Abstract
We previously showed that near-infrared laser photobiomodulation (PBM) (810 nm, CW, 18 J/cm2, 25 mW/cm2) delivered to the mouse daily for 3-days after a controlled cortical impact traumatic brain injury (TBI) gave a significant improvement in neurological/cognitive function. However the same parameters delivered 14X daily gave significantly less benefit. This biphasic dose response intrigued us, and we decided to follow the mice that received 3X or 14X laser treatments out to 56-days post-TBI. We found the 14X group showed worse neurological function than the no-treatment TBI group at 2-weeks, but started to improve steadily during the next 6-weeks, and by 56-days were significantly better than the no-treatment TBI mice, but still worse than the 3X mice. A marker of activated glial cells (GFAP) was significantly increased in the brain regions (compared to both untreated TBI and 3X groups) at 4-weeks in the 14X group, but the GFAP had fallen to low levels in both 3X and 14X groups by 8-weeks. We conclude that an excessive number of laser-treatments delivered to mice can temporarily inhibit the process of brain repair stimulated by tPBM, but then the inhibitory effect ceases, and brain repair can resume. The mechanism may be temporary induction of reactive gliosis.
Keywords: Traumatic brain injury, controlled cortical impact mouse model, photobiomodulation, LLLT, biphasic dose response, Morris water maze, glial fibrillary acidic protein, reactive gliosis
Graphical abstract
1. Introduction
Traumatic brain injury (TBI) is a serious condition, and is one of the leading causes of mortality and morbidity especially in military personnel, athletes, and in the younger population. In the US, each year roughly 1.7 million people sustain a TBI of whom about 52,000 die [1]. Currently, at least 5.3 million people live with disabilities as a consequence of TBI in the US [2]. Each year, more than 50,000 hospitalizations occur due to TBI [3]. According to data from Center for Disease Control, the major causes for TBI are falls in children and elderly, and motor vehicle accidents in young people. TBI can be classified as mild, moderate and severe according to the impact of the injury and the score in the Glasgow Coma Scale (GCS). Severe and moderate TBI, is a major public health problem throughout the world [4]. Despite the burden of the disease, treatment approaches have not (so far) been satisfactory.
The pathophysiology of TBI is still poorly understood and includes the primary impact (the head trauma) which results in development of secondary brain injury [5]. Activation of several cellular processes and tissue pathways causes inflammation, brain edema, increased intracranial pressure and impaired cerebral perfusion. When all these processes are combined, the end point is likely to be progressive cerebral ischemia, which might cause diminished glucose and oxygen transport to neurons that leads to lack of energy production. Neurons without energy will lose the ability to maintain membrane ion channels, which in turn cause enhanced voltage and NMDA-dependent depolarizing postsynaptic potentials [6]. Moreover, NMDA receptor activation causes rises in intracellular calcium, which results in mitochondrial dysfunction, inflammation and apoptosis [7]. Cortical spreading depression is increased due to the intracellular calcium overload which is also seen in the brain after stroke [8].
Transcranial low-level light laser therapy (LLLT) (also known as photobiomodulation, PBM) has been investigated in many different disease models (including stroke and TBI [9]) and has been shown to alleviate the symptoms related to TBI in various animal studies [10–19]. LLLT is associated with increased cellular ATP levels and improved mitochondrial respiration [20] that might reverse deficits occasioned by TBI. Recently we showed [18] that mice treated with 1 or 3 daily applications of LLLT (CW 810 nm laser, 18 J/cm2 at 25 mW/cm2, 1 cm diameter spot) had significant improvements in neurological function; however 14 daily laser treatments did not provide any benefit over TBI untreated control 28 days after TBI. This biphasic dose response intrigued us, and we decided to follow the mice that received 3X or 14X laser treatments out to 56 days post-TBI. We wished to determine whether the putative benefits of LLLT had been completely abrogated by the excessive number of LLLT applications, or could it be possible that the benefits had only been delayed rather than abolished. In our current study, we treated mice with controlled cortical impact (CCI) model of severe TBI, with sham, 3 and 14 daily applications of LLLT. We observed the effects of different treatment repetitions on neurobehavioral function, memory and learning as measured by Morris Water Maze, and immunofluorescence of brain sections measuring cell density and glial activation at 28 and 56 days.
2. Materials and methods
2.1 Animals
Ethics Statement
All animal experiments were approved by the Subcommittee on Research Animal Care of the Massachusetts General Hospital (protocol no: 2010N000202) and the guidelines of the National Institutes of Health (NIH) were strictly followed.
Male BALB/c mice aged 6–8 week (weight 20–25 g; Charles River Laboratories, Wilmington, MA) were used for this study. Mice were divided into 4 groups each consisting of 24 mice: Sham surgical procedure with no actual TBI, TBI without treatment, TBI with 3-laser treatments, TBI with 14-laser treatments. Six mice from each group were sacrificed at days 7, 14, 28 and 56 for measurement of lesion size and immunohistochemistry studies. The neurobehavioral cohort (NSS and MWM) was comprised of 6 mice from each treatment group. Animals were housed 1 mouse per cage, maintained on a 12-hour dark 12-hour light cycle with food and water provided ad libitum.
2.2 Mouse model of traumatic brain injury
Mice underwent focal controlled cortical impact model of TBI under 1.5% isoflurane anesthesia. Briefly, following the anesthesia induction the top of the head was shaved and depilated with Nair. A 1 cm midline incision was made along with longitudinal suture to expose the skull and a 5 mm temporoparietal craniotomy performed carefully with a 4 mm trephine attached to an electric portable drill. Skull flap removed gently and TBI was performed using a 3 mm tipped pneumatic control device (AmScien Instruments LLC Richmond, Virginia, Model AMS 201) with high pressure 150 psi, low pressure 30 psi, rod speed 4.8 m/sec, rising duration 8.41 ms and set impact depth of 2 mm. These setting were previously proven by our lab to cause moderate to severe brain trauma [16–18]. The craniotomy opening was immediately sealed with skull wax after TBI induction and the skin was sutured. Mice were placed back in their cages after recovering from anesthesia and postop care was given with food and water provided. Mice in sham procedure group encountered each step including craniotomy except the actual TBI impact. In order to confirm the brain injury, NSS was assessed 1 hour after the procedure and only mice with severe brain injury (NSS 7–8) were included for further experiments. Mice with NSS > 7, depressed skull fracture or hemorrhage were excluded from the study.
2.3 Laser treatment
Mice received the first laser treatment 4 hour post-TBI after being lightly anesthetized and immobilized by gently taping their paws to a plastic plate. Mice in the 3 and 14-laser groups were exposed to 810 nm continuous wave laser, max output of 3.5 W (Dio-Dent Micro 810, HOYA ConBio, Fremont, CA) on top of the head with a spot size of 1 cm that covered the entire skull. The duration of the laser irradiation was 12 min with 18 J/cm2 fluence delivered at an irradiance of 25 mW/cm2. Mice in 3-laser group received laser treatment on days 1, 2, 3 and mice in 14-laser group were irradiated daily for 14 consequent days. Sham-TBI and TBI with sham treatment groups underwent all steps except that no actual laser treatment was given.
2.4 Neurobehavioral testing
2.4.1 NSS
Neurological severity score (NSS) has been regularly used to evaluate the functional neurological status of the mice after TBI [21]. NSS test uses 10 tasks, which assess motor function, alertness and balance (Table 1). Each failed task contributes one point and these can add up to a total score of 10. NSS score of less than 5 indicated mild TBI, 5–6 moderate TBI, 7–8 severe TBI and more than 8 signified mice sustaining very severe TBI. In our experiments, mice were tested 1 hour post-TBI and once weekly throughout week 8. Only the mice with severe TBI (NSS 7–8) included in this study.
Table 1.
Neurological Severity Scoring (NSS). One point given for each task failed. Zero points indicates no dysfunction (normal healthy mice) whereas 10 points means very severe injury.
| Task | NSS |
|---|---|
| Presence of mono or hemi-paresis | 1 |
| Inability to walk on a 3 cm wide beam | 1 |
| Inability to walk on a 2 cm wide beam | 1 |
| Inability to walk on a 1 cm wide beam | 1 |
| Inability to balance on a 1 cm wide beam | 1 |
| Inability to balance on a 0.5 cm wide round stick | 1 |
| Failure to exit a 30 cm diameter circle in 2 min | 1 |
| Inability to walk straight | 1 |
| Loss of startle behavior | 1 |
| Loss of seeking behavior | 1 |
| Total | 10 |
2.4.2 Morris water maze
The Morris water maze test was employed following a previously described protocol [17] where the experimental set up for the MWM task consists of a circular swimming pool (200 cm diameter) filled with water (30 cm height) kept at 24 °C. The target zone is the quadrant where the escape platform of 15 cm diameter was placed in a fixed position either visible (1 cm above the water) or hidden (1 cm under the water surface). The other three quadrants were left, right and opposite zones and all four quadrants were marked with different color signs affixed to the walls above the pool to act as navigation aids. Acquisition training consisted of trial blocks of two daily-trials for 10 days, commencing at four different positions from the border of the maze in a semi-random order and with 15 minutes inter-trial intervals. If the platform was not found in 120 s, the mouse was placed on the platform for 15 s and then returned to its cage. After the acquisition training, the actual trial started: the trial on the first day was latency to the visible platform; trials at second day through sixth day were latency to the hidden platform; the test at the seventh day was a probe trial where the platform was completely removed. MWM evaluations lasted from days 21–27 and from days 49–55 post-TBI.
2.5 Histomorphology and immunohistochemistry
Mice were anesthetized with isoflurane and transcardially perfused with 0.9% saline then with 0.4% phosphate-buffered paraformaldehyde. Brains were removed and embedded in paraffin following fixation in paraformaldehyde solution for 3 days. Serial 5 mm thick coronal blocks were taken and microtome used to cut 10 µ thick sections from the top, middle and bottom of the thick sliced blocks. Paraffinized sections immersed in xylene and graded ethanol for deparaffinization. Some of the slides stained with Hematoxylin-eosin (H&E) for lesion size measurement and pictures taken by using AutoPix (Arcturus, Mountain View, CA). Percent injured area was calculated by dividing the lesion size by a number equal to double the area measured for the noninjured hemisphere (total brain area).
Antigen retrieval was carried out with citrate buffer solution in microwave oven at 98 °C and sections then incubated in 5% BSA/0.1% TritonX-100 in PBS. Sections incubated in following primary antibodies overnight at 4 degree Celsius: monoclonal mouse anti GFAP (Millipore, Cat.# 04-106). Primary antibodies were removed by washing the slides multiple times with dH2O and PBS. Rabbit anti-mouse antibody labeled with PE (1 : 500, Sigma-Aldrich) was used for secondary immune-staining. Subsequently, sections were cover-slipped with DAPI containing mounting media (Fisher Scientific). Confocal microscope (Olympus America Inc., Center Valley, PA) was used to detect GFAP stained cells (red) and DAPI stained cells (blue). Image J software (National Institute of Health, Bethesda, MD) was used for quantification. Ratios of GFAP positive cells to DAPI stained cells were calculated to normalize for the number of the cells in each imaging field.
2.6 Statistical analysis
Data are presented as mean ± SD. Neurobehavioral tests analyzed using repeated measures analysis of variance (ANOVA) whereas we used one-way ANOVA for immunohistochemistry studies. Tukey post-hoc test was used for pairwise comparisons. Significance was defined as p < 0.05. SPSS statistics V17.0 (IBM, Armonk, NY) was used for analysis.
3. Results
3.1 Neurobehavioral evaluation by NSS
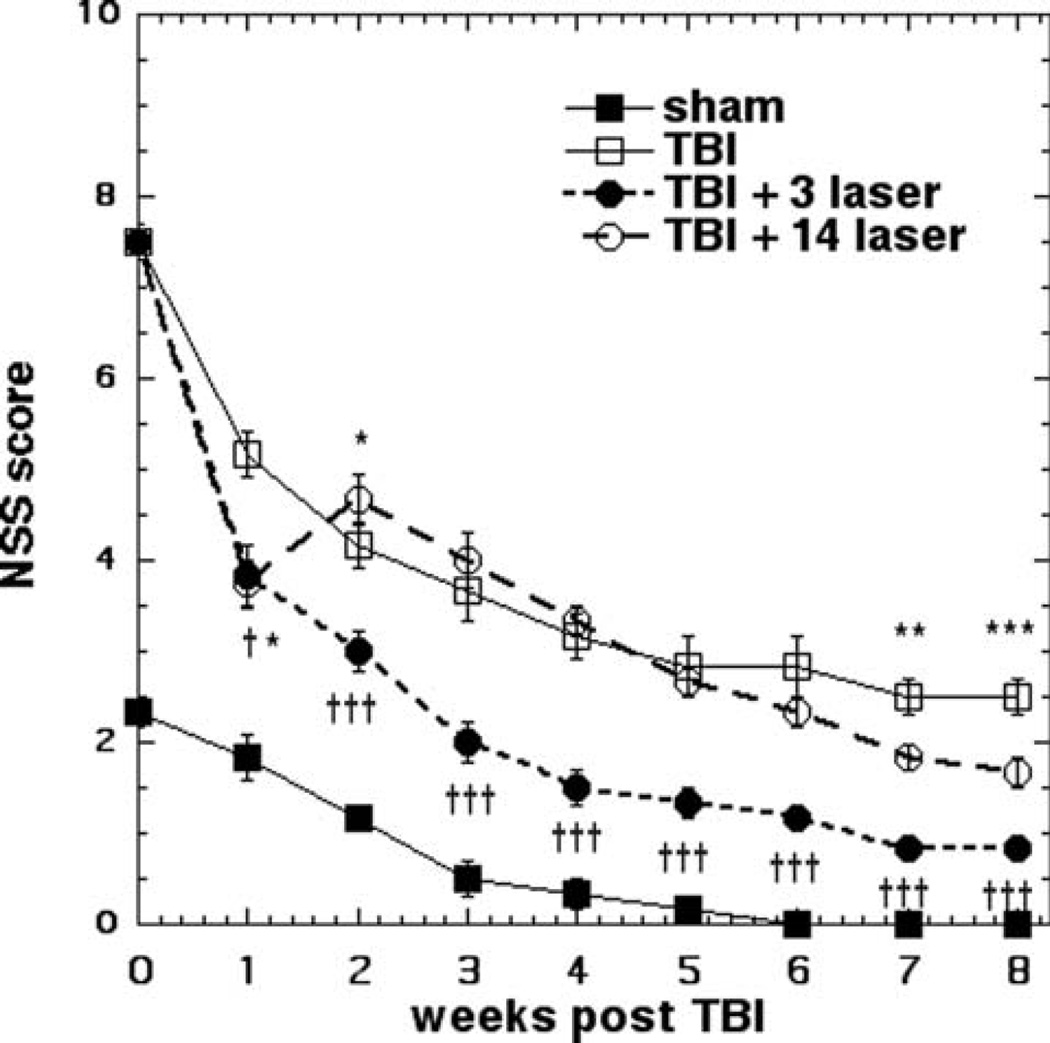
The impact of LLLT on the neurological functioning after TBI was assessed by measuring the NSS every week (Figure 1). We included a group of mice that had received a sham TBI procedure (surgery but no brain impact) as this procedure does produce a deficit in performance. Animals in each group other than 14-day laser treatment, showed a slow but steady improvement in NSS as time passed. The mice that received 3 LLLT showed highly significant (p < 0.001) improvements compared to the untreated TBI group starting at week 1 and relatively increasing until week 8. The mice that received 7 daily LLLT treatments (first week of the planned 14 LLLT) had exactly the same NSS score as the mice that only received 3 daily LLLT treatments when measured at week 1. However this particular group of mice then went on to receive another 7 daily LLLT treatments (on days 8–14). This group of mice showed a worsening of neurological functioning (higher NSS score) on day 14 and the score was significantly worse than the 3-day laser treatment group at week-2 (p < 0.05). The 14-day treatment group went on to show an improvement in NSS at each time point after week 2, but this was not significantly different compared to untreated TBI mice until week 6 after which the 14-day treated group had a better performance than the untreated group which was statistically significant (p < 0.01 at week 7, p < 0.001 at week 8). However the 3-day LLLT group performed significantly better than the 14-day LLLT even at week 8 (p < 0.001).
Figure 1.
NSS scores. Mean ± SD (n = 6) NSS scores of mice measured each week for 8 weeks in 4 groups consisting of: sham control mice, untreated (sham) TBI mice, mice treated once per day for 3 days and once per day for 14 days with 810 nm laser (18 J/cm2 delivered at 25 mW/cm2). *p < 0.05; p < 0.01; ***p < 0.001 vs. TBI. † <0.05, ††† <0.001 vs. 14 LLLT.
3.2 MWM
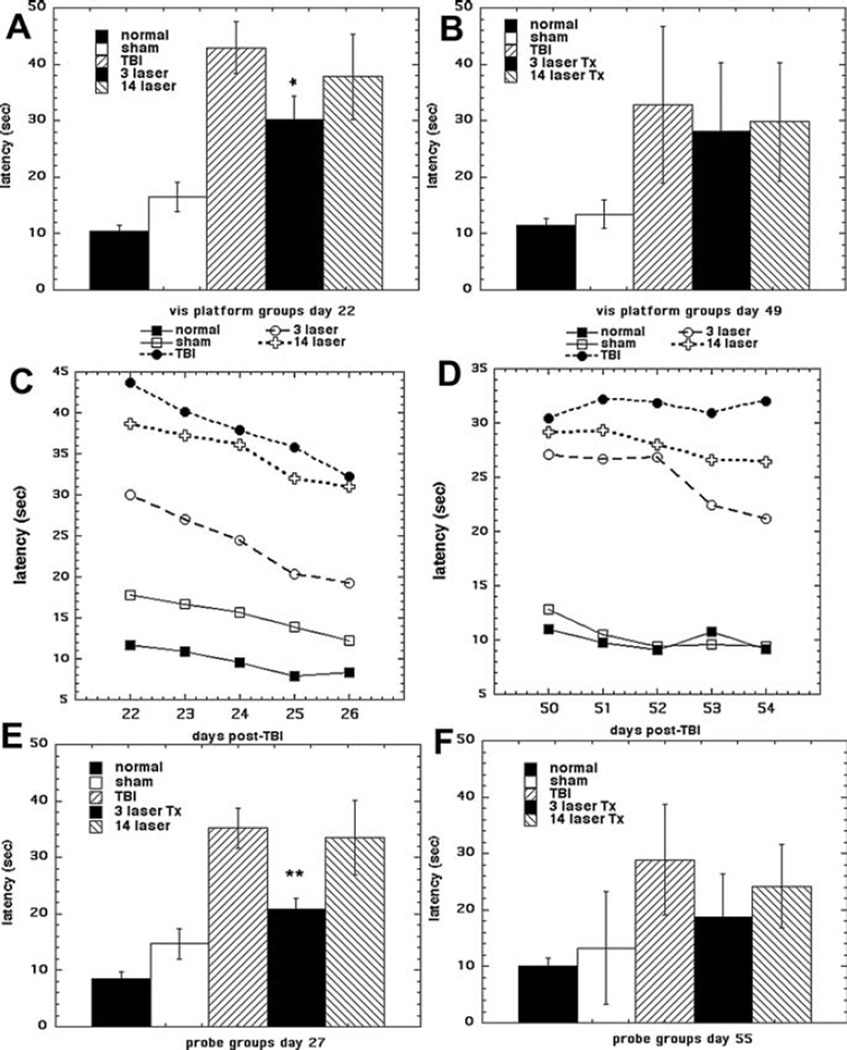
The Morris water maze test (MWM) has been widely used to assess spatial learning and memory [22]. The MWM consists of an initial visible platform trial, a series of five consecutive daily trials to the hidden platform, and a final probe test to the absent platform. In our experiment, we performed MWM at two different time points (over days 21–27 and days 49–55). We included groups of normal mice and sham TBI mice, as in the case of MWM normal mice have a measurable latency time. Figure 2 shows the results. The visible platform results at day 21 are shown in Figure 2A and at day 49 are shown in Figure 2B. At day 21 the 3 LLLT group had significantly lower latency time compared to 14 LLLT group (p < 0.05). However the difference had disappeared at day 49, and in fact there was no significant difference between any of the 3 TBI groups. The hidden platform trials are shown in Figure 2C (days 22–26) and Figure 2D (days 50–54). At the first set of trials the 3 LLLT group showed a much shorter latency time than the 14 LLLT group and the untreated TBI group, with a more pronounced downward trend showing a better learning capability. At the second set of trials the latencies of the 3 LLLT and 14 LLLT groups were much closer together and were both lower than the untreated TBI group. The data from the probe trial are shown in Figure 2E (day 27) and Figure 2F (day 55). The results are similar to the visible platform data in Figures 2A, B. At day 27 the 3 LLLT group had lower latency than the 14 LLLT group, which was the same as the untreated TBI group. At day 55 the latency between the three TBI groups was not significantly different from each other.
Figure 2.
Morris water maze. The effect of tLLLT on cognitive performance of mice in 5 groups (n = 6) consisting of: normal mice, sham control mice, untreated (sham) TBI mice, mice treated once per day for 3 days and once per day for 14 days with 810 nm laser. The Morris water maze testing was conducted over two 7-day periods from days 21–27 (A, C, E) and days 49–55 (B, D, F). Data points are mean latency (sec) ± SD (n = 6). A, B visible platform test; C, D hidden platform test; E, F probe test. *p < 0.05 vs. untreated TBI and 14 LLLT; **p < 0.01 vs. untreated TBI and 14 LLLT.
3.3 Lesion size
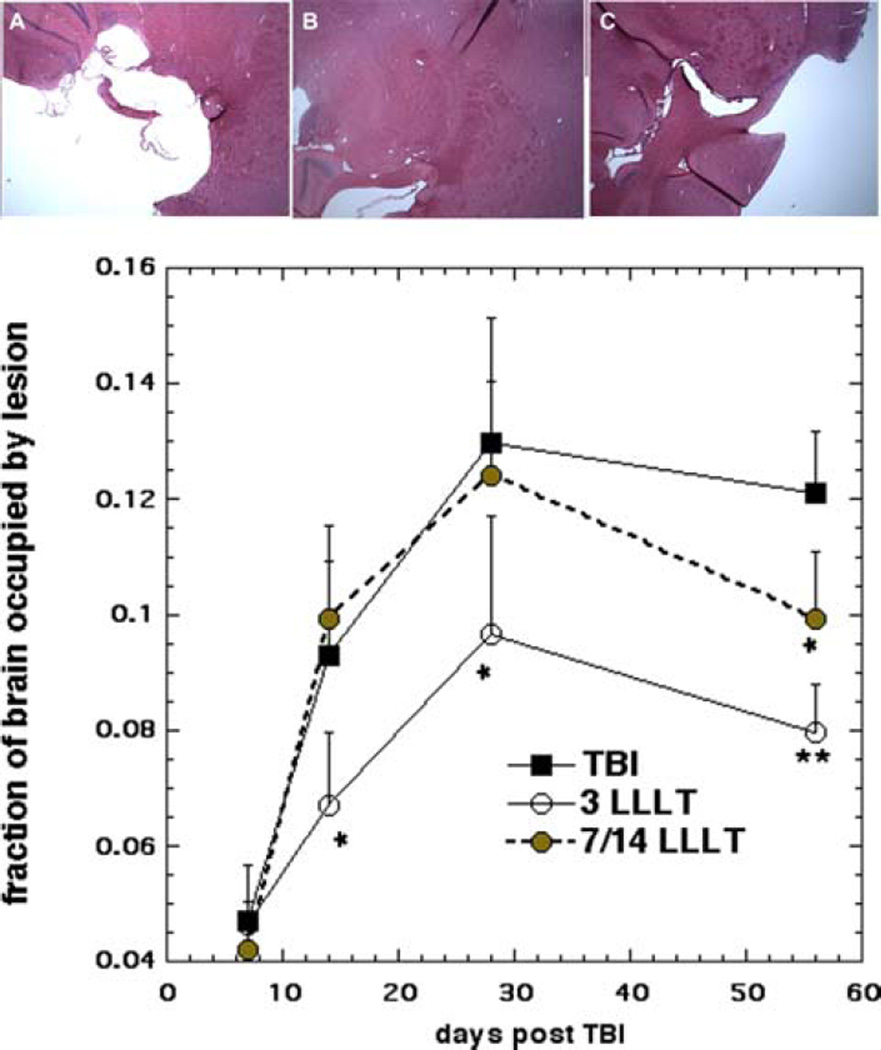
Figure 3A shows an example of H&E sections taken at week shows the lesion size measured in H&E stained sections at four different time points (1, 2, 4, 8 weeks). At week 1 the lesion size was similar between the 3 groups. However the lesion size expanded markedly after week 1 until week-4. There was no difference between treatment groups and sham treated group at week-1 (p > 0.05). However, the 3-LLT group had smaller lesion size compared to 14-day treatment and untreated group at week-2 (p < 0.05). Both laser treated groups (3 and 14-day) demonstrated shrinkage in the mean lesion area at week 8. The lesion size in the 14 LLLT group was smaller than the untreated group at week 8 (p < 0.05). These findings follow a parallel course with the neurobehavioral and cognitive function data (NSS and MWM).
Figure 3.
(A–C) Sample H&E stained sections from mouse brains. Mice were sacrificed at 56 days after having received; (A) zero laser treatments, TBI; (B) 3 laser treatments; or (C) 14 laser treatments. (D) Lesion size measurement. Mice (n = 4) were sacrificed at 1 week, 2 weeks, 4 weeks and 8 weeks, and lesion size determined from H&E stained sections. Data points are mean ± SD (n = 4) of the fraction of total brain occupied by lesion. *p < 0.05 vs. untreated TBI and 14 LLLT; *p < 0.05 vs. untreated TBI; **p < 0.01 vs untreated TBI.
3.4 GFAP staining
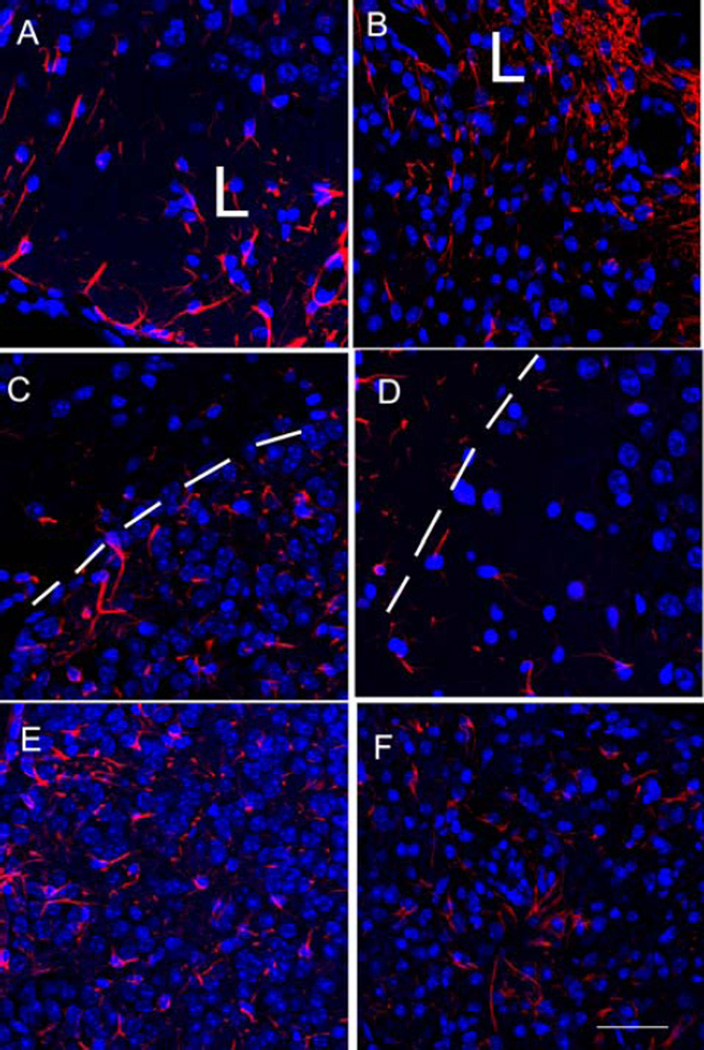
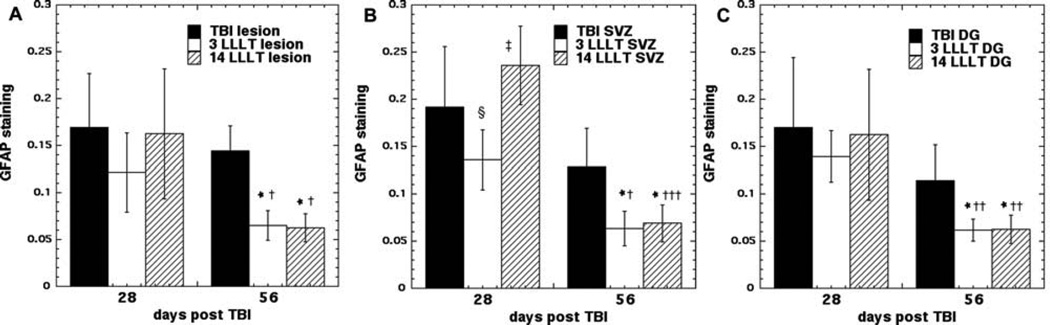
Glial fibrillary acidic protein (GFAP) is an intermediate filament (IF) protein expressed in some glial cells, mainly astrocytes, and ependymal cells in the central nervous system. GFAP constitutes the main structure of the cytoskeleton of astrocytes in addition to nestin, vimentin and synemin [23, 24]. GFAP expression is increased in stroke [25] and TBI [26] and is thought to be detrimental to normal brain function [27]. Figure 4 shows representative examples of red GFAP staining (with blue DAPI staining of nuclei to control for cell numbers) in sections taken from the subventricular zone (SVZ) in groups of mice with TBI that were untreated, or treated with 3 and 14 LLLT applications and sacrificed days 28 and 56 post-TBI. Figure 5 shows the mean ratios of GFAP to DAPI staining measured in the perilesional cortex, SVZ and dentate gyrus (DG) at weeks 4 and 8. At week 4 the untreated TBI mice had higher mean GFAP/DAPI ratios in the lesion cortex and in the SVZ compared to the 3 LLLT treated mice (p > 0.05). However the 14 LLLT group had even much higher ratios compared to the 3 LLLT group in lesion and SVZ (p < 0.05), and the ratios were also even higher than the untreated TBI group (p < 0.05). At week 8 the levels in the untreated group had reduced by a small amount. The levels in the 3 LLLT group had reduced by a greater amount, and the difference compared to the untreated TBI group was now more significant (p < 0.01). However the most pronounced drop at week 8 occurred in the 14 LLLT group, where the ratios were now significantly lower than the untreated group (instead of being higher at week 4), and there was now no significant difference compared to the 3 LLLT group.
Figure 4.
Protein expression of GFAP in the mouse brain. Representative immunofluorescence micrographs showing protein expression of GFAP (red) at 400× magnification in the subventricular zone of mice sacrificed at 28 or 56 days post-TBI. DAPI was used to stain the nuclei blue. (A) Untreated TBI mouse euthanized at day 28 (B) Untreated TBI mouse euthanized at day 56 (C) 3X LLLT mouse euthanized at day 28. (D) 3X LLLT mouse euthanized at day 36. (E) 14X LLLT mouse euthanized at day 28. (F) 14X LLLT mouse euthanized at day 56. Scale bar = 100 µm. L indicates side of the sectioin neasrest to the lesion. White dotted line indicates the granular layer of the dentate gyrus.
Figure 5.
Quantification of GFAP expression in three different areas of the mouse brain: (A) perilesional cortex; (B) SVZ, (C) dentate gyrus of hippocampus (DG). Mice (n = 4) were sacrificed at days 28 or 56 and red (GFAP)/blue (DAPI) fluorescence ratios measured using Image J. Data points are mean ± SD. *p < 0.05 vs. untreated TBI; † p < 0.05, †† p < 0.01, ††† p < 0.01 vs. same treatment at 28 days; § p < 0.05 vs. untreated TBI at 28 days; ‡ p < 0.05 vs. 3 LLLT at 28 days.
4. Discussion
Our previous studies showed that transcranial application of NIR laser to the head of mice was remarkably effective in mitigating the deleterious effects of acute serious/moderate TBI [28]. Not only could tLLLT improve neurological function (NSS) [29, 30] and learning and memory (MWM) [17], but it could also beneficially affect several histological markers of brain repair [16, 17]. We demonstrated this efficacy in both a closed-head TBI mouse model [30] and in a CCI (open brain) TBI mouse model [16–18]. In a recent study [18], we compared the effects of three different treatment repetition regimens; a single LLLT application delivered at 4 hours post-TBI; 3 LLLT applications delivered on days 1–3 post TBI; 14 daily LLLT applications delivered on days 1–14. We reasoned that a human patient with acute TBI would be unlikely to receive just a single application of LLLT, and therefore wished to see whether several repeated applications would be better than a single application. In order to do this we reduced the dose of LLLT per treatment from 36 J/cm2 of 810 nm laser delivered at 50 mW/cm2 (which we had previously shown to be highly effective as a single treatment at 4 hours post TBI [29]) to 18 J/cm2 of 810 nm laser delivered at 25 mW/cm2 which took the same amount of time to deliver (12 minutes) [18]. In a different application of LLLT (for arthritis of the knee in rats) we had previously shown [31] that the illumination time is an important parameter when optimizing a LLLT regimen. In the study [18] comparing 1, 3 and 14 daily LLLT applications we were somewhat surprised to find that 3 LLLT application were not only significantly better than a single LLLT application, but also better than 14 consecutive daily applications. This could be interpreted as a worrying finding, as the clinical application of LLLT for acute TBI would be more difficult if there were a real possibility of causing long-term damage to the brain by an injudicious selection of treatment parameters.
We found (as expected) that 3-day LLLT treatment improved neuromuscular performance and cognitive function at 4 weeks, and this improvement continued up to 8 weeks. However, when the LLLT was repeated for 14 days, there was a decline in cognitive function at 2 weeks and it was not until week 4 that the NSS caught up with the untreated TBI group. However the 14 LLLT group improved at a relatively faster rate from week 4 up to week 8. Although the 14 LLLT group did not catch up to the 3 LLLT group, it does appear that the detrimental effect of the excessive 14 LLLT applications was only temporary rather than permanent. Therefore we believe that there are two processes occurring in the brain with the 14 LLLT applications. There is the beneficial effect of LLLT on brain repair, and there is also, at the same time, a counteracting process of reactive gliosis occurring as shown by the rise in GFAP staining at 4 weeks. Presumably this process of reactive gliosis acts to temporarily inhibit the ongoing process of brain repair. However the reactive gliosis is only temporary in nature, and when it fades at weeks 5–8, it allows the light-stimulated process of brain repair to resume, albeit not enough to allow the 14 LLLT group to catch up with the 3 LLLT group. We have shown that the 3 LLLT treatment acts in several ways to encourage brain repair in acute TBI mice. It induces neurogenesis in the SVZ and the DG, together with increases in BDNF expression (both at 1 week) and increases synaptogesis in the perilesional cortex at week 4.
Reactive astrogliosis, is a physiological response of astrocytes seen in many brain disorders, including TBI, ischemic stroke, and diseases characterized by neuroinflammation, and neurodegeneration [32]. Glial cells actively interact with neurons and influence synaptic development and neuroplasticity through an array of secreted and contact-dependent signals [33]. Compared with normal nonreactive astrocytes, reactive astrocytes show altered expression of many genes and exhibit distinct functions [34]. Increased expression of GFAP has been the most commonly employed molecular marker of reactive astrocytes [35]. It is thought that reactive gliosis can act as somewhat of a “double edged sword” in brain trauma [32]. The beneficial function includes protecting neurons from dying [36], detoxifying reactive oxygen species [37], synthesizing VEGF to encourage formation of new blood vessels [38], and limiting leukocyte infiltration [39]. On the other hand, the deleterious effects of reactive gliosis include limiting synaptic regeneration [40], limiting regeneration of axons [41], and decreasing formation of neuroprogenitor cells [42]. Since the literature shows that reactive gliosis can inhibit the processes we previously identified as being stimulated by tLLLT in TBI mice (neurogenesis and synaptogenesis) our hypothesis appears at least plausible.
We did not measure markers of microglial activation in the brain slices, although in retrospect, it would have been a very good idea. Whalen’s group [12] showed that application of tLLLT (800 nm) to mice with CCI TBI produced improvements in MWM performance. Importantly they also showed that the expression of ionized calcium binding adaptor molecule 1 (iba-1, considered to be the best marker for activated microglia) was significantly reduced by LLLT in brain sections at 2 days post-TBI.
In recent years the existence of the biphasic dose response (sometimes known as the “Arndt-Schulz law” or hormesis [43]) in LLLT and photobiomodulation has become increasingly appreciated as a real and significant factor in designing LLLT studies [44, 45]. This biphasic dose response has been demonstrated with several of the important parameters that are relevant to LLLT. Demidova et al. [46] showed (in a mouse model of excisional wound healing) that different fluences delivered at a constant power density (irradiance) showed a peak effectiveness at 2 J/cm2, with both lower (1 J/cm2) and higher (10 J/cm2) fluences showing a lower efficacy, and a very high dose (50 J/cm2) actually showing an inhibitory effect. Oron et al. found [47] that the same fluence (0.3 J/cm2) delivered directly to the rat heart at 5 mW/cm2, had a better effect at reducing infarct size after heart attack, than the same fluence delivered at a lower irradiance (2.5 mW/cm2) or at a higher irradiance (20 mW/cm2). As mentioned above, Xuan et al. found [18] that repeating the tLLLT application (for mouse TBI) 3 times daily was more effective than a single repetition, or than 14 daily repetitions. However in the present case it appears that the deleterious effect of exceeding the optimum value in the dose response curve (number of treatments) is not exactly caused by too much of the same mechanism (this could be considered as over-stimulation) involved in the beneficial effect of a lesser number of applications, but rather may be caused by a second different mechanism (reactive gliosis) superimposed on top of the first mechanism (photostimulation of brain repair).
Other investigators have reported that relatively minor or unexpected differences in the laser parameters or the experimental design can make differences in the therapeutic response in animal models of TBI. Oron et al. found [14] that the improvement seen in mice with TBI that had been treated 6 hours post-TBI was significantly better than the improvement seen in mice that had been treated 8 hours post-TBI. The fact that this difference was due to a relatively small increase in time between TBI and commencement of therapy was surprising. Khuman et al. only found [12] a real benefit in mice with TBI when they were treated with laser therapy through the craniotomy hole after the brain impact had been delivered CCI TBI, and not when the light was delivered to the closed head. Giacci et al. found [48] that photobiomodulation was effective in two out of four in vivo rat models of CNS injury: partial optic nerve transection (effective), light-induced retinal degeneration (effective), TBI (ineffective) and spinal cord injury (SCI, ineffective). They attributed these differences to lesser penetration of light in the case of TBI and SCI. Wu et al. [30] found that only two wavlengths (660 nm and 810 nm) were effective in tLLLT for closed-head TBI in a mouse model, while two different wavelengths (732 nm and 980 nm) were ineffective. A similar observation of an initially detrimental effect of light turning into a beneficial effect in the longer term was observed in a study by Rutar et al. that dealt with light-induced retinal damage in Sprague-Dawley rats [49].
Photobiomodulation or tLLLT is increasingly being tested in patients with TBI (usually chronic). Naeser and collaborators have treated a series of patients with chronic TBI using a LED array (500 mW, CW, red (633 nm)/NIR (870 nm) LED cluster with a power density of 25.8 mW/cm2 (area of 19.29 cm2) was applied to the forehead for a typical duration of 10 minutes (13.3 J/cm2) [50], and (in a later study) to the forehead and other parts of the scalp [51]. Patients exhibited improvements in many of their symptoms (cognitive and psychological) [52]. Henderson and Morries have treated a series of chronic TBI patients with LLLT using a high power laser (810 nm, 10 Hz, power up to 15 W) with a fairly small spot and constant movement of the spot on the forehead and temporal area to avoid the tissue overheating [53–55]. The fluence delivered to the skin of patients ranged from 55 J/cm2 to 81 J/cm2. Patients showed improvements in cognitive and psychological symptoms. The investigators attributed their success to their high power density giving better light penetration depth into the brain.
In conclusion we have shown that an excessive number (14) of daily treatments of transcranial NIR LLLT in a mouse model of TBI loses the benefits provided by a lesser number of treatments (three), at least in the short term. However this deleterious effect is not permanent, and by 8 weeks post-TBI, even the excessive number of LLLT treatments is showing some positive benefit. The mechanism of this delaying effect appears to be that a temporary induction of reactive gliosis after 14 LLLT inhibits the brain repair processes already stimulated by the laser. The results may go some way to reassure the field, that even if the optimum dose of LLLT for TBI is inadvertently exceeded, no permanent damage is likely to have been caused.
Acknowledgments
This work was supported by Air Force Office of Scientific Research grant FA9550-13-1-0068, by US Army Medical Research Acquisition Activity grant W81XWH-09-1-0514, and by US Army Medical Research and Materiel Command grant W81XWH-13-2-0067. MRH was supported by US NIH grant R01AI050875. We are grateful to Jie Zhao of the Photopathology Core of Wellman Center for Photomedicine for help with pathology.
References
- 1.Coronado VG, Xu L, Basavaraju SV, McGuire LC, Wald MM, Faul MD, Guzman BR, Hemphill JD C. Centers for Disease and Prevention. MMWR Surveill Summ. 2011;60:1–32. [PubMed] [Google Scholar]
- 2.Thurman DJ, Alverson C, Dunn KA, Guerrero J, Sniezek JE. J Head Trauma Rehabil. 1999;14:602–615. doi: 10.1097/00001199-199912000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Taylor CA, Greenspan AI, Xu L, Kresnow MJ. J Head Trauma Rehabil. 2015;30:150–159. doi: 10.1097/HTR.0000000000000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leo P, McCrea M. In: Epidemiology, in Translational Research in Traumatic Brain Injury. Laskowitz D, Grant G, editors. Boca Raton (FL): 2016. [PubMed] [Google Scholar]
- 5.Laskowitz D, Grant G. In: Translational Research in Traumatic Brain Injury. Laskowitz D, Grant G, editors. Boca Raton (FL): 2016. [PubMed] [Google Scholar]
- 6.Ross ST, Soltesz I. J Neurophysiol. 2000;83:2916–2930. doi: 10.1152/jn.2000.83.5.2916. [DOI] [PubMed] [Google Scholar]
- 7.Bramlett HM, Dietrich WD. J Cereb Blood Flow Metab. 2004;24:133–150. doi: 10.1097/01.WCB.0000111614.19196.04. [DOI] [PubMed] [Google Scholar]
- 8.Aiba I, Shuttleworth CW. J Physiol. 2012;590:5877–5893. doi: 10.1113/jphysiol.2012.234476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hashmi JT, Huang YY, Osmani BZ, Sharma SK, Naeser MA, Hamblin MR. PM & R. 2010;2:S292–S305. doi: 10.1016/j.pmrj.2010.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ando T, Xuan W, Xu T, Dai T, Sharma SK, Kharkwal GB, Huang YY, Wu Q, Whalen MJ, Sato S, Obara M, Hamblin MR. PLoS One. 2011;6:e26212. doi: 10.1371/journal.pone.0026212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dong T, Zhang Q, Hamblin MR, Wu MX. J Cereb Blood Flow Metab. 2015;35:1435–1444. doi: 10.1038/jcbfm.2015.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khuman J, Zhang J, Park J, Carroll JD, Donahue C, Whalen MJ. J Neurotrauma. 2012;29:408–417. doi: 10.1089/neu.2010.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oron A, Oron U, Streeter J, de Taboada L, Alexandrovich A, Trembovler V, Shohami E. J Neurotrauma. 2007;24:651–656. doi: 10.1089/neu.2006.0198. [DOI] [PubMed] [Google Scholar]
- 14.Oron A, Oron U, Streeter J, De Taboada L, Alexandrovich A, Trembovler V, Shohami E. J Neurotrauma. 2012;29:401–407. doi: 10.1089/neu.2011.2062. [DOI] [PubMed] [Google Scholar]
- 15.Wu Q, Xuan W, Ando T, Xu T, Huang L, Huang YY, Dai T, Dhital S, Sharma SK, Whalen MJ, Hamblin MR. Lasers Surg Med. 2012;44:218–226. doi: 10.1002/lsm.22003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xuan W, Agrawal T, Huang L, Gupta GK, Hamblin MR. J Biophotonics. 2015;8:502–511. doi: 10.1002/jbio.201400069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xuan W, Vatansever F, Huang L, Hamblin MR. J Biomed Opt. 2014;19:108003. doi: 10.1117/1.JBO.19.10.108003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xuan W, Vatansever F, Huang L, Wu Q, Xuan Y, Dai T, Ando T, Xu T, Huang YY, Hamblin MR. PLoS ONE. 2013;8:e53454. doi: 10.1371/journal.pone.0053454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Q, Zhou C, Hamblin MR, Wu MX. J Cereb Blood Flow Metab. 2014;34:1391–1401. doi: 10.1038/jcbfm.2014.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chung H, Dai T, Sharma SK, Huang YY, Carroll JD, Hamblin MR. Ann Biomed Eng. 2012;40:516–533. doi: 10.1007/s10439-011-0454-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petraglia AL, Plog BA, Dayawansa S, Chen M, Dashnaw ML, Czerniecka K, Walker CT, Viterise T, Hyrien O, Iliff JJ, Deane R, Nedergaard M, Huang JH. J Neurotrauma. 2014;31:1211–1224. doi: 10.1089/neu.2013.3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.D’Hooge R, De Deyn PP. Brain Res Brain Res Rev. 2001;36:60–90. doi: 10.1016/s0165-0173(01)00067-4. [DOI] [PubMed] [Google Scholar]
- 23.Middeldorp J, Hol EM. Prog Neurobiol. 2011;93:421–443. doi: 10.1016/j.pneurobio.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 24.Sullivan SM. J Neurochem. 2014;130:729–732. doi: 10.1111/jnc.12754. [DOI] [PubMed] [Google Scholar]
- 25.Huang L, Wu ZB, Zhuge Q, Zheng W, Shao B, Wang B, Sun F, Jin K. Int J Med Sci. 2014;11:344–348. doi: 10.7150/ijms.8140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vos PE, Jacobs B, Andriessen TM, Lamers KJ, Borm GF, Beems T, Edwards M, Rosmalen CF, Vissers JL. Neurology. 2010;75:1786–1793. doi: 10.1212/WNL.0b013e3181fd62d2. [DOI] [PubMed] [Google Scholar]
- 27.Messing A, Brenner M. Glia. 2003;43:87–90. doi: 10.1002/glia.10219. [DOI] [PubMed] [Google Scholar]
- 28.Huang YY, Gupta A, Vecchio D, de Arce VJ, Huang SF, Xuan W, Hamblin MR. J Biophotonics. 2012;5:827–837. doi: 10.1002/jbio.201200077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ando T, Xuan W, Xu T, Dai T, Sharma SK, Kharkwal GB, Huang YY, Wu Q, Whalen MJ, Sato S, Obara M, Hamblin MR. PLoS ONE. 2011;6:e26212–e26220. doi: 10.1371/journal.pone.0026212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu Q, Xuan W, Ando T, Xu T, Huang L, Huang YY, Dai T, Dhital S, Sharma SK, Whalen MJ, Hamblin MR. Lasers Surg Med. 2012;44:218–226. doi: 10.1002/lsm.22003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Castano AP, Dai T, Yaroslavsky I, Cohen R, Apruzzese WA, Smotrich MH, Hamblin MR. Lasers Surg Med. 2007;39:543–550. doi: 10.1002/lsm.20516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pekny M, Pekna M. Physiol Rev. 2014;94:1077–1098. doi: 10.1152/physrev.00041.2013. [DOI] [PubMed] [Google Scholar]
- 33.Jones OD. Neuroscience. 2015;309:113–124. doi: 10.1016/j.neuroscience.2015.08.035. [DOI] [PubMed] [Google Scholar]
- 34.Castejon OJ. Folia Neuropathol. 2015;53:173–192. doi: 10.5114/fn.2015.54419. [DOI] [PubMed] [Google Scholar]
- 35.Eng LF, Ghirnikar RS, Lee YL. Neurochem Res. 2000;25:1439–1451. doi: 10.1023/a:1007677003387. [DOI] [PubMed] [Google Scholar]
- 36.Li L, Lundkvist A, Andersson D, Wilhelmsson U, Nagai N, Pardo AC, Nodin C, Stahlberg A, Aprico K, Larsson K, Yabe T, Moons L, Fotheringham A, Davies I, Carmeliet P, Schwartz JP, Pekna M, Kubista M, Blomstrand F, Maragakis N, Nilsson M, Pekny M. J Cereb Blood Flow Metab. 2008;28:468–481. doi: 10.1038/sj.jcbfm.9600546. [DOI] [PubMed] [Google Scholar]
- 37.Dringen R, Brandmann M, Hohnholt MC, Blumrich EM. Neurochem Res. 2015;40(12):2570–2582. doi: 10.1007/s11064-014-1481-1. [DOI] [PubMed] [Google Scholar]
- 38.Beck H, Plate KH. Acta Neuropathol. 2009;117:481–496. doi: 10.1007/s00401-009-0483-6. [DOI] [PubMed] [Google Scholar]
- 39.Christopherson KS, Ullian EM, Stokes CC, Mullowney CE, Hell JW, Agah A, Lawler J, Mosher DF, Bornstein P, Barres BA. Cell. 2005;120:421–433. doi: 10.1016/j.cell.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 40.Wilhelmsson U, Li L, Pekna M, Berthold CH, Blom S, Eliasson C, Renner O, Bushong E, Ellisman M, Morgan TE, Pekny M. J Neurosci. 2004;24:5016–5021. doi: 10.1523/JNEUROSCI.0820-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Overman JJ, Clarkson AN, Wanner IB, Overman WT, Eckstein I, Maguire JL, Dinov ID, Toga AW, Carmichael ST. Proc Natl Acad Sci USA. 2012;109:E2230–E2239. doi: 10.1073/pnas.1204386109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goldshmit Y, Frisca F, Pinto AR, Pebay A, Tang JK, Siegel AL, Kaslin J, Currie PD. Brain Behav. 2014;4:187–200. doi: 10.1002/brb3.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Calabrese EJ. Environ Pollut. 2013;182:452–460. doi: 10.1016/j.envpol.2013.07.046. [DOI] [PubMed] [Google Scholar]
- 44.Huang YY, Chen AC, Carroll JD, Hamblin MR. Dose Response. 2009;7:358–383. doi: 10.2203/dose-response.09-027.Hamblin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang YY, Sharma SK, Carroll JD, Hamblin MR. Dose Response. 2011;9:602–618. doi: 10.2203/dose-response.11-009.Hamblin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Demidova-Rice TN, Salomatina EV, Yaroslavsky AN, Herman IM, Hamblin MR. Lasers Surg Med. 2007;39:706–715. doi: 10.1002/lsm.20549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oron U, Yaakobi T, Oron A, Hayam G, Gepstein L, Rubin O, Wolf T, Ben Haim S. Lasers Surg Med. 2001;28:204–211. doi: 10.1002/lsm.1039. [DOI] [PubMed] [Google Scholar]
- 48.Giacci MK, Wheeler L, Lovett S, Dishington E, Majda B, Bartlett CA, Thornton E, Harford-Wright E, Leonard A, Vink R, Harvey AR, Provis J, Dunlop SA, Hart NS, Hodgetts S, Natoli R, Van Den Heuvel C, Fitzgerald M. PLoS One. 2014;9:e104565. doi: 10.1371/journal.pone.0104565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rutar M, Natoli R, Kozulin P, Valter K, Gatenby P, Provis JM. Invest Ophthalmol Vis Sci. 2011;52:5347–5358. doi: 10.1167/iovs.10-7119. [DOI] [PubMed] [Google Scholar]
- 50.Naeser MA, Saltmarche A, Krengel MH, Hamblin MR, Knight JA. Photomed Laser Surg. 2011;29:351–358. doi: 10.1089/pho.2010.2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Naeser MA, Zafonte R, Krengel MH, Martin PI, Frazier J, Hamblin M, Knight JA, Meehan W, Baker EH. J Neurotrauma. 2014;31(11):1008–1017. doi: 10.1089/neu.2013.3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Naeser MA, Hamblin MR. Photomed Laser Surg. 2015;33(9):443–446. doi: 10.1089/pho.2015.3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Henderson TA, Morries LD. Adv Mind Body Med. 2015;29:27–33. [PubMed] [Google Scholar]
- 54.Henderson TA, Morries LD. Neuropsychiatr Dis Treat. 2015;11:2191–2208. doi: 10.2147/NDT.S78182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morries LD, Cassano P, Henderson TA. Neuropsychiatr Dis Treat. 2015;11:2159–2175. doi: 10.2147/NDT.S65809. [DOI] [PMC free article] [PubMed] [Google Scholar]