferro A, et al. Chronic neuropathological and neurobehavioral changes in a repetitive mild traumatic brain injury model. Ann Neurol. 2014;75:241–254. doi: 10.1002/ana.24064. [DOI] [PubMed] [Google Scholar]
- Mrak RE, Sheng JG, Griffin WS. Glial cytokines in Alzheimer's disease: review and pathogenic implications. Hum Pathol. 1995;26:816–823. doi: 10.1016/0046-8177(95)90001-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muccigrosso MM, Ford J, Benner B, et al. Cognitive Deficits Develop 1 month after Diffuse Brain Injury and are Exaggerated by Microglia-Associated Reactivity to Peripheral Immune Challenge. Brain Behav Immun. 2016 doi: 10.1016/j.bbi.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesic O, Xu GY, McAdoo D, High KW, Hulsebosch C, Perez-Pol R. IL-1 receptor antagonist prevents apoptosis and caspase-3 activation after spinal cord injury. J Neurotrauma. 2001;18:947–956. doi: 10.1089/089771501750451857. [DOI] [PubMed] [Google Scholar]
- Netea MG. Training innate immunity: the changing concept of immunological memory in innate host defence. Eur J Clin Invest. 2013;43:881–884. doi: 10.1111/eci.12132. [DOI] [PubMed] [Google Scholar]
- Newell E, Shellington DK, Simon DW, et al. Cerebrospinal Fluid Markers of Macrophage and Lymphocyte Activation After Traumatic Brain Injury in Children. Pediatr Crit Care Med. 2015;16:549–557. doi: 10.1097/PCC.0000000000000400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- Norden DM, Fenn AM, Dugan A, Godbout JP. TGFbeta produced by IL-10 redirected astrocytes attenuates microglial activation. Glia. 2014;62:881–895. doi: 10.1002/glia.22647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norden DM, Godbout JP. Review: microglia of the aged brain: primed to be activated and resistant to regulation. Neuropathol Appl Neurobiol. 2013;39:19–34. doi: 10.1111/j.1365-2990.2012.01306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norden DM, Trojanowski PJ, Villanueva E, Navarro E, Godbout JP. Sequential activation of microglia and astrocyte cytokine expression precedes increased iba-1 or GFAP immunoreactivity following systemic immune challenge. Glia. 2016;64:300–316. doi: 10.1002/glia.22930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osier N, Carlson SW, DeSana AJ, Dixon CE. Chronic Histopathological and Behavioral Outcomes of Experimental Traumatic Brain Injury in Adult Male Animals. Journal of neurotrauma. 2014 doi: 10.1089/neu.2014.3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota A, Ikeda T, Xia XY, Xia YX, Ikenoue T. Hypoxic-ischemic tolerance induced by hyperthermic pretreatment in newborn rats. J Soc Gynecol Investig. 2000;7:102–105. [PubMed] [Google Scholar]
- Oz M, Lorke DE, Hasan M, Petroianu GA. Cellular and molecular actions of Methylene Blue in the nervous system. Med Res Rev. 2011;31:93–117. doi: 10.1002/med.20177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palin K, Moreau ML, Sauvant J, Orcel H, Nadjar A, Duvoid-Guillou A, Dudit J, Rabie A, Moos F. Interleukin-6 activates arginine vasopressin neurons in the supraoptic nucleus during immune challenge in rats. Am J Physiol Endocrinol Metab. 2009;296:E1289–1299. doi: 10.1152/ajpendo.90489.2008. [DOI] [PubMed] [Google Scholar]
- Pineau I, Sun L, Bastien D, Lacroix S. Astrocytes initiate inflammation in the injured mouse spinal cord by promoting the entry of neutrophils and inflammatory monocytes in an IL-1 receptor/MyD88-dependent fashion. Brain Behav Immun. 2010;24:540–553. doi: 10.1016/j.bbi.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Popovich PG, Guan Z, Wei P, Huitinga I, van Rooijen N, Stokes BT. Depletion of hematogenous macrophages promotes partial hindlimb recovery and neuroanatomical repair after experimental spinal cord injury. Exp Neurol. 1999;158:351–365. doi: 10.1006/exnr.1999.7118. [DOI] [PubMed] [Google Scholar]
- Powell ND, Sloan EK, Bailey MT, et al. Social stress up-regulates inflammatory gene expression in the leukocyte transcriptome via beta-adrenergic induction of myelopoiesis. Proc Natl Acad Sci U S A. 2013;110:16574–16579. doi: 10.1073/pnas.1310655110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Probert L, Eugster HP, Akassoglou K, Bauer J, Frei K, Lassmann H, Fontana A. TNFR1 signalling is critical for the development of demyelination and the limitation of T-cell responses during immune-mediated CNS disease. Brain. 2000;123(Pt 10):2005–2019. doi: 10.1093/brain/123.10.2005. [DOI] [PubMed] [Google Scholar]
- Qian J, Zhu L, Li Q, Belevych N, Chen Q, Zhao F, Herness S, Quan N. Interleukin-1R3 mediates interleukin-1-induced potassium current increase through fast activation of Akt kinase. Proc Natl Acad Sci U S A. 2012;109:12189–12194. doi: 10.1073/pnas.1205207109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez K, Niraula A, Sheridan JF. GABAergic modulation with classical benzodiazepines prevent stress-induced neuro-immune dysregulation and behavioral alterations. Brain Behav Immun. 2016;51:154–168. doi: 10.1016/j.bbi.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramlackhansingh AF, Brooks DJ, Greenwood RJ, et al. Inflammation after trauma: microglial activation and traumatic brain injury. Ann Neurol. 2011;70:374–383. doi: 10.1002/ana.22455. [DOI] [PubMed] [Google Scholar]
- Reboldi A, Coisne C, Baumjohann D, et al. C-C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat Immunol. 2009;10:514–523. doi: 10.1038/ni.1716. [DOI] [PubMed] [Google Scholar]
- Riediger T, Cordani C, Potes CS, Lutz TA. Involvement of nitric oxide in lipopolysaccharide induced anorexia. Pharmacol Biochem Behav. 2010;97:112–120. doi: 10.1016/j.pbb.2010.04.015. [DOI] [PubMed] [Google Scholar]
- Rimaniol AC, Haik S, Martin M, Le Grand R, Boussin FD, Dereuddre-Bosquet N, Gras G, Dormont D. Na+-dependent high-affinity glutamate transport in macrophages. J Immunol. 2000;164:5430–5438. doi: 10.4049/jimmunol.164.10.5430. [DOI] [PubMed] [Google Scholar]
- Rosczyk HA, Sparkman NL, Johnson RW. Neuroinflammation and cognitive function in aged mice following minor surgery. Exp Gerontol. 2008;43:840–846. doi: 10.1016/j.exger.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo MV, McGavern DB. Immune Surveillance of the CNS following Infection and Injury. Trends Immunol. 2015;36:637–650. doi: 10.1016/j.it.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter MW, Beggs S. Sublime Microglia: Expanding Roles for the Guardians of the CNS. Cell. 2014;158:15–24. doi: 10.1016/j.cell.2014.06.008. [DOI] [PubMed] [Google Scholar]
- Samoilova EB, Horton JL, Hilliard B, Liu TS, Chen Y. IL-6-deficient mice are resistant to experimental autoimmune encephalomyelitis: roles of IL-6 in the activation and differentiation of autoreactive T cells. J Immunol. 1998;161:6480–6486. [PubMed] [Google Scholar]
- Sawicki C, McKim D, Wohleb E, Jarrett B, Reader B, Norden D, Godbout J, Sheridan J. Social defeat promotes a reactive endothelium in a brain region-dependent manner with increased expression of key adhesion molecules, selectins and chemokines associated with the recruitment of myeloid cells to the brain. Neuroscience. 2014 doi: 10.1016/j.neuroscience.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer DP, Stevens B. Phagocytic glial cells: sculpting synaptic circuits in the developing nervous system. Curr Opin Neurobiol. 2013;23:1034–1040. doi: 10.1016/j.conb.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider H, Pitossi F, Balschun D, Wagner A, del Rey A, Besedovsky HO. A neuromodulatory role of interleukin-1beta in the hippocampus. Proc Natl Acad Sci U S A. 1998;95:7778–7783. doi: 10.1073/pnas.95.13.7778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnieder TP, Trencevska I, Rosoklija G, Stankov A, Mann JJ, Smiley J, Dwork AJ. Microglia of prefrontal white matter in suicide. Journal of neuropathology and experimental neurology. 2014;73:880–890. doi: 10.1097/NEN.0000000000000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonberg DL, Popovich PG, McTigue DM. Oligodendrocyte generation is differentially influenced by toll-like receptor (TLR) 2 and TLR4-mediated intraspinal macrophage activation. J Neuropathol Exp Neurol. 2007;66:1124–1135. doi: 10.1097/nen.0b013e31815c2530. [DOI] [PubMed] [Google Scholar]
- Schuitemaker A, van der Doef TF, Boellaard R, et al. Microglial activation in healthy aging. Neurobiol Aging. 2012;33:1067–1072. doi: 10.1016/j.neurobiolaging.2010.09.016. [DOI] [PubMed] [Google Scholar]
- Semple BD, Bye N, Rancan M, Ziebell JM, Morganti-Kossmann MC. Role of CCL2 (MCP-1) in traumatic brain injury (TBI): evidence from severe TBI patients and CCL2−/− mice. J Cereb Blood Flow Metab. 2010;30:769–782. doi: 10.1038/jcbfm.2009.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrats J, Schiltz JC, Garcia-Bueno B, van Rooijen N, Reyes TM, Sawchenko PE. Dual roles for perivascular macrophages in immune-to-brain signaling. Neuron. 2010;65:94–106. doi: 10.1016/j.neuron.2009.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan R, Szmydynger-Chodobska J, Warren OU, Mohammad F, Zink BJ, Chodobski A. A New Panel of Blood Biomarkers for the Diagnosis of Mild Traumatic Brain Injury/Concussion in Adults. J Neurotrauma. 2016;33:49–57. doi: 10.1089/neu.2014.3811. [DOI] [PubMed] [Google Scholar]
- Shechter R, Miller O, Yovel G, et al. Recruitment of beneficial M2 macrophages to injured spinal cord is orchestrated by remote brain choroid plexus. Immunity. 2013;38:555–569. doi: 10.1016/j.immuni.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shultz SR, Bao F, Weaver LC, Cain DP, Brown A. Treatment with an anti-CD11d integrin antibody reduces neuroinflammation and improves outcome in a rat model of repeated concussion. Journal of neuroinflammation. 2013;10:26. doi: 10.1186/1742-2094-10-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sica A, Schioppa T, Mantovani A, Allavena P. Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression: potential targets of anti-cancer therapy. Eur J Cancer. 2006;42:717–727. doi: 10.1016/j.ejca.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Sierra A, Gottfried-Blackmore AC, McEwen BS, Bulloch K. Microglia derived from aging mice exhibit an altered inflammatory profile. Glia. 2007;55:412–424. doi: 10.1002/glia.20468. [DOI] [PubMed] [Google Scholar]
- Sokolova A, Hill MD, Rahimi F, Warden LA, Halliday GM, Shepherd CE. Monocyte chemoattractant protein-1 plays a dominant role in the chronic inflammation observed in Alzheimer's disease. Brain Pathol. 2009;19:392–398. doi: 10.1111/j.1750-3639.2008.00188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolowski JD, Mandell JW. Phagocytic clearance in neurodegeneration. Am J Pathol. 2011;178:1416–1428. doi: 10.1016/j.ajpath.2010.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sroga JM, Jones TB, Kigerl KA, McGaughy VM, Popovich PG. Rats and mice exhibit distinct inflammatory reactions after spinal cord injury. J Comp Neurol. 2003;462:223–240. doi: 10.1002/cne.10736. [DOI] [PubMed] [Google Scholar]
- Stein M, Keshav S, Harris N, Gordon S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J Exp Med. 1992;176:287–292. doi: 10.1084/jem.176.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stichel CC, Luebbert H. Inflammatory processes in the aging mouse brain: participation of dendritic cells and T-cells. Neurobiol Aging. 2007;28:1507–1521. doi: 10.1016/j.neurobiolaging.2006.07.022. [DOI] [PubMed] [Google Scholar]
- Talley Watts L, Long JA, Boggs RC, Manga H, Huang S, Shen Q, Duong TQ. Delayed Methylene Blue Improves Lesion Volume, Multi-Parametric Quantitative Magnetic Resonance Imaging Measurements, and Behavioral Outcome after Traumatic Brain Injury. J Neurotrauma. 2016;33:194–202. doi: 10.1089/neu.2015.3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Noda Y, Hasegawa T, Nabeshima T. A concussive-like brain injury model in mice (I): impairment in learning and memory. J Neurotrauma. 1997;14:851–862. doi: 10.1089/neu.1997.14.851. [DOI] [PubMed] [Google Scholar]
- Tansey MG, Goldberg MS. Neuroinflammation in Parkinson's disease: its role in neuronal death and implications for therapeutic intervention. Neurobiol Dis. 2010;37:510–518. doi: 10.1016/j.nbd.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarr AJ, Liu X, Reed NS, Quan N. Kinetic characteristics of euflammation: the induction of controlled inflammation without overt sickness behavior. Brain Behav Immun. 2014;42:96–108. doi: 10.1016/j.bbi.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasaki K, Ruetzler CA, Ohtsuki T, Martin D, Nawashiro H, Hallenbeck JM. Lipopolysaccharide pre-treatment induces resistance against subsequent focal cerebral ischemic damage in spontaneously hypertensive rats. Brain Res. 1997;748:267–270. doi: 10.1016/s0006-8993(96)01383-2. [DOI] [PubMed] [Google Scholar]
- Torres-Platas SG, Cruceanu C, Chen GG, Turecki G, Mechawar N. Evidence for increased microglial priming and macrophage recruitment in the dorsal anterior cingulate white matter of depressed suicides. Brain Behav Immun. 2014;42:50–59. doi: 10.1016/j.bbi.2014.05.007. [DOI] [PubMed] [Google Scholar]
- Turtzo LC, Lescher J, Janes L, Dean DD, Budde MD, Frank JA. Macrophagic and microglial responses after focal traumatic brain injury in the female rat. J Neuroinflammation. 2014;11:82. doi: 10.1186/1742-2094-11-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tynan RJ, Naicker S, Hinwood M, Nalivaiko E, Buller KM, Pow DV, Day TA, Walker FR. Chronic stress alters the density and morphology of microglia in a subset of stress-responsive brain regions. Brain Behav Immun. 2010a doi: 10.1016/j.bbi.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Tynan RJ, Naicker S, Hinwood M, Nalivaiko E, Buller KM, Pow DV, Day TA, Walker FR. Chronic stress alters the density and morphology of microglia in a subset of stress-responsive brain regions. Brain Behav Immun. 2010b;24:1058–1068. doi: 10.1016/j.bbi.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Ullian EM, Harris BT, Wu A, Chan JR, Barres BA. Schwann cells and astrocytes induce synapse formation by spinal motor neurons in culture. Mol Cell Neurosci. 2004;25:241–251. doi: 10.1016/j.mcn.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Velazquez A, Ortega M, Rojas S, Gonzalez-Olivan FJ, Rodriguez-Baeza A. Widespread microglial activation in patients deceased from traumatic brain injury. Brain Inj. 2015;29:1126–1133. doi: 10.3109/02699052.2015.1018325. [DOI] [PubMed] [Google Scholar]
- Vitaliano PP, Murphy M, Young HM, Echeverria D, Borson S. Does caring for a spouse with dementia promote cognitive decline? A hypothesis and proposed mechanisms. J Am Geriatr Soc. 2011;59:900–908. doi: 10.1111/j.1532-5415.2011.03368.x. [DOI] [PubMed] [Google Scholar]
- Walsh JT, Hendrix S, Boato F, et al. MHCII-independent CD4+ T cells protect injured CNS neurons via IL-4. J Clin Invest. 2015;125:2547. doi: 10.1172/JCI82458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter S, Letiembre M, Liu Y, et al. Role of the toll-like receptor 4 in neuroinflammation in Alzheimer's disease. Cell Physiol Biochem. 2007;20:947–956. doi: 10.1159/000110455. [DOI] [PubMed] [Google Scholar]
- Wang G, Zhang J, Hu X, et al. Microglia/macrophage polarization dynamics in white matter after traumatic brain injury. J Cereb Blood Flow Metab. 2013;33:1864–1874. doi: 10.1038/jcbfm.2013.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil ZM, Gaier KR, Karelina K. Injury timing alters metabolic, inflammatory and functional outcomes following repeated mild traumatic brain injury. Neurobiol Dis. 2014;70:108–116. doi: 10.1016/j.nbd.2014.06.016. [DOI] [PubMed] [Google Scholar]
- Werner C, Engelhard K. Pathophysiology of traumatic brain injury. Br J Anaesth. 2007;99:4–9. doi: 10.1093/bja/aem131. [DOI] [PubMed] [Google Scholar]
- Witcher KG, Eiferman DS, Godbout JP. Priming the inflammatory pump of the CNS after traumatic brain injury. Trends Neurosci. 2015;38:609–620. doi: 10.1016/j.tins.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, Fenn AM, Pacenta AM, Powell ND, Sheridan JF, Godbout JP. Peripheral innate immune challenge exaggerated microglia activation, increased the number of inflammatory CNS macrophages, and prolonged social withdrawal in socially defeated mice. Psychoneuroendocrinology. 2012;37:1491–1505. doi: 10.1016/j.psyneuen.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, Hanke ML, Corona AW, Powell ND, Stiner LM, Bailey MT, Nelson RJ, Godbout JP, Sheridan JF. beta-Adrenergic receptor antagonism prevents anxiety-like behavior and microglial reactivity induced by repeated social defeat. J Neurosci. 2011a;31:6277–6288. doi: 10.1523/JNEUROSCI.0450-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, Hanke ML, Corona AW, Powell ND, Stiner LM, Bailey MT, Nelson RJ, Godbout JP, Sheridan JF. β-Adrenergic receptor antagonism prevents anxiety-like behavior and microglial reactivity induced by repeated social defeat. J Neurosci. 2011b;31:6277–6288. doi: 10.1523/JNEUROSCI.0450-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, McKim DB, Shea DT, Powell ND, Tarr AJ, Sheridan JF, Godbout JP. Re-establishment of anxiety in stress-sensitized mice is caused by monocyte trafficking from the spleen to the brain. Biol Psychiatry. 2014a;75:970–981. doi: 10.1016/j.biopsych.2013.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, McKim DB, Sheridan JF, Godbout JP. Monocyte trafficking to the brain with stress and inflammation: a novel axis of immune-to-brain communication that influences mood and behavior. Front Neurosci. 2014b;8:447. doi: 10.3389/fnins.2014.00447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, Powell ND, Godbout JP, Sheridan JF. Stress-induced recruitment of bone marrow-derived monocytes to the brain promotes anxiety-like behavior. J Neurosci. 2013;33:13820–13833. doi: 10.1523/JNEUROSCI.1671-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock T, Morganti-Kossmann MC. The role of markers of inflammation in traumatic brain injury. Front Neurol. 2013;4:18. doi: 10.3389/fneur.2013.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynne AM, Henry CJ, Huang Y, Cleland A, Godbout JP. Protracted downregulation of CX3CR1 on microglia of aged mice after lipopolysaccharide challenge. Brain Behav Immun. 2010;24:1190–1201. doi: 10.1016/j.bbi.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Morgan TE, Rozovsky I, Finch CE. Aging and glial responses to lipopolysaccharide in vitro: greater induction of IL-1 and IL-6, but smaller induction of neurotoxicity. Exp Neurol. 2003;182:135–141. doi: 10.1016/s0014-4886(03)00057-8. [DOI] [PubMed] [Google Scholar]
- Xiong Y, Mahmood A, Chopp M. Animal models of traumatic brain injury. Nat Rev Neurosci. 2013;14:128–142. doi: 10.1038/nrn3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Blumbergs PC, Jones NR, Manavis J, Sarvestani GT, Ghabriel MN. Early expression and cellular localization of proinflammatory cytokines interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in human traumatic spinal cord injury. Spine (Phila Pa 1976) 2004;29:966–971. doi: 10.1097/00007632-200405010-00004. [DOI] [PubMed] [Google Scholar]
- Yang L, Jones NR, Blumbergs PC, Van Den Heuvel C, Moore EJ, Manavis J, Sarvestani GT, Ghabriel MN. Severity-dependent expression of pro-inflammatory cytokines in traumatic spinal cord injury in the rat. J Clin Neurosci. 2005;12:276–284. doi: 10.1016/j.jocn.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Ye SM, Johnson RW. An age-related decline in interleukin-10 may contribute to the increased expression of interleukin-6 in brain of aged mice. Neuroimmunomodulation. 2001;9:183–192. doi: 10.1159/000049025. [DOI] [PubMed] [Google Scholar]
- Youm YH, Grant RW, McCabe LR, et al. Canonical Nlrp3 inflammasome links systemic low-grade inflammation to functional decline in aging. Cell metabolism. 2013;18:519–532. doi: 10.1016/j.cmet.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Bailey WM, Braun KJ, Gensel JC. Age decreases macrophage IL-10 expression: Implications for functional recovery and tissue repair in spinal cord injury. Exp Neurol. 2015a;273:83–91. doi: 10.1016/j.expneurol.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Bailey WM, Kopper TJ, Orr MB, Feola DJ, Gensel JC. Azithromycin drives alternative macrophage activation and improves recovery and tissue sparing in contusion spinal cord injury. J Neuroinflammation. 2015b;12:218. doi: 10.1186/s12974-015-0440-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Lapointe BM, Clark SR, Zbytnuik L, Kubes P. A requirement for microglial TLR4 in leukocyte recruitment into brain in response to lipopolysaccharide. J Immunol. 2006;177:8103–8110. doi: 10.4049/jimmunol.177.11.8103. [DOI] [PubMed] [Google Scholar]
- Ziebell JM, Taylor SE, Cao T, Harrison JL, Lifshitz J. Rod microglia: elongation, alignment, and coupling to form trains across the somatosensory cortex after experimental diffuse brain injury. J Neuroinflammation. 2012;9:247. doi: 10.1186/1742-2094-9-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziv Y, Ron N, Butovsky O, Landa G, Sudai E, Greenberg N, Cohen H, Kipnis J, Schwartz M. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat Neurosci. 2006;9:268–275. doi: 10.1038/nn1629. [DOI] [PubMed] [Google Scholar]
- Zong S, Zeng G, Wei B, Xiong C, Zhao Y. Beneficial effect of interleukin-1 receptor antagonist protein on spinal cord injury recovery in the rat. Inflammation. 2012;35:520–526. doi: 10.1007/s10753-011-9341-5. [DOI] [PubMed] [Google Scholar]