Abstract
PURPOSE
We examined the relationship between glycemic control and urinary tract infections (UTI) in women with type 1 diabetes mellitus.
MATERIALS AND METHODS
Women enrolled in the Epidemiology of Diabetes Interventions and Complications (EDIC) study, the observational follow-up of the Diabetes Control and Complications Trial (DCCT) were surveyed to assess the rate of physician-diagnosed UTI in the preceding 12 months. The relationship between HbA1c levels and number of UTIs in the previous 12 months was assessed using a multivariable Poisson regression model.
RESULTS
At EDIC Year 17, 572 women were evaluated: mean age 50.7 ± 7.2 years, mean body mass index (BMI) 28.6 ± 5.9 kg/m2, type 1 diabetes duration 29.8 ± 5.0 years, and mean HbA1c 8.0 ± 0.9%. Of these, 86 (15.0%) reported at least one physician diagnosed UTI during the past 12 months. Higher HbA1c levels were significantly associated with number of UTIs, such that for every unit increase (1%) in recent HbA1c level, there was a 21% (p=0.02) increase in UTI frequency in the previous 12 months after adjusting for race, hysterectomy status, urinary incontinence (UI), sexual activity in the past 12 months, peripheral and autonomic neuropathy and nephropathy.
CONCLUSIONS
The frequency of UTI increases with poor glycemic control in women with type 1 diabetes. This relationship is independent of other well-described predictors of UTI and suggests that factors directly related to glycemic control may influence the risk of lower urinary tract infections.
Keywords: Urinary tract infection, Diabetes, Risk Factors
INTRODUCTION
Urinary tract infections (UTI) affect millions of women each year.1 Diabetes mellitus is a comorbidity that predisposes patients to more frequent and complex UTI.2 Several epidemiologic studies support this tenet, demonstrating a 1.2 to 2.2 fold increased risk of lower UTI in subjects with diabetes compared to those without diabetes.3-6 The relationship between traditional measures of diabetes severity such as duration of disease, level of glycemic control and presence of diabetic complications among women with diabetes and risk of UTI, however, is not clear. Glycated hemoglobin (HbA1c) and diabetic micro-vascular and neuropathic complications have not been associated with symptomatic UTI, while duration of diabetes and insulin use have been variably correlated.4, 7, 8 These studies were limited by their inclusion of women with primarily type 2 diabetes and single measurement of glycemic control. Consequently, data on factors associated with symptomatic UTI for women with type 1 diabetes mellitus are limited.
We examined the relationship between HbA1c and UTI in women with type 1 diabetes using data from the Diabetes Control and Complications Trial (DCCT) and its observational follow-up, the Epidemiology of Diabetes Interventions and Complications (EDIC) study. This study has collected detailed information on women with type 1 diabetes since 1983. Information regarding UTI was collected in 2010 (EDIC year 17) as part of an ancillary study of urologic complications of diabetes (UroEDIC). We hypothesized that worse glycemic control, as measured by HbA1c levels, in type 1 diabetes was associated with an increased rate of UTI.
MATERIALS AND METHODS
Population and Setting
The DCCT was a multicenter, randomized clinical trial designed to compare the effects of intensive and conventional diabetes therapy on the development and progression of early microvascular complications of type 1 diabetes.9, 10 From 1983-1989, 1441 subjects (680 women) aged 13-39 years were recruited. The DCCT included a primary prevention cohort and a secondary intervention cohort. The primary prevention cohort consisted of 348 women with no retinopathy, a urinary albumin excretion rate <40 mg/24 hours, and diabetes duration of 1–5 years at baseline. The secondary intervention cohort consisted of 332 women who, at baseline, had non-proliferative retinopathy, urinary albumin excretion rate ≤200 mg/24 hours, and diabetes duration of 1–15 years. Individuals were excluded if they had hypertension, history of symptomatic ischemic heart disease, or symptomatic peripheral neuropathy. At the end of the trial in 1993, the DCCT proved that intensive therapy significantly reduced the risk of diabetic microvascular complications compared with conventional treatment.11 Intensive treatment was subsequently recommended for all subjects.
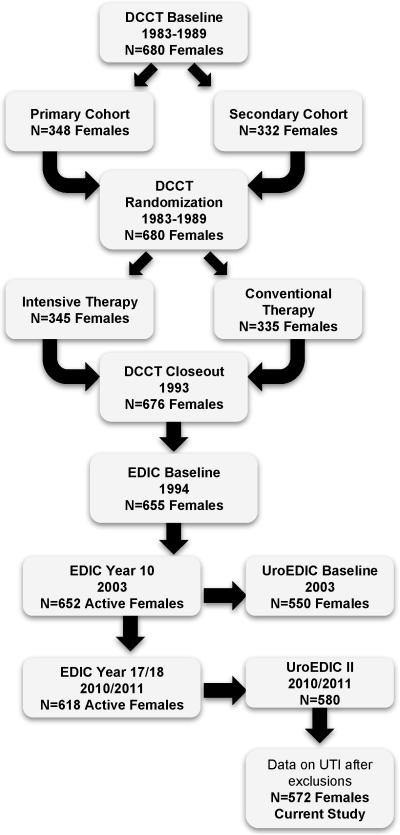
In 1994, 655 (96%) of the surviving 680 women volunteered to participate in EDIC; mean age 35 ± 7 years. During 2010, EDIC year 17, 580 of 618 active female participants (94%), mean age 51 ± 7 years, agreed to participate in UroEDIC, an ancillary study to examine the presence of urological complications, including urinary incontinence, lower urinary tract symptoms, urinary tract infections and sexual dysfunction. Among the 580 women, 575 women had UTI information. Three individuals reported 4, 6, and 9 UTI infections and the following HbA1c levels: 8.5, 8.0 and 8.2, respectively. Given the single observation for these counts and influential status (inclusion altered association estimate), they were excluded resulting in 572 women for the current analyses (Figure 1). All DCCT/EDIC procedures were reviewed and approved by institutional review boards at the participating center and all participants provided written informed consent.
Figure 1.
Flow of female participants in DCCT/EDIC/UroEDIC
Urinary Tract Infection Measurements
Urinary tract infections (UTI) were quantified by the response to the question: “How many times in the last 12 months were you diagnosed by a physician with a bladder infection?” This question is the same as that used in the Third National Health and Nutrition Examination Survey (NHANES).12
Diabetes Measurements
Each EDIC subject underwent an annual history, physical examination, and laboratory evaluation including HbA1c which was measured at baseline and quarterly during DCCT and annually in EDIC.13 For purposes of this analysis, recent glycemic control was the HbA1c measured at the EDIC year 16 visit (1 year prior to UTI assessment), and long term glycemic control was the time-weighted mean of all HbA1c values obtained during DCCT and through EDIC year 16. Retinopathy was established by fundus photographs that were obtained periodically and graded centrally using the Early Treatment Diabetic Retinopathy Study scale.14, 15 Albumin excretion rate (AER) was measured in alternate years during EDIC. Nephropathy was defined as microalbuminuria (AER 30-300 mg/24hr) or albuminuria (AER >300 mg/24hr). Peripheral neuropathy was determined at year 17 using the Michigan Neuropathy Screening Instrument (MNSI) and defined as >6 positive responses on the MNSI questionnaire or a score of >2 on the MNSI physical exam. Cardiovascular autonomic neuropathy definition: either R-R variation <15 or R-R variation between 15-19.9 plus either a Valsalva ratio ≤1.5 or a supine-to-standing drop of 10 mmHg in diastolic blood pressure measured at EDIC year 16/17.16
Other Measurements
Other well established factors related to UTI measured during EDIC Year 17 (unless stated otherwise) include: menopausal status, oral contraceptive use, parity, sexual activity and urinary incontinence (UI). Natural menopause definition: cessation of menses for 1 year in the absence of gynecologic surgery. Surgical menopause was defined by report of hysterectomy and/or bilateral oophorectomy. Parity definition: number of live births as of EDIC Year 10. UI was assessed at EDIC years 10 and 17 by a self-administered questionnaire using validated instruments from previous studies.17 UI was defined as weekly or more frequent incontinence. Women were also queried about engaging in sexual activity in the past 12 months at EDIC years 10 and 17.
Statistical Analysis
The distribution of socio-demographic and clinical characteristics, markers of diabetes control, treatment and diabetic complications were compared by UTI and HbA1c status at EDIC year 17 using the Chi-Square test for categorical variables (Fisher's was used when sample sizes were small) and the Wilcoxon rank-sum test for continuous variables. A multivariable Poisson regression model was built to estimate the effects of glycemic control (% HbA1c) on UTI count. All variables significant with an alpha level of 0.1 in bivariate analyses along with sexual activity in the last 12 months (yes or no) were included in the model. To determine the individual impact of both recent and long term glycemic control on UTI count, both HbA1c variables (recent EDIC year 16 HbA1c and time-weighted mean HbA1c over DCCT/EDIC) were included in the initial model. Time-weighted HbA1c did not have a significant independent association with UTI count after inclusion of recent HbA1c levels and therefore was excluded from the final model. The percent change in UTI count per 1 unit increase in each covariate was reported using the expression, [EXP(β)-1]*100. All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
RESULTS
At EDIC year 17, 572 women, aged 50.7 ± 7.2 years, had mean body mass index (BMI) of 28.6 ± 5.9 kg/m2, with type 1 diabetes duration of 29.8 ± 5.0 years, and mean HbA1c of 8.0 ± 0.9%. Eighty-six (15.0%) women reported at least one physician diagnosed UTI in the past 12 months. Fifty-nine women (10%) reported 1 UTI, 22 women (4%) reported 2 UTIs, and 5 women (1%) reported 3 UTIs in the last 12 months. Current age, BMI, menopausal status and sexual activity in the last 12 months were not significantly associated with UTI status in unadjusted analysis (Table 1). Women reporting UTI in the last 12 months were more likely to be non-white (8% vs. 3%; p=0.02) and there was an increased report of prior hysterectomy (24% vs. 17%; p=0.1) compared to women not reporting UTI. DCCT treatment (intensive vs. conventional), cohort (primary vs. secondary), and insulin dose were not associated with UTI at EDIC year 17. Recent HbA1c levels were higher among women reporting UTIs compared to women not reporting UTIs. Additionally, nephropathy, peripheral neuropathy and autonomic neuropathy were all significantly more prevalent among women reporting UTIs at EDIC year 17 (Table 1). Mean age, BMI, duration of diabetes and insulin dose were all higher in women with increased HbA1c levels (>7.8% vs. ≤7.8%) (data not shown).
Table 1.
Characteristics of Participants at EDIC Year 17 by UTI Status*
| No (n=486) | Yes (n=86) | p-value | |
|---|---|---|---|
| Sociodemographic/Clinical | |||
| Attained Age (years) Mean (SD) | 50.6±7.1 | 50.9±7.7 | 0.7 |
| Non-White Race No. (%) | 14 (3) | 7 (8) | 0.02 |
| Currently Married No. (%) | 329 (70) | 56 (67) | 0.6 |
| College Education No. (%) | 280 (60) | 44 (53) | 0.2 |
| Current Drinker No. (%)† | 185 (39) | 33 (40) | 0.9 |
| Current Smoker No. (%)† | 55 (12) | 11 (13) | 0.7 |
| Body Mass Index (BMI) (kg/m2) Mean (SD) | 28.7±6.1 | 27.8±4.4 | 0.7 |
| BMI Category (kg/m2) No. (%) | |||
| Normal (<25) | 131 (28) | 20 (24) | 0.2 |
| Overweight (25-29) | 177 (38) | 40 (49) | |
| Obese (≥30) | 153 (33) | 22 (27) | |
| Oral contraceptive use No. (%) | 22 (5) | 2 (2) | 0.3 |
| Postmenopausal No. (%) | 271 (57) | 51 (61) | 0.5 |
| Hysterectomy No. (%) | 79 (17) | 20 (24) | 0.1 |
| Parity (n of live births) No. (%)‡ | |||
| 0 | 156 (32) | 22 (26) | 0.4 |
| 1 | 91 (19) | 16 (19) | |
| ≥2 | 239 (49) | 48 (56) | |
| Sexually active in last 12 months | 375 (78) | 65 (76) | 0.8 |
| Urinary Incontinence (at least weekly) | 108 (23) | 26 (31) | 0.1 |
| Diabetes Control and Treatment | |||
| DCCT cohort No. (% primary prevention) | 246 (51) | 43 (50) | 0.9 |
| Treatment arm No. (% intensive) | 259 (53) | 43 (50) | 0.6 |
| Duration of diabetes (years) | 29.8±5.0 | 29.6±5.1 | 0.7 |
| Current Hemoglobin A1c (%) | 7.9±1.2 | 8.4±1.3 | 0.002 |
| DCCT/EDIC time-weighted mean HbA1c | 8.0±0.9 | 8.3±1.0 | 0.008 |
| Insulin Dose (units/kg/day) | 0.67±0.29 | 0.68±0.28 | 0.9 |
| Diabetic Complications (% with complication) | |||
| Retinopathy No. (%)§ | 81 (17) | 14 (16) | 0.9 |
| Nephropathy No. (%)∥ | 67 (14) | 22 (28) | 0.004 |
| Peripheral Neuropathy No. (%)¶ | 185 (40) | 40 (49) | 0.1 |
| Autonomic Neuropathy No. (%)# | 170 (37) | 41 (49) | 0.03 |
| Coronary Calcium>0 No. (%)** | 80 (19) | 15 (20) | 0.8 |
Note: Data are presented as Means (SD) or N (%), p-values based on chi-square or Wilcoxan rank-sum test.
UTI defined as ≥1 episode in past 12 months.
Smoking defined as “currently smokes cigarettes or ever smoked in the past 12 months (any amount).” Drinking defined as “consumed an average of at least one alcoholic beverage per week during the past 12 months.”
Parity defined as number of live births as of EDIC Year 10.
Defined through EDIC year 14 using the Early Treatment Diabetic Retinopathy Study on a scale of 0-23 (<12 Non Proliferative or None, ≥12 Proliferative).
Defined at EDIC years 17/18 by Albumin Excretion Rate (mg/24hr) (AER≥30).
Defined at EDIC year 17 by the Michigan Neuropathy Screening Instrument >6 responses on the questionnaire or a score of >2 on the exam.
Autonomic testing completed in EDIC year 16/17 and abnormal finding defined as R-R variation<15 or RR variation<20 in combination with a Valsalva ratio≤1.5 or a decrease of >10 mm Hg in diastolic BP upon standing.
Coronary calcium deposit during EDIC years 7-9.
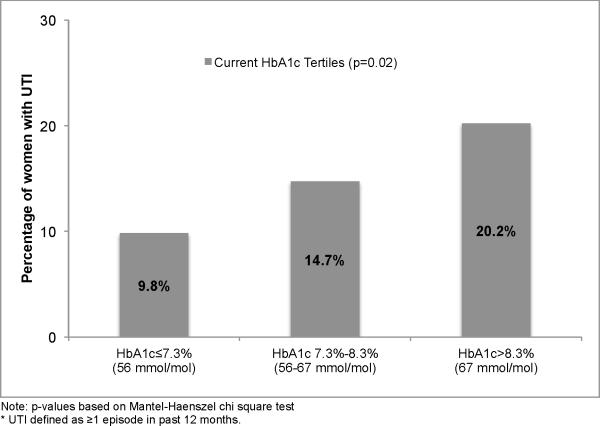
The percentage of women reporting UTIs increased with increasing tertiles of recent HbA1c levels (Figure 2). A multivariable Poisson regression analysis demonstrated a 21% increase in UTI infection count per 1% increase (e.g. 7% to 8%) in recent HbA1c level (p=0.02) after adjustment for race, hysterectomy status, UI, sexual activity in the past 12 months, peripheral and autonomic neuropathy and nephropathy (Table 2). A separate multivariable Poisson regression model examining DCCT/EDIC time-weighted mean HbA1c levels revealed a 31.8% (p=0.03) increase in UTI count per 1% increase in HbA1c, however, the association of the time-weighted mean HbA1c with UTI risk was no longer significant when also adjusted for the recent HbA1c.
Figure 2.
Percentage of women with UTI* at EDIC Year 17 per tertile of current EDIC Year 16 HbA1c
Table 2.
Adjusted Poisson Model of # of Urinary Tract Infections at EDIC Year 17
| Covariates | Parameter Estimate (SE) | % change in UTI count for unit increase in covariate* | p-value |
|---|---|---|---|
| Unadjusted | |||
| Current HbA1c (at EDIC Year 16) (%) | 0.23 (0.06) | 38.0% | 0.0001 |
| Current HbA1c (at EDIC Year 16) (mmol/mol) | 0.02 (0.006) | ||
| Adjusted | |||
| Non-White race | 0.37 (0.44) | 45.1% | 0.4 |
| Hysterectomy | 0.46 (0.24) | 58.6% | 0.06 |
| Urinary Incontinence (≥weekly) | 0.27 (0.23) | 31.1% | 0.2 |
| Sexually Active in last 12 months | 0.19 (0.26) | 20.8% | 0.5 |
| Peripheral Neuropathy† | 0.04 (0.23) | 4.3% | 0.9 |
| Autonomic Neuropathy‡ | 0.08 (0.22) | 8.2% | 0.7 |
| Nephropathy§ | 0.28 (0.27) | 32.4% | 0.3 |
| Current Hemoglobin A1c (at EDIC Year 16) (%) | 0.19 (0.08) | 21.0% | 0.02 |
| Current Hemoglobin A1c (at EDIC Year 16) (mmol/mol) | 0.02 (0.008) |
The percent change in UTI count per 1 unit increase in covariate was reported using the equation, [EXP(β)-1]*100.
Defined at EDIC year 17 by the Michigan Neuropathy Screening Instrument >6 responses on the questionnaire or a score of >2 on the exam.
Autonomic testing completed in EDIC year 16/17 and abnormal finding defined as R-R variation<15 or RR variation<20 in combination with a Valsalva ratio≤1.5 or a decrease of >10 mm Hg in diastolic BP upon standing.
Nephropathy defined as AER≥30 mg/24hr at EDIC years 17/18.
DISCUSSION
We observed a significant relationship between recent HbA1c levels and rate of UTI in women with type 1 diabetes. The findings show that for every one-unit increase in recent HbA1c, there was a 21% increase in number of UTI and the association was independent of known risk factors for UTI such as race, hysterectomy status, UI, sexual activity in the past 12 months, peripheral and autonomic neuropathy and nephropathy, suggesting that glycemia itself may independently affect UTI in this population.
Although diabetes is associated with higher risk for UTI, other studies to date have failed to identify an association between level of glycemic control and UTI.4, 6, 7 For example, evaluation of post-menopausal women with predominantly type 2 diabetes who had a culture-confirmed UTI did not demonstrate an increased risk associated with increased HbA1c.4 Another study found an increased risk of UTI in women taking insulin and those with longer duration of diabetes, but no relationship between HbA1c and UTI.6 Finally, an evaluation of a cohort of women in their 40's with type 1 and type 2 diabetes found that glycemic control was also not a significant risk factor for UTI (RR 1.27, 95% CI 0.90-1.79).7
There are several possible explanations for the difference between prior studies and our results. First, prior studies included women almost exclusively with type 2 diabetes and were limited to a single measure of HbA1c. It is unclear if women with type 1 diabetes are more prone to UTI risk compared to their type 2 counterparts. Most UTIs reported in prior studies were found to be due to typical uropathogens suggesting that both type 1 and type 2 diabetes facilitate the same route of infection.6 A more plausible explanation is that HbA1c levels overall were lower in these cohorts which may impair ability to detect associations.6 In a prior analysis of the EDIC cohort in 2003, when mean HbA1c levels were lower, we also did not observe an association between HbA1c levels and UTI.8 It is possible our current findings of an association can be attributed to the effect of increased glycemia being more manifest in older subjects. Interestingly, in a recent study that included 190 patients with diabetes with available HbA1c values within 3 months before collection of isolates related to colonization of the urinary tract, those women with the highest HbA1c values were at greatest risk for urosepsis.18
There is pathophysiological support for the findings that poor glycemic control may directly and indirectly increase risk for UTI. First, higher glucose concentrations in the urine may promote the growth of pathogenic bacteria, act as a culture medium and promote bacterial adherence to the urinary tract.19-21 Second, genitourinary damage due to diabetes may result in impaired bladder emptying 22 and decreased bladder sensation 23 resulting in conditions conducive to UTI. When both recent and long-term levels of glycemic control were evaluated, only recent levels of HbA1c were independently associated with UTI suggesting a glucotoxic effect on outcome. Finally, overall infection risk in patients with diabetes is higher.3
Sexual activity was not associated with UTI in the recent study. This is in contrast with the bulk of the literature which includes sexual activity as a generally accepted risk factor for UTI1 but also with findings previously reported in this cohort.8 These discrepancies can be attributed in part to aging of the cohort as associations between sexual activity and UTI have been primarily supported in younger women.1 Finally, we observed an effect of hysterectomy on UTI rate in women with type 1 diabetes. Alterations in the normal anatomy of the pelvis after hysterectomy may contribute to increased risk of UTIs.24 Further studies examining these factors in older women with diabetes are warranted.
There are several important clinical implications for our findings. First, the overall burden of UTI in this study (15%) is lower than that observed for other populations of women with type 1 diabetes (20%).7 While this could be a function of the variation in the diagnosis and definition of UTI across studies, it is also possible that this is a result of improved glycemic control, which may have contributed to the prevention of infection. Second, our data suggest that better glycemic control is associated with lower rate of UTI. These findings provide a rationale for counseling women with type 1 diabetes about UTIs and glycemic control. Offering the knowledge that they can potentially decrease their risk of UTI by improving blood glucose control might motivate some women to improve their self-care. Finally, clinicians are increasingly required to address all potential risk factors for UTI, not only for patient safety, but also because of impact on quality indicators and potential financial penalties for healthcare providers and payers.25-27
Strengths of our study include large sample size, minimal loss to follow-up and frequent validated measurement of key covariates. However, there are several limitations of the study including the reliance on patient-reported “physician diagnosed” UTI. For practical reasons, we could not confirm these self-reports so first-hand provider evaluation, laboratory testing, and treatment assessments were not available. Without these data, it is not possible to know if recall bias may have affected our findings. However, recent studies suggest that diagnostic tests such as urinalysis and urine cultures are rarely necessary in the setting of uncomplicated cystitis.28 We have no reason to believe that any possible misclassification as a result of recall bias would have differed between specific exposure groups. Information on sexual activity and its frequency and parity were not collected on a repeated basis. While the cohort has been followed for a number of decades, participants are still relatively young and most are Caucasian. As DCCT/EDIC participants are generally a highly motivated group of individuals with generally good health behaviors, results may not apply to a broader population with type 1 diabetes.
CONCLUSIONS
Our findings demonstrate that urinary tract infection is associated with poor glycemic control in women with type 1 diabetes. This relationship is independent of other well-described risk factors for UTI and suggests that factors directly related to glycemic control may affect UTIs. These results provide a rationale for counseling women with type 1 diabetes about the increased risk of UTI and the importance of maintaining good glycemic control.
Supplementary Material
ACKNOWLEDGMENTS
A complete list of participants in the DCCT/EDIC Research Group is presented in the Supplementary Material published online for the article in N Engl J Med 2015, 372:1722-33.
Industry contributors have had no role in the DCCT/EDIC study but have provided free or discounted supplies or equipment to support participants’ adherence to the study: Abbott Diabetes Care (Alameda, CA), Animas (Westchester, PA), Bayer Diabetes Care (North America Headquarters, Tarrytown, NY), Becton Dickinson (Franklin Lakes, NJ), Eli Lilly (Indianapolis, IN), Extend Nutrition (St. Louis, MO), Insulet Corporation (Bedford, MA), Lifescan (Milpitas, CA), Medtronic Diabetes (Minneapolis, MN), Nipro Home Diagnostics (Ft. Lauderdale, FL), Nova Diabetes Care (Billerica, MA), Omron (Shelton, CT), Perrigo Diabetes Care (Allegan, MI), Roche Diabetes Care (Indianapolis, IN) , and Sanofi-Aventis (Bridgewater NJ).
Funding/Support: The DCCT/EDIC has been supported by cooperative agreement grants (1982-93, 2012-2017), and contracts (1982-2012) with the Division of Diabetes Endocrinology and Metabolic Diseases of the National Institute of Diabetes and Digestive and Kidney Disease (current grant numbers U01 DK094176 and U01 DK094157), and through support by the National Eye Institute, the National Institute of Neurologic Disorders and Stroke, the General Clinical Research Centers Program (1993-2007), and Clinical Translational Science Center Program (2006-present), Bethesda, Maryland, USA. Additional support for this DCCT/EDIC collaborative study (UroEDIC) was provided by an R01 grant (2009-2013) with the National Institute of Diabetes and Digestive and Kidney Disease (5R01DK083927-03). Sara M. Lenherr's efforts were funded by NIH/NIDDK T32 DK07782.
Footnotes
Trial Registration: clinicaltrials.gov NCT00360815 and NCT00360893.
REFERENCES
- 1.Foxman B. Urinary tract infection syndromes: occurrence, recurrence, bacteriology, risk factors, and disease burden. Infect Dis Clin North Am. 2014;28:1. doi: 10.1016/j.idc.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Stapleton A. Urinary tract infections in patients with diabetes. Am J Med. 2002;113(Suppl 1A):80S. doi: 10.1016/s0002-9343(02)01062-8. [DOI] [PubMed] [Google Scholar]
- 3.Muller LM, Gorter KJ, Hak E, et al. Increased risk of common infections in patients with type 1 and type 2 diabetes mellitus. Clin Infect Dis. 2005;41:281. doi: 10.1086/431587. [DOI] [PubMed] [Google Scholar]
- 4.Boyko EJ, Fihn SD, Scholes D, et al. Diabetes and the risk of acute urinary tract infection among postmenopausal women. Diabetes Care. 2002;25:1778. doi: 10.2337/diacare.25.10.1778. [DOI] [PubMed] [Google Scholar]
- 5.Shah BR, Hux JE. Quantifying the risk of infectious diseases for people with diabetes. Diabetes Care. 2003;26:510. doi: 10.2337/diacare.26.2.510. [DOI] [PubMed] [Google Scholar]
- 6.Boyko EJ, Fihn SD, Scholes D, et al. Risk of urinary tract infection and asymptomatic bacteriuria among diabetic and nondiabetic postmenopausal women. Am J Epidemiol. 2005;161:557. doi: 10.1093/aje/kwi078. [DOI] [PubMed] [Google Scholar]
- 7.Geerlings SE, Stolk RP, Camps MJ, et al. Risk factors for symptomatic urinary tract infection in women with diabetes. Diabetes Care. 2000;23:1737. doi: 10.2337/diacare.23.12.1737. [DOI] [PubMed] [Google Scholar]
- 8.Czaja CA, Rutledge BN, Cleary PA, et al. Urinary tract infections in women with type 1 diabetes mellitus: survey of female participants in the epidemiology of diabetes interventions and complications study cohort. J Urol. 2009;181:1129. doi: 10.1016/j.juro.2008.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diabetes Control and Complications Trial Research Group Effect of intensive diabetes treatment on the development and progression of long-term complications in adolescents with insulin-dependent diabetes mellitus: Diabetes Control and Complications Trial. J Pediatr. 1994;125:177. doi: 10.1016/s0022-3476(94)70190-3. [DOI] [PubMed] [Google Scholar]
- 10.Writing Team for the Diabetes, Control, Complications Trial/Epidemiology of Diabetes, Interventions, Complications Study Sustained effect of intensive treatment of type 1 diabetes mellitus on development and progression of diabetic nephropathy: the Epidemiology of Diabetes Interventions and Complications (EDIC) study. JAMA. 2003;290:2159. doi: 10.1001/jama.290.16.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 12.Third National Health and Nutrition Examination Survey . Reference Manuals and Reports: Manual for Medical Technicians and Laboratory Procedures Used for NAHNES III (CD-ROM) Hyattsville, MD: 1988-1994. [Google Scholar]
- 13.Epidemiology of Diabetes Interventions and Complications (EDIC) Design, implementation, and preliminary results of a long-term follow-up of the Diabetes Control and Complications Trial cohort. Diabetes Care. 1999;22:99. doi: 10.2337/diacare.22.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diabetes Control and Complications Trial Research Group The effect of intensive diabetes treatment on the progression of diabetic retinopathy in insulin-dependent diabetes mellitus: The diabetes control and complications trial. Archives of Ophthalmology. 1995;113:36. doi: 10.1001/archopht.1995.01100010038019. [DOI] [PubMed] [Google Scholar]
- 15.Early Treatment Diabetic Retinopathy Study Research Group Early photocoagulation for diabetic retinopathy. ETDRS report number 9. Opthalmology. 1991;98:766. [PubMed] [Google Scholar]
- 16.Pop-Busui R, Low PA, Waberski BH, et al. Effects of prior intensive insulin therapy on cardiac autonomic nervous system function in type 1 diabetes mellitus: the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study (DCCT/EDIC). Circulation. 2009;119:2886. doi: 10.1161/CIRCULATIONAHA.108.837369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sandvik H, Hunskaar S, Vanvik A, et al. Diagnostic classification of female urinary incontinence: an epidemiological survey corrected for validity. J Clin Epidemiol. 1995;48:339. doi: 10.1016/0895-4356(94)00147-i. [DOI] [PubMed] [Google Scholar]
- 18.Wang MC, Tseng CC, Wu AB, et al. Bacterial characteristics and glycemic control in diabetic patients with Escherichia coli urinary tract infection. J Microbiol Immunol Infect. 2013;46:24. doi: 10.1016/j.jmii.2011.12.024. [DOI] [PubMed] [Google Scholar]
- 19.Chen SL, Jackson SL, Boyko EJ. Diabetes mellitus and urinary tract infection: epidemiology, pathogenesis and proposed studies in animal models. J Urol. 2009;182:S51. doi: 10.1016/j.juro.2009.07.090. [DOI] [PubMed] [Google Scholar]
- 20.Daneshgari F, Liu G, Birder L, et al. Diabetic bladder dysfunction: current translational knowledge. J Urol. 2009;182:S18. doi: 10.1016/j.juro.2009.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geerlings SE, Meiland R, van Lith EC, et al. Adherence of type 1-fimbriated Escherichia coli to uroepithelial cells: more in diabetic women than in control subjects. Diabetes Care. 2002;25:1405. doi: 10.2337/diacare.25.8.1405. [DOI] [PubMed] [Google Scholar]
- 22.Hosking DJ, Bennett T, Hampton JR. Diabetic autonomic neuropathy. Diabetes. 1978;27:1043. doi: 10.2337/diab.27.10.1043. [DOI] [PubMed] [Google Scholar]
- 23.Peleg AY, Weerarathna T, McCarthy JS, et al. Common infections in diabetes: pathogenesis, management and relationship to glycaemic control. Diabetes Metab Res Rev. 2007;23:3. doi: 10.1002/dmrr.682. [DOI] [PubMed] [Google Scholar]
- 24.Raz R, Gennesin Y, Wasser J, et al. Recurrent Urinary Tract Infections in Postmenopausal Women. Clinical Infectious Diseases. 2000;30:152. doi: 10.1086/313596. [DOI] [PubMed] [Google Scholar]
- 25.Burke JP. Infection control - a problem for patient safety. N Engl J Med. 2003;348:651. doi: 10.1056/NEJMhpr020557. [DOI] [PubMed] [Google Scholar]
- 26.Saint S, Meddings JA, Calfee D, et al. Catheter-associated urinary tract infection and the Medicare rule changes. Ann Intern Med. 2009;150:877. doi: 10.7326/0003-4819-150-12-200906160-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.CMS.gov. [December 2014];Hospital-Acquired Conditions. 2014 Aug; Available at http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/HospitalAcqCond/Hospital-Acquired_Conditions.html.
- 28.Bent S, Saint S. The optimal use of diagnostic testing in women with acute uncomplicated cystitis1. The American Journal of Medicine. 2002;113:20. doi: 10.1016/s0002-9343(02)01056-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.