Abstract
Background
Sirolimus, an immunosuppressant agent used in renal transplantation, can prevent allograft rejection. Identification of the therapeutic index (ratio of minimum toxic concentration to minimum therapeutic concentration) for immunosuppresants is necessary to optimize the care of patients and set standards for bioequivalence evaluation of sirolimus products. However, the therapeutic index for sirolimus has been inconsistently defined, potentially due to inconsistencies in sirolimus exposure-response relationships.
Methods
The authors used retrospective therapeutic drug monitoring data from the electronic health records of patients treated in a tertiary healthcare system from 2008 to 2014, to: 1) develop a population pharmacokinetic model, 2) use the model to simulate sirolimus concentrations, and 3) characterize the exposure-response relationship. Using Wilcoxon rank-sum and Fisher’s exact tests, the authors determined relationships between sirolimus exposure and adverse events (anemia, leukopenia, thrombocytopenia, hyperlipidemia, decline in renal function) and the composite efficacy endpoint of graft loss or rejection.
Results
The developed 2-compartment population pharmacokinetic model showed appropriate goodness of fit. In a late-phase (>12 months), post-renal transplant population of 27 inpatients, the authors identified statistically significant relationships between 83 simulated peak and trough sirolimus concentrations and outcomes: graft loss or rejection (p=0.018), and decline in renal function (p=0.006), respectively.
Conclusions
Use of therapeutic drug monitoring results and pharmacokinetic modeling permitted correlation of sirolimus concentrations with graft loss or rejection, and decline in renal function. However, the method was limited in its assessment of other adverse events. To better evaluate sirolimus exposure-response relationships, the method should be applied to a larger sample of newly-transplanted patients with a higher propensity toward adverse events or efficacy failure.
Keywords: sirolimus, pharmacokinetics, pharmacodynamics, anemia, thrombocytopenia, adverse event, efficacy
INTRODUCTION
Sirolimus is an immunosuppressive agent that inhibits antibody production and T-lymphocyte activation and proliferation. Approved by the Food and Drug Administration (FDA) since 1999, sirolimus is indicated for the prevention of allograft rejection in patients 13 years and older with a transplanted kidney.1 According to existing evidence, sirolimus is efficacious as a sole immunosuppressive agent in preventing organ rejection, when administered at doses of 1 to 4.2 mg/day.2,3 The product label currently recommends doses of 1 to 6 mg/day, with up to 7 mg/day administered to those with high immunologic risk.3
Despite a narrow recommended dosing range, previous studies indicate significant intra- and inter-individual variability in sirolimus pharmacokinetic (PK) parameters and resultant exposures in patients on chronic treatment with sirolimus.4,5 Because of the observed variability in sirolimus exposure, current recommendations suggest dose titration to achieve trough concentrations of 12 to 24 μg/L, often dependent on time since transplantation.2,3,6 Importantly, titration of sirolimus dosing based on trough concentrations alone does not take into account drug toxicity. Previous investigators have identified significant adverse events (AEs), including bone marrow suppression, hyperlipidemia, diabetes mellitus, renal dysfunction, pneumonitis, and others, that occur despite administration within the recommended dose range and achievement of therapeutic sirolimus concentrations.7-12
Previous investigators have not consistently defined the therapeutic index (the ratio of minimum toxic concentration to minimum therapeutic concentration) of sirolimus.2-4 The current FDA product specific draft guidance for sirolimus states that the range between sirolimus therapeutic and toxic whole blood concentrations is narrow. However, a definitive conclusion on the therapeutic index was not reported. Therefore, we aimed to determine whether the use of sirolimus concentrations obtained through therapeutic drug monitoring in a cohort of renal transplant patients could be used to develop a population PK model for sirolimus. We aimed to use such a model to link simulated exposures with efficacy or AEs in the same cohort of patients, and to determine whether this combined methodology will better define sirolimus exposure-response relationships that could help define the therapeutic index of sirolimus.
MATERIALS AND METHODS
Database/Patient population
We included patients ≥18 years of age treated with oral sirolimus to prevent allograft rejection following renal transplantation. Eligible participants were inpatient or seen at an outpatient clinic in the Duke University Health System between January 2008 and July 2014, and had at least 1 recorded sirolimus concentration in the medical record. We excluded patients without any electronically available dosing records. We identified qualifying patients and data using an electronic data warehouse containing information from all operational systems serving the medical center’s hospitals and clinics. We reviewed and extracted the following data from the data warehouse: patient demographics, drug levels, and other lab results. We verified drug dosing and changes in dosing, concomitant medications of interest, safety data, and clinical outcomes through direct review of electronic medical records. The Duke University Institutional Review Board and FDA Research Involving Human Subjects Committee approved the study with a waiver of informed consent.
Standard procedures for analysis of sirolimus blood samples
Sirolimus samples obtained within the Duke University Health System were collected in whole blood EDTA containers and transferred to the Mayo Clinic Laboratories in Rochester, MN. Blood samples underwent protein precipitation, and the resultant supernatant was analyzed by high performance liquid chromatography-tandem mass spectrometry. The lower limit of quantification for the assay was 2 ng/mL and the precision of the assay was <10% throughout the analytical range. (Mayo Clinic Laboratories, personal communication)
Population PK analysis
We analyzed sirolimus whole blood PK data with a nonlinear mixed effects modeling approach using the software NONMEM (version 7.2, Icon Solutions, Ellicott City, MD, USA). We used first-order conditional estimation method with interaction (FOCE-I) for all model runs and performed run management using Pirana (version 2.8.1).13 We then performed visual predictive checks and bootstrap methods with Perl-speaks-NONMEM (version 3.6.2).14 We performed data manipulation and visualization using the software STATA (version 13.1, College Station, TX), R (version 3.0.2, R Foundation for Statistical Computing, Vienna, Austria) and RStudio (version 0.97.551, RStudio, Boston, MA, USA) packages, including lattice, Xpose and ggplot2.15
We evaluated one- and two-compartment PK models in NONMEM. We assessed between-subject variability (BSV) for PK model parameters using an exponential relationship. In addition to BSV, we assessed between-occasion variability (BOV) for PK model parameters using an exponential relationship. Proportional, additive, and combined (proportional plus additive) residual error models were evaluated.
We visually inspected the potential effect of covariates on PK parameters by creating scatter and box plots (continuous and categorical variables, respectively) for the following covariates: age, body weight, serum creatinine, hematocrit, gender, race, and presence of ≥1 concomitant medication of interest with known PK interaction with sirolimus (i.e., diltiazem, cyclosporine, erythromycin, ketoconazole, rifampin, verapamil). We normalized continuous covariates to the population median. For continuous covariates, we used power function to describe covariate relationships on PK parameters. We used a forward inclusion (p<0.05 and delta objective function value (Δ OFV) >3.8) and backward elimination (p<0.001 and ΔOFV >10.8) approach to evaluate statistical significance of relevant covariates. Missing clinical data was imputed using the last value carried forward.
Population PK model evaluation and validation
During the population PK model building process, successful minimization, diagnostic plots, plausibility and precision of parameter estimates, as well as objective function and shrinkage values, were used to assess model appropriateness. We evaluated parameter precision other than the absorption rate constant and lag time for the final population PK model using non-parametric bootstrapping (1000 replicates) to generate the 95% confidence intervals for parameter estimates. We performed standardized visual predictive check, using the final model to generate 1000 Monte Carlo simulation replicates per time point of sirolimus exposure. We then compared subject-level simulated results with observed values by calculating and plotting the percentile of each observed concentration in relation to its 1000-simulated observations.16 The dosing and covariate values used to generate the simulations in the visual predictive check were the same as those used in the study population.
Relationship between sirolimus exposure and efficacy or AEs
We identified the following AEs of interest based on the drug FDA label: anemia (male: hematocrit <0.39; female: hematocrit <0.35), thrombocytopenia (platelets <150 ×103/ml), leukopenia (white blood cell count <3.2 ×103/ml), hypercholesterolemia (total cholesterol >199 mg/dL), hypertriglyceridemia (triglycerides >149 mg/dL), and decline in renal function (25% increase in serum creatinine during an admission or clinician documentation noting worsening function). Efficacy was assessed through the composite outcome of graft loss, and biopsy-proven rejection. We used our population PK model to simulate sirolimus exposure, including concentrations at the time of hematologic or cholesterol lab draws, and maximum, trough, and average concentration for each day of an AE assessment (i.e., lab draw) or efficacy diagnosis (i.e., serum chemistry or biopsy-proven rejection). Simulations accounted for any changes in drug dosing during hospitalization. For each inpatient, we characterized observed and simulated concentrations as meeting goal, less than goal, or greater than goal according to the timing of the simulated sirolimus exposure in relationship to the time since transplant. More specifically, for patients 0 to <4 months post- transplant, an acceptable goal trough concentration is 10 to 15 μg/L; from 4 to 12 months post-transplant, the goal is 16 to 24 μg/L; and for patients beyond 12 months post-transplant, the goal is 12 to 20 μg/L.3 Patients without a well-defined date of transplant in the electronic health record (EHR) were assigned to the late post-transplant group (>12 months).
Because we could not confirm patient-reported dosing for outpatients, we did not simulate exposure in this population. However, we determined whether individually observed concentrations were within the goal range based on time since transplant.
For inpatients and outpatients, we performed Wilcoxon rank sum analyses, Fisher’s exact tests, and linear regression where appropriate, to determine the distribution of specific efficacy or AEs among patients who met, those who were above, and those who were below goal concentrations. Finally, we performed a sensitivity analysis using similar statistical methods. In this analysis, we used lower thresholds to define anemia (hematocrit ≤ 0.25) and thrombocytopenia (≤ 100 × 103/ml) to determine whether those predefined thresholds influenced our results.
RESULTS
Inpatient cohort characteristics and observed data
A total of 83 sirolimus blood concentrations were available from 27 adult renal transplant patients who were admitted to a Duke University Health System hospital during the study period (Table 1). On average, patients in our cohort were 49 months post-transplant (median [range] 34 months [0-202]), and the median sirolimus dose was 2 mg/day (range [1, 6]) for observed concentrations (Table 1). We identified a wide range of concentrations collected during the study period (median [range] 7.9 ng/mL [2.5-64.4]); however, 3 concentrations were below the limit of quantification and not included in the analyses. Only 2 inpatients were also taking ≥1 of the CYP3A4/5 interacting drugs of interest during the study period. Based on recommended sirolimus concentrations according to time since transplant, 9 patients (15 [18%] concentrations) had observed concentrations within the goal range. Twenty-four patients (61 (73%) concentrations) were below the goal range, and 5 patients (7 (8%) concentrations) were above the goal range. Only 1 patient had all concentrations within the goal range; 20 patients had all concentrations below or within the goal concentration range; the remaining 6 patients each had some concentrations below, within, and above the target range.
Table 1.
Inpatient demographics
| Variable | Median (range) or N (%) |
|---|---|
| N | 27 |
| Age (years) | 56 (31 - 67) |
| Body weight (kg) | 74 (51 - 124) |
| Female | 12 (44) |
| Race | |
| White | 16 (59) |
| Black or African American | 10 (37) |
| Unknown or not reported | 1 (4) |
| Sirolimus dose (mg/day) | 2 (1, 6) |
| Time post-transplant (months) | 34 (0-202) |
| Hematocrit | 0.30 (0.22-0.44) |
| Platelet count (× 103/ml) | 179 (39-570) |
| White blood cell count (× 103/ml) | 7.2 (1.2-22.3) |
| Serum creatinine (mg/dl) | 2.0 (0.7-9.0) |
Outpatient cohort characteristics and observed data
During the study period, a total of 513 sirolimus concentrations were available from 96 patients seen in a Duke University Health System outpatient clinic, and the median [range] time since transplant was 59 months [1-237]. Based on recommended sirolimus concentrations according to time since transplant, 29 patients (47 (9%) concentrations) were within the goal range, 75 patients (434 (84%) concentrations) were below the goal range, and 8 patients (8 (2%) concentrations) were above the goal range. Twenty-four (5%) observed concentrations (from 16 patients) were below the limit of quantification and were not included in further analyses.
Population PK model development
Based on visual inspection of PK data and review of the literature, we chose a two-compartment model with first-order absorption and elimination. Because few samples from our population were obtained within the first 2-3 hours after dose, thereby precluding characterizing the absorption phase following drug administration, we used the parameter estimates of the absorption rate constant (2.18 h−1) and lag time (0.24) from a previous model and fixed them in our model.17 The model adequately described the observed sirolimus concentrations from the inpatient cohort. On preliminary scatter plots, BSV in clearance appeared to be influenced by covariates, age and body weight. During the univariable covariate screen for clearance, we observed a statistically significant drop in OFV with the age covariate (see Table, Supplemental Digital Content 1, which summarizes the covariate model building process for sirolimus). The goodness of fit also improved after we included age in the final model. Scatter plots suggested no statistically significant correlations between covariates and volume of distribution.
Population PK model evaluation
The final model parameter estimates had good precision (Table 2). The relative standard errors around the parameter point estimates were 9 to 39%. The median bootstrap estimates were within 5% of original population estimates for all parameters. The 2.5th and 97.5th percentiles were narrow for all the parameters except BOV, which had a wider range. There were no obvious trends or model misspecification identified in the goodness of fit diagnostic plots for the final model (see Figure, Supplemental Digital Content 2, which expresses the final population PK model diagnostic plots). The standardized visual predictive check revealed a good fit of the observed sirolimus concentrations, as evidenced by the uniform distribution of calculated observation percentiles for each time point and only 9.8% of observed concentrations outside of the 90% prediction interval.
Table 2.
Parameter estimates for the final sirolimus population PK model
| Parameter | Estimate | RSE (%) |
Parameter estimates reported in the literaturea,b |
|---|---|---|---|
| Structural Model | |||
| Apparent oral clearance (L/h)c | 7.4 | 9 | 14.1 24; 8.91 17 |
| Central volume of distribution (L) | 128 | 29 | 219 24; 112.9 17 |
| Apparent distributional clearance (L/h) | 27.8 | 39 | 37.8 24 |
| Peripheral volume of distribution (L) | 278 | 29 | 297 24; 45217 |
| Exponent of age effect on clearancec | −1.02 | 28 | |
| Absorption rate constant (1/h) | 2.18d | - | 2.18 17 |
| Absorption lag time (h) | 0.24d | - | 0.24 17 |
| Between subject variability (%CV) | |||
| Apparent oral clearance (L/h) | 22.7 | 32 | 23.8, 22; 13.2 17; 48.424 |
| Between-occasion Variability (%CV) | |||
| Apparent oral clearance (L/h) | 26.1 | 36 | |
| Residual Variability (%) | |||
| Proportional error | 33.8 | 14 | |
Values from reference 18 are presented as mean values
Renal transplant patients
Apparent oral clearance (L/h) = (Individual age in years/Median age of the population)
Parameter values for absorption rate constant and lag time were obtained from a previously published model and fixed in our model
Relationship between observed or simulated sirolimus exposures and efficacy or AEs among inpatients and outpatients
Our analyses identified 2 notable findings in the inpatient population. Graft loss or rejection was more common among those with simulated trough concentrations above goal compared to those with simulated trough concentrations below goal (coeff [95% confidence interval]: 0.32 [0.16, 0.48]). Decline in renal function was more common among those with simulated peak concentrations at the recommended trough goal compared to peak concentrations below the goal range (0.11 [0.04, 0.18]). Initially, we observed statistically significant associations between sirolimus concentrations and anemia (p=0.017 for observed concentrations) or thrombocytopenia (0.029 for observed, 0.040 for simulated trough, 0.002 for simulated average concentrations); however, the associations were no longer statistically significant when we further evaluated these relationships with sensitivity testing using lower thresholds for classification of anemia and thrombocytopenia. We did not identify other statistically significant associations between the distribution of AEs and drug concentrations. We found no statistically significant associations between concentrations and AEs among outpatients.
DISCUSSION
Our combined method proved useful in the development of a population PK model using EHR data, simulation of drug exposure, and determination of some exposure-AE relationships in our population. Our PK model provided reasonable parameter estimates of volume of distribution and clearance compared to those in the literature. Observed differences between our parameter estimate for volume of distribution and existing estimates, may be related to unmeasured differences in population characteristics, including: 1) administration with food and food content, 2) administered drug formulation, and 3) body fat composition, given the high partition coefficient of sirolimus that increases the likelihood of a higher volume of distribution with high body fat composition.18-21 Differences in apparent oral clearance between our population and others may be related to variability in cyclosporine use and exposure, differences in extent of CYP3A4 and CYP3A5 expression, other disease states (e.g., liver disease and cancer), and other drug interactions.18,19,22,23 Unlike other studies that have developed population PK models, we identified age as an important covariate for apparent clearance. This finding may reflect the relatively more advanced median age of patients in our study (59, range 29 to 72 compared to 48.6, range 20 to 69), and potential influences of age on CYP3A4 activity.17,24,25
We successfully used our model to simulate drug exposures not generally identified with routine therapeutic drug monitoring. While the majority of patients in our cohort were without adverse event despite concentrations below goal range, our combined methodology allowed us to identify increased frequency of graft loss or rejection among those with simulated trough concentrations above compared to those at goal. Given the average time since transplant of patients in our cohort, we suspect the observed relationship between elevated concentrations and the composite efficacy outcome, reflects patients with graft dysfunction due to chronic allograft nephropathy who may have other reasons for poor drug clearance (i.e., liver dysfunction). Alternatively, such patients may have recently transitioned from a calcineurin inhibitor to sirolimus in order to attempt graft rescue or to minimize additional nephrotoxicity, but have not yet achieved target dosing.26-28
We also identified a relationship between decline in renal function and peak sirolimus concentrations within recommended trough goals. The association between inadequate immunosuppression and decline in renal function for renal transplant patients is well documented and often represents graft dysfunction secondary to rejection.29-31 Conversely, a decline in renal function associated with high sirolimus concentrations may represent drug toxicity.32 Although previous studies have documented limited renal toxicity associated with sirolimus, few have evaluated toxicity with sirolimus concentrations or determined the effect of sirolimus independent of cyclosporine.32,33 Our observed association between peak concentrations and decline in renal function is a unique finding in our data. Toxicity of immunosuppressants and other drugs (e.g., antibiotics) is usually attributed to: 1) cumulative exposures, 2) average exposures, or 3) area under the concentration time curves.34,35 Although many potential reasons exist for renal dysfunction in hospitalized adult patients, a true relationship between peak sirolimus concentrations and nephrotoxicity, particularly in patients in the late transplant phase, could impact the post-dose timing of therapeutic drug monitoring.
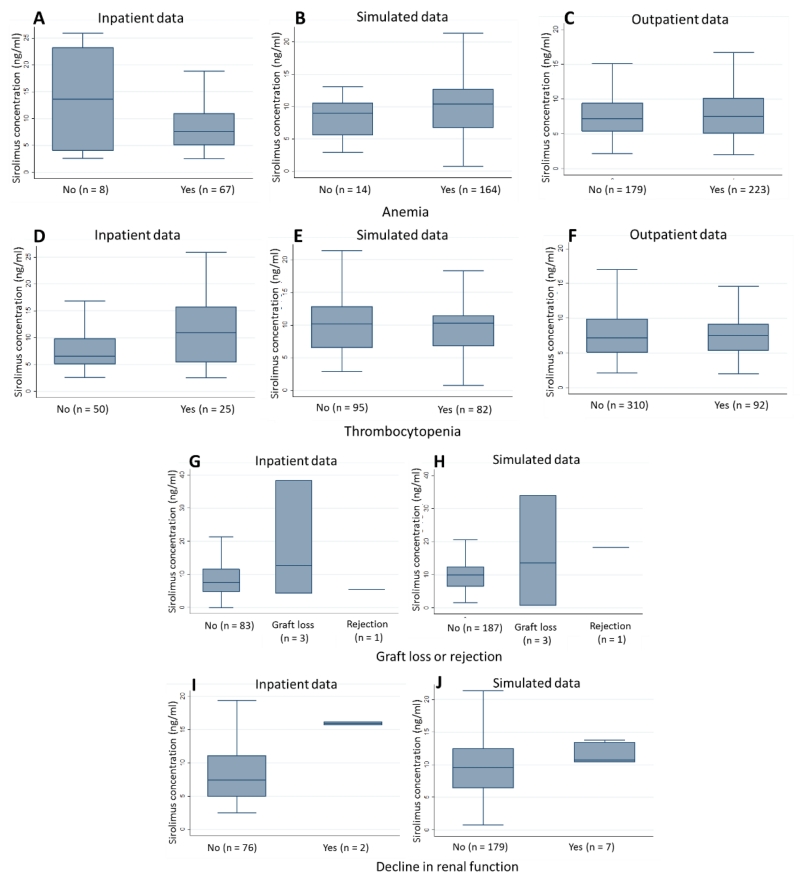
Although useful in some aspects, our combined methodology was limited in its ability to fully characterize the exposure-AE relationship for all AEs of interest. For example, application of our method to hematologic AEs likely produced spurious results. We only observed relationships in the inpatient population with a relatively small sample size and numerous other reasons for thrombocytopenia and anemia (Figure1); results were no longer present with sensitivity testing; and the directionality of results were contradictory and appeared biologically implausible, or confounded by other clinical factors. Further, much of the existing evidence suggests that hematologic abnormalities are most commonly present in the first 2 weeks after transplant and would be much less likely in our late-transplant population.36 Our use of electronic health records also limited our ability to identify other potential AEs such as gingival hyperplasia or evaluate other likely relevant covariates (e.g., serum albumin) in our PK model.
Figure 1.
Boxplots of sirolimus exposure in patients with and without anemia from inpatient data (A), simulated data (B), and outpatient data (C). Boxplots of sirolimus exposure in patients with and without thrombocytopenia from inpatient data (D), simulated data (E), and outpatient data (F). Boxplots of sirolimus exposure in patients with and without graft loss or rejection from inpatient data (G) and simulated data (H). Boxplots of sirolimus exposure in patients with and without decline in renal function from inpatient data (I) and simulated data (J). Sirolimus exposure was defined as measured sirolimus concentrations on the day of AEs in inpatient and outpatient data. Sirolimus exposure was defined as average sirolimus concentration on the day of AE in simulated data.
Estimation of the sirolimus exposure-response relationships for all known sirolimus AEs was likely limited by our study population. Although reflective of prescribing practice for sirolimus at our hospital during the specified time period, our sample size was small and mostly >1 year post transplant. These factors likely decreased the propensity to observe AEs, limited our ability to evaluate effects of interacting drugs in our PK model, and limited the number of patients with efficacy failure. Second, our study does not account for trends over time in sirolimus exposure that may be more definitively associated with AEs. Next, we targeted trough concentrations identified in the product label and literature for patients not receiving concomitant immunosuppression; goal concentrations used in real-world practice are often dependent upon both the time since transplant and the baseline risk for rejection of the individual patient.(3) Based on retrospective review, we were unable to identify individual patient risk status. Given the limited number of patients who had a sirolimus concentration and were therefore included in our cohort, our population may be biased towards patients suspected to have sirolimus concentrations out of the targeted range. Further, the goals for peak and average sirolimus concentrations have not previously been established; comparison of these values to trough values allowed a consistent point of reference, but may not reflect the most relevant point of reference for peak and average concentrations, given variable effects of drug PK on each of these values.
CONCLUSIONS
A combined approach using therapeutic drug monitoring and other data from the EHR, and PK modeling identified important exposure-AE relationships and revealed that the majority of patients did well with concentrations below goal range, but was limited in its ability to characterize all exposure-response relationships. If applied to a larger cohort who is at high risk for drug toxicity and efficacy failure, our combined approach may prove to be an efficient and inexpensive way to establish the therapeutic index of drugs, particularly when prospective data is limited.
Supplementary Material
ACKNOWLEDGEMENTS
Funding for this manuscript was made possible, in part, by the Food and Drug Administration through grant 1U01FD004858-01. Views expressed in written materials or publications and by speakers and moderators do not necessarily reflect the official policies of the Department of Health and Human Services; nor does any mention of trade names, commercial practices, or organization imply endorsement by the United States Government.
Source of Funding: K.Z. is funded by KL2TR001115-03 from the Duke Clinical and Translational Science Awards and by the nonprofit Thrasher Research Fund (www.thrasherresearch.org). K.D.H. is funded by KL2TR001115-03 from the Duke Clinical and Translational Science Awards, by the nonprofit Mend a Heart Foundation and by the Gilead Sciences Cardiovascular Scholars program. LK is funded by 5T32HD043029-13. D.G. is funded by K23HD083465 from the National Institute for Child Health and Human Development (NICHD) and by the nonprofit Thrasher Research Fund (www.thrasherresearch.org). C.P.H. receives salary support for research from the National Center for Advancing Translational Sciences of the National Institutes of Health (NIH) (UL1TR001117). C.M. receives support for research from the NICHD (HHSN275201000003I), the FDA (3U01FD004858-01), and from industry for drug development in adults (https://dcri.org/about-us/conflict-of-interest). M.C.W. receives support for research from the NIH (1R01-HD076676-01A1), the National Center for Advancing Translational Sciences of the NIH (UL1TR001117), the NIAID (HHSN272201500006I and HHSN272201300017I), the NICHD (HHSN275201000003I), the FDA (1U01FD004858-01), the Biomedical Advanced Research and Development Authority (HHSO100201300009C), the nonprofit Thrasher Research Fund (www.thrasherresearch.org), and from industry for drug development in adults and children (www.dcri.duke.edu/research/coi.jsp).
Footnotes
Conflicts of Interest
For the remaining authors none were declared.
REFERENCES
- 1.Report of the Committee for the Assessment of Biometric Aspects of Controlled Trials of Hypoglycemic Agents. JAMA. 1975;231:583–608. [PubMed] [Google Scholar]
- 2.Kuypers DR. Benefit-risk assessment of sirolimus in renal transplantation. Drug Saf. 2005;28(2):153–181. doi: 10.2165/00002018-200528020-00006. [DOI] [PubMed] [Google Scholar]
- 3. [Accessed August 1, 2006];EuroQol Web. 2006 http://gs1.q4matics.com/EuroqolPublishWeb/
- 4.Kahan BD, Napoli KL, Kelly PA, et al. Therapeutic drug monitoring of sirolimus: correlations with efficacy and toxicity. Clin Transplant. 2000 Apr;14(2):97–109. doi: 10.1034/j.1399-0012.2000.140201.x. [DOI] [PubMed] [Google Scholar]
- 5.Kelly PA, Napoli K, Kahan BD. Conversion from liquid to solid rapamycin formulations in stable renal allograft transplant recipients. Biopharmaceutics & drug disposition. 1999 Jul;20(5):249–253. doi: 10.1002/(sici)1099-081x(199907)20:5<249::aid-bdd181>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 6.MacDonald A, Scarola J, Burke JT, Zimmerman JJ. Clinical pharmacokinetics and therapeutic drug monitoring of sirolimus. Clin Ther. 2000;22(Suppl B):B101–121. doi: 10.1016/s0149-2918(00)89027-x. [DOI] [PubMed] [Google Scholar]
- 7.MacDonald AS. A worldwide, phase III, randomized, controlled, safety and efficacy study of a sirolimus/cyclosporine regimen for prevention of acute rejection in recipients of primary mismatched renal allografts. Transplantation. 2001 Jan 27;71(2):271–280. doi: 10.1097/00007890-200101270-00019. [DOI] [PubMed] [Google Scholar]
- 8.Kahan BD, Julian BA, Pescovitz MD, Vanrenterghem Y, Neylan J, Rapamune Study Group Sirolimus reduces the incidence of acute rejection episodes despite lower cyclosporine doses in caucasian recipients of mismatched primary renal allografts: a phase II trial. Transplantation. 1999 Nov 27;68(10):1526–1532. doi: 10.1097/00007890-199911270-00016. [DOI] [PubMed] [Google Scholar]
- 9.Vitko S, Wlodarczyk Z, Kyllonen L, et al. Tacrolimus combined with two different dosages of sirolimus in kidney transplantation: results of a multicenter study. Am J Transplant. 2006 Mar;6(3):531–538. doi: 10.1111/j.1600-6143.2005.01193.x. [DOI] [PubMed] [Google Scholar]
- 10.Lo A, Egidi MF, Gaber LW, et al. Observations regarding the use of sirolimus and tacrolimus in high-risk cadaveric renal transplantation. Clin Transplant. 2004 Feb;18(1):53–61. doi: 10.1111/j.1399-0012.2004.00116.x. [DOI] [PubMed] [Google Scholar]
- 11.Lee HS, Huh KH, Kim YS, et al. Sirolimus-induced pneumonitis after renal transplantation: a single-center experience. Transplantation proceedings. 2012 Jan;44(1):161–163. doi: 10.1016/j.transproceed.2011.11.059. [DOI] [PubMed] [Google Scholar]
- 12.Weiner SM, Sellin L, Vonend O, et al. Pneumonitis associated with sirolimus: clinical characteristics, risk factors and outcome--a single-centre experience and review of the literature. Nephrol Dial Transplant. 2007 Dec;22(12):3631–3637. doi: 10.1093/ndt/gfm420. [DOI] [PubMed] [Google Scholar]
- 13.Keizer RJ, van Benten M, Beijnen JH, Schellens JH, Huitema AD. Pirana and PCluster: a modeling environment and cluster infrastructure for NONMEM. Computer methods and programs in biomedicine. 2011 Jan;101(1):72–79. doi: 10.1016/j.cmpb.2010.04.018. [DOI] [PubMed] [Google Scholar]
- 14.Lindbom L, Ribbing J, Jonsson EN. Perl-speaks-NONMEM (PsN)--a Perl module for NONMEM related programming. Computer methods and programs in biomedicine. 2004 Aug;75(2):85–94. doi: 10.1016/j.cmpb.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 15.Jonsson EN, Karlsson MO. Xpose--an S-PLUS based population pharmacokinetic/pharmacodynamic model building aid for NONMEM. Computer methods and programs in biomedicine. 1999 Jan;58(1):51–64. doi: 10.1016/s0169-2607(98)00067-4. [DOI] [PubMed] [Google Scholar]
- 16.Wang DD, Zhang S. Standardized visual predictive check versus visual predictive check for model evaluation. Journal of clinical pharmacology. 2012 Jan;52(1):39–54. doi: 10.1177/0091270010390040. [DOI] [PubMed] [Google Scholar]
- 17.Ferron GM, Mishina EV, Zimmerman JJ, Jusko WJ. Population pharmacokinetics of sirolimus in kidney transplant patients. Clin Pharmacol Ther. 1997 Apr;61(4):416–428. doi: 10.1016/S0009-9236(97)90192-2. [DOI] [PubMed] [Google Scholar]
- 18.Zimmerman JJ, Ferron GM, Lim HK, Parker V. The effect of a high-fat meal on the oral bioavailability of the immunosuppressant sirolimus (rapamycin) Journal of clinical pharmacology. 1999 Nov;39(11):1155–1161. [PubMed] [Google Scholar]
- 19.Zimmerman JJ. Exposure-response relationships and drug interactions of sirolimus. AAPS J. 2004;6(4):e28. doi: 10.1208/aapsj060428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yee GC. Recent advances in cyclosporine pharmacokinetics. Pharmacotherapy. 1991;11(5):130S–134S. [PubMed] [Google Scholar]
- 21.Yatscoff R, LeGatt D, Keenan R, Chackowsky P. Blood distribution of rapamycin. Transplantation. 1993 Nov;56(5):1202–1206. doi: 10.1097/00007890-199311000-00029. [DOI] [PubMed] [Google Scholar]
- 22.Jiao Z, Shi XJ, Li ZD, Zhong MK. Population pharmacokinetics of sirolimus in de novo Chinese adult renal transplant patients. Br J Clin Pharmacol. 2009 Jul;68(1):47–60. doi: 10.1111/j.1365-2125.2009.03392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu K, Cohen EE, House LK, et al. Nonlinear population pharmacokinetics of sirolimus in patients with advanced cancer. CPT Pharmacometrics Syst Pharmacol. 1:e17. doi: 10.1038/psp.2012.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Djebli N, Rousseau A, Hoizey G, et al. Sirolimus population pharmacokinetic/pharmacogenetic analysis and bayesian modelling in kidney transplant recipients. Clinical pharmacokinetics. 2006;45(11):1135–1148. doi: 10.2165/00003088-200645110-00007. [DOI] [PubMed] [Google Scholar]
- 25.George J, Byth K, Farrell GC. Age but not gender selectively affects expression of individual cytochrome P450 proteins in human liver. Biochemical pharmacology. 1995 Aug 25;50(5):727–730. doi: 10.1016/0006-2952(95)00192-3. [DOI] [PubMed] [Google Scholar]
- 26.Diekmann F, Campistol JM. Conversion from calcineurin inhibitors to sirolimus in chronic allograft nephropathy: benefits and risks. Nephrol Dial Transplant. 2006 Mar;21(3):562–568. doi: 10.1093/ndt/gfi336. [DOI] [PubMed] [Google Scholar]
- 27.Sayin B, Karakayali H, Colak T, et al. Conversion to sirolimus for chronic allograft nephropathy and calcineurin inhibitor toxicity and the adverse effects of sirolimus after conversion. Transplant Proc. 2009 Sep;41(7):2789–2793. doi: 10.1016/j.transproceed.2009.07.094. [DOI] [PubMed] [Google Scholar]
- 28.Yates PJ, Nicholson ML. The aetiology and pathogenesis of chronic allograft nephropathy. Transpl Immunol. 2006 Nov;16(3-4):148–157. doi: 10.1016/j.trim.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 29.De Geest S, Borgermans L, Gemoets H, et al. Incidence, determinants, and consequences of subclinical noncompliance with immunosuppressive therapy in renal transplant recipients. Transplantation. 1995 Feb 15;59(3):340–347. [PubMed] [Google Scholar]
- 30.Kahan BD, Welsh M, Schoenberg L, et al. Variable oral absorption of cyclosporine. A biopharmaceutical risk factor for chronic renal allograft rejection. Transplantation. 1996 Sep 15;62(5):599–606. doi: 10.1097/00007890-199609150-00010. [DOI] [PubMed] [Google Scholar]
- 31.Johnson EM, Canafax DM, Gillingham KJ, et al. Effect of early cyclosporine levels on kidney allograft rejection. Clin Transplant. 1997 Dec;11(6):552–557. [PubMed] [Google Scholar]
- 32.Letavernier E, Bruneval P, Mandet C, et al. High sirolimus levels may induce focal segmental glomerulosclerosis de novo. Clin J Am Soc Nephrol. 2007 Mar;2(2):326–333. doi: 10.2215/CJN.03751106. [DOI] [PubMed] [Google Scholar]
- 33.Saunders RN, Metcalfe MS, Nicholson ML. Rapamycin in transplantation: a review of the evidence. Kidney Int. 2001 Jan;59(1):3–16. doi: 10.1046/j.1523-1755.2001.00460.x. [DOI] [PubMed] [Google Scholar]
- 34.Swartling M, Gupta R, Dudas V, Guglielmo BJ. Short term impact of guidelines on vancomycin dosing and therapeutic drug monitoring. Int J Clin Pharm. 2012 Apr;34(2):282–285. doi: 10.1007/s11096-012-9614-6. [DOI] [PubMed] [Google Scholar]
- 35.Schiff J, Cole E, Cantarovich M. Therapeutic monitoring of calcineurin inhibitors for the nephrologist. Clin J Am Soc Nephrol. 2007 Mar;2(2):374–384. doi: 10.2215/CJN.03791106. [DOI] [PubMed] [Google Scholar]
- 36.Murgia MG, Jordan S, Kahan BD. The side effect profile of sirolimus: a phase I study in quiescent cyclosporine-prednisone-treated renal transplant patients. Kidney Int. 1996 Jan;49(1):209–216. doi: 10.1038/ki.1996.28. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.